Abstract
Activation of group II metabotropic glutamate receptors (mGlu2 and mGlu3) has been implicated as a potential therapeutic strategy for treating both motor symptoms and progressive neurodegeneration in Parkinson's disease (PD). Modulation of excitatory transmission in the basal ganglia represents a possible mechanism by which group II mGlu agonists could exert antiparkinsonian effects. Previous studies have identified reversible effects of mGlu2/3 activation on excitatory transmission at various synapses in the basal ganglia, including the excitatory synapse between the subthalamic nucleus (STN) and the substantia nigra pars reticulata (SNr). Using whole-cell patch clamp studies of GABAergic SNr neurons in rat midbrain slices, we have found that a prolonged activation of group II mGlus by the selective agonist LY379268 induces a long-term depression (LTD) of evoked excitatory postsynaptic current (EPSC) amplitude. Bath application of LY379268 (100 nM, 10 minutes) induced a marked reduction in EPSC amplitude, and excitatory transmission remained depressed for at least 40 minutes after agonist washout. The effect of LY379268 was concentration-dependent and was completely blocked by the group II mGlu-preferring antagonist LY341495 (500 nM). To determine the relative contributions of mGlu2 and mGlu3 to the LTD induced by LY379268, we tested the ability of LY379268 (100 nM) to induce LTD in wild type mice and mice lacking mGlu2 or mGlu3. LY379268 induced similar LTD in wild type mice and mGlu3 knockout mice, whereas LTD was absent in mGlu2 knockout mice, indicating that mGlu2 activation is necessary for the induction of LTD in the SNr. These studies suggest a novel role for mGlu2 in the long-term regulation of excitatory transmission in the SNr and invite further exploration of mGlu2 as a therapeutic target for treating the motor symptoms of PD.
Keywords: metabotropic glutamate receptor, substantia nigra pars reticulata, long-term depression, synaptic plasticity, basal ganglia, Parkinson's disease
Introduction
The basal ganglia are a highly interconnected group of subcortical nuclei that play important roles in movement and multiple types of learning. Pathological disruption of neurotransmission in the basal ganglia leads to several disorders of the central nervous system, including Parkinson's disease (PD) and other movement disorders. PD is a chronic neurodegenerative disorder that is characterized by primary motor symptoms including bradykinesia, tremor, rigidity, and postural instability [12]. The primary pathology giving rise to motor impairments in PD patients is degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc). Loss of dopaminergic innervation of the striatum as well as extrastriatal basal ganglia structures causes major physiological disruptions in the basal ganglia-thalamocortical motor circuitry. Dopamine replacement therapies, including the dopamine precursor L-DOPA and dopamine receptor agonists, are the primary pharmacological strategies used for symptomatic treatment of parkinsonian motor symptoms [5]. While these treatments alleviate motor symptoms for several years in most patients, the eventual development of motor complications, psychiatric side effects, and end-of-dose “wearing off” of the drug effects limits their long-term efficacy in most PD patients. These drawbacks highlight the need for nondopaminergic therapeutic strategies for the management of the motor symptoms of PD.
Recent studies suggest that the loss of dopaminergic modulation of striatal activity in PD patients leads to increased inhibitory tone at the striatopallidal synapse in the indirect pathway of the basal ganglia [9]. This leads to disinhibition of the subthalamic nucleus (STN), changes in STN firing patterns, and an increase in excitatory drive from the STN to the basal ganglia output nuclei, which include the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr, the major basal ganglia output nucleus in rodents) [3, 18, 21, 22, 30]. Because aberrant glutamatergic transmission is associated with pathological alterations in basal ganglia function, glutamate receptors have been suggested as promising therapeutic targets for treating PD [13]. A family of G protein-coupled glutamate receptors termed metabotropic glutamate receptors (mGlus) are expressed throughout the basal ganglia and play important roles in regulating basal ganglia function [6]. The mGlus include 8 subtypes (mGlu1–mGlu8) that have been divided into three major groups based on sequence homology, second messenger coupling, and pharmacological profiles [23]. Group I mGlus (mGlu1 and mGlu5) are often postsynaptically localized, whereas group II (mGlu2 and mGlu3) and group III mGlus (mGlus 4, 6, 7, and 8) are often expressed on presynaptic terminals where they regulate neurotransmitter release. Activation of group II and group III mGlus has been implicated as a therapeutic strategy for PD due to their potential to reduce neurotransmitter release at pathologically overactive synapses such as the excitatory synapse between the STN and the SNr [4, 13, 31].
