Abstract
The ability of comprehend and produce speech after stroke depends on whether the areas of the brain that support language have been damaged. Here we review two different ways to predict language outcome after stroke. The first depends on understanding the neural circuits that support language. This model-based approach is a challenging endeavor because language is a complex cognitive function that involves the interaction of many different brain areas. The second approach does not require an understanding of why a lesion impairs language, instead, predictions are made on the basis of how previous patients with the same lesion recovered. This requires a database storing the speech and language abilities of a large population of patients who have, between them, incurred a comprehensive range of focal brain damage. In addition it requires a system that converts an MRI scan from a new patient into a 3D description of the lesion and then compares this lesion to all others on the database. The outputs of this system are the longitudinal language outcomes of corresponding patients in the database. This will provide a new patient, their carers and the clinician team managing them the range of likely recovery patterns over a variety of language measures.
Aphasia is a disorder caused by damage to the areas of the brain that support our ability to comprehend and produce speech. It is usually caused by stroke, tumour, traumatic brain injury or degenerative brain disease and leads to difficulties in speaking, understanding, reading and writing. Communication problems severely impair quality of life. Even everyday tasks can become impossible, such as making telephone calls, shopping or having a conversation. Every year, millions of people suffer the consequences of brain damage. In the UK alone, there are around 250,000 people with aphasia and many of them are under the age of 65. Surprisingly, however, the relationship between the site of brain damage and the type of aphasia remain poorly understood.1-5 In short, there is still no way of predicting when, how or whether a patient with language difficulties will recover. This makes it difficult to decide which course of therapy is best suited to an individual patient. For current procedures in assessing and treating aphasia, see Boxes 1 and 2.
Box 1. Causes and signs of aphasia in the clinic.
Causes
Stroke and dementia are the two main causes of aphasia. Although additional cognitive deficits can occur with stroke, they are more common in dementia. Other neurological conditions can present with an isolated aphasic syndrome; such patients will usually have other associated symptoms or signs e.g. migraine, brain tumours, head injury, multiple sclerosis, inflammatory or infective CNS disorders.
Examination
A clinician can determine whether or not a patient needs formal speech language assessments by initiating conversation with questions such as “How did you get here today?” Patients can also be asked to describe a picture (e.g. from a newspaper), and name items at hand (e.g. shirt, button, cuff, seam). If speech output is low, patients can be asked to count or repeat back single words or phrases. Assessment and diagnosis are based on the rate of speech (high, low or normal) and its structural content (is it grammatically well formed? Is there an overreliance on content words (nouns)); its functional content (is the patient getting concepts across or not); errors (phonemic, semantic, jargon).
Writing usually mirrors speech output in pure aphasic disorders but when an articulatory disorder is superimposed e.g. dysphonia, dysarthria, speech apraxia, written output may be better than speech output (NB: right hand function will often be compromised so assess for content rather than neatness).
Comprehension of speech can be assessed with one, two and three stage commands: e.g. “Point at the ceiling, then the floor and then the door.” If very severely affected (usually in the acute phase post-stroke), arrange three common objects and ask the patient to point to one of them. Reading is usually impaired in aphasic disorders (central alexia). Reading aloud may be diagnostically helpful in some patients with dementia; surface alexia (mispronouncing irregular or exception words, e.g. yacht read as “yatched”) can occur early in semantic dementia.123
Box 2. Treating Aphasia.
