1. Introduction
Assessing the potential risk of incorporated particles to human health, an important part within hazard identification and exposure is their dosimetry. Dosimetry represents the rational base for any subsequent toxicological evaluations. Only sufficient knowledge of both dosimetry and toxicological responses allows for comprehensive hazard identification. Here we will compare the current knowledge on the dosimetry of inhaled engineered nanoparticles (NP; 1-100nm diameter) versus that of submicrometer and micron-sized particles (μP; > 100nm diameter). Dosimetry starts with the estimate of an incorporated dose of particles which needs to be distinguished and derived from the exposure dose. While μP exposure has extensively been discussed previously1,2, NP exposure during the entire life-time is still under discussion. Currently occupational NP exposure during NP generation and processing dominates the debate. This is particularly true for the inhalation route. However, since many NP and μP are used in a large and increasing number of products of large diversity in food, pharmacology, personal care and other day-by-day goods with rapidly growing tendency, it appears to be necessary to gain a general estimate of the total NP and μP exposure by oral intake of consumers at large.
Depending on the route of intake the incorporated dose may be rather complex as it is the case for inhaled particles. Their deposition probability in the various regions of the respiratory tract depends on three sets of properties: (1) physico-chemical parameters of the inhaled particles themselves, (2) the breathing conditions and (3) the geometry of the respiratory tract including changes in diseased lungs. Once particles have been deposited onto the respiratory tract epithelia, multiple serial interactions and mechanisms with (1) proteins and biomolecules of body and cellular fluids, and (2) with cellular membranes and organ barriers will be initiated. These will determine the fate of a particle and its chemical and physical modifications (including protein binding to the surface and metabolic reactions) within the lungs as the organ of intake (primary organ), as well as after translocation into circulation and accumulation in secondary organs or excretion out of the body. The multitude of actions determines the biokinetics of the initially incorporated particle and its metabolic compounds in the body over time. In this paper we focus on the biokinetics of NP versus μP administered to lungs and specifically discuss quantitative biokinetics as a comprehensive approach. We will discuss the rather comprehensive data set for rodents and compare to the scarce, but more appropriate data of dogs and monkeys as human models.
2. Particle characterization and administration to the lung
The generation of a well characterized aerosol containing the selected model particles and their inhalation during a well-controlled exposure study provide the gold standard of particle dosimetry and toxicology to the respiratory tract. Since often neglected it needs to be emphasized that there is a natural dose limit on the particle number concentration of a sufficiently stable aerosol during exposure due to thermodynamic properties of the aerosol. At particle concentrations beyond 106 cm−3, coagulation dynamics under inhalable aerosol conditions causes rapid decrease of the particle number concentration accompanied by a shift of the aerosol size distribution to larger sizes. While this limit is usually not reached for μP aerosols even at mass concentrations of up to several 10 mg/m3, this is an important limit for NP aerosols. The number concentration limit of 106 cm−3 of e.g. 20nm, or100nm unit-density-NP or 500nm unit-density-μP corresponds to mass concentrations of 4.2 μg/m3, 525 μg/m3 and 66 mg/m3, respectively. Note the upper mass concentration for 500nm μP occurs only very seldom. Accordingly the dose rate to the respiratory tract is determined by this physical phenomenon and the breathing parameters: breathing frequency and tidal volume. In contrast, particle doses used for intratracheal instillation etc. allow for extremely high dose rates conflicting with maximally possible dose rates during inhalation. For the above mentioned aerosols the maximum hourly dose rate is 0.5 μg/h, 60μg/h and 60mg/h, respectively; (presuming an hourly inhaled volume of 360 L/h and a deposition probability of 0.3. This hourly dose deposits on about 5 1010 epithelial cells3. Hence, corresponding in vitro doses to 106 cultured cells would be limited to 0.5 ng, 60 ng and 7.5 μg, respectively, while usually in vitro doses exceed 1-10 μg. Furthermore, particle doses administered as a bolus during <30 sec frequently exceed drastically the maximal hourly inhaled dose by a factor 120.
Another limit is the particle size: for rodents which are obligatory nose breathers virtually no particles larger than 3μm in aerodynamic diameter can reach the lungs because these particles deposit already in the nose4.
3. Methodologies for determining biokinetics of inhaled particles
Many of the recent biokinetics studies attempted to administer a given particle dose followed by time-sequential analyses of particle retention in a few selected organs and tissues of interest. This selective approach provides limited information on the overall biokinetics of the administered particle material, as it usually describes the fate of only a small fraction of the administered particles – sometimes < 30%. In fact, it leaves important open questions about the excreted fraction and eventually major accumulated fractions in sites like the skeleton or soft tissue or other organs not analyzed. Therefore, a comprehensive understanding of the entire biokinetics is hampered by this reductionist analysis. In addition, biokinetics studies are commonly embedded in larger toxicological studies which should be based on a broad range of administered particle doses. In these cases even the lower dose of the selected particle dose range already causes significant biological and eventually toxic responses, which may affect the biokinetic fate of the particles. While these biokinetics studies are important for dosing and evaluation of toxic responses, they need to be compared to a baseline study under physiological (healthy) conditions. Therefore, administered particle doses used in biokinetics studies should aim for as low levels as possible, being necessary for a careful analysis in all relevant target organs and the remaining body (including excretion) to determine the particle biokinetics under physiological conditions. Such dose considerations are often neglected.
