Abstract
The study of successful versus failed zoonotic infections may provide important clues of how viral infection is naturally prevented. In this issue of Cell Host & Microbe, a collaborative group led by Frank Kirchhoff uncovers an important piece of the pandemic HIV-1 puzzle.
Phylogenetic analyses indicate that HIV-1 arose from a zoonotic infection from chimpanzees. Moreover, HIV-1 has been transmitted to humans on at least three separate occasions, and each of those zoonotic events has given rise to an independent group of HIV-1 sequences referred to as HIV-1 M (main), O (outlier), and N (non-M, non-O). Remarkably, the frequency of these viruses in the current human population is vastly different, with around 30 million people living with HIV-1 M group infection and about 10,000 with O group infection. Only a handful of N group infections have ever been described. Thus, O and N group viruses are not pandemic, and in the absence of HIV-1 M group infections, HIV/AIDS would be a rare tropical disease. It is obviously of great importance to understand the differences between the viruses that underlie their different frequencies. What are the features of pandemic HIV-1 that make it so much more successful? Now, Sauter and colleagues show that, although pandemic HIV-1 M group has successfully adapted its Vpu protein to antagonize the human antiviral restriction factor tetherin, the O group viruses have not. Could the ability to escape tetherin explain the different frequencies of pandemic and nonpandemic HIV-1 strains?
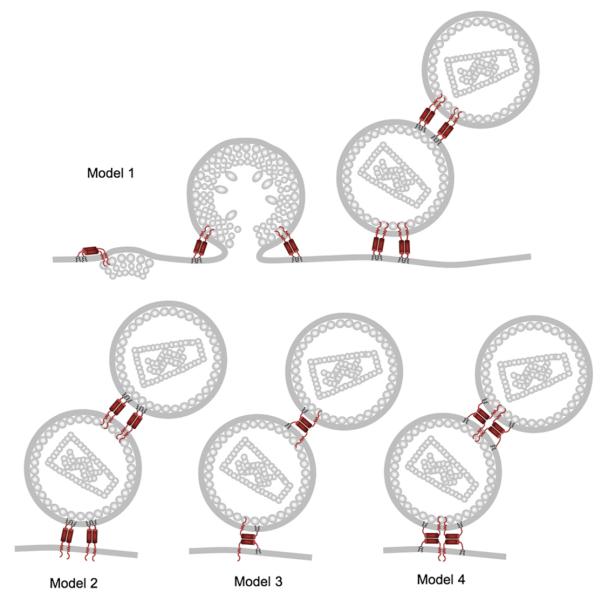
Mammalian cells encode interferon-inducible antiviral restriction factors as a means of protecting themselves from viral infection. The recently identified BST-2/CD317/HM1.24/tetherin (Neil et al., 2008; Van Damme et al., 2008) is an excellent example of one such protein that can restrict infectivity of retroviruses, filoviruses, and arena viruses. Tetherin has an N-terminal extracellular domain, a C-terminal GPI anchor, and a coiled coil in the middle that promotes dimerization (Figure 1). It is thus attached to the cell membrane at each end, and tetherin dimers are proposed to form a protein tether linking assembled virions to infected cells, leading to their endocytosis and degradation in lyzosomes (Neil et al., 2008; Van Damme et al., 2008). Two recent studies use electron microscopy and tetherin mutagenesis to show that it is probably tetherin itself that forms the tether between the virus and the cell membrane (Fitzpatrick et al., 2009; Perez-Caballero et al., 2009). Perez-Cabellero and colleagues elegantly demonstrate the simplicity of the tethering mechanism by generating an artificial tetherin with full activity by fusing a transmembrane region to a coiled coil with a GPI anchor at the C terminus. Although this artificial molecule had no sequence similarity to tetherin, tethered virions observed by electron microscopy appeared similar to those tethered by tetherin (Perez-Caballero et al., 2009). Fitzpatrick and colleagues use electron microscopy and antitetherin labeling to show that tetherin appears in the virion tethers and in the membrane of the few released virions that manage to escape (Fitzpatrick et al., 2009). These two studies favor different possibilities regarding the configuration of the tetherin dimers that constitute the tethers (Figure 1), but we predict that it will be difficult to rule out the possibility that tetherin can effectively tether in more than one configuration.
Figure 1. Models for Tetherin's Antiretroviral Tethering Activity.
Tetherin restricts retroviral release by tethering newly formed virions to the cell surface. The protease-sensitive tether probably consists solely of dimeric tetherin protein. Shown are models for the various tetherin protein configurations. Currently, Perez-Caballero et al. (2009) and Fitzpatrick et al. (2009) favor different models, but we expect that it will be difficult to rule out the existence of more than one of these examples. The figure is reprinted from Perez-Caballero et al. (2009).
In order to replicate in cells that express tetherin, primate lentiviruses encode accessory proteins Vpu and Nef that specifically antagonize tetherin (Jia et al., 2009; Neil et al., 2008; Sauter et al., 2009; Zhang et al., 2009). It appears that a virus can have one or more tetherin antagonists, either Vpu or Nef, or even the envelope glycoprotein. Recent data demonstrate that the envelope glycoproteins from HIV-2 and Tantalus monkey SIV antagonize tetherin by sequestration in the Trans Golgi Network (TGN) (Gupta et al., 2009; Le Tortorec and Neil, 2009). It is not yet clear how Nef antagonizes tetherin, but HIV-1 Vpu leads to tetherin degradation. Importantly, tetherin shows evidence of positive (adaptive) selection (McNatt et al., 2009), presumably as a result of evolutionary pressure applied by antagonistic viral proteins that counteract its inhibition of viral replication. This has led to the species-specific tetherin sensitivity to viral countermeasures. For example, Tantalus monkey tetherin cannot be abrogated by HIV-1 Vpu due to variation in the tetherin transmembrane region (McNatt et al., 2009). Similarly, SIV Nefs are able to overcome simian tetherins, but not human tetherin, due to sequence variation in the cytoplasmic tail of tetherin (Jia et al., 2009; McNatt et al., 2009; Sauter et al., 2009; Zhang et al., 2009).
So how do the various HIV-1 and chimpanzee SIV (SIVcpz) strains compare in their ability to antagonize tetherin? Sauter and colleagues show that, like a variety of SIVs, SIVcpz uses Nef to antagonize tetherin. As we now know from several studies, HIV-1 uses its Vpu protein (McNatt et al., 2009; Neil et al., 2008). This suggests that SIVcpz-derived HIV-1 has had to adapt its Vpu in order to effectively transmit to humans. However, adaptation to use Vpu to antagonize tetherin has not been achieved by the significantly less prevalent HIV-1 O group viruses. Adaptation was required due to sequence variation between the various tetherin proteins. Importantly, the human tetherin protein uniquely has a 4 amino acid deletion in its cytoplasmic tail that makes it insensitive to Nef from a variety of SIVs, including SIVcpz (Sauter et al., 2009; Zhang et al., 2009). Replacing these amino acids makes human tetherin almost fully sensitive to antagonism by SIVcpz Nef and moderately sensitive to O group Nefs (Sauter et al., 2009). We suggest that the tetherin tail deletion was selected by previous encounters with viral antagonists, perhaps even Nef/Vpu-like molecules from long-lost infections. The high level of relatedness between humans and chimpanzees has led us to believe that the species barriers to zoonotic viral infection between chimpanzees and humans are rather low. This is the first example of an adaptation made by HIV-1 to improve replication in humans or human-human transmission. The difference in prevalence between M and O suggests that this adaptation has been critical for the establishment of the human pandemic. The chimp-human species barrier may be low, but tetherin seems to have made an important contribution by virtue of the loss of 4 amino acids in its cytoplasmic tail.
What of N group virus' ability to antagonize tetherin? N group infections are extremely rare; fewer than 20 have ever been described. Sauter and colleagues tested Vpu from three N group viruses and found that one of them antagonized tetherin quite well, whereas the other two did so but rather poorly. It is hard to conclude that N group Vpus can generally antagonize tetherin from these observations, but certainly, N group Vpus are better at this than are O group Vpus. On the other hand, the N group Vpus were unable to downregulate cell surface expression of the T cell receptor CD4. This is thought to be a critical Vpu function that is essential for preventing super-infection and facilitating infectious viral release. These observations suggest that N group viruses may have other problems or at least have alternate, perhaps less effective, strategies for manipulating their new human host.
Does the relative success or failure of these host virus interactions have an impact on disease? This is hard to say, but there is little evidence that they do. O and M group infections appear to lead to similarly high frequencies of clinical AIDS, high plasma viral loads, and similar frequencies of mother-to-child transmissions. Furthermore, rhesus macaque SIV with a human Nef gene, which is apparently unable to antagonize rhesus tetherin, can cause disease in rhesus macaques, although it is less pathogenic. Thus, the ability to antagonize tetherin may be more important for transmission than pathogenesis. However, more work is required, and we propose that the continued comparison of the molecular details of host virus interactions between common and rare types of HIV and their hosts will reveal important information.
The central message from Sauter's important study is that SIVcpz-derived HIV-1 has switched from using Nef to using Vpu to counteract tetherin during zoonosis from chimpanzees to humans. It appears that this change may have made a significant contribution to its ability to spread through the human population. Importantly, primate lentiviruses distantly related to HIV-1, such as SIVgsn, SIVmus, and SIVmon, also use Vpu to antagonize tetherin, suggesting that primate lentiviruses have switched which proteins they use to antagonize tetherin on several occasions. We are reminded of the plasticity of lentiviral protein function and the power of selective pressure. The Red Queen hypothesis suggests an ongoing evolutionary conflict between hosts and their pathogens, and in this case, we have a vivid example of how tetherin and primate lentiviruses have swapped the advantage throughout their evolution. Currently, HIV-1 M group appears to have the lead.
REFERENCES
- Fitzpatrick K, Skasko MA, Deerink TJ, Crum J, Ellisman MH, Guatelli JC. PLoS Pathog. 2009 doi: 10.1371/journal.ppat.1000701. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mlcochova P, Pelchen-Matthews A, Petit SJ, Mattiuzzo G, Pillay D, Takeuchi Y, Marsh M, Towers GJ. Proc. Natl. Acad. Sci. USA. 2009 doi: 10.1073/pnas.0907075106. in press. Published online October 28, 2009. 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. PLoS Pathog. 2009;e1000429;5 doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tortorec A, Neil SJ. J. Virol. 2009;83:11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatt MW, Zang T, Hatziioannou T, Bartlett M, Fofana IB, Johnson WE, Neil SJ, Bieniasz PD. PLoS Pathog. 2009;5:e1000300. doi: 10.1371/journal.ppat.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Perez-Caballero D, Zang T, Ebrahimi A, McNatt M, Gregory D, Johnson MC, Bieniasz PD. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D, Schindler M, Specht A, Landford WN, Münch J, Kim K-A, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, et al. Cell Host Microbe. 2009;6(this issue):409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. Cell Host Microbe. 2009;6:54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]