Speech therapy
Treatment of aphasia is effective, particularly in patients with a non-progressive cause.124-131 Speech therapy does improve ‘real-world’ outcomes but the main issue is ‘dose’ or amount of therapy. A meta-analysis comparing ‘positive’ with ‘negative’ studies showed that up to 90 hours or more may be needed, the negative studies averaged 44 (SD 8) hours of therapy in total.126 This amount of therapy can be difficult to achieve in many health care systems. The intensity and timing of therapy has not been clearly established.132 The general principles of “the earlier the better” and “the more the merrier” would seem reasonable, but some patients, especially those with disabling stroke, are not physically or psychologically ready to engage with therapy in the acute phase. Recovery curves tend to be asymptotic133 but there is now plenty of evidence that patients can benefit from intensive interventions in the late or chronic phase, even several years (> 5) after the causative event.134-136
Drug therapy
The use of drugs to promote recovery either alone or in conjunction with speech therapy is an important area of ongoing research. Although early studies appeared discouraging, more recent, perhaps better controlled studies, have shown effects for cholinergic and dopaminergic agents and those that antagonize glutamatergic NMDA receptors.137-139
Magnetic resonance imaging (MRI) provides detailed information on brain structure and has motivated new research on the relationship between lesion site and language outcome. The aim of our article is to: (1) review what we already know from patient population studies about the lesion sites that impair speech comprehension and production; (2) introduce a new data-led system (PLORAS) for predicting language outcome and recovery after stroke; and (3) discuss future directions for research. We focus on the diagnosis and prognosis of language disorders in the post-acute phase after stroke, when oedema has reduced and perfusion of the ischemic penumbra has stabilized.6 Although the general principles may be applied to patients with traumatic brain injury and tumours (pre- and post-neurosurgical intervention), we do not review the longitudinal changes in language function that occur in degenerative diseases.7-12
1. What do we already know?
Our current understanding of the brain regions that support language was founded on the pioneering work of Paul Broca13-15 and Carl Wernicke16-19 in the late nineteenth century. Based on postmortem autopsies, Broca reported damage in the posterior half of the inferior frontal gyrus in a patient who was only able to say “tan” and later Wernicke identified damage in the left posterior temporal cortex in a patient who had difficulty comprehending speech. The area associated with producing speech was subsequently referred to as Broca’s area and the area associated with comprehending speech was subsequently referred to as Wernicke’s area. Broca’s area is neuroanatomically defined as the pars opercularis (Brodmann’s area 44, anterior to the precentral sulcus) and the pars triangularis (Brodmann’s area 45, between the ascending and horizontal limbs of the sylvian fissure).20 In contrast, Wernicke’s area is neuroanatomically defined as the posterior part of Brodmann area 22 which encircles the auditory cortex on the Sylvian fissure where the temporal and parietal lobes meet. Although its anatomical and functional boundaries are not well defined,17 separate neural subsystems within classic Wernicke’s area have been identified.21, 22
The importance of Broca’s and Wernicke’s areas for producing and understanding speech has been appreciated for a century and a half but the oversimplification of this categorization is also well documented.21, 23-26 In brief, speech comprehension and production difficulties can arise from damage to many different areas; normal speech comprehension and production both involve Broca’s and Wernicke’s area,22, 27-31 and damage to Broca’s or Wernicke’s areas does not always impair language function.24, 32, 33
In a fascinating recent report, Dronkers et al. (2007)14 had the unique opportunity to conduct a high resolution structural MRI scan on the 19th Century brains that Paul Broca based his original theory on. Broca did not dissect these brains. He based his hypothesis on their surface characteristics and then preserved the brains for future investigation. The recent MRI investigations, reported by Dronkers et al.,14 and earlier reports of CT scans by Signoret et al.,34 revealed that the lesions in Broca’s patients were much more extensive than the posterior half of the left inferior frontal gyrus. Damage was also observed deep in the inferior parietal lobe, the anterior superior temporal lobe, the claustrum, putamen, globus pallidus, head of the caudate nucleus and the internal and external capsules. The insula was found to be completely destroyed as was the entire length of the superior longitudinal fasciculus, along with other frontal-parietal periventricular white matter and the medial subcallosal fasciculus. In brief, damage was noted throughout the left hemisphere, both cortically and subcortically.