Anatomical studies using group II mGlu-specific antibodies have demonstrated that group II mGlus are present on presynaptic glutamatergic axon terminals in the SNr [4]. We have previously shown that brief application of group II mGlu agonists reversibly reduces the amplitude of synaptically evoked excitatory postsynaptic currents (EPSCs) recorded from GABAergic SNr neurons [4]. The antibodies and group II mGlu agonists used in these studies did not distinguish between mGlu2 and mGlu3; therefore, it is not known if one or both group II mGlu subtypes are required to regulate excitatory transmission in the SNr. In addition, the effects of prolonged activation of group II mGlus at this synapse, which may occur under both normal and pathological conditions, have not been fully elucidated.
Methods
To further explore these questions, we examined the regulation of synaptic transmission in Sprague-Dawley rats (14-18 days old, Charles River, Wilmington, MA) and mGlu2 and mGlu3 knockout mice and wild type littermates (21-30 days old, bred by Taconic Farms, Cambridge City, IN) [17]. Animals were housed under a 12 hour light/dark cycle (lights on at 6 AM to 6 PM) with free access to food and water in accordance with American Association for the Accreditation of Laboratory Animal Care guidelines. Procedures were approved by the Institutional Animal Care and Use Committee of Vanderbilt University and conformed to the guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals. On the morning of the study, animals were decapitated under isoflurane anesthesia and brains were rapidly removed and submerged into ice-cold cutting solution (in mM: sucrose, 187; KCl, 3; CaCl2, 2; MgSO4, 1.9; KH2PO4, 1.2; glucose, 20; NaHCO3, 26; equilibrated with 95% O2/5% CO2). Parasaggital midbrain slices 275-300 μm thick were prepared using a Vibratome (Vibratome 3000, St. Louis, MO) and then transferred to artificial cerebrospinal fluid (ACSF; in mM: NaCl, 124; KCl, 2.5; MgSO4,1.3; NaH2PO4,1.0; CaCl2, 2.0; glucose, 20; NaHCO3, 26; glutathione, 0.005, and sodium pyruvate, 0.5; equilibrated with 95% O2/5% CO2) and allowed to recover at 32°C for 30 minutes. Slices were then incubated at room temperature in ACSF for at least 30 minutes prior to recordings.
During recordings, slices were submerged in a brain slice chamber and perfused with oxygenated room temperature ACSF at a rate of 1.5-2 ml/min, and glutathione and sodium pyruvate were excluded from perfusate. Neurons were visualized using an Olympus BX51WI upright microscope (Olympus, Lake Success, NY) coupled with a 40× water immersion objective and Hoffman optics. Borosilicate glass pipettes were pulled using a Flaming/Brown micropipette puller (model P-97; Sutter Instruments, Novato, CA) to produce patch electrode resistances of 3.0-7.0 MΩ when filled with intracellular solution (in mM: potassium gluconate, 140; HEPES, 10: NaCl, 10; EGTA, 0.6; GTP, 0.2; and ATP, 2; pH adjusted to 7.4 with 0.5N KOH). Whole cell patch clamp recordings were made from putative GABAergic neurons in the SNr using a Warner 505B amplifier (Warner Instruments) or an Axon Multiclamp 700B amplifier (Molecular Devices). GABAergic projection neurons were identified according to previously established electrophysiological characteristics [26]. EPSCs were recorded at a holding potential of −60 mV (unless otherwise noted) and evoked every 10 seconds using a concentric bipolar tungsten electrode placed in the cerebral peduncle rostral to and outside of the SNr. Experiments were performed in the presence of (-)-bicuculine methobromide (20 μM, Tocris Bioscience, Ellisville, MO or Ascent Scientific, Weston-SuperMare, UK) to block GABAA-mediated inhibitory currents unless otherwise noted. The voltage-clamp signal was low-pass-filtered at 1 kHz, digitized at 10 kHz, and acquired using a Clampex9.2/DigiData 1332 system (Molecular Devices, Sunnyvale, CA). Holding current, input resistance, and access resistance were monitored throughout all experiments. Stable baseline EPSC amplitudes were recorded for at least 5 minutes prior to bath application of drugs. (1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) was generously provided by Dr. James Monn (Eli Lilly, Indianapolis, IN). (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495) was purchased from Tocris Bioscience. All drugs were prepared as frozen stocks and diluted in ACSF immediately prior to the experiment.
All data analysis was performed using Clampfit software (v9.2, Molecular Devices, Sunnyvale, DA), GraphPad Prism (GraphPad Software Inc, San Diego, CA), and Excel (Microsoft Corp., Redmond, WA). EPSC amplitudes were averaged every 6 sweeps and normalized to the average of the baseline EPSC amplitudes. Data are expressed as mean ± SEM. Statistical comparisons were performed using a Student's unpaired t-test with significance level set to p < 0.05.
Results
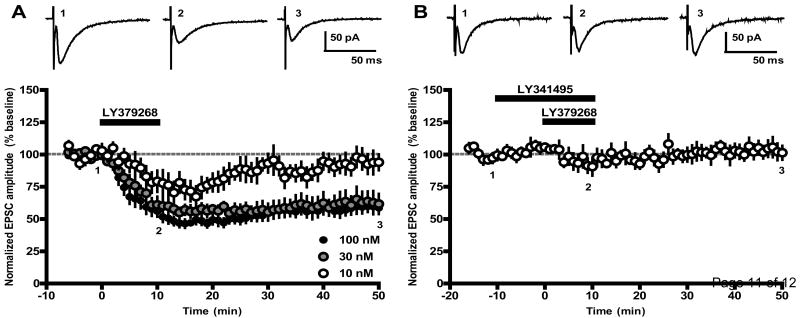
After stable baseline measurement, LY379268 (10, 30, or 100 nM) was bath applied to slices for 10 minutes, and recordings were continued for at least 40 minutes after drug washout. LY379268 (100 nM) induced a robust, long-term depression (LTD) of EPSC amplitudes (58.7 ± 4.5% of baseline 40 minutes after LY379268 washout, n = 14; Fig. 1A, black circles). A similar level of depression was observed after application of 30 nM LY379268 (61.7 ± 7.8% of baseline 40 minutes after LY379268 washout, n = 7; Fig. 1A, gray circles). In contrast, 10 nM LY379268 produced a slightly less robust acute depression of EPSC amplitudes that peaked 17 minutes after onset of LY379268 application (67.3 ± 8.1% of baseline) but failed to induce LTD (94.0 ± 6.1% of baseline 40 minutes after LY379268 washout, n = 6; Fig. 1A, open circles). LY379268 did not alter holding current or input resistance at any concentration tested (data not shown).
Fig 1.
Activation of group II mGlus induces long-term depression of excitatory transmission in the SNr. (A) Time course of the EPSC amplitude following 10 minute bath application of LY379268 (10-100 nM) shows that induction of LTD by LY379268 is concentration-dependent. Data represent mean ± SEM for 6-14 cells per drug treatment. Representative sample traces from a single experiment with 100nM LY379268 were taken from time points indicated by numbers and are the average of 6 individual traces. (B) Pre-incubation of slices with the mGlu2/3 antagonist LY341495 (500 nM, 10 minutes prior to LY379268 application) blocks induction of LTD (n = 5).
To ensure that the effects of LY379268 were mediated by group II mGlus, slices were pretreated with the group II mGlu-preferring antagonist LY341495 (500 nM) 10 minutes prior to application of LY379268 (100 nM). LY341495 completely blocked the ability of LY379268 to reduce EPSC amplitudes (101.4 ± 5.4% of baseline 40 minutes after drug washout, n = 5; Fig. 1B), confirming that the LTD induced by LY379268 depends upon group II mGlu activation.
Because inhibitory transmission mediated by GABA is a critical determinant of basal ganglia output, we performed an additional experiment in the absence of the GABAA receptor antagonist bicuculine to ensure that group II mGluR activation is able to induce LTD in the presence of normal GABA transmission. In order to accurately measure EPSC amplitudes in the absence of bicuculine, we voltage-clamped the recorded cells near the reversal potential of chloride (-75 mV). Similar to results obtained in the presence of bicuculine, we found that a 10 minute bath application of LY379268 (100 nM) induced robust LTD in all recorded neurons (46.8 ± 11.4% of baseline 40 minutes after LY379268 washout, n = 3; data not shown).
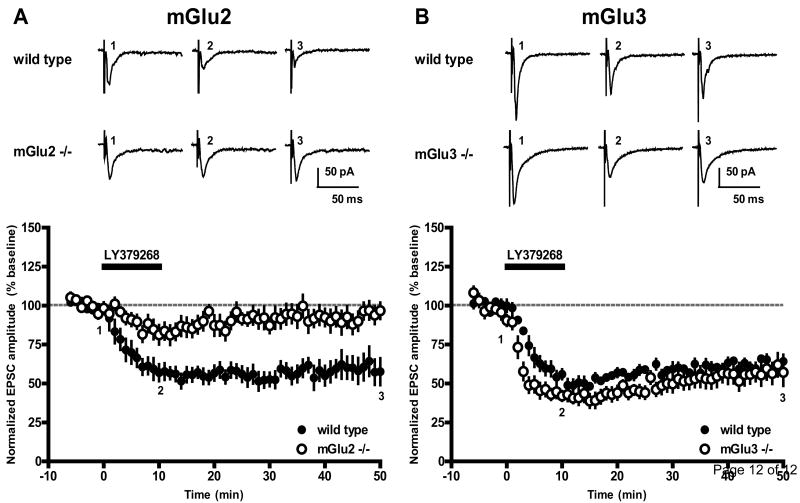
Due to a lack of antagonists that distinguish between mGlu2 and mGlu3, we employed mGlu2 and mGlu3 knockout mice to determine which of the receptor subtypes mediates LY379268-induced LTD. In slices obtained from wild type littermate controls, LY379268 (100 nM, 10 minutes) induced LTD that was not significantly different from the depression observed in slices obtained from rats (57.6 ± 8.7% of baseline 40 minutes after LY379268 washout for mGlu2 control animals, n = 7, vs. 58.7 ± 4.5% for rats, n = 14, p = 0.90; 64.2 ± 4.4% of baseline 40 minutes after LY379268 washout for mGlu3 control animals, n = 7, vs. 58.7 ± 4.5% for rats, n = 14, p = 0.45; Fig. 2A and 2B, closed circles). Likewise, no difference in the magnitude of depression of EPSC amplitude was observed between the two wild type control groups (p = 0.49). In mice lacking mGlu2, LY379268 failed to induce LTD (96.7 ± 5.3% of baseline 40 minutes after LY379268 washout, n = 8, vs. 57.6 ± 8.7% for wild type controls, n = 7, p = 0.002; Fig. 2A, open circles). Interestingly, a small reversible depression was observed in slices obtained from mGlu2 knockout mice (81.0 ± 6.3% of baseline 12 minutes after onset of LY379268 application). This depression may be mediated by mGlu3 activation, or may be due to unknown off-target activity. LY379268-induced LTD was unaffected in slices obtained from mice lacking mGlu3 (57.2 ± 8.7% of baseline 40 minutes after LY379268 washout, n = 6, vs. 64.2 ± 4.4% for wild type littermate controls, n = 7, p = 0.47; Fig. 2B, open circles). However, a moderate increase in the rate of EPSC amplitude depression was observed in slices obtained from mGlu3 knockout mice when compared with wild type littermate controls (p < 0.05 for minutes 2-4, 6, 8, and 10 of LY379268 application). This finding may suggest that in slices from wild type animals, mGlu3 activation moderately opposes the initial EPSC depression induced by LY379268 application. Alternatively, an upregulation of mGlu2 or change in mGlu2 function in slices obtained from mGlu3 knockout mice could be responsible for this phenomenon.
