Abstract
The body map in the parietal neocortex is built by inputs from the brainstem and thalamic somatosensory nuclei. Receptor density in the sensory periphery and neural activity play a major role in allocation of cortical tissue to different components of the somatosensory body map. Here we present evidence that neural activity mediated via N-methyl-D-aspartate (NMDA) receptors plays a major role in parcellation of the cortical body map subdivisions. In mice with genetically lowered NMDA receptor function along the trigeminal pathway, subcortical trigeminal nuclei shrink and, consequently, the face representation area of the primary somatosensory cortex diminishes in size. In contrast, dorsal column subcortical paw representation areas that are not as severely affected by the genetic manipulation of NMDA receptors do not show any areal changes, yet their cortical projection zones expand. Our findings indicate that both subcortical and cortical mechanisms contribute to cortical parcellation of body map subdivisions in an NMDA receptor-dependent manner.
Indexing terms: barrels, barreloids, trigeminal system, transgenic mice
A unique feature of the mammalian neocortex is its areal specification. Somatosensory, auditory, and visual sensory epithelia are mapped within precise cortical regions that are remarkably similar in all mammals. The locations of the primary sensory cortices are set by gradients of differential gene expression during early development of the telencephalic vesicles (Ragsdale and Grove, 2001; Grove and Fukuchi-Shimogori, 2003; López-Bendito and Molnár, 2003). However, these intrinsically allocated cortical areas can shift or change their properties when thalamocortical inputs or sensory stimulation and distributions of receptors in the periphery are altered (for reviews see Fox, 2002, Kaas, 2002; Kaas and Catania, 2002; Rauschecker, 2002; Wall et al., 2002). The sensory periphery is mapped onto the neocortex across multiple synapses through subcortical structures. Another conspicuous feature of the sensory maps in the neocortex (and associated subcortical nuclei) is the patterning of pre- and postsynaptic inputs that replicate a specific feature of the sensory periphery. Ocular dominance columns in layer IVc of the primate visual cortex and “whisker barrels” in layer IV of the rodent somatosensory cortex are well-known examples. In layer IV of the primary somatosensory cortex of nocturnal rodents, thalamocortical axon arbors and their postsynaptic partners form discrete modules (“barrels”) that replicate the patterned distribution of whiskers on the snout in a one-to-one fashion (Woolsey and Van der Loos, 1970; Killackey, 1973). Similar modules are also present in the forepaw and hind paw representation areas (Belford and Killackey, 1978; Dawson and Killackey, 1987).
Patterned cortical maps (barrel fields) are established during a critical period in development and depend on an intact sensory periphery and subcortical somatosensory structures (for reviews see Woolsey, 1990; O’Leary et al., 1994; Killackey et al., 1995). Whisker and digit-related patterns are first established in the brainstem somatosensory nuclei, then in the ventroposteromedial/ventroposterolateral nuclei (VPM/VPL) of the dorsal thalamus, and finally in the neocortex. Such cellular modules are called “barreloids” in the VPM/VPL, and “barrelettes” in the brainstem (Woolsey and Van der Loos, 1970; Van der Loos, 1976; Ma and Woolsey, 1984). The instructive role of the sensory periphery in sculpting central neural patterns has been demonstrated by lesion studies performed with perinatal rodents and with mice selectively bred for aberrant numbers of whiskers (Welker and Van der loos, 1986; Woolsey, 1990; O’Leary et al., 1994; Killackey et al., 1995). Several lines of evidence also indicate that somatosensory periphery-related neural maps and patterns are conveyed to target cells by the afferents at each synaptic relay station (Erzurumlu and Jhaveri, 1990, 1992a,b; Senft and Woolsey, 1991).