Given what we now know about the size of the lesions in Broca’s patients, it is impossible to say from these classic cases which areas of brain damage were responsible for the speech production difficulties. All we can infer is that some part of the lesion was necessary for prior language ability but we can not say which part it was from a single case study. The critical point here is that the location and extent of lesions do not correspond to the size and shape of functional areas but are instead determined by cerebrovascular factors (ie the blood supply that has been interfered with) in the case of ischemic stroke, the physical location of the trauma in the case of head injury or the type and extent of pathology in the case of neurodegeneration.35, 36
This leads to two important points that are emphasized throughout this article. First, a predictive model of language outcome after brain damage needs to be based on data from populations of patients so that the effects of lesions that are most consistent across patients can be identified. This is not possible with small samples that might not be representative of the general aphasic population but could instead be “rare” or “interesting” cases sampled from the extremes of the clinical spectrum. The second point is that both functional imaging and lesion studies have shown that many brain regions support both speech comprehension and production,37, 38 see Figure 1. Therefore aphasia is likely to result from many different lesion sites and the association of damage to one region does not exclude the involvement of other regions.38, 39 For example, speech production involves many cognitive processing steps including: access to conceptual knowledge, linking conceptual knowledge to the sounds of words, selecting the most appropriate word, access to the articulatory plans associated with that word, motor programming, initiation of movement, co-ordination of the timing and direction of movements of the speech articulators and respiratory control. Each of these subcomponents may be supported by a different brain region or network of regions. Consequently, speech production difficulty could arise from damage to many different regions.40
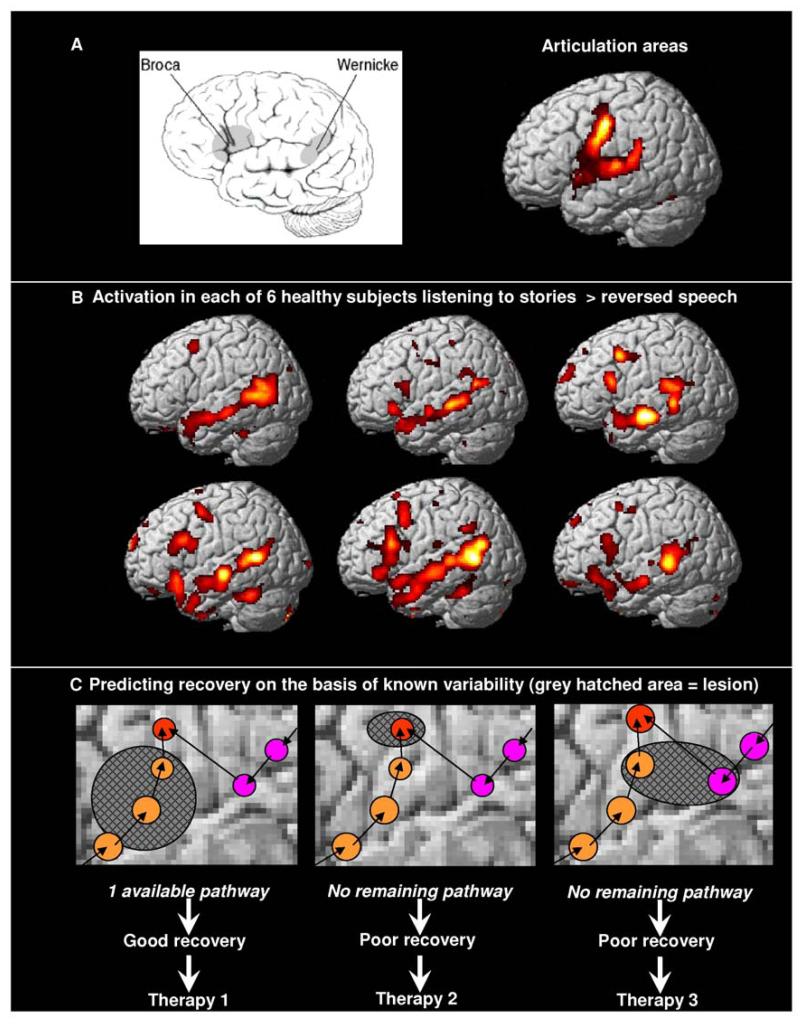
Figure 1. Brain activation during speech processing and the predicted effects of lesions.