Fig 2.
LY379268-induced LTD requires mGlu2 activation. (A) Time course of the EPSC amplitude following 10 minute bath application of LY379268 (100 nM) reveals that LY379268 induced LTD in wild type littermate controls (closed circles, n = 7) but failed to induce LTD in mGlu2 knockout mice (open circles, n = 8). Data represent mean ± SEM. Representative sample traces from a single experiment were taken from time points indicated by numbers and are the average of 6 individual traces. (B) LY379268 (100 nM, 10 minutes) induced LTD in both wild type littermate controls (closed circles, n = 7) and mGlu3 knockout mice (open circles, n = 6).
Discussion
Although activation of mGlus has been shown to induce LTD at synapses in other brain regions, our current findings represent the first demonstration of mGlu-mediated long term synaptic plasticity within the indirect pathway of the basal ganglia. While long-term depression induced by pharmacological activation of group II mGlus has not been described previously at the STN-SNr synapse, this phenomenon has been observed at excitatory synapses in several other brain regions, including the basolateral amygdala [16], the striatum [14], the nucleus accumbens [27, 28], the bed nucleus of the stria terminalis [10], and the medial prefrontal cortex [1, 11, 24, 25]. Depending on the synapse, group II mGlu-mediated LTD can be induced and expressed either presynaptically or postsynaptically. Previous studies evaluating mRNA and protein distribution of group II mGlus in the basal ganglia indicate a presynaptic localization of group II mGlus at the STN-SNr synapse, whereas no evidence of postsynaptic group II mGlu expression has been detected in SNr neurons [4, 29]. In addition, application of the group II mGluR agonist LY354740 does not alter the frequency or amplitude of spontaneous miniature EPSCs recorded from GABAergic SNr neurons, nor does it affect the membrane properties of SNr neurons [4], suggesting that the effect of group II mGlu agonists on excitatory transmission is mediated by activation of a presynaptic receptor. Thus, it is likely that the induction of LTD in SNr neurons is at least partially mediated by a presynaptic mechanism, although potential contributions of postsynaptic receptors cannot be ruled out. Because mGlu3 is also expressed in astrocytes [29], downstream effects of glial mGlu3 activation could also underlie alterations in neurotransmission induced by group II mGlu agonists. However, the finding that activation of mGlu2 receptors is sufficient to induce LTD in the mouse SNr suggests that glial mGlu3 receptors are not likely to play a role in this form of synaptic plasticity.
From a therapeutic perspective, it is possible that inducing LTD at the STN-SNr synapse could be beneficial in the treatment of the motor symptoms of PD by reducing excessive excitatory transmission to the basal ganglia output nuclei. Interestingly, previous studies have shown that intranigral or intracerebroventricular administration of group II mGlu agonists reverses reserpine-induced akinesia in rats [8, 20] and systemic administration of LY354740 reverses the catalepsy and muscle rigidity induced by the dopamine receptor antagonist haloperidol [4, 15]. Induction of LTD in the SNr therefore represents a novel mechanism by which group II mGlu agonists may alleviate PD-like motor deficits in preclinical models, although several other potential mechanisms of action may also contribute to the antiparkinsonian effects observed after group II mGlu administration [13]. While studies evaluating the behavioral effects of group II mGlu agonists in preclinical models of PD have used agonists that activate both mGlu2 and mGlu3, our finding that mGlu2 activation is sufficient to induce LTD in the mouse SNr suggests that selective activation of mGlu2 using a subtype-selective agonist or positive allosteric modulator may have therapeutic benefit for the primary motor symptoms of PD as well. However, recent studies demonstrating that mGlu3 activation slows nigrostriatal degeneration in toxin-based models of PD-like neurodegeneration suggest that coactivation of both mGlu2 and mGlu3 receptors may yield both symptomatic relief as well as disease modification [2, 7].
In addition to the potential therapeutic benefits of group II mGlu activation for treating PD, synaptic plasticity within the indirect pathway of the basal ganglia could play a role in basal ganglia-dependent learning and memory processes. Types of action learning such as memorization of skilled movement sequences, goal-directed learning, and habit learning are known to be dependent on the basal ganglia, particularly involving the dorsal striatum [19]. While much attention has been focused on the relevance of various forms of corticostriatal synaptic plasticity to basal ganglia-dependent forms of learning, the potential contribution of extrastriatal forms of plasticity has not been established. Future studies in vivo will therefore be necessary to further explore the potential physiological and therapeutic relevance of synaptic plasticity in the SNr.
*. Highlights.
Pharmacological activation of group II metabotropic glutamate receptors with LY379268 induces long-term depression of synaptically evoked excitatory transmission in the rat substantia nigra pars reticulata.
LY379268 induces long-term depression in wild type and mGlu3 knockout mice.
LY379268 does not induce long-term depression in mGlu2 knockout mice.
Acknowledgments
This work was funded by NIH grants NS48334, NS067737, and MH74953. The authors thank Drs. James Monn and David McKinzie of Eli Lilly and Company for generously providing LY379268 and mGlu2 and mGlu3 knockout mice. We also thank Dr. Meredith Noetzel for assistance with animal management.
Abbreviations
- PD
Parkinson's disease
- SNc
substantia nigra pars compacta
- STN
subthalamic nucleus
- GPi
globus pallidus internal segment
- SNr
substantia nigra pars reticulata
- mGlu
metabotropic glutamate receptor
- EPSC
excitatory postsynaptic current
- ACSF
artificial cerebrospinal fluid
- LTD
long-term depression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barbara JG, Auclair N, Roisin MP, Otani S, Valjent E, Caboche J, Soubrie P, Crepel F. Direct and indirect interactions between cannabinoid CB1 receptor and group II metabotropic glutamate receptor signalling in layer V pyramidal neurons from the rat prefrontal cortex. Eur J Neurosci. 2003;17:981–90. doi: 10.1046/j.1460-9568.2003.02533.x. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia G, Molinaro G, Riozzi B, Storto M, Busceti CL, Spinsanti P, Bucci D, Di Liberto V, Mudo G, Corti C, Corsi M, Nicoletti F, Belluardo N, Bruno V. Activation of mGlu3 receptors stimulates the production of GDNF in striatal neurons. PLoS One. 2009;4:e6591. doi: 10.1371/journal.pone.0006591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–20. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 4.Bradley SR, Marino MJ, Wittmann M, Rouse ST, Awad H, Levey AI, Conn PJ. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia Nigra pars reticulata. J Neurosci. 2000;20:3085–94. doi: 10.1523/JNEUROSCI.20-09-03085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JJ, Swope DM. Pharmacotherapy for Parkinson's disease. Pharmacotherapy. 2007;27:161S–173S. doi: 10.1592/phco.27.12part2.161S. [DOI] [PubMed] [Google Scholar]
- 6.Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–98. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- 7.Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, Mugnaini M, Nicoletti F, Bruno V. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 2007;27:8297–308. doi: 10.1523/JNEUROSCI.1889-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson L, Chadha A, Megalou M, Duty S. The group II metabotropic glutamate receptor agonist, DCG-IV, alleviates akinesia following intranigral or intraventricular administration in the reserpine-treated rat. Br J Pharmacol. 2000;129:541–6. doi: 10.1038/sj.bjp.0703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 10.Grueter BA, Winder DG. Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:1302–11. doi: 10.1038/sj.npp.1300672. [DOI] [PubMed] [Google Scholar]
- 11.Huang CC, Hsu KS. The role of NMDA receptors in regulating group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. Neuropharmacology. 2008;54:1071–8. doi: 10.1016/j.neuropharm.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 13.Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8:475–91. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn L, Alonso G, Robbe D, Bockaert J, Manzoni OJ. Group 2 metabotropic glutamate receptors induced long term depression in mouse striatal slices. Neurosci Lett. 2001;316:178–82. doi: 10.1016/s0304-3940(01)02397-7. [DOI] [PubMed] [Google Scholar]
- 15.Konieczny J, Ossowska K, Wolfarth S, Pilc A. LY354740, a group II metabotropic glutamate receptor agonist with potential antiparkinsonian properties in rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:500–2. doi: 10.1007/pl00005284. [DOI] [PubMed] [Google Scholar]
- 16.Lin HC, Wang SJ, Luo MZ, Gean PW. Activation of group II metabotropic glutamate receptors induces long-term depression of synaptic transmission in the rat amygdala. J Neurosci. 2000;20:9017–24. doi: 10.1523/JNEUROSCI.20-24-09017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linden AM, Shannon H, Baez M, Yu JL, Koester A, Schoepp DD. Anxiolytic-like activity of the mGLU2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology (Berl) 2005;179:284–91. doi: 10.1007/s00213-004-2098-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Ford-Dunn HL, Hayward GN, Nandi D, Miall RC, Aziz TZ, Stein JF. The oscillatory activity in the Parkinsonian subthalamic nucleus investigated using the macro-electrodes for deep brain stimulation. Clin Neurophysiol. 2002;113:1667–72. doi: 10.1016/s1388-2457(02)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–61. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray TK, Messenger MJ, Ward MA, Woodhouse S, Osborne DJ, Duty S, O'Neill MJ. Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson's disease. Pharmacol Biochem Behav. 2002;73:455–66. doi: 10.1016/s0091-3057(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 21.Ni Z, Bouali-Benazzouz R, Gao D, Benabid AL, Benazzouz A. Intrasubthalamic injection of 6-hydroxydopamine induces changes in the firing rate and pattern of subthalamic nucleus neurons in the rat. Synapse. 2001;40:145–53. doi: 10.1002/syn.1036. [DOI] [PubMed] [Google Scholar]
- 22.Ni ZG, Bouali-Benazzouz R, Gao DM, Benabid AL, Benazzouz A. Time-course of changes in firing rates and firing patterns of subthalamic nucleus neuronal activity after 6-OHDA-induced dopamine depletion in rats. Brain Res. 2001;899:142–7. doi: 10.1016/s0006-8993(01)02219-3. [DOI] [PubMed] [Google Scholar]
- 23.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otani S, Auclair N, Desce JM, Roisin MP, Crepel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J Neurosci. 1999;19:9788–802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otani S, Daniel H, Takita M, Crepel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002;22:3434–44. doi: 10.1523/JNEUROSCI.22-09-03434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–57. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- 27.Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ. Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci. 2002;22:4346–56. doi: 10.1523/JNEUROSCI.22-11-04346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbe D, Bockaert J, Manzoni OJ. Metabotropic glutamate receptor 2/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. Eur J Neurosci. 2002;16:2231–5. doi: 10.1046/j.1460-9568.2002.02273.x. [DOI] [PubMed] [Google Scholar]
- 29.Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–18. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vila M, Perier C, Feger J, Yelnik J, Faucheux B, Ruberg M, Raisman-Vozari R, Agid Y, Hirsch EC. Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by metabolic and electrophysiological measurements. Eur J Neurosci. 2000;12:337–44. doi: 10.1046/j.1460-9568.2000.00901.x. [DOI] [PubMed] [Google Scholar]
- 31.Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata. J Neurophysiol. 2001;85:1960–8. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]