Somatotopic maps are established via a number of axon guidance cues, independent of neural activity (Erzurumlu et al., 1990, Maier et al., 1999; Vanderhaeghen et al., 2000; Fukuchi-Shimogori and Grove, 2001; Grove and Fukuchi-Shimogori, 2003; López-Bendito and Molnár, 2003). On the other hand, neural patterning within “somatotopic” maps is controlled by activity-mediated mechanisms (for review see Erzurumlu and Kind, 2001). The results of numerous pharmacological-blockade and genetic-manipulation studies support the conclusion that N-methyl-D-aspartate receptor (NMDAR)-mediated neural activity plays a major role in refinement of neural connections and plasticity of vertebrate sensory pathways (Cline et al., 1987; Hahm et al., 1991; Simon et al., 1992; Li et al., 1994; Kutsuwada et al., 1996; Iwasato et al., 1997, 2000). Given the demonstrated role of NMDAR-mediated activity in patterning of somatotopic maps, we investigated whether NMDAR-dependent patterning within subcortical and cortical maps plays a role in the allocation of cortical tissue to the representation of different portions of the body map. We present evidence, from mice with genetic alterations of NMDAR function, that patterned components of the cortical body map subdivisions have advantages over other subdivisions that do not develop patterns, by claiming more cortical territory. We conclude that NMDAR signaling plays a major role in setting up cortical map subdivision boundaries.
MATERIALS AND METHODS
Generation and genotyping of NMDAR1 knockdown mice
NMDAR1 knockdown (NR1KD) mice were generated as described previously (Iwasato et al., 1997). Briefly, transgenic construct carrying a putative NR2D promoter region and NR1-1a/pA was microinjected into fertilized eggs. By crossing NR1+/− mice (Li et al., 1994) with founders, the NR1 transgene was incorporated into the NR1+/− strain. NR1−/− mice carrying the NR1 transgene had a 70% reduction of NR1 expression. These mice were introduced as NR1−/− LTg+/+ mice (Iwasato et al., 1997); here we refer to them as NMDAR1 knockdown (NR1KD) mice. In the present experiments, wild-type NR1 mice with the transgene were used as controls. Mice were genotyped by tail-sample polymerase chain reaction (PCR) as described elsewhere (Iwasato et al., 1997). The mouse colonies were maintained on a 12:12-hour light:dark schedule, and food and water were provided ad libitum. All animal handling was in accordance with a protocol approved by the Louisiana State University Health Sciences Center Animal Use and Care Committee.
Histology
Postnatal (P5 and P14) mice were given an overdose of sodium pentobarbital and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. Brains were removed and cut between the inferior and superior colliculi. The forebrains were split in half along the sagittal plane. The left cortex from each half was then removed and flattened between two glass slides. All samples were kept in the fixative overnight. After cryoprotection in 30% sucrose in PB for 2 days, 90-μm-thick serial coronal sections through the brainstem trigeminal nuclei and the thalamus and 60-μm tangential sections of flattened cortex were taken using a freezing microtome. Sections were kept in serial order and reacted for cytochrome oxidase (CO) histochemistry, which is a highly reliable marker for visualizing somatosensory body maps and patterns within them. Briefly, sections were incubated with PB containing 0.5 mg/ml cytochrome C (Sigma, St. Louis, MO; type III), 0.5 mg/ml diaminobenzidine (DAB; Sigma), and 40 mg/ml sucrose (Sigma) for 3– 4 hours at 37°C in a shaker incubator. Sections were then rinsed in PB, mounted on subbed slides, and coverslipped with an aqueous medium containing gelatin and glycerol.
Area and volumetric measurements and data analysis
For area measurements, CO-stained flattened cortex sections were visualized under a light microscope, and the images were acquired with a Cool Snap digital camera (US Photometrics, Tucson, AZ). Areas of interest were outlined and measured using the MetaVue image-analysis program (Universal Imaging, Downington, PA). For each region, area was determined as a percentage of the entire section to account for any difference in overall brain size. One potential problem with areal measurements relates to individual variability in the size of the somatosensory maps and within the same animal between the two hemispheres. Riddle and Purves (1995) reported variations for the adult rat and related these variations to experience-dependent changes, but no such notable variations were found in the barrel cortex during the first postnatal week. For each case, we normalized the areal boundaries to the overall area of the section outline. Thus, the differences between cases are normalized, and the comparisons are made with the entire body map area. These measurements could not be performed in an investigator-blind fashion, because the phenotypes are obvious. Volumetric measurements of subcortical somatosensory-related regions were obtained from serial sections of 90 μm thickness. The relative volume was calculated by measuring the specific (nuclear or body map subdivision) area and the entire area of each section. This was then multiplied by 90 (section thickness in micrometers). Cumulative values from serial sections were determined and divided by the value of the total volume of sections. This also allowed us to circumvent the variability in plane of sections.
All samples were analyzed with a Nikon Diaphot 300 microscope. Photomicrographs were captured with a Cool Snap digital camera and MetaVue software. The images were adjusted for brightness and contrast in Adobe Photoshop 7.0 on a PC. No other alterations were made.
RESULTS
Sensory inputs from the large whiskers (mystacial vibrissae) and sinus hairs on the face are transmitted via the maxillary division of the trigeminal nerve to the principal sensory nucleus of the trigeminal nerve (PrV) in the brainstem. In this nucleus, pre- and postsynaptic neural elements form whisker-specific patterns (barrelettes). Similarly, inputs from the hind and forepaws are carried by the corresponding dorsal root ganglion (DRG) axons to the dorsal column nuclei (DCN; gracile and cuneate nuclei), where digit-related patterns develop. A schematic representation of the trigeminal and dorsal column-lemniscal pathways is presented in Figure 1. PrV and DCN axons convey these patterns to the contralateral thalamus (VPM and VPL, respectively) via the medial lemniscus. In VPM/VPL, the entire body map is put together with the patterning of neural elements (barreloids) corresponding to large whiskers, sinus hairs, and digits. Thalamocortical axons convey this body map and patterning to layer IV of the primary somatosensory cortex (SI), where divisions of the body map are clearly separated from one another by septal regions, and patterned areas develop barrels.
Fig. 1.
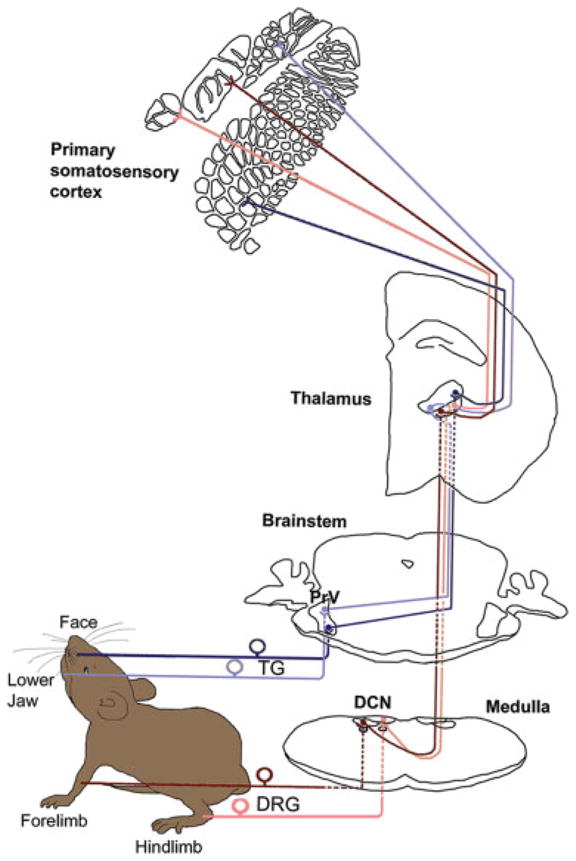
Mouse somatosensory pathways. Trigeminal ganglion–trigeminal lemniscal pathway (marked with blue) carries the orofacial inputs to the VPM and subsequently to the facial areas of the primary somatosensory (SI) cortex. The pathway schematized by dark blue color carries sensory information from the face; the pathway marked with light blue carries information from the lower jaw. Dorsal root ganglion– dorsal column lemniscal pathway is marked in red. Sensory information from hindlimb (light red) and forelimb (dark red) are first transmitted to the dorsal column nuclei (gracile nucleus and cuneate nucleus, respectively), then to the contralateral VPL, and then to the paw representation areas of the SI cortex.
CO histochemistry reveals whisker and digit-related patterns at all levels of the somatosensory neuraxis from the trigeminal and dorsal column nuclei to the VPM/VPL and finally in the SI neocortex. Comparisons of these patterns and deficits in control and NR1KD mice are shown in Figure 2. At all stations, the patterns are best visualized between P5 and P14. In NR1KD mice, DCN patterns are normal, but PrV and VPM show no patterns, so the face region of the cortical map is devoid of any patterning, whereas DCN-VPL projection zones (paw components) form patterns (arrowheads in Fig. 2E). In these mice, the orientation of the cortical and subcortical maps and their components do not show any apparent changes, confirming previous findings indicating that this aspect of map formation is determined by early gene expression and specific axon guidance molecules independent of neural activity (for review see López-Bendito and Molnár, 2003). These results suggest that, with relative lower NR1 level, whisker-specific patterns fail to develop in the PrV, the VPM, and the face component of the cortical body map, whereas higher levels of NR1 expression in caudal brainstem allow development of digit-related patterns in the DCN and, consequently, in the VPL and paw components of the cortical body map (Iwasato et al., 1997).
Fig. 2.
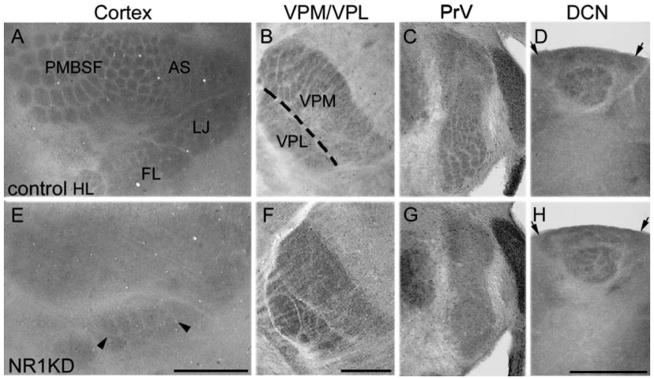
Somatosensory maps in the neocortex, thalamus, PrV, and DCN in control (A–D), and NR1KD (E–H) mice as visualized with cytochrome oxidase histochemistry. A: In flattened P14 cortex, the major whisker representation area, the posterior medial barrel sub-field (PMBSF), anterior snout (AS), lower jaw (LJ), forelimb (FL), and hindlimb (HL) areas are indicated for the wild-type cortex. In NR1KD neocortex (E), paw regions show patterns (arrowheads), whereas the face region is devoid of any patterning. Subcortical somatosensory nuclei are shown in B–D for control mice and in F–H for NR1KD mice. In NR1KD mice, digit-related barreloids are present in VPL but there is no patterning in VPM (F). Barrelettes are absent in the PrV of NR1KD mice (G, arrows) but digit-related patterns are present in the DCN (H, arrows). Scale bar = 1 mm in A (applies to A,E); 500 μm in F (applies to B,C,F,G); 500 μm in H (applies to D,H).
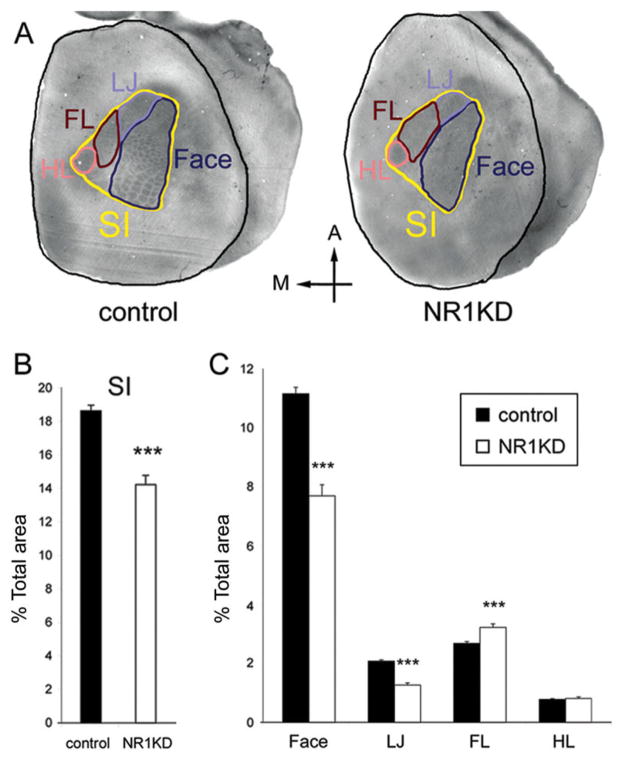
To test the hypothesis that NMDARs play a major role in competitive interactions between trigeminal and dorsal column pathways during territorial settlement of cortical somatosensory map components, we measured the areas devoted to different components of the cortical body map in NR1KD mice and compared them with controls. Area measurements from flattened cortices of P14 control and NR1KD mice revealed that flattened cortical areas were similar to each other in both phenotypes. The percentage of the entire body map (SI) of NR1KD was significantly smaller (24%) than that of controls (Fig. 3B; P < 0.001, t-test). The percentage of the face and lower jaw (LJ) components of the cortical body map of NR1KD mice were significantly (P < 0.001, t-test) smaller (31% and 39%) than in controls. In contrast, the forelimb (FL) region of NR1KD was significantly (P < 0.001, t-test) enlarged (20%; Fig. 3C).
Fig. 3.
Cortical area measurement of P14 control and NR1KD mice. A: The component regions of the body map were outlined in different colors: black, the entire neocortical area; yellow, the primary somatosensory cortex (SI); dark blue, face area; light blue, lower jaw (LJ); dark red, forelimb (FL); light red, hindlimb (HL). B: The percentage of primary somatosensory region (SI) in the entire flattened neocortex. The SI of NR1KD was significantly (P < 0.0001) smaller (24%) than control. C: The percentage of different regions in SI. Both face and lower jaw (LJ) regions of NR1KD were significantly smaller (31% and 34%, respectively; P < 0.0001) than control. In contrast, the forelimb (FL) region of NR1KD was significantly (P < 0.001) enlarged (20%). The hindlimb (HL) region in both animals shows no difference in size. Results are mean ± SEM. ***P < 0.001, Student’s t-test.
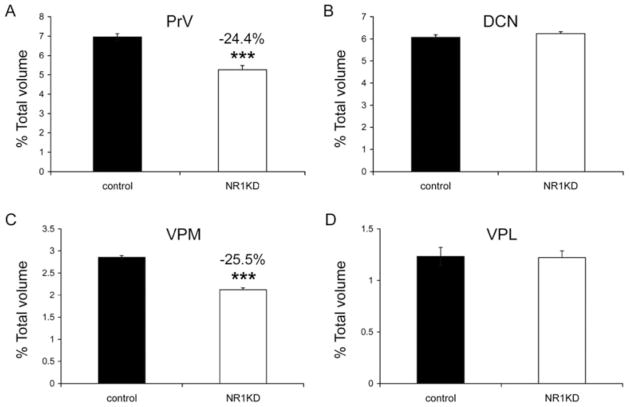
To determine whether cortical space allocation to different body map components is a simple reflection of the subcortical areal allocations, we measured the volumes of PrV, DCN, VPM, and VPL from serial sections stained with CO histochemistry (Fig. 4). Whisker and digit-related subcortical patterns are best seen in P5 mice, and these patterns become obscured as fibers through the somatosensory nuclei become myelinated after P7. We collected CO-stained serial brainstem and thalamus sections from P5 control and NR1KD mice. The relative volumes of PrV and DCN, as well as VPM and VPL, were calculated. The volumetric analyses showed that NR1KD mice have smaller PrV (24.4%) than controls (Fig. 4A), whereas the volumes of DCN in both mice were indistinguishable (Fig. 4B). The relative volumes of VPM and VPL were also compared. NR1KD mice have smaller VPM (25.5%) than control mice (Fig. 4C); however, the volumes of VPL in both genotypes were indistinguishable (Fig. 4D). Thus, in NR1KD mice, the overall sizes of the PrV, VPM, and face and lower jaw representation areas in the SI cortex are reduced, whereas DCN and VPL do not show a size change, although the cortical representation of the DCN-VPL expands.
Fig. 4.
Volumetric measurements of subcortical somatosensory nuclei. A: Compared with the PrV in control P5 mice, P5 NR1KD mice have smaller (24.4%) PrV. B: The relative volume of DCN shows no significant difference between control and NR1KD mice. C,D: Relative volumes of VPM and VPL. NR1KD mice have smaller (25.5%) VPM than control mice (C); however, the volumes of VPL in the two genotypes are indistinguishable (D). Results are mean ± SEM. ***P < 0.001, Student’s t-test.
DISSCUSSION
At each level of the somatosensory neuraxis, neurons have distinct receptive field properties and are activated by a specific locus on the face or body. Groups of cells with similar receptive field properties collectively form a body map that is first put together in the VPM/VPL and then transformed into the larger scale body map seen in the SI cortex. From the dorsal root and trigeminal ganglia all the way to the neocortex, there is anatomical convergence and divergence of inputs, which determine the progressive expansion of the neural volume devoted to body representation. Our results show that neural activity plays an important role in parcellation of the somatosensory body map in the developing neocortex. Studies from nonhuman primates and humans have revealed much about how these maps are altered by sensory experience or by injury to the sensory periphery or along any specific leg of the pathway leading to the neocortex (for an extensive review see Wall et al., 2002). Clearly, neural activity and competition at any given level play a role in these map changes, and here we argue that NMDAR-mediated activity plays a major role in this process during development (see Fox et al., 1999).
If NMDARs play a role in final allocation of body map subdivisions in the neocortex, what are the underlying mechanisms? In recent years, numerous studies have revealed that several genes and transcription factors are expressed in a region- and lamina-specific fashion in the developing telencephalic vesicle and that these intrinsic cortical markers might determine the positioning and areal expanse of somatosensory, visual, and auditory maps independent of the incoming sensory inputs from the periphery (Ragsdale and Grove, 2001; Rakic, 2001, 2002; Sestan et al., 2001; Bishop et al., 2002; Garel et al., 2003). It is now known that Fgf8, ephrin A5, ephrin B3, some members of the cadherins, and GAP-43 play a role in the delineation of somatosensory cortical areas in rodents (Huntley and Benson, 1999; Maier et al., 1999; Prakash et al., 2000; Vanderhaeghen et al., 2000; Fukuchi-Shimogori and Grove, 2001; Hevner et al., 2001, 2002; Mann et al., 2002; Poskanzer et al., 2003; Yun et al., 2003). Thus, molecular markers intrinsic to the somatosensory cortex play a significant role in positioning the body map and its components by directing thalamocortical axon pathfinding and termination zones. In contrast, but not necessarily contradictory to these findings, stands a large volume of data from rodents to primates showing alterations, expansions, or shrinkage of specific sensory map regions following sensory denervation or deprivation both during development and in adulthood (for reviews see Fox, 2002; Rauschecker, 2002; Wall et al., 2002). The results from the latter series of studies most likely reflect alterations in neural activity and/or selective survival of the afferent pools that contribute to these maps (Wall et al., 2002). Our current vision for somatotopic map formation in the cortex is such that intrinsic molecular guidance cues set the stage for areal and laminar specification of the cortex and proper targeting of thalamocortical axons into these zones and their subdivisions. Once these maps and their components are set, activity-dependent mechanisms, in particular those via the NMDARs, play a major role in periphery-related patterning and final adjustment of areal representation. We do not think that the early somatosensory cortex-specific areal markers would be altered in our mutant mice, because they are not downstream from NMDAR activation. Furthermore, in NR1KD mice, the positioning of map subdivisions and orientation does not change. Thus secondary effects on cortical boundary molecules can be discounted.
Physiological recording/imaging studies delineating the boundaries of somatosensory maps in the neocortex of mice with altered NMDAR function would be informative. Such studies could reveal how these maps are initially set up and how, in older animals, they undergo plastic changes following experience (e.g., overuse, conditioned learning), sensory deprivation, or peripheral and central injuries. In addition, single-cell recordings would reveal receptive field properties of neurons in different components of the cortical body map and whether somatotopic precision is abolished in body map components that have shrunk and lost periphery-related patterns.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: NS 039050 (to R.S.E.).
We thank Ms. Barri King for breeding and genotyping mice and Dr. Takuji Iwasato for providing the transgenic mice.
LITERATURE CITED
- Belford GR, Killackey HP. Anatomical correlates of the forelimb in the ventrobasal complex and the cuneate nucleus of the neonatal rat. Brain Res. 1978;158:450– 455. doi: 10.1016/0006-8993(78)90688-1. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Rubenstein JL, O’Leary DDM. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT, Debski EA, Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987;84:4325–4342. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DR, Killackey HP. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. J Comp Neurol. 1987;256:246–256. doi: 10.1002/cne.902560205. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992a;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Emergence of connectivity in the embryonic rat parietal cortex. Cereb Cortex. 1992b;2:336–352. doi: 10.1093/cercor/2.4.336. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of “barrels” in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S, Benowitz LI. Transient patterns of GAP-43 expression during the formation of barrels in the rat somatosensory cortex. J Comp Neurol. 1990;292:443– 456. doi: 10.1002/cne.902920310. [DOI] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799– 814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Fox K, Henley J, Isaac J. Experience-dependent development of NMDA receptor transmission. Nat Neurosci. 1999;2:279–299. doi: 10.1038/7203. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Groove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- Hahm JO, Langdon RB, Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature. 1991;35:568–570. doi: 10.1038/351568a0. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Miyashita-Lin E, Rubenstein JL. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: evidence that cortical and thalamic axons interact and guide each other. J Comp Neurol. 2002;447:8–17. doi: 10.1002/cne.10219. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Benson DL. Neural (N)-cadherin at developing thalamocortical synapses provides an adhesion mechanism for the formation of somatopically organized connections. J Comp Neurol. 1999;407:453– 471. [PubMed] [Google Scholar]
- Iwasato T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knöpfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Sensory loss and cortical reorganization in mature primates. Prog Brain Res. 2002;138:167–176. doi: 10.1016/S0079-6123(02)38077-4. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Catania KC. How do features of sensory representations develop? Bioessays. 2002;24:334–343. doi: 10.1002/bies.10076. [DOI] [PubMed] [Google Scholar]
- Killackey HP. Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res. 1973;51:326–331. doi: 10.1016/0006-8993(73)90383-1. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402– 407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427– 437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Molnár Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Ma PM, Woolsey TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Res. 1984;306:374–379. doi: 10.1016/0006-8993(84)90390-1. [DOI] [PubMed] [Google Scholar]
- Maier DL, Mani S, Donovan SL, Soppet D, Tessarollo L, McCasland JS, Meiri KF. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci U S A. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Ray S, Harris W, Holt C. Topographic mappings in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron. 2002;35:461– 473. doi: 10.1016/s0896-6273(02)00786-9. [DOI] [PubMed] [Google Scholar]
- O’Leary DDM, Ruff NL, Dyck RH. Development, critical period plasticity, and adult reorganizations of mammalian somatosensory systems. Curr Opin Neurobiol. 1994;4:535–544. doi: 10.1016/0959-4388(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash N, Vanderhaeghen P, Cohen-Cory S, Frisen J, Flanagan JG, Frostig RD. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J Neurosci. 2000;20:5841–5847. doi: 10.1523/JNEUROSCI.20-15-05841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale CW, Groove E. Patterning the mammalian cerebral cortex. Curr Opin Neurobiol. 2001;11:50–58. doi: 10.1016/s0959-4388(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurobiology. Neurocreationism—making new cortical maps. Science. 2001;294:1011–1012. doi: 10.1126/science.294.5544.1011. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolving concepts of cortical radial and areal specification. Prog Brain Res. 2002;136:265–280. doi: 10.1016/s0079-6123(02)36023-0. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical map plasticity in animals and humans. Prog Brain Res. 2002;138:73– 88. doi: 10.1016/S0079-6123(02)38072-5. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Purves D. Individual variation and lateral asymmetry of the rat primary somatosensory cortex. J Neurosci. 1995;15:4184–4195. doi: 10.1523/JNEUROSCI.15-06-04184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft SL, Woolsey TA. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991;1:308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- Sestan N, Rakic P, Donoghue MJ. Independent parcellation of the embryonic visual cortex and thalamus revealed by combinatorial Eph/ephrin gene expression. Curr Biol. 2001;11:39– 43. doi: 10.1016/s0960-9822(00)00043-9. [DOI] [PubMed] [Google Scholar]
- Simon DK, Prusky GT, O’Leary DDM, Constantine-Paton M. N-methyl-D-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc Natl Acad Sci U S A. 1992;89:10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 1976;2:1– 6. doi: 10.1016/0304-3940(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Lu Q, Prakash N, Frisen J, Walsh CA, Frostig RD, Flanagan JG. Mapping label required for normal scale of body representation in the cortex. Nat Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Rev. 2002;39:181–215. doi: 10.1016/s0165-0173(02)00192-3. [DOI] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J Neurosci. 1986;6:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA. Peripheral alteration and somatosensory development. In: Coleman EJ, editor. Development of sensory systems in mammals. New York: Wiley; 1990. pp. 461–516. [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Yun ME, Johnson RR, Antic A, Donoghue MJ. EphA family gene expression in the developing mouse neocortex: regional patterns reveal intrinsic programs and extrinsic influence. J Comp Neurol. 2003;456:203–216. doi: 10.1002/cne.10498. [DOI] [PubMed] [Google Scholar]