4. Quantitative biokinetics
To overcome the issues discussed above quantitative biokinetics may be an attractive and comprehensive alternative. It aims for an overall quantitative estimate of the total amount of the administered particles within and out of the body. Quantitative biokinetics can be performed after the administration of NP or μP by any route of delivery: inhalation, intratracheal instillation or aspiration to the respiratory tract; oral ingestion or gavage to the gastro-intestinal tract; intravenous or intra-arterial injection to the blood circulation, or dermal applications to the skin. The concept is simple, aiming to estimate the total amount of particles administered and retained in the entire body at certain time points after exposure including total excretion, as shown in Figure 1. Hence, not only the distributions of particles in organs and tissues of interest are determined, but particles in the organs and tissues of the remaining carcass and those excreted are also included. With this, a 100% balance of the administered particles is achieved and a complete, yet detailed, quantitative analysis of their biokinetics is obtained5. Note when particles undergo chemical and physical changes such as particle decomposition and/or disintegration, the biokinetics fate of the various fractions may differ from each other and they may lose their particulate structure into molecules and ions and newly formed complexes. Although this approach of quantitative biokinetics is principally applicable to any animal species, ethical, economic and practical reasons usually limit the analytical attempt to small laboratory animals.
Figure 1.
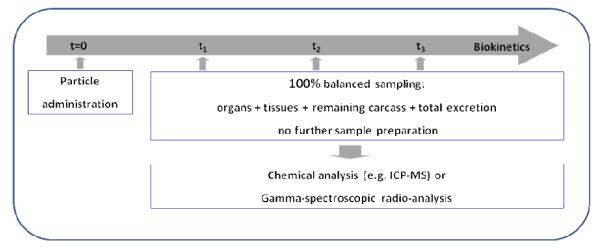
Concept of quantitative particle biokinetics assessment. Particles are administered at time t=0. From this time point on, the entire urinary and fecal excretions are collected separately. Animals are euthanized at times t1, t2, t3, etc., and organs and tissues of interest and the entire remaining carcass are sampled (100% balanced sampling). Special sample preparation is required before chemical analysis. When particles are radio-labeled, total organ samples will be analyzed directly using gamma spectrometry without any further preparation.
Analytical chemistry e.g. inductively coupled plasma mass spectroscopy (ICP-MS) provide suitable methods to quantify chemical elements of particles in the collected specimens. Thereby, special attention has to be paid to sample preparation, because of the extremely low mass of NP retained in organs. In addition, endogenous species of the chemical element of the particle may contribute to the natural “background” concentration. Gamma radio-spectroscopy may provide an elegant highly sensitive analytical alternative, allowing for a direct analysis of complete organs and tissues without any pre-treatment. In this case, the particles need to be labeled using a radio-isotope being firmly integrated into the particle matrix without any allowance for leaching. While this requirement is hard to fulfill, when a radio-isotope is not blended into the particles during production but chemically engineered onto the particle surface, stable labeling is obtained, when the radio-isotope belongs to the same chemical element as the particle matrix. This, however, is often difficult to achieve. Nuclear reactions within already existing particles upon neutron, proton or any other ion beam irradiation, allow radio-activation of one to a few atoms per particle. Mostly, the amount of radioactivity is sufficiently high for subsequent radio-analysis, when not more than a single atom per NP was converted by the nuclear reaction while more radio-isotopes in μP are required due to their larger particle mass. Note however, that usually the ratio between the radioactive and the stable isotopes of a particle is much less than 10−5, representing a negligible “impurity” fraction by the radioisotope, which is unlikely to affect the chemical properties and stability of the particle matrix. Firm radio-labeling may not always be possible with any given particle because of possible nuclear reactions. However, an important advantage of the gamma-spectrometric analysis is the option of using extremely low particle masses necessary for determining particle biokinetics at the physiological background level. The use of high sensitivity, low-background gamma detectors of well-type geometry allows the radioactivity determination of about 0.1 Bq in a total organ or the entire remaining carcass. Such low detection limits allow for analyses of particle doses in the picogram-range per organ or tissue. Furthermore, when administering an initial particle dose of 10-50 kBq and a mass of 1-10 μg per rodent then two important aims can be achieved:
(a) The dose range from administration to organs of lowest radioactivity can cover a dynamic range of more than five orders of magnitude and
(b) The administered particle mass is likely not to trigger patho-physiological responses of the organism.
Yet, since the radio-spectrometric method similarly like the chemical analytical methods do not distinguish between the particulate state and chemical speciation, those important particle relevant parameters including the firm labeling of the radio-isotope need to be estimated by additional (auxiliary) studies specifically designed to answer these questions. Since particles being cleared out of the lungs by mucociliary transport may enter the gastrointestinal tract additional auxiliary studies need to cover particle biokinetics after oral ingestion.
Total organs and all body fluids are collected without any cross contamination. Tissue samples, the remaining carcass and the entire excretion are collected such that the entire organism is sampled and weighed in wet state. The application of clean dissection techniques is essential to avoid cross contamination, in particular in inhalation studies, where fur contamination occurs upon whole body exposures. Systematic changes of dissection tools and equipment are mandatory. In addition, whole body vascular perfusion is recommended to empty the blood vessels within the organs to solely determine particle retention in the parenchyma.
5. Current knowledge of particle biokinetics
5.1. Particle retention in the lungs
After particle inhalation and deposition on the lung epithelium the retention of particles starts with their wetting by surfactant and the epithelial lining fluid and subsequent displacement from the air into the aqueous phase regardless of particle shape, surface topography and surface free energy6,7. Though experimentally demonstrated yet for μP of 1 - 6 μm in diameter, the same is expected for NP, since this process becomes even more efficient with decreasing particle size.
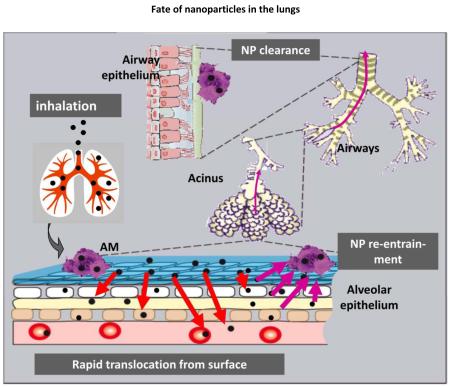
In general, the majority of μP remains on the epithelial surface in airways and alveoli of rodent lungs and are accessible to broncho-alveolar lavage (BAL), see Figure 28-12. However, already 24 hours after inhalation most NP are no longer accessible to BAL (Figure2).
Figure 2.
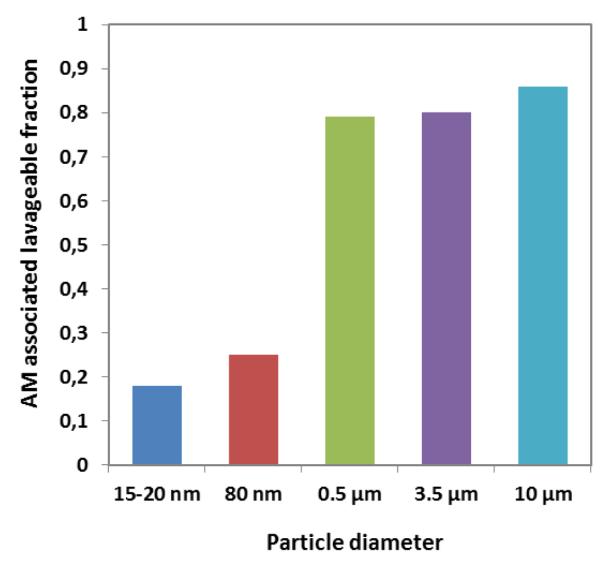
Alveolar-macrophage (AM) associated fractions of instilled μP (0.5, 3 and 10 μm polystyrene particles) and inhaled NP (radio-labeled 20 and 80nm iridium NP) found in BAL of rats 24 hours after administration13,14. The low fractions of the iridium NP are similar to those of 20nm gold NP, titanium dioxide NP and elemental carbon NP (data not shown).
Their retention time in the lung depends on the deposition site and on the interaction of the particles with the inner lung surface. It is short for particles deposited in conducting airways due to efficient mucociliary and cough clearance (time span hours up to one day), but it increases with airway generation number, as a consequence of increasing pathway length and decreasing mucus transport velocity towards distal airways. While the retention of μP generally does not exceed 24–48 h in rodent airways, there is evidence for prolonged retention of NP and μP in airways of dogs and man4,15,16. The probability of long term particle retention in the airways is inversely correlated to particle size and a maximal fraction of up to 80% was found for particles ≤ 100 nm16,17.
Possible explanations for the long term retained particles in the airways are: (1) the particles were no longer accessible to mucociliary clearance either because they penetrated through the mucus layer deep into the periciliary phase6, or (2) they deposited in areas without any mucus coverage, since patchy mucus layers can exist in small airways18. In both cases, particles can penetrate between cilia to further interact with cells of the inner lung surface, i.e. macrophages, dendritic and epithelial cells19. Hence, the probability for particle relocation beyond the epithelial barrier is enhanced.
5.2. Particle clearance from the peripheral lungs
There are three major transport pathways of biopersistent particles out of the peripheral lungs: (a) alveolar-macrophage-mediated transport from the alveoli to the ciliated airways for mucociliary transport to the larynx and subsequent swallowing into the gastro-intestinal tract, (b) particle transport towards lung-associated lymph nodes; (c) translocation into blood circulation for subsequent accumulation in secondary organs. Note, the clearance of rapidly or moderately soluble particles is not addressed but may be an important clearance mechanism for many particles4,13. In the following we will discuss the first clearance pathway in detail distinguishing between NP versus μP. We will not address the second transport pathway towards lymph nodes as we and others have reported on that previously4,5,13,20.
6. Particle relocation from the alveolar epithelium into the pulmonary tissues of rodents
6.1. Differences in biokinetics between NP and μP in rodents
NP relocation into rat epithelial and interstitial tissues (pulmonary tissues) was described after inhalation of aerosolized submicrometer agglomerates of 20nm primary TiO2 versus the inhalation of aerosolized submicrometer agglomerates of primary 200-300nm TiO2 particles11. When compared to submicrometer agglomerates of primary 200-300nm μP an increased NP fraction was determined in pulmonary tissues by chemical analyses. NP versus 200-300nm μP samples were compared which had been obtained from exhaustive BAL and the according lavaged lungs. After the 12-week exposure the authors estimated that about 50% more NP mass than larger particles were relocated into pulmonary tissues. They suggest that dis-agglomeration of the agglomerates of the primary 20nm TiO2 had occurred on the epithelial surface prior to relocation into rat pulmonary tissues which did not occur to the 200-300nm TiO2 particle agglomerates. Similar results were found for intratracheally instilled submicrometer agglomerates of primary 20nm TiO2 or Al2O3 NP versus instilled submicrometer agglomerates of primary 200-300nm TiO2 or Al2O3 particles10. However, in these studies the extent of dis-agglomeration could not be quantified.
In a more recent rat inhalation study using freshly generated iridium NP aerosols of 20nm or 80nm median diameter (geometric standard deviation (GSD) 1.6) about 80% and 75%, respectively, of the NP (deposited on the alveolar epithelial surface) were relocated into epithelial and pulmonary tissues within 24 hours and no longer accessible to BAL, Figure 2 21,22. These fractions (relative to the contemporary lung burden) remained unchanged over the next six months since only about 20% were eliminated by BAL, Figure 3.
Figure 3.
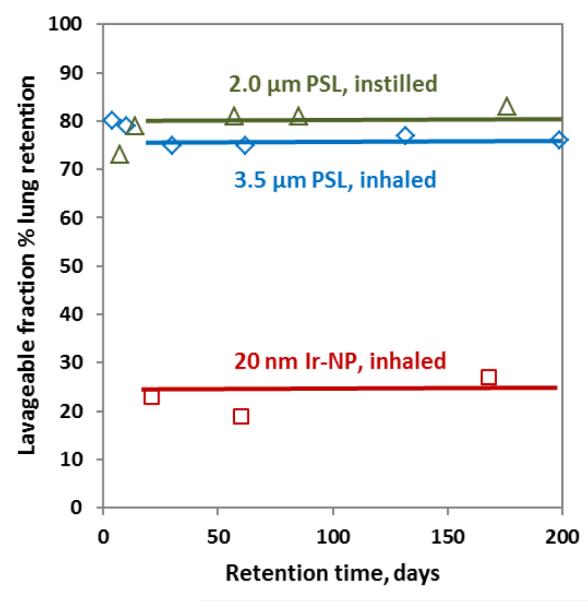
Fractions of μP (either inhaled 3.5 μm 85Sr-labeled polystyrene particles (μPSL) or intratracheally instilled fluorescent 2 μm μPSL versus inhaled NP (radio-labeled 20nm iridium NP) found in broncho-alveolar lavage fluids of rats at various time points from day 3 through six months after administration8,11,14,21,22. All fractions are relative to the actual lung burden.
Twenty-four hours after inhalation we found similar BAL versus lavaged lungs fractionations of freshly generated 25nm elemental carbon and 20nm TiO2 NP aerosols23. Three different NP materials (iridium, elemental carbon, titanium dioxide) of similar size (20nm), similar polydispersity and NP morphology were studied – they all were chain-aggregates/agglomerates of primary particles <5nm. They were inhaled as 20nm NP aerosols by rats and they all showed rapid internalization into the interstitium, emphasizing the importance of NP size. Note, at a deposited NP dose of about 1 μg per rat lung after the 1-hour inhalation a retrograde agglomeration on the alveolar epithelial surface is basically excluded since the projected area of all NP covers only 10−12 of the rat lung surface area.
In contrast, about 80% of inhaled or instilled μP were accessible to BAL during months after administration and the remaining small fraction of 20% not accessible to BAL was relocated into pulmonary tissues, Figure 3. Different to the interstitial relocation of NP, the majority of μP (fluorescent 2 μm and radio-labeled 3.5 and 10 μm polystyrene particles) remained on the alveolar epithelial surface of rodent lungs and more than 80% of the lung-retained fraction were collected by exhaustive BAL throughout the entire retention time of six months, Fig 38,11. Similar results were obtained for micron-sized radio-labeled fused-clay particles in hamster lungs9. All these studies confirmed that until more than six months after μP instillation or inhalation an 80%-fraction of the contemporary rodent lung particle burden remains on the alveolar epithelium being accessible to BAL throughout this period.
Note the μP burden as a fraction of the initial dose declines rather rapidly due to continuous alveolar-macrophage mediated clearance towards the ciliated airways and larynx. The initial rate starts at 1-3%/d but it declines exponentially with time such that after about 200 days >90% of the μP are cleared4,24.
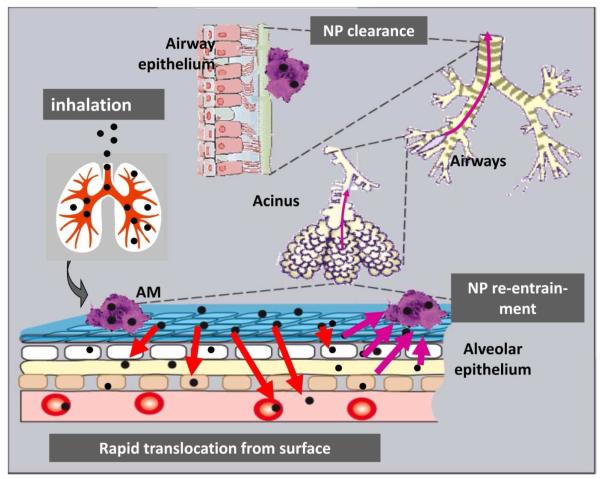
Surprisingly, and very similar to the μP clearance kinetics, more than 90% of the initial 20nm iridium NP deposit had been cleared from the alveolar region during the six month retention period. The NP fraction was also cleared by alveolar-macrophage-mediated transport as confirmed by BAL analysis21,22. So, these long-term retained NP apparently re-appeared from interstitial spaces back onto the epithelium for subsequent clearance by lung-surface macrophages. Although not yet confirmed, the most likely mechanism of NP re-appearance on the epithelial surface is presumed to be via macrophage-migration in the interstitium. Even more surprising, despite the high interstitial NP retention, the clearance kinetics, i.e. the daily cleared NP fractions towards the larynx followed the same kinetics as those of the daily cleared μP fractions, which were primarily retained on the epithelium5,21. So the earlier discussed clearance rate mediated by rodent lung-surface macrophages of 1-3%/d towards the larynx4,24 does not only include alveolar macrophages but also macrophages from the interstitium re-entering the epithelium and migrating towards the ciliated airways. This is depicted schematically in Figure 4.
Figure 4.
Pathways of relocation of inhaled NP after deposition on the alveolar epithelium of the rodent lungs: rapid transport into the epithelium and interstitial spaces for long-term retention; upon clearance NP can re-entrain back onto the epithelium for macrophage-mediated transport towards ciliated airways and the larynx22.
6.2. Differences in the biokinetics of rodents versus dogs and monkeys
There seem to be significant differences in μP biokinetics between rodents and human lungs. Yet not all necessary experimental data are available on the human μP biokinetics. Therefore the canine and simian biokinetics may serve as more appropriate models since the kinetics of μP lung retention and clearance is very similar in these three species as discussed earlier4,24. Macrophage-mediated μP clearance kinetics from the human, simian and canine lungs was shown to be one order of magnitude slower than in rodents; the initial rate is 0.1 – 0.2%/d of the contemporary lung burden and declines exponentially with time. Furthermore, from early canine biokinetics studies25-27 on lung retention, clearance and serial, partial broncho-alveolar lavage (BAL) of insoluble, radio-labeled μP over one year we gained evidence that μP did not remain on the epithelium like in rodents but had been re-located into the interstitium with a half-life of 150 d. Yet long-term μP clearance was dominated by macrophage-mediated transport to the ciliated airways and larynx – similar to the long-term NP clearance from the rodent lungs.
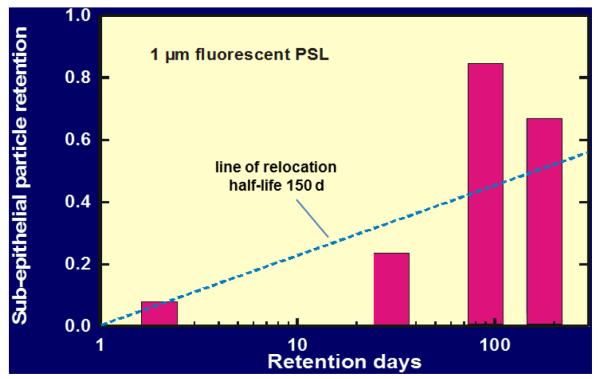
In order to identify μP transport from the epithelium towards interstitial spaces in dogs we performed a morphological study: after a short-term inhalation of 1 μm fluorescent polystyrene particles (μPSL) we analyzed the morphological location of the μPSL in lung tissues by fluorescence microscopy28. Indeed μPSL retention in interstitial spaces increased during six months to about 70% of all lung retained μPSL, as shown in Figure 5. These morphologic data are in reasonable agreement with the previous biokinetics data. These had shown a μP relocation kinetics into pulmonary tissues which increased exponentially with a half-life of 150 d, see Figure 5.
Figure 5.
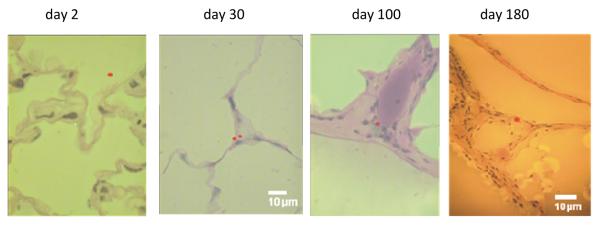
Upper panel shows the increasing particle fraction in the canine lung interstitium during a six-month retention period after a short-term inhalation of 1μm fluorescent polystyrene particles (μPSL)28. The line corresponds to the particle relocation kinetics with a half-life of 150 d into pulmonary tissues as previously determined in biokinetics studies25-27. Lower panels show morphologic locations of μPSL in lung tissues during the six-month period (μPSL, false color).
Further evidence for species differences in particle relocation and accumulation in pulmonary tissues comes from a long-term exposure study to various dusts to monkeys and rats: Diesel soot, coal dust and a combination of both29. Though a similar retention pattern as in canine pulmonary tissues (see above) was observed in monkeys, particle retention in rats was much more prominent on the alveolar epithelium, similar to the data shown in Figure 3. While the coal dust aerosol (<7μm diameter) was micron-sized, the Diesel soot exhaust consisted of agglomerated sub-micron and nanoparticles. The fact that the coal dust particles and the Diesel exhaust particles were retained similarly in pulmonary tissues of monkeys demonstrates the access of NP, submicron and micron-sized particles to pulmonary tissues. The various data discussed here, suggest that the NP relocation towards pulmonary tissues is similar in rodents, dogs and monkeys and most likely in man, - in contrast to the biokinetics of μP in rodents with negligible access to pulmonary tissues.