A. Left Schematic illustration of Broca’s area and Wernicke’s area. Right: fMRI activation for reciting the phrase “1,2,3.” This articulation task activates the same set of regions in the left and right hemisphere (right hemisphere not shown).
B. fMRI activation in each of 6 healthy subjects listening to stories relative to listening to meaningless reversed speech.140 During this speech comprehension task, all 6 subjects consistently activate Wernicke’s area in the posterior superior temporal cortex. In addition, activation is consistently observed in the anterior superior temporal cortex and inconsistently observed in Broca’s area.
C. Schematic and hypothetical illustration of the effects of lesions (grey hatched areas) when there are two alternative pathways (orange and pink) to the same output (red). The effect of the lesion depends on whether one of the pathways remains intact. Although this is more likely after a small lesion, it is not so much the size of the lesion that matters but where the lesion occurs. For example, the middle configuration has a small lesion that knocks out an area that is critical to all both pathways whereas the left configuration has a large lesion that leaves one pathway intact. The lesion in the right configuration illustrates how damage to two pathways can have a much more devastating effect than damage to one pathway. The effect of the lesion on recovery will influence the most appropriate type of therapy.
An appreciation that many brain regions are required for speech production explains why patients can have poor speech production without damage to Broca’s area4, 24 but it does not explain why some patients have good speech production abilities in the presence of damage to Broca’s area.4, 24 A concern here is that the mapping of language functions to brain structures might vary so much from patient to patient that any kind of meaningful prediction at the individual level would be impossible. This concern is lessened, however, by the consistent localization of language areas identified by functional neuro-imaging studies (e.g. fMRI) of speech comprehension and production across different groups of participants.41 Moreover, fMRI studies have shown that the main sources of between subject variability in task-dependent brain activity do not appear to be random or infinite but instead reflect a limited number of different ways of doing the same task.42, 43 If alternative neural systems are available to support speech production (see Figure 1C), then selective disruption to Broca’s area may only have a transient effect on speech production.44
With the points above in mind, we now consider the lesion sites that have recently been associated with language impairments in large populations of patients rather than un-integrated single case studies. The inclusion criteria for our literature search were as follows. First we searched pubmed for research papers reporting population studies of the lesion sites that impaired performance on speech production or speech comprehension tasks. Our web searches therefore included various combinations of the following terms: “stroke”, “MRI”, “aphasia”, “language”, “speech”, “comprehension”, “production”, “VLSM”, “VBM”, “voxel based”. We also searched the reference lists of identified papers for further leads. Second, we only included studies that used in vivo radiological investigations with high resolution MRI to provide detailed information on the structural integrity of the brain. This increased the precision with which the language related areas could be identified. However, because the development of MRI is relatively recent in the history of lesion studies, our literature search was limited to the last 15 years. Third, we focused on studies that analyzed data from the whole brain rather than damage to predefined regions of interest. This is because studies that include data from the whole brain are more likely to find areas that are (i) most consistently identified with a particular language dysfunction; and (ii) not predicted a priori. Our choice to exclude smaller studies that used regions of interest does not imply that we think these studies have no value. To the contrary, there is great value in studies that focus on a single anatomical region (e.g. subcortical regions45-49), particularly when the region of interest is rarely damaged (e.g. the cerebellum50, 51); or when the effect of damage to one region is compared to another.52 In brief, our inclusion criteria reflect our interest in population studies because the findings can be generalized to new patients.
The biggest surprise from this literature search was that we only found 13 studies that met our inclusion criteria.32, 53-64 Table 1 lists the identified lesion sites in the vertical column and the language process/task in the horizontal column, highlighting (in black) which combinations of lesions and language difficulties have been reported. Although the results can be interpreted from several different perspectives, we focus here on using these results to help predict language outcome from lesion site. This requires us to reverse the line of inference. Rather than search the brain for lesion sites where damage is related to performance on a particular behavioral measure; the alternative approach identifies the language functions that are impaired after damage to a particular brain region.
Table 1. Lesion sites associated with speech comprehension and production impairments.
Blacked out cells indicate lesion-symptom relationship with reference source, see key below.
| LESION SITE | COMPREHENSION | PRODUCTION | SYNTAX | ||||
|---|---|---|---|---|---|---|---|
| LOBE | REGION | Nonfluent | Apraxia | Repeat | Persev’ | Gram’ | |
| Insula | B4, B6 | D6,Bo7 | K0 | ||||
| Frontal | K0 | ||||||
| IFG / sensorimotor | B7 | Bo7, K7 | |||||
| iFG/mFG dorsal | B3, D4, A7a | ||||||
| iFG/mFG ventral | D4 | A7a | |||||
| sup. long. fasciculus | B4 | ||||||
| Parietal | B3 S4a | K0, B6 | |||||
| angular g | |||||||
| post. temp-parietal | A7a | ||||||
| Temporal | post. temp | Bo7 | W4 | ||||
| ant. temp | Bo7 | W4 | |||||
| post. sup. temp | K0, S4a,B7,S9 | A7a | |||||
| ant. sup. temp | D4 | ||||||
| middle temp | B3,D4,S4a,B7,S9 | ||||||
| Subcortical | putamen | K0, B6 | |||||
| caudate | K0 | ||||||
Blacked out cells indicate a lesion-symptom relationship
Abbreviations
Repeat Auditory repetition
Gram’ grammaticality
Persev’ perseveration
sup. long. fasciculus superior longitudinal fasciculus
IFG inferior frontal gyrus
mFG middle frontal gyrus
angular g. angular gyrus.
temp-parietal temporo-parietal
temp. temporal
sup. temp superior temporal
post. posterior
ant. Anterior
Source
A7a Amici (2007a)53
A7b Amici (2007b)54
B3 Bates (2003)55
B6 Baldo et al. (2006)56
B7 Baldo et al. (2007)57
Bo7 Borovsky et al. (2007)58
D4 Dronkers(2004)59
D6 Dronkers (1996)60
K0 Kreisler (2000)32
K7 Kinkingnéhun (2007)61
S4 Saygin auditory (2004)62
S9 Specht et al. (2009)63
W4 Wilson and Saygin (2004)64
The first point to note from Table 1 is that the results replicate a long history of previous lesion reports based on series of single patient studies.24, 65-72 Thus, our literature search found that the most critical lesion sites causing language difficulties were located in the left hemisphere; all studies of auditory speech comprehension identified the importance of the left posterior superior temporal and/or left middle temporal regions (Wernicke’s area); and non-fluent speech production was associated with damage to the left inferior/middle frontal gyri and the underlying white matter (all in the vicinity of Broca’s area). This replication of previous findings is encouraging because it validates the neuroimaging techniques that have been developed for population studies of patients. In addition to replicating previous findings, the findings summarized in Table 1 highlight two important results. One is that speech comprehension is consistently associated with left frontal as well as left temporal damage.73 The other observation is that the area most frequently associated with nonfluent articulation is the left insula, rather than Broca’s area. As noted above, however, the strong association of non-fluent speech with left insula lesions does not exclude the importance of Broca’s area for fluent speech.40
Population studies of the lesions causing speech comprehension and production difficulties are still in their early days. The next step is to determine how consistent the group results are, at the individual level. This will provide an estimation of the proportion of patients with damage to a particular region who did or did not have a particular language impairment. As multiple areas of damage can be associated with the same language dysfunction, post hoc investigation of the individual lesions is also important for determining whether low scores are associated with damage to (i) multiple regions in all the affected patients; (ii) different subregions in different patients; or (iii) different combinations of subregions. This will require “multi-lesion” analyses that take into account the size, distribution and combination of all damaged regions.74-78 Critically, however, the success of these multi-lesion analyses will depend on increasing the population size and ensuring as much diversity as possible in the lesion site and behavioral scores36, 61 because a region supporting a language function will only be identified if the sample includes patients with and without damage to that region. For example, studies of progressive aphasia and semantic dementia have shown that anterior temporal lobe damage impairs both verbal and nonverbal comprehension79-81 but population studies of stroke patients have less data pertaining to the loss of the anterior temporal cortex because this region is in the border-zone between the middle and posterior cerebral artery territories, and is thus very rarely completely damaged by ischaemic stroke. Therefore the lack of a relationship between language dysfunction and a region in a lesion study does not imply that the area was not important for language.
In summary, future studies are needed to (i) combine data from large samples of patients, (ii) include heterogeneous lesion sites and behavior; (iii) account for the extent of damage (i.e. the number and combination of regions damaged) as well as the site of damage and (iv) report post hoc analyses of the individual variability in language outcome for each region and combination of regions that are damaged, along with (v) the degree of damage to each area.
2. Predicting Language Outcome and Recovery After Stroke
The aphasic patient wants to know what chance they have of recovery and what therapies will speed up the recovery process. These questions can be answered from two different perspectives. The first is based on a theoretical model of how language is implemented in the brain. This “model-led” approach requires an understanding of the full set of regions involved in each language task, the function of each subregion and alternative neural systems for the same language function. At present, there is no way of knowing when our understanding of language networks will be sufficient to predict language outcome after brain damage. In contrast, the second approach is based on data from other patients who have similar brain damage. This “data-led” approach will allow the clinician to inform the aphasic patient how other patients, with the same symptoms and distribution of stroke damage, progressed in the years following their stroke. The data-led approach is likely to be more rapidly available than the model-led approach because it does not necessitate an understanding of why damage has impaired language function. It simply requires proof of efficacy, safety, and reproducibility.
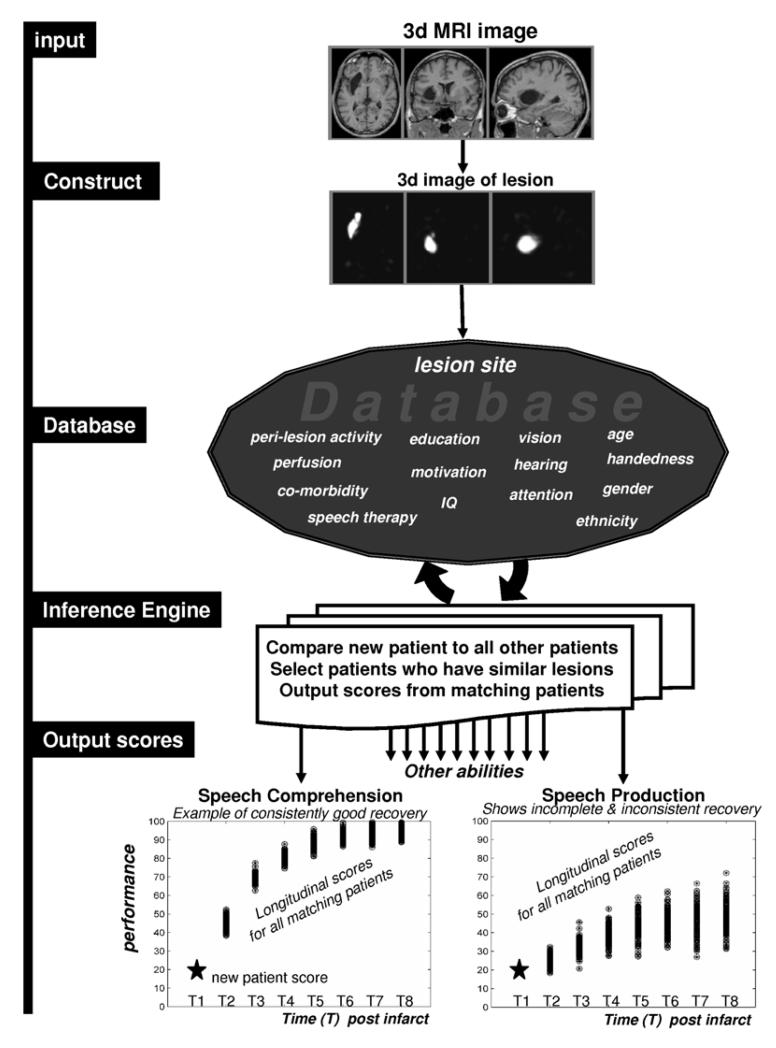
A data-led system for predicting language outcome and recovery after stroke requires a database of structural MRI and behavior from standardized assessments in hundreds of stroke patients. It also requires software to measure and compare lesions in different patients (e.g. 61, 82-86). It will then be possible to estimate the expected language outcome of a new patient by: (1) conducting a high resolution MRI scan; (2) comparing the lesion site with that of all the other patients in the database; (3) selecting patients in the database who are most similar to the new patient, in terms of their lesions and presenting symptoms; and (4) extracting the language scores, over time, for these “similar” patients, see Figure 2. This procedure would provide information of the following type: “85% of previous patients with the same lesion made a full recovery in 1 year”. As language involves many different skills that can break down independently of one another (e.g. articulation, comprehension, reading etc.) a different output will be needed for each type of function. For example, there might be a 90% likelihood of regaining one function, a 10% likelihood of regaining another function, and a 50% likelihood of regaining a third function. This information would be useful to patients, relatives and therapists.
Figure 2. Schematic illustration of the procedures and database needed to estimate recovery of language after brain damage.
The input is a high resolution structural MRI scan of a new patient. This is converted to a 3D image that indexes the degree of damage at every voxel (2mm3) in the brain.94 The lesion image is compared to those from all the other patients already in the database. Patients in the database are then selected if they have a very similar lesion to the new patient. The outputs are the language scores for the new patient plotted, over time to estimate the time course of recovery for the new patient. The outputs differ with the type of language function and will also depend on patient demographics, co-morbidity and degree and type of therapeutic intervention.
The accuracy of the language outcome predictions will depend on multiple factors. For some lesions and behaviors, the predictive accuracy should be close to 100%. For example, it is highly likely that a right handed patient will have prolonged speech and language difficulties following extensive left middle cerebral artery damage. Conversely, it is highly unlikely that a lesion to the visual cortex will cause difficulties in the comprehension or production of speech. For many other lesion sites and behaviors, the predictive accuracy may be low because the effect of the same lesion site on language function varies from patient to patient (see Figure 2, lower right). Factors that might underlie the inconsistency across patients with similar lesions include: co-morbidity, age, handedness, gender, hearing, vision, education, pre-morbid learning ability, motivation to relearn, attention, working memory, multi-lingual experience, ethnicity, social and cultural background, and the availability of speech therapy and pharmacological intervention.87-90 However, there will also be intrinsic variability in functional anatomy because the gyral and sulcul “landmarks” in structural MRI scans do not necessarily correspond to the functionally relevant cytoarchitectural structure. In this case, the predictive validity will be apparent from the degree to which language outcome varies across patients in the database.
The accuracy of the language outcome predictions will also depend on how accurately the lesion has been measured. Brain damage is typically distributed in a complicated fashion because damage to one brain region impacts upon the structure and function of neighboring and distantly connected regions.91, 92 Therefore, a single neurological infarct can result in damage to multiple and distributed regions. Each of these regions may cause their own functional impairment but, as shown in Figure 1C, the combination of multiple regions of damage may have a much greater effect on behavior than the sum of the effect of each region alone.74, 75, 93 The measurement of the lesion site therefore needs to define the damage as a 3 dimensional volume (the lesion) in a 3 dimensional space (stereotaxic coordinates) without losing information on the relative degree of damage in each part of the lesion.84, 94 High resolution and high definition imaging are more likely to detect regions where damage is subtle or invisible to the eye. Other factors that may improve the description of the lesion include information on perfusion, diaschisis, oedema and neuronal activity in the peri-lesional tissue.6, 95-100
Given that speech and language performance after brain damage may depend on so many different variables, how feasible is the data-led prediction approach depicted in Figure 2? There is no doubt that the answer to this question will depend on the language function being tested and the location of the lesion site. For example, brain damage following stroke is more common in some vascular territories than others,101 therefore the database will have more information on the effects of frequently occurring than rare lesions. It is also encouraging to note that recent studies of large populations of stroke patients show that the strongest predictor of language dysfunction is the lesion site (e.g. 32, 102). For example, Yang and colleagues studied a sample of 1198 stroke patients, of which 325 were aphasic. They report that 288/325 (88.6%) of the patients with aphasia had damage to known left hemisphere language areas, such as Broca’s and Wernicke’s areas and only 9 patients had no language difficulties following damage to Broca’s and Wernicke’s areas.102 If these data were entered into a relational database of the sort described in Figure 2, it could also be used to investigate the factors that explain inconsistencies across patients.
Other relevant factors concern the difficulty and expense of recruiting, scanning and testing patients with diverse lesion sites at different time points; the computing power needed to analyze, integrate and store the data; and the methodological advances that are required to combine and compare data from different patients. Most of these obstacles have now either been overcome or could be overcome with sufficient resources and collaboration. For example, computing power has risen at an exponential rate;103 international and web-based communication is now cheaper and easier;104 and methodological advances in neuro-imaging105 have increased our ability to (i) combine brain images from different patients into a common anatomical space84, 94, 106 and (ii) analyze thousands of regions at the same time. Furthermore, recent advances in neuro-imaging analysis and computing power are now sufficient to support large scale databases of neuroimaging data.107-109 Indeed, multi-centre collaborations have already generated a great deal of enthusiasm and expertise, including the Traumatic Brain Injury Model Systems National Database (TBIMS110), the Alzheimer’s Disease Neuroimaging Initiative (ADNI111), and the Brain Development Cooperative Group (BDCG112).
In this context, we draw attention to our new data-led system that aims to Predict Language Outcome and Recovery After Stroke (PLORAS). At the end of 2009, we have 330 patients on the database and the software to output the scores from patients who have the same lesion as a new patient. The next stage is to translate these procedures to a larger scale and make them available over the web. We welcome any clinical or research collaborators interested in testing or contributing to our PLORAS system.
3. Future directions for research
In the previous section, we highlighted the value of a data-led system for predicting language outcome and recovery after stroke (PLORAS). Here we emphasize the importance of future research that will refine the predictions by accounting for sources of inter-patient variations. The most important of these are likely to be demographic details such as age at stroke, handedness, educational attainment, co-morbidity, motivation, vision, hearing, and attention. Randomized, blinded, controlled trials of treatment efficacy will also help to distinguish predictions for recovery that occurs spontaneously or depends on the type and duration of interventional therapy.
We can also finesse our predictions with information as to which brain areas and white matter connections are most important for language. This involves integrating data from lesion studies with that from other methodologies including functional imaging,113-118 structural studies of white matter connections,119 perfusion,96 analysis of cognitive ontologies120 and recent advances in our understanding of how brain regions interact within distinct brain networks.76-78, 121, 122
To summarize, it is now feasible to create a database for the clinical translation of neuroscience and the idea of freely sharing imaging data across units is gaining momentum. We have initiated such as scheme for Predicting Language Outcome and Recovery After Stroke (PLORAS). As the database increases, and the results of future research are integrated, the time course of recovery will be adjusted to account for demographic details such as age at stroke, educational attainment, co-morbidity and the type and duration of interventional therapy. The predictive validity of this scheme is therefore proportional to the size of the database108, 109 which in turn will depend on inter-center collaborations.
Currently, there is no way of accurately predicting recovery from aphasia.
Many factors influence recovery but the main determinant is lesion site.
We introduce a new system (PLORAS) to predict language outcome on the basis of lesion site.
Predictive validity will depend on collaborative efforts to develop an international database.
Review criteria Pubmed search: lesions, aphasia, speech, language, recovery, voxel based morphometry, voxel based lesion-symptom mapping, database.
Author biographies
Professor Cathy Price is a Wellcome Trust Senior Research Fellow and principal investigator of language studies at the Wellcome Trust Centre for Neuroimