Abstract
Germ cell tumors (GCTs) of the testis are rare, but are the most common cancer in young men. GCTs may consist of one predominant histologic pattern or may represent a mixture of multiple histologic types. For treatment purposes, two broad categories are recognized: 1) pure seminoma and 2) others, which together are termed nonseminomatous GCTs (NSGCTs). In general, seminoma tends to be less aggressive, to be diagnosed at an earlier stage, and to spread predictably along lymphatic channels to the retroperitoneum before spreading hematogenously to the lung or other organs. Compared with NSGCTs, seminoma is exquisitely sensitive to radiation therapy and platinum-based chemotherapy. NSGCTs are usually mixed tumors and teratoma often exists at the sites of metastasis with other GCT elements; cure often requires chemotherapy to kill the chemosensitive-components and surgery to remove the teratomatous components. The main factors contributing to excellent cure rates of GCTs are careful staging at diagnosis; adequate early treatment using chemotherapeutic combinations, with or without radiotherapy and surgery; and very strict follow-up and salvage therapy. We review several clinical studies and summarize the current trends in the management of GCTs.
Keywords: Neoplasms, Testis, Therapeutics
INTRODUCTION
Testicular cancer represents 1% to 1.5% of male neoplasia and 5% of urologic tumors in general, with 3 to 6 new cases occurring per 100,000 males per year in Western society [1]. Also, a clear trend has been seen toward an increased testicular cancer incidence in the past 30 years in most industrialized countries [2]. The peak incidence is in the third decade of life for nonseminoma and in the fourth decade for pure seminoma. Familial clustering has been observed, particularly among siblings [3]. The epidemiologic risk factors for the development of testicular cancer are a history of cryptorchidism or undescended testis, Klinefelter syndrome, a familial history of testicular cancer among first-degree relatives (father or brothers), the presence of a contralateral tumor or testicular intraepithelial neoplasia, and infertility [4-6]. Testicular cancer has excellent cure rates. The main factors contributing to this are careful staging at diagnosis; adequate early treatment using chemotherapeutic combinations, with or without radiotherapy (RT) and surgery; and very strict follow-up and salvage therapy. The aim of this review was to summarize the current trends in the management of germ cell tumors (GCTs).
DIAGNOSIS
1. Clinical examination
Testicular cancer generally affects young men in the third or fourth decade of life. It normally appears as a painless, unilateral mass in the scrotum or the casual finding of an intrascrotal mass [7]. In approximately 20% of cases, the first symptom is scrotal pain, and 27% of patients with testicular cancer will have local pain [8]. In about 10% of cases, a testicular cancer can mimic orchidoepididymitis, with a consequent delay in correct diagnosis [1].
2. Serum tumor markers (STMs)
STMs are prognostic factors and contribute to diagnosis and staging [9]. The following markers should be determined, alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), and lactate dehydrogenase (LDH). However, negative marker levels do not exclude the diagnosis of a GCT. Globally, an increase in these markers occurs in 51% of GCT cases [10]. The mean serum half-life of AFP and hCG is 5 to 7 days and 2 to 3 days, respectively [11]. AFP increases in 50% to 70% of patients with nonseminomatous germ cell tumors (NSGCTs), and an increase in hCG is seen in 40% to 60% of patients with NSGCTs. LDH is a less specific marker, and its concentration is proportional to the tumor volume. STM should be re-evaluated after orchiectomy to determine the half-life kinetics. Postorchiectomy markers are important to classify the patient according to the International Germ Cell Cancer Collaborative Group (IGCCCG) risk classification. The persistence of elevated STMs after orchiectomy indicates the presence of metastatic disease, but normalization of marker levels after orchiectomy does not rule out the presence of tumor metastases. Other markers studied include placental alkaline phosphatase (PLAP), which can be of value in monitoring patients with pure seminoma. Cytogenetic and molecular markers are available in specific centers but, at present, only contribute to research studies. Measurement of serum AFP, hCG, and LDH levels is mandatory, and measurement of PLAP is optional.
3. Imaging study
Ultrasonography (US) must be performed for any doubtful case. Physical examination will reveal the features of the mass and must always be performed in conjunction with a general examination to find possible distant metastases, a palpable abdominal mass, or gynecomastia. A correct diagnosis must be established in all patients with an intrascrotal mass [12]. Currently, diagnostic US serves to confirm the presence of a testicular mass and to explore the contralateral testis. Its sensitivity in detecting a testicular cancer is almost 100%, and it has an important role in determining whether a mass is intra- or extratesticular [12]. Retroperitoneal and mediastinal lymph nodes are best assessed by using computed tomography (CT). Magnetic resonance imaging (MRI) produces similar results to CT scanning in the detection of retroperitoneal nodal enlargement [13]. A chest CT scan is the most sensitive method of evaluating the thorax and mediastinal nodes. Other examinations, such as brain or spinal CT, bone scan, or liver US, should be performed if suspicion of metastases to these organs exists. MRI offers greater sensitivity and specificity than does US for diagnosing tumors [14,15].
4. Inguinal exploration and orchiectomy
Every patient with a suspected testicular mass must undergo inguinal exploration with exteriorization of the testis within its tunics. Immediate orchiectomy with division of the spermatic cord at the internal inguinal ring must be performed if a tumor is found. If the diagnosis is not clear, a testicular biopsy should be taken under spermatic cord clamping for frozen section histologic examination. In cases of disseminated disease, such as life-threatening metastases in the lung with pulmonary insufficiency, it is recommended to start with up-front chemotherapy, and orchiectomy can be delayed until clinical stabilization has occurred. In synchronous bilateral testicular cancer, metachronous contralateral tumors, or a tumor in a solitary testis with normal preoperative testosterone levels, organ-preserving surgery can be performed when the tumor volume is less than 30% of the testicular volume and the surgical rules are respected [16].
STAGING
The staging system recommended is the 2009 tumor-node-metastasis (TNM) staging of the International Union Against Cancer [17]. Staging includes determination of the anatomic disease extent; assessment of the STMs, including the nadir values of hCG, AFP, and LDH after orchiectomy; a clear definition of the regional nodes; and N category modifications related to the nodal size. According to the 2009 TNM classification, stage I testicular cancer includes the following substages: stage IA, pT1N0M0S0; stage IB, pT2-T4N0M0S0; and stage IS, any pT/TxN0M0S1-S3. Patients with stage IA disease have primary tumors limited to the testis and epididymis, with no evidence of microscopic vascular or lymphatic invasion by tumor cells on microscopy, no sign of metastases on clinical examination or imaging, and postorchiectomy STM levels within normal limits. Patients with stage IB have a more locally invasive primary tumor, but no sign of metastatic disease. Patients with stage IS have persistently elevated STM levels after orchiectomy, which is evidence of subclinical metastatic disease. If the STM levels are declining according to the expected half-time decay after orchiectomy, the patient is usually followed up until normalization. In large, population-based patient series, 75% to 80% of patients with seminoma and about 55% of patients with NSGCT have stage I disease at diagnosis [18,19]. True stage IS is found in about 5% of patients with nonseminoma. If staging retroperitoneal lymph node dissection were performed in patients with stage IS disease, nearly all patients would be found to have pathologic stage II disease [18]. In 1997, the IGCCCG defined a prognostic factor-based staging system for metastatic testis tumors by using the identification of some clinical, independent adverse factors. This staging system has been incorporated into the TNM classification and uses histologic type, primary tumor location, metastasis location, and prechemotherapy serum marker levels as prognostic factors to categorize patients according to a good, intermediate, or poor prognosis (Table 1) [20].
TABLE 1.
International germ cell cancer collaborative group risk classification for advanced germ cell tumors (GCTs)
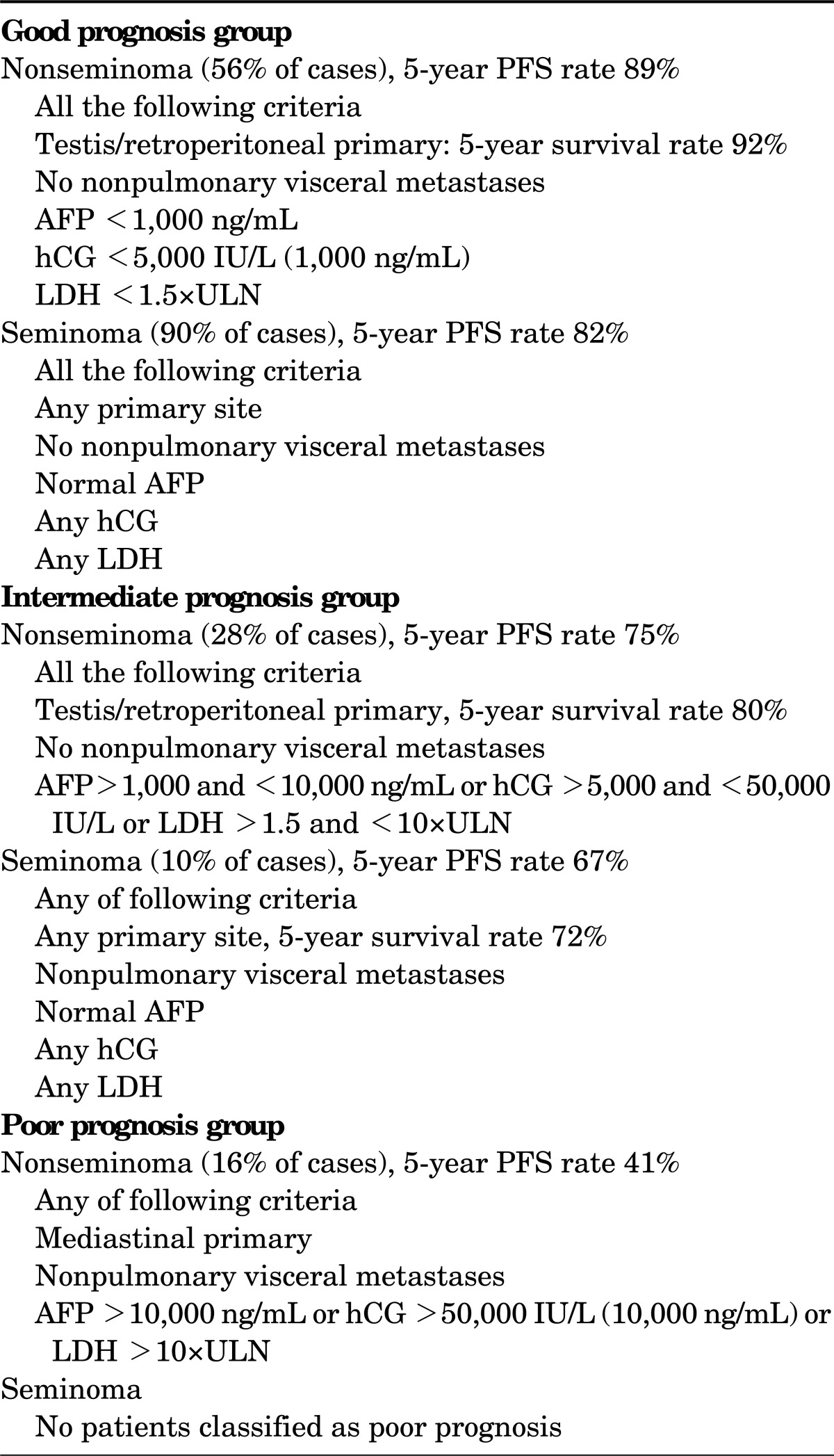
PFS, progression-free survival; AFP, alfa-fetoprotein; hCG, human chorionic gonadotropin; LDH, lactate dehydrogenase; ULN, upper limit of normal (modified from J Clin Oncol 1997;15:594-603, with permission of the American Society of Clinical Oncology [20]).
MANAGEMENT
For treatment purposes, the distinction between seminoma and NSGCT holds great importance. Compared with NSGCT, seminoma has a relatively favorable natural history. In general, seminoma tends to be less aggressive, to be diagnosed at an earlier stage, and to spread predictably along lymphatic channels to the retroperitoneum before spreading hematogenously to the lung or other organs. Seminoma is also associated with a lower incidence of occult metastasis and a lower risk of systemic relapse after treatment of the retroperitoneum, which has important implications for the use of chemotherapy. Compared with NSGCT, seminoma is exquisitely sensitive to radiation therapy and platinum-based chemotherapy [21]. RT is a standard treatment option for stage I and IIA-B seminoma, but has no role in the treatment of NSGCTs. NSGCTs are usually mixed tumors and teratoma often exists at metastatic sites with other GCT elements; cure often requires chemotherapy to kill the chemosensitive components and surgery to remove the teratomatous components [22,23].
MANAGEMENT OF STAGE I SEMINOMA
Although RT was previously the standard for patients with clinical stage I seminoma, recognition has been growing since the early 1990s that adjuvant RT is associated with an increased risk of late side effects, including second non-germ cell malignancies and cardiovascular disease [24-29]. Concerns regarding the late toxicity of RT, the success of surveillance of stage I nonseminomatous GCTs, and improvements in diagnostic imaging have led to an assessment of close surveillance after orchiectomy for stage I seminoma with treatment reserved for those with relapse. In addition, adjuvant carboplatin chemotherapy has been shown to give results similar to RT. With any of these approaches including surveillance, RT, or carboplatin chemotherapy, 5-year disease-specific survival rates of above 99% can be expected [30].
1. Surveillance
A risk-adapted approach to management has been reported by the Spanish Germ Cell Cancer Cooperative Study Group, with surveillance reserved for good prognosis patients and adjuvant therapy for patients with 1 or 2 adverse prognostic factors [31]. Prognostic factors for relapse were patient age (≤30 years vs. >30 years), tumor diameter (≤40 mm vs. >40 mm), histologic subtype (classical vs. anaplastic), pathologic T (pT) stage (pT1 or pT2 vs. pT3 or pT4), vascular invasion, rete testis invasion, and preoperative hCG levels [31]. That study confirmed that low-risk patients had a small risk of relapse. At relapse, most patients can be successfully treated with retroperitoneal RT alone. One concern regarding the routine use of surveillance was the potential for the increased use of chemotherapy. However, data from the Princess Margaret Hospital indicate that the 10-year actuarial risk of requiring chemotherapy at any point in the treatment of patients was 4.6% in patients treated by surveillance and 3.9% in those who underwent adjuvant RT, which suggests that the increase in the use of chemotherapy in patients followed up by surveillance is not significant [32]. However, an optimal follow-up strategy for patients on surveillance has not yet been determined.
2. Adjuvant RT
Adjuvant retroperitoneal RT was the standard treatment of stage I seminoma for 60 years. The overall survival rate in most series in the modern era has been 92% to 99% at 10 years, with few, if any, deaths from seminoma. In large, single, or multi-institutional series, the relapse rate has varied from 0.5% to 5% [33-35]. The most common sites of relapse after adjuvant RT are the mediastinum, lung, and left supraclavicular fossa. In patients with stage I disease treated to the para-aortic nodes alone, relapses are also seen in the pelvic nodes. Chemotherapy is the treatment of choice for supra-diaphragmatic relapse and gives close to a 100% cure rate. Most relapses occur within 2 years of RT. Follow-up efforts should therefore concentrate on the first 2 years after RT and include clinical examination, chest radiography, and CT of the pelvis.
3. Adjuvant chemotherapy
Another strategy that has been investigated to reduce the long-term toxicity of adjuvant RT for stage I seminoma is the use of adjuvant chemotherapy. An update of the study was presented at the American Society of Clinical Oncology 2008 annual meeting. After a median follow-up of 6.5 years, the 5-year relapse rates were 4% and 5.3%, respectively, for RT and chemotherapy. An unexpected finding in that study was a reduction in the observed number of second primary GCTs in patients treated with adjuvant chemotherapy, with a 5-year event rate of 1.96% with RT versus 0.54% with chemotherapy. One major unanswered question about carboplatin chemotherapy in this setting is whether there are late effects of treatment. As with RT, platinum-based chemotherapy has been associated with an increased risk of cancer and heart disease. Although the total chemotherapy dose used in the treatment of stage I seminoma is low compared with the chemotherapy dose given for more advanced-stage disease, only long-term follow-up studies will inform us whether long-term health issues are associated with 1 or 2 doses of carboplatin. The vast majority of relapses occur within the first 3 years, and follow-up efforts should thus concentrate on this period with less frequent visits thereafter.
MANAGEMENT OF STAGE II SEMINOMA
At workup after orchiectomy, about 15% to 20% of patients have radiologically involved para-aortic lymph nodes. The number of patients with stage II disease has been too small to mount phase III studies of treatment, and treatment decisions must be determined from reports from single institutions where patients have been treated in a uniform fashion. The most important prognostic factor in stage II seminoma is the bulk of the retroperitoneal tumor. The lymph node size was the only factor that predicted recurrence in 95 patients with stage II seminoma treated with RT at the Princess Margaret Hospital from 1981 to 1999 [36]. The 5-year relapse-free rate in 79 patients with nodal disease of less than 5 cm (stage IIA-IIB) was 91% (7 of 79 patients) compared with 44% (9 of 16 patients) in patients with bulkier disease (stage IIC). Of these patients, 13 were treated with chemotherapy at relapse, and 9 were free of disease at the last follow-up visit. However, the high failure rate after RT in patients with bulky retroperitoneal disease, that not all patients with recurrence can be salvaged, and the apparently better outcome of similar patients who were treated with chemotherapy at diagnosis mandates primary chemotherapy, instead of RT, for this population. Staging should not be the only parameter used to decide the treatment of retroperitoneal disease in patients with stage II seminoma. The tumor bulk must also be considered. In patients with such bulky disease, chemotherapy, rather than RT, should be used [36]. The technique of RT for stage II seminoma is similar to that used for stage I disease. The treatment volume includes the gross tumor and the para-aortic and ipsilateral common and external iliac lymph nodes. The radiation dose is typically 25 Gy in 20 daily fractions, plus a boost of an additional 10 Gy to the gross lymphadenopathy [36]. The use of combination carboplatin and RT in stage IIA-IIB seminoma has been suggested by Gilbert et al. [37]. They described a series of 62 patients treated with 1 to 2 courses of carboplatin 4 to 6 weeks before RT. Since 1997, 29 patients have been treated with 1 course of carboplatin before RT to the para-aortic nodes alone, and no relapses were observed. This approach is attractive in that it offers the potential of reducing the treatment volume with RT, at the same time improving the results compared with RT alone. However, this approach cannot be accepted as routine practice without additional study, especially because the use of combined modality therapy has been shown to increase the risk of second non-GCT and cardiovascular disease in long-term survivors [38]. If chemotherapy is recommended as the primary treatment or for relapse after RT, 3 cycles of bleomycin, etoposide, and cisplatin (BEP) or 4 courses of etoposide and cisplatin (EP) should be considered as standard options.
RESIDUAL MASS AFTER RADIOTHERAPY OR CHEMOTHERAPY
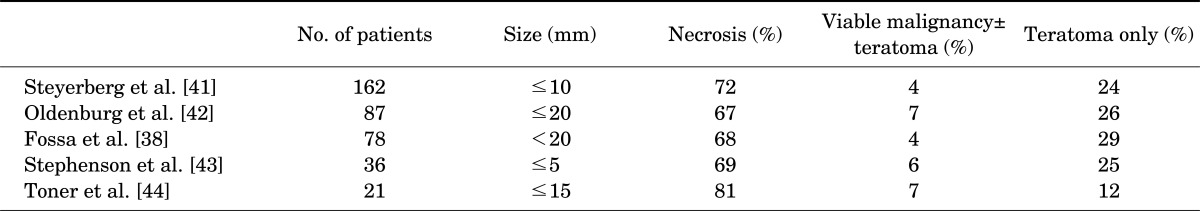
After treatment, patients with stage II disease require follow-up imaging of the abdomen until complete disease regression has occurred. A stable, persistent mass often represents fibrosis or necrosis, and only a few such masses will contain active tumor. However, the possibility of a nonseminomatous component to explain the residual mass must be kept in mind, even in patients whose primary tumors show pure seminoma [39]. The Memorial Sloan-Kettering Cancer Center group published their data from 55 of 104 patients who had demonstrated residual masses after chemotherapy [40]. Of these 55 patients, 32 (58%) had undergone formal retroperitoneal lymph node dissection, and 23 (42%) had multiple intraoperative biopsies performed because the residual mass was deemed unresectable. Among patients with a mass of more than 3 cm (n = 27), 8 (30%) had a residual viable tumor. Of the 8 recurrences, 2 were teratoma and 6 were seminoma. Given this high proportion of persistent malignancy, the Memorial Sloan-Kettering Cancer Center investigators have recommended resection or biopsy of masses of 3 cm or larger. Numerous studies have demonstrated that, on average, patients with residual masses of 2 cm or smaller have a 30% and 6% incidence of teratoma and viable malignancy, respectively (Table 2). In patients with disseminated seminoma, postchemotherapy masses of smaller than 3 cm may be safely observed, whereas patients with masses of larger than 3 cm should be evaluated with positron emission tomography or CT 2 months after completion of chemotherapy, with very selective administration of postchemotherapy retroperitoneal lymph node dissection (PC-RPLND). Late relapse occurring more than 2 years after chemotherapy is rare, and surgery remains the mainstay of therapy in cases of resectable masses independent of tumor markers. Surgery should always be considered for resectable masses following salvage therapies or in chemoresistant disease to maximize the chance of cure.
TABLE 2.
Histology of postchemotherapy residual masses less than 20 mm
MANAGEMENT OF STAGE I NSGCT
An estimated 20% to 30% of patients with clinical stage I NSGCTs have occult metastasis, with the retroperitoneum being the most common site. Thus, any treatment after orchiectomy represents overtreatment for most patients. The long-term, cancer-specific survival rate approaches 100% for all patients, regardless of the initial treatment strategy. Thus, efforts to reduce treatment-related toxicity are paramount in the treatment of these patients.
1. Surveillance
The rationale for surveillance for clinical stage I NSGCT is based on the majority of patients being cured by orchiectomy, thus avoiding unnecessary treatment-related morbidity and cost. Patients receiving active surveillance should undergo frequent evaluations in the first 2 years with chest imaging, CT abdominopelvic imaging, STM determinations, and clinical assessment. Continued surveillance over 5 years with chest imaging, STM determinations, and clinical assessment is recommended. Surveillance is not recommended for those who are anticipated to be poorly compliant. The standard treatment of relapse is induction chemotherapy, although primary RPLND can be considered for patients with nonbulky (<5 cm) retroperitoneal disease and normal serum AFP and hCG levels and with the availability of experienced surgeons [45-51].
2. RPLND
The rationale for RPLND with clinical stage I NSGCT is based on the retroperitoneum being the most common site of occult metastasis without systemic disease and the high cure rates after RPLND alone in patients with occult retroperitoneal metastasis and teratoma. A full, bilateral template dissection has been associated with the lowest risk of abdominopelvic recurrence (<2%) and a high rate of antegrade ejaculation (>90%) when nerve-sparing techniques have been used. Bilateral template RPLND with nerve sparing is recommended in patients who desire future paternity [52-54]. However, attempts at nerve sparing should not compromise the completeness of the resection. Also, patients should be informed of the risk of relapse after RPLND and the potential benefits and risks of these approaches.
3. Primary chemotherapy
The rationale for primary chemotherapy for clinical stage I NSGCT is based on the low risk of relapse after RPLND in pathologic stage II patients receiving 2 cycles of adjuvant cisplatin-based chemotherapy. Two cycles of cisplatin-based primary chemotherapy is recommended for clinical stage I NSGCT [55,56]. The durable efficacy and safety of these studies have established 2 cycles of chemotherapy as the standard regimen when given as the primary treatment of clinical stage I NSGCT and as adjuvant treatment after RPLND for pathologic stage II disease. Routine abdominopelvic CT should be included in the surveillance of patients after chemotherapy.
MANAGEMENT OF STAGE IS NSGCT
Patients with no clinical evidence of metastatic NSGCT after orchiectomy other than persistently elevated or increasing AFP or HCG levels should receive chemotherapy, just as for advanced disease, usually with either BEP×3 cycles or EP×4 cycles [57-59]. Studies of primary RPLND for clinical stage IS NSGCT have reported that 37% to 100% of patients subsequently required chemotherapy for retroperitoneal metastasis, persistently elevated STM, or relapse [57-59]. A general consensus has been reached that these patients should receive induction chemotherapy. An elevated STM level at a single point after orchiectomy does not necessarily indicate stage IS disease. In this situation, the marker might be decreasing according to its expected biological half-life, and the measurements should be repeated to clarify the situation.
MANAGEMENT OF STAGE IIA AND IIB NSGCT
Patients with elevated postorchiectomy AFP or HCG levels should receive induction chemotherapy. Induction chemotherapy and primary RPLND are acceptable treatment options for patients with clinical stage IIA with normal postorchiectomy AFP and HCG levels. Also, induction chemotherapy is the preferred treatment in patients with clinical stage IIB with normal postorchiectomy AFP and HCG levels [60,61]. Patients should be informed of both treatments, including the potential short- and long-term treatment-related toxicity and the risk and nature of any additional treatments. The decision to proceed with induction chemotherapy or RPLND should be determined by patient preference and the specific expertise of the treating physician and institution.
MANAGEMENT OF POSTCHEMOTHERAPY RESIDUAL MASSES IN NSGCT
Approximately one-third of patients who undergo chemotherapy for metastatic NSGCT have residual retroperitoneal disease. Patients who obtain a complete serologic remission and radiographic residual mass in the transverse axial CT of diameter <1 cm after chemotherapy have a 6% to 9% risk of relapse [55].These patients are considered by most experts to be at low risk of relapse. There is universal agreement that patients with residual radiographic masses >1 cm following initial chemotherapy require resection [55]. Patients with a completely resected teratoma in only the PC-RPLND specimen have a >90% chance of cure, whereas patients with viable GCT should be considered for additional therapy [62].
MANAGEMENT OF ADVANCED SEMINOMA AND NSGCT
Patients with advanced GCT can achieve long-term, disease-free survival when chemotherapy is combined with expert and judicious resection of residual disease. Patients with advanced GCTs should choose a chemotherapy regimen on the basis of their IGCCCG risk classification (Table 1). BEP×3 cycles or BEP×4 cycles is the standard therapy for good-risk patients with advanced GCT. BEP×4 cycles is the standard therapy for poor- and intermediate-risk patients with GCT. Using these treatments, we can achieve durable remissions of approximately 90%, 75%, and 45% in patients with good, intermediate, and poor risk, respectively [62-68].
1. Treatment of good prognosis GCT
Three cycles of BEP is the current standard for patients with good prognosis GCT, because of the report by Einhorn et al. [62] in 1989. Demonstrating the equivalent efficacy of 3 cycles of BEP to 4 cycles of BEP with less toxicity in good-risk patients. The outcome of several other randomized trials that included patients with seminoma and nonseminoma led to the establishment of this regimen as the standard of care for patients with good prognosis GCT [63-65]. However, in the case of contraindications against, or the risks associated with, using bleomycin, EP given for 4 cycles is an acceptable alternative for patients with good prognosis seminoma or NSGCT.
2. Treatment of Intermediate Prognosis GCT
From the data in randomized trials using BEP within the IGCCCG prognostic analysis, as well as randomized trials comparing cisplatin, vinblastine, bleomycin (PVB), and BEP, 4 cycles of BEP should be regarded as the standard treatment of intermediate prognosis seminoma and nonseminoma. A small trial of the EORTC of intermediate prognosis patients compared BEP×4 cycles and etoposide, ifosfamide, cisplatin (VIP)×4 cycles and showed no difference in the response rate, disease-free survival, or overall survival [66]. Therefore, the exchange of bleomycin with ifosfamide does not improve the outcome of this specific patient population. However, that study showed that in the case of contraindications against, or risks with, the use of bleomycin, this drug could be substituted with ifosfamide without losing efficacy at the expense of having some more bone marrow toxicity.
3. Treatment of Poor Prognosis GCT
As for intermediate prognosis, the randomized trials comparing PVB and BEP for 4 cycles have established that BEP is more active in this poor prognosis population and should be the standard of care. The comparison of the standard with double-dose platinum or a sequential alternating protocol with PVB or bleomycin, vincristine, and cisplatin, as well as VIP×4, did not show any improvement with these more complex or alternative protocols compared with the standard BEP×4 cycles [66-68]. Additional efforts to improve the outcomes of patients with poor-risk GCT have largely been unsuccessful. Given its efficacy and toxicity, BEP×4 remains the standard of care for poor prognosis patients.
CONCLUSIONS
For the management of seminoma and NSGCTs, clear standards have been defined on the basis of the results of prospective clinical trials. Careful management according to these guidelines will give the patient the greatest chance for a high cure rate or at least an optimal outcome.
Footnotes
The authors have nothing to disclose.
References
- 1.Schottenfeld D, Warshauer ME, Sherlock S, Zauber AG, Leder M, Payne R. The epidemiology of testicular cancer in young adults. Am J Epidemiol. 1980;112:232–246. doi: 10.1093/oxfordjournals.aje.a112989. [DOI] [PubMed] [Google Scholar]
- 2.Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol. 2003;170:5–11. doi: 10.1097/01.ju.0000053866.68623.da. [DOI] [PubMed] [Google Scholar]
- 3.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242–253. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 4.Forman D, Oliver RT, Brett AR, Marsh SG, Moses JH, Bodmer JG, et al. Familial testicular cancer: a report of the UK family register, estimation of risk and an HLA class 1 sib-pair analysis. Br J Cancer. 1992;65:255–262. doi: 10.1038/bjc.1992.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westergaard T, Olsen JH, Frisch M, Kroman N, Nielsen JW, Melbye M. Cancer risk in fathers and brothers of testicular cancer patients in Denmark. A population-based study. Int J Cancer. 1996;66:627–631. doi: 10.1002/(SICI)1097-0215(19960529)66:5<627::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Dieckmann KP, Loy V, Buttner P. Prevalence of bilateral testicular germ cell tumours and early detection based on contralateral testicular intra-epithelial neoplasia. Br J Urol. 1993;71:340–345. doi: 10.1111/j.1464-410x.1993.tb15955.x. [DOI] [PubMed] [Google Scholar]
- 7.Germa-Lluch JR, Garcia del Muro X, Maroto P, Paz-Ares L, Arranz JA, Guma J, et al. Clinical pattern and therapeutic results achieved in 1490 patients with germ-cell tumours of the testis: the experience of the Spanish Germ-Cell Cancer Group (GG) Eur Urol. 2002;42:553–562. doi: 10.1016/s0302-2838(02)00439-6. [DOI] [PubMed] [Google Scholar]
- 8.Skakkebaek NE. Possible carcinoma-in-situ of the testis. Lancet. 1972;2:516–517. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- 9.Klein EA. Tumor markers in testis cancer. Urol Clin North Am. 1993;20:67–73. [PubMed] [Google Scholar]
- 10.Wanderas EH, Tretli S, Fossa SD. Trends in incidence of testicular cancer in Norway 1955-1992. Eur J Cancer. 1995;31A:2044–2048. doi: 10.1016/0959-8049(95)00321-5. [DOI] [PubMed] [Google Scholar]
- 11.Peyret C. Tumeurs du testicule. Synthèse et recommandations en onco-urologie. Prog Urol. 1993;2:60–64. [Google Scholar]
- 12.Martin JM, Panzarella T, Zwahlen DR, Chung P, Warde P. Evidence-based guidelines for following stage 1 seminoma. Cancer. 2007;109:2248–2256. doi: 10.1002/cncr.22674. [DOI] [PubMed] [Google Scholar]
- 13.Ellis JH, Bies JR, Kopecky KK, Klatte EC, Rowland RG, Donohue JP. Comparison of NMR and CT imaging in the evaluation of metastatic retroperitoneal lymphadenopathy from testicular carcinoma. J Comput Assist Tomogr. 1984;8:709–719. doi: 10.1097/00004728-198408000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Thurnher S, Hricak H, Carroll PR, Pobiel RS, Filly RA. Imaging the testis: comparison between MR imaging and US. Radiology. 1988;167:631–636. doi: 10.1148/radiology.167.3.3283834. [DOI] [PubMed] [Google Scholar]
- 15.Mattrey RF. Magnetic resonance imaging of the scrotum. Semin Ultrasound CT MR. 1991;12:95–108. [PubMed] [Google Scholar]
- 16.Heidenreich A, Weissbach L, Holtl W, Albers P, Kliesch S, Kohrmann KU, et al. Organ sparing surgery for malignant germ cell tumor of the testis. J Urol. 2001;166:2161–2165. doi: 10.1016/s0022-5347(05)65526-7. [DOI] [PubMed] [Google Scholar]
- 17.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7th ed. Chichester (UK): Wiley-Blackwell; 2009. [Google Scholar]
- 18.Zagars GK. Management of stage I seminoma: radiotherapy. In: Horwich A, editor. Testicular cancer: investigation and management. 2nd ed. London: Chapman & Hall Medical; 1999. p. 99. [Google Scholar]
- 19.Klepp O, Flodgren P, Maartman-Moe H, Lindholm CE, Unsgaard B, Teigum H, et al. Early clinical stages (CS1, CS1Mk+ and CS2A) of non-seminomatous testis cancer. Value of pre- and post-orchiectomy serum tumor marker information in prediction of retroperitoneal lymph node metastases. Swedish-Norwegian Testicular Cancer Project (SWENOTECA) Ann Oncol. 1990;1:281–288. doi: 10.1093/oxfordjournals.annonc.a057749. [DOI] [PubMed] [Google Scholar]
- 20.International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 21.Warde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20:4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Bokemeyer C, Kuczyk MA, Serth J, Hartmann JT, Schmoll HJ, Jonas U, et al. Treatment of clinical stage I testicular cancer and a possible role for new biological prognostic parameters. J Cancer Res Clin Oncol. 1996;122:575–584. doi: 10.1007/BF01221188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C, et al. Risk factors for relapse in clinical stage I non-seminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol. 2003;21:1505–1512. doi: 10.1200/JCO.2003.07.169. [DOI] [PubMed] [Google Scholar]
- 24.Zagars GK, Ballo MT, Lee AK, Strom SS. Mortality after cure of testicular seminoma. J Clin Oncol. 2004;22:640–647. doi: 10.1200/JCO.2004.05.205. [DOI] [PubMed] [Google Scholar]
- 25.van Leeuwen FE, Stiggelbout AM, van den Belt-Dusebout AW, Noyon R, Eliel MR, van Kerkhoff EH, et al. Second cancer risk following testicular cancer: a follow-up study of 1,909 patients. J Clin Oncol. 1993;11:415–424. doi: 10.1200/JCO.1993.11.3.415. [DOI] [PubMed] [Google Scholar]
- 26.van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MW, Ribot JG, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370–4378. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 27.van den Belt-Dusebout AW, Nuver J, de Wit R, Gietema JA, ten Bokkel Huinink WW, Rodrigus PT, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2006;24:467–475. doi: 10.1200/JCO.2005.02.7193. [DOI] [PubMed] [Google Scholar]
- 28.Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 29.Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21:1513–1523. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- 30.Oliver RT, Mason MD, Mead GM, von der Maase H, Rustin GJ, Joffe JK, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293–300. doi: 10.1016/S0140-6736(05)66984-X. [DOI] [PubMed] [Google Scholar]
- 31.Aparicio J, Germa JR, Garcia del Muro X, Maroto P, Arranz JA, Saenz A, et al. Risk-adapted management for patients with clinical stage I seminoma: the Second Spanish Germ Cell Cancer Cooperative Group study. J Clin Oncol. 2005;23:8717–8723. doi: 10.1200/JCO.2005.01.9810. [DOI] [PubMed] [Google Scholar]
- 32.Tolan S, Vesprini D, Jewett MA, Warde PR, O'Malley M, Panzarella T, et al. No role for routine chest radiography in stage I seminoma surveillance. Eur Urol. 2010;57:474–479. doi: 10.1016/j.eururo.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Santoni R, Barbera F, Bertoni F, De Stefani A, Livi L, Paiar F, et al. Stage I seminoma of the testis: a bi-institutional retrospective analysis of patients treated with radiation therapy only. BJU Int. 2003;92:47–52. doi: 10.1046/j.1464-410x.2003.04273.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones WG, Fossa SD, Mead GM, Roberts JT, Sokal M, Horwich A, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I testicular seminoma: a report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN 18525328) J Clin Oncol. 2005;23:1200–1208. doi: 10.1200/JCO.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Fossa SD, Horwich A, Russell JM, Roberts JT, Cullen MH, Hodson NJ, et al. Medical Research Council Testicular Tumor Working Group. Optimal planning target volume for stage I testicular seminoma: a Medical Research Council randomized trial. J Clin Oncol. 1999;17:1146. doi: 10.1200/JCO.1999.17.4.1146. [DOI] [PubMed] [Google Scholar]
- 36.Chung PW, Gospodarowicz MK, Panzarella T, Jewett MA, Tew-George B, et al. Stage II testicular seminoma: patterns of recurrence and outcome of treatment. Eur Urol. 2004;45:754–759. doi: 10.1016/j.eururo.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert DC, Vanas NJ, Beesley S, Bloomfield D, Money-Kyrle J, Norman A, et al. Treating IIA/B seminoma with combination carboplatin and radiotherapy. J Clin Oncol. 2009;27:2101–2102. doi: 10.1200/JCO.2008.21.5269. [DOI] [PubMed] [Google Scholar]
- 38.Fossa SD, Gilbert E, Dores GM, Chen J, McGlynn KA, Schonfeld S, et al. Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst. 2007;99:533–544. doi: 10.1093/jnci/djk111. [DOI] [PubMed] [Google Scholar]
- 39.Mosharafa AA, Foster RS, Leibovich BC, Bihrle R, Johnson C, Donohue JP. Is post-chemotherapy resection of seminomatous elements associated with higher acute morbidity? J Urol. 2003;169:2126–2128. doi: 10.1097/01.ju.0000060121.33899.4b. [DOI] [PubMed] [Google Scholar]
- 40.Herr HW, Sheinfeld J, Puc HS, Heelan R, Bajorin DF, Mencel P, et al. Surgery for a post-chemotherapy residual mass in seminoma. J Urol. 1997;157:860–862. [PubMed] [Google Scholar]
- 41.Steyerberg EW, Keizer HJ, Fossa SD, Sleijfer DT, Toner GC, Schraffordt Koops H, et al. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic nonseminomatous germ cell tumor: multivariate analysis of individual patient data from six study groups. J Clin Oncol. 1995;13:1177–1187. doi: 10.1200/JCO.1995.13.5.1177. [DOI] [PubMed] [Google Scholar]
- 42.Oldenburg J, Alfsen GC, Lien HH, Aass N, Waehre H, Fossa SD. Postchemotherapy retroperitoneal surgery remains necessary in patients with nonseminomatous testicular cancer and minimal residual tumor masses. J Clin Oncol. 2003;21:3310–3317. doi: 10.1200/JCO.2003.03.184. [DOI] [PubMed] [Google Scholar]
- 43.Stephenson AJ, Bosl GJ, Motzer RJ, Bajorin DF, Stasi JP, Sheinfeld J. Nonrandomized comparison of primary chemotherapy and retroperitoneal lymph node dissection for clinical stage IIA and IIB nonseminomatous germ cell testicular cancer. J Clin Oncol. 2007;25:5597–5602. doi: 10.1200/JCO.2007.12.0808. [DOI] [PubMed] [Google Scholar]
- 44.Toner GC, Neerhut GJ, Schwarz MA, Thursfield VJ, Sandeman TF, Giles GG, et al. The management of testicular cancer in Victoria, 1988-1993. Urology Study Committee of the Victorian Co-operative Oncology Group. Med J Aust. 2001;174:328–331. doi: 10.5694/j.1326-5377.2001.tb143306.x. [DOI] [PubMed] [Google Scholar]
- 45.Abratt RP, Pontin AR, Barnes RD, Reddi BV. Adjuvant chemotherapy for stage I non-seminomatous testicular cancer. S Afr Med J. 1994;84:605–607. [PubMed] [Google Scholar]
- 46.Amato RJ, Ro JY, Ayala AG, Swanson DA. Risk-adapted treatment for patients with clinical stage I nonseminomatous germ cell tumor of the testis. Urology. 2004;63:144–148. doi: 10.1016/j.urology.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 47.Bohlen D, Borner M, Sonntag RW, Fey MF, Studer UE. Long-term results following adjuvant chemotherapy in patients with clinical stage I testicular nonseminomatous malignant germ cell tumors with high risk factors. J Urol. 1999;161:1148–1152. [PubMed] [Google Scholar]
- 48.Chevreau C, Mazerolles C, Soulie M, Gaspard MH, Mourey L, Bujan L, et al. Long-term efficacy of two cycles of BEP regimen in high-risk stage I nonseminomatous testicular germ cell tumors with embryonal carcinoma and/or vascular invasion. Eur Urol. 2004;46:209–214. doi: 10.1016/j.eururo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Cullen MH, Stenning SP, Parkinson MC, Fossa SD, Kaye SB, Horwich AH, et al. Short-course adjuvant chemotherapy in high-risk stage I nonseminomatous germ cell tumors of the testis: a Medical Research Council report. J Clin Oncol. 1996;14:1106–1113. doi: 10.1200/JCO.1996.14.4.1106. [DOI] [PubMed] [Google Scholar]
- 50.Dearnaley DP, Fossa SD, Kaye SB, Cullen MH, Harland SJ, Sokal MP, et al. Adjuvant bleomycin, vincristine and cisplatin (BOP) for high-risk stage I non-seminomatous germ cell tumours: a prospective trial (MRC TE17) Br J Cancer. 2005;92:2107–2113. doi: 10.1038/sj.bjc.6602624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliver RT, Ong J, Shamash J, Ravi R, Nagund V, Harper P, et al. Long-term follow-up of Anglian Germ Cell Cancer Group surveillance versus patients with Stage 1 nonseminoma treated with adjuvant chemotherapy. Urology. 2004;63:556–561. doi: 10.1016/j.urology.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 52.Donohue JP, Foster RS. Retroperitoneal lymphadenectomy in staging and treatment. The development of nerve-sparing techniques. Urol Clin North Am. 1998;25:461–468. doi: 10.1016/s0094-0143(05)70035-5. [DOI] [PubMed] [Google Scholar]
- 53.Eggener SE, Carver BS, Sharp DS, Motzer RJ, Bosl GJ, Sheinfeld J. Incidence of disease outside modified retroperitoneal lymph node dissection templates in clinical stage I or IIA nonseminomatous germ cell testicular cancer. J Urol. 2007;177:937–942. doi: 10.1016/j.juro.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 54.Jewett MA, Kong YS, Goldberg SD, Sturgeon JF, Thomas GM, Alison RE, et al. Retroperitoneal lymphadenectomy for testis tumor with nerve sparing for ejaculation. J Urol. 1988;139:1220–1224. doi: 10.1016/s0022-5347(17)42869-2. [DOI] [PubMed] [Google Scholar]
- 55.Ondrus D, Hornak M, Matoska J, Kausitz J, Belan V. Primary chemotherapy in the management of low stage (IIA and IIB) non-seminomatous germ cell testicular tumours. Int Urol Nephrol. 1992;24:299–304. doi: 10.1007/BF02549539. [DOI] [PubMed] [Google Scholar]
- 56.Pont J, Albrecht W, Postner G, Sellner F, Angel K, Holtl W. Adjuvant chemotherapy for high-risk clinical stage I non-seminomatous testicular germ cell cancer: long-term results of a prospective trial. J Clin Oncol. 1996;14:441–448. doi: 10.1200/JCO.1996.14.2.441. [DOI] [PubMed] [Google Scholar]
- 57.Davis BE, Herr HW, Fair WR, Bosl GJ. The management of patients with nonseminomatous germ cell tumors of the testis with serologic disease only after orchiectomy. J Urol. 1994;152:111–113. doi: 10.1016/s0022-5347(17)32830-6. [DOI] [PubMed] [Google Scholar]
- 58.Saxman SB, Nichols CR, Foster RS, Messemer JE, Donohue JP, Einhorn LH. The management of patients with clinical stage I nonseminomatous testicular tumors and persistently elevated serologic markers. J Urol. 1996;155:587–589. [PubMed] [Google Scholar]
- 59.Culine S, Theodore C, Terrier-Lacombe MJ, Droz JP. Primary chemotherapy in patients with nonseminomatous germ cell tumors of the testis and biological disease only after orchiectomy. J Urol. 1996;155:1296–1298. [PubMed] [Google Scholar]
- 60.Lerner SE, Mann BS, Blute ML, Richardson RL, Zincke H. Primary chemotherapy for clinical stage II nonseminomatous germ cell testicular tumors: selection criteria and long-term results. Mayo Clin Proc. 1995;70:821–828. doi: 10.1016/S0025-6196(11)63938-4. [DOI] [PubMed] [Google Scholar]
- 61.Socinski MA, Garnick MB, Stomper PC, Fung CY, Richie JP. Stage II nonseminomatous germ cell tumors of the testis: an analysis of treatment options in patients with low volume retroperitoneal disease. J Urol. 1988;140:1437–1441. doi: 10.1016/s0022-5347(17)42067-2. [DOI] [PubMed] [Google Scholar]
- 62.Einhorn LH, Williams SD, Loehrer PJ, Birch R, Drasga R, Omura G, et al. Evaluation of optimal duration of chemotherapy in favorable-prognosis disseminated germ cell tumors: a Southeastern Cancer Study Group protocol. J Clin Oncol. 1989;7:387–391. doi: 10.1200/JCO.1989.7.3.387. [DOI] [PubMed] [Google Scholar]
- 63.Bokemeyer C, Kohrmann O, Tischler J, Weissbach L, Rath U, Haupt A, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with 'good-risk' metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015–1021. doi: 10.1093/oxfordjournals.annonc.a010493. [DOI] [PubMed] [Google Scholar]
- 64.Horwich A, Sleijfer DT, Fossa SD, Kaye SB, Oliver RT, Cullen MH, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844–1852. doi: 10.1200/JCO.1997.15.5.1844. [DOI] [PubMed] [Google Scholar]
- 65.Horwich A, Oliver RT, Wilkinson PM, Mead GM, Harland SJ, Cullen MH, et al. A medical research council randomized trial of single agent carboplatin versus etoposide and cisplatin for advanced metastatic seminoma. MRC Testicular Tumour Working Party. Br J Cancer. 2000;83:1623–1629. doi: 10.1054/bjoc.2000.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Wit R, Stoter G, Sleijfer DT, Neijt JP, ten Bokkel Huinink WW, de Prijck L, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78:828–832. doi: 10.1038/bjc.1998.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nichols CR, Williams SD, Loehrer PJ, Greco FA, Crawford ED, Weetlaufer J, et al. Randomized study of cisplatin dose intensity in poor-risk germ cell tumors: a Southeastern Cancer Study Group and Southwest Oncology Group protocol. J Clin Oncol. 1991;9:1163–1172. doi: 10.1200/JCO.1991.9.7.1163. [DOI] [PubMed] [Google Scholar]
- 68.Kaye SB, Mead GM, Fossa S, Cullen M, de Wit R, Bodrogi I, et al. Intensive induction-sequential chemotherapy with BOP/VIP-B compared with treatment with BEP/EP for poor-prognosis metastatic nonseminomatous germ cell tumor: a Randomized Medical Research Council/European Organization for Research and Treatment of Cancer study. J Clin Oncol. 1998;16:692–701. doi: 10.1200/JCO.1998.16.2.692. [DOI] [PubMed] [Google Scholar]