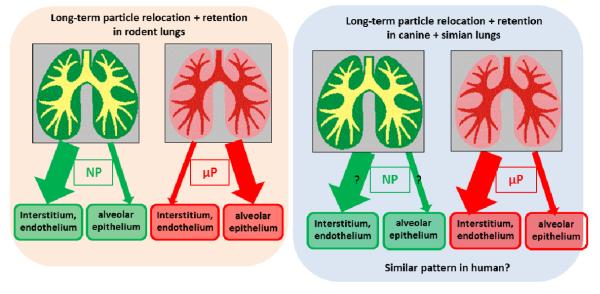
In Figure 6 the long-term relocation and retention of NP versus μP are depicted showing the differences between rodent lungs versus canine and simian lungs. We speculate that the human pattern may be quite similar to the latter.
Figure 6.
Graphical scheme of long-term relocation and retention of NP versus μP showing the differences between rodent lungs versus canine and simian lungs. We hypothesize that the human pattern may be quite similar to that of the large animal species.
As mentioned above we hypothesize that the canine and simian are valid models for the biokinetics of inhaled particles in humans because of the congruent human, simian and canine data. Unfortunately no detailed long-term NP clearance kinetics data are known for the human, simian or canine lungs. In fact, it appears unlikely that NP are long-term retained on the epithelium when μP are relocated to the interstitium. Yet the kinetics of macrophage-mediated clearance seems to be independent of the location of the retained particle as the rodent data showed.
So, we can summarize the various findings on particle retention and biokinetics:
A clear difference in the location of retained μP between the canine and simian versus rodent lungs,
A ten-fold difference in the kinetics of macrophage-mediated clearance from human, canine and simian versus rodent lungs,
But long-term macrophage-mediated clearance seems not to depend on the particle retention site either on the epithelium or in the interstitium of all species studied.
7. Predominant NP lung retention and limited translocation towards the circulation
As discussed above insoluble NP in rodent lungs are predominantly long-term retained in interstitial spaces of the alveolar region. Yet there is only limited translocation towards the circulation. This is indeed very surprising since we found significant fractions of 20nm titanium dioxide NP in endothelial cells of rat lungs within 24 hours after inhalation30. Unfortunately there is no direct proof for this NP accumulation in endothelial cells of large animal species and man but the study of Nikula and coworkers29 provides indirect evidence as discussed above.
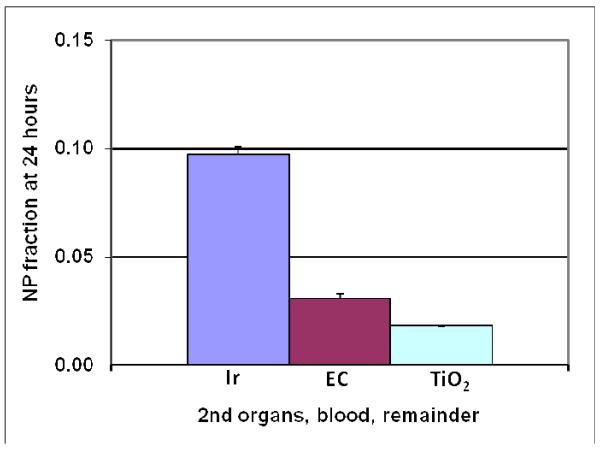
Yet the limited NP translocation in rat lungs strongly depends on the physico-chemical NP properties. For instance, after a 1-2-hour inhalation of 20nm NP of different materials (iridium, elemental carbon and titanium dioxide) by healthy adult rats the totally translocated NP fraction across the air-blood-barrier after 24 hours varies significantly by one order of magnitude from almost 10% for iridium NP to about 1% of for titanium dioxide NP; see Figure 7. Iridium:14,21; elemental carbon:23; titanium dioxide: Kreyling & Semmler-Behnke, personal communication.
Figure 7.
Twenty-four-hour translocated fractions of alveolar NP deposit towards blood and subsequent organs and tissues; three different materials (iridium, elemental carbon and titanium dioxide) were inhaled as freshly generated 20nm NP aerosols for 1-2 hours by healthy adult rats. The iridium NP translocation is significantly higher than those of elemental carbon and titanium dioxide NP. Iridium:14,21; elemental carbon:23; titanium dioxide: Kreyling & Semmler-Behnke, personal communication.
Of the translocated NP fractions in Figure 6 the highest organ fraction was found in the liver followed by spleen and kidneys followed by the heart, uterus and brain. This was true for all three NP. Interestingly even higher fractions of translocated NP of all three materials than in organs were found in soft tissue (blood content excluded) and the skeleton. Particularly, the latter is of interest since the NP were transported via blood circulation such that the skeletal NP retention is expected to be directly in the bone marrow. In addition, low but detectable NP concentrations in the blood over several weeks after inhalation of iridium and titanium dioxide NP suggest not completely stationary conditions of the translocated and accumulated NP in the organism but some clearance from the primary and secondary organs needs to be taken into account resulting in re-translocation and further accumulation in the various organs, soft tissue and the skeleton.
8. Summary
Biokinetics of inhaled biopersistent NP and μP is maintained by a multitude of highly dynamic processes which depend not only on physico-chemical properties of the particles but also on a manifold of cellular and molecular responses and interactions. As a result particles do not necessarily remain on their site of deposition after inhalation but undergo numerous transport processes within the various anatomical sites of the lungs and out of the lungs. While μP prominently stay long-term on the rodent alveolar epithelium but NP relocate into rodent interstitial spaces, both μP and NP gradually relocate into the canine and simian interstitium. Hence, these transport processes and their kinetics may differ between rodents versus dogs and monkeys and probably human. As a result, they significantly affect μP and NP particle dosimetry and toxicology, and may offer new opportunities in the new field of nano-medicine for diagnosis and therapy.
Conspectus.
Researchers need to study the biokinetics of inhaled biopersistent nano- and micron-sized particles (NPs and μPs) to assess their toxicity and to develop understand their potential risks. When particles are inhaled, they do not necessarily remain on their sites of deposition in the respiratory tract. Instead they can undergo numerous transport processes within the various tissues of the lungs, including clearance from the lungs. In this context we would like to understand how the biokinetic studies performed in animals can be extrapolated to humans. Interestingly, the particle retention is much shorter in rodent lungs and declines much faster than it does in human, simian and canine lungs.
The predominant long-term clearance pathway for both NPs and μPs in humans and other animal species is macrophage-mediated particle transport from the peripheral lungs towards ciliated airways and the larynx. However, the transport rate is ten times higher in rodents than in other species. In addition to particle clearance out of the lung, we also observe particle redistribution from the epithelium toward and within the interstitium and lymph nodes of the lung and particle translocation to blood circulation leading to subsequent accumulation in secondary organs. While μPs have limited access to interstitial spaces in the rodent lungs, NPs rapidly relocate in the epithelium and the underlying interstitium. By contrast, indirect evidence shows that both NP and μP are relocated into the epithelium and interstitial spaces of the human, simian and canine lungs.
Only NPs translocate into the circulatory system and subsequently accumulate in the secondary organs and tissues of the body. Translocated NP fractions are rather low but they depend strongly on the physicochemical properties of the NP and their surface properties. Growing evidence indicates that the binding and conjugation of proteins to NP play an essential role on the translocation across cellular membranes and organ barriers.
In summary, particle biokinetics result from a multitude of highly dynamic processes which depend not only on physicochemical properties of the particles but also on a multitude of cellular and molecular responses and interactions. Given the rather small accumulation in secondary organs after acute inhalation exposures, it appears likely that adverse effects caused by NP accumulated in secondary organs may only occur after chronic exposure over extended time periods. Therefore adverse health effects in secondary organs such as the cardiovascular-system that are associated with chronic exposure of ambient urban air pollution are less likely to result from particle translocation. Instead, chronic particle inhalation could trigger or modulate the autonomous nervous system or the release of soluble mediators into circulation leading to adverse health effects.
Acknowledgement
This work was partially supported by the European Union (EU) FP5 FIGD-CT-2000-00053, EU FP6 PARTICLE_RISK 012912 (NEST), EU FP7 NeuroNano (NMP4-SL-2008-214547) and ENPRA (NMP4-SL-2009-228789), US-NIH RO1HL070542.
BIOGRAPHICAL INFORMATION
Wolfgang G. Kreyling (PhD) is a biophysicist at the Institute of Lung Biology and Disease of the Helmholtz Center Munich (HMGU). His research interests range from aerosol sciences and nanoparticle technology to lungs biophysics reaching from the characterization of ambient aerosols to particle dosimetry and nanoparticle lung interactions on the level of the entire organism, cells like alveolar macrophages, and molecular compounds.
Manuela Semmler-Behnke (DVM) was a postdoctoral research fellow at the Institute of Lung Biology and Disease of the Helmholtz Center Munich (HMGU). Currently she works at the Bavarian Health and Food Safety Authority. Her research interests are deposition and biokinetics of inhaled engineered nanoparticles in rodents from neonates to adults or applications after different routes of entry (lungs, blood, gastrointestinal tract).
Shinji Takenaka (DVM, PhD) is a pathologist at the Institute of Lung Biology and Disease, CPC, Helmholtz Center Munich, Germany. His research focuses on health effects of air pollutants in experimental animals after long-term exposure to common air pollutants or industrial products. Formerly he worked at the National Institute for Environmental Studies, Tsukuba, Japan, and the former Fraunhofer Institute of Toxicology and Aerosol Research, Schmallenberg/Hannover, Germany. At present, he studies the ultrastructural localization of inhaled nanoparticles in the lungs of rodents.
Winfried Möller (PhD) is a biophysicist at the Institute of Lung Biology and Disease (iLBD) of the Helmholtz Zentrum München. He developed the generation of ferromagnetic radio-labelled iron oxide micro and nano particles for human inhalation studies. Using these test aerosols he conducted several human inhalation studies on deposition and clearance. He currently investigates micro- and nano-particle based targeted drug delivery to the upper airways and the paranasal sinuses.
References
- (1).ICRP Human respiratory tract model for radiological protection. A report of a Task Group of the International Commission on Radiological Protection. Ann. ICRP. 1994;24:1–482. [PubMed] [Google Scholar]
- (2).US-EPA Particulate Matter (PM) Standards Revision - 2006. Vol. 2006. US Government; 2006. [Google Scholar]
- (3).Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am. J. Respir. Cell Mol. Biol. 1992;6:235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- (4).Kreyling WG, Scheuch G. Clearance of particles deposited in the lungs. In: Gehr P, Heyder J, editors. Particle-Lung Interactions. Marcel Bekker,Inc.; New York; Basel: 2000. pp. 323–376. [Google Scholar]
- (5).Geiser M, Kreyling W. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010;7(No. 2) doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Schürch S, Gehr P, Im Hof V, Geiser M, Green F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respir. Physiol. 1990;80:17–32. doi: 10.1016/0034-5687(90)90003-h. [DOI] [PubMed] [Google Scholar]
- (7).Geiser M, Matter M, Maye I, Im Hof V, Gehr P, Schurch S. Influence of airspace geometry and surfactant on the retention of man-made vitreous fibers (MMVF 10a) Environ. Health Perspect. 2003;111:895–901. doi: 10.1289/ehp.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lehnert BE, Valdez YE, Tietjen GL. Alveolar macrophage-particle relationships during lung clearance. Am. J. Respir. Cell Mol. Biol. 1989;1:145–154. doi: 10.1165/ajrcmb/1.2.145. [DOI] [PubMed] [Google Scholar]
- (9).Ellender M, Hodgson A, Wood KL, Moody JC. Effect of bronchopulmonary lavage on lung retention and clearance of particulate material in hamsters. Environ. Health Perspect. 1992;97:209–213. doi: 10.1289/ehp.9297209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ferin J, Oberdörster G, Soderholm SC, Gelein R. Pulmonary tissue access of ultrafine particles. J. Aerosol Med. 1991;4:57–68. [Google Scholar]
- (11).Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994;102(Suppl 5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Oberdörster G, Ferin J, Soderholm SC, Gelein R, Cox C, Baggs R, Morrow PE. Increased Pulmonary Toxicity of Inhaled Ultrafine Particles: Due to Lung overload Alone? Ann. Occup. Hyg. 1994;38(Suppl.1):295–302. [Google Scholar]
- (13).Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdörster G, Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- (15).Kreyling WG, Blanchard JD, Godleski JJ, Haeussermann S, Heyder J, Hutzler P, Schulz H, Sweeney TD, Takenaka S, Ziesenis A. Anatomic localization of 24- and 96-h particle retention in canine airways. J. Appl. Physiol. 1999;87:269–284. doi: 10.1152/jappl.1999.87.1.269. [DOI] [PubMed] [Google Scholar]
- (16).Möller W, Kreyling WG, Schmid O, Semmler-Behnke M, Schulz H. Deposition, Retention and Clearance, and Translocation of Inhaled Fine and Nano-Sized Particles in the Respiratory Tract. In: Gehr P, Mühlfeld C, Rothen-Rutishauser B, Plank F, editors. Particle-Lung Interactions. 2nd ed Vol. 241. Informa Healthcare USA; New York: 2009. pp. 79–107. [Google Scholar]
- (17).Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Haussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit. Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- (18).Van As A, Webster I. The morphology of mucus in mammalian pulmonary airways. Environ. Res. 1974;7:1–12. [Google Scholar]
- (19).Blank F, Rothen-Rutishauser B, Gehr P. Dendritic cells and macrophages form a transepithelial network against foreign particulate antigens. Am. J. Respir. Cell Mol. Biol. 2007;36:669–677. doi: 10.1165/rcmb.2006-0234OC. [DOI] [PubMed] [Google Scholar]
- (20).Donaldson K, Murphy F, Duffin R, Poland C. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010;7(No. 5) doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Semmler M, Seitz J, Erbe F, Mayer P, Heyder J, Oberdorster G, Kreyling WG. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal. Toxicol. 2004;16:453–459. doi: 10.1080/08958370490439650. [DOI] [PubMed] [Google Scholar]
- (22).Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, Oberdorster G, Kreyling WG. Efficient elimination of inhaled nanoparticles from the alveolar region: evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ. Health Perspect. 2007;115:728–733. doi: 10.1289/ehp.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdorster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009;21:55–60. doi: 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- (24).Kreyling WG. Interspecies comparison of lung clearance of “insoluble” particles. J. Aerosol Med. 1990;3:S93–S110. [Google Scholar]
- (25).Heyder J, Beck-Speier I, Ferron GA, Josten M, Karg E, Kreyling WG, Lenz AG, Maier KL, Reitmeier P, Ruprecht L, Takenaka S, Wohland T, Ziesenis A, Schulz H. Long-term responses of canine lungs to acidic particles. Inhal. Toxicol. 2009;21:920–932. doi: 10.1080/08958370802651994. [DOI] [PubMed] [Google Scholar]
- (26).Kreyling WG, Dirscherl P, Ferron GA, Heilmann P, Josten M, Miaskowski U, Neuner M, Reitmeir P, Ruprecht L, Schumann Health effects of sulfur-related environmental air pollution. III. Nonspecific respiratory defense capacities. Inhal. Toxicol. 1999;11:391–422. doi: 10.1080/089583799197069. et, a. [DOI] [PubMed] [Google Scholar]
- (27).Kreyling WG, Ferron GA, Fürst G, Heilmann P, Neuner M, Ruprecht L, Schumann G, Takenaka S, Heyder J. Long-term exposure of dogs to a sulfite aerosol: III. Macrophage mediated long-term particle clearance. Inhal. Toxicol. 1992;4:197–233. [Google Scholar]
- (28).Kreyling WGT, S., Schumann G, Ziesenis A. Particles are predominantly transported from the canine epithelium towards the interstitial spaces and not to larynx! Analogy to human lungs? Am. J. Respir. Crit. Care Med. 2001;163:A166. [Google Scholar]
- (29).Nikula KJ, Avila KJ, Griffith WC, Mauderly JL. Sites of particle retention and lung tissue responses to chronically inhaled diesel exhaust and coal dust in rats and cynomolgus monkeys. Environ. Health Perspect. 1997;105(Suppl. 5):1231–1234. doi: 10.1289/ehp.97105s51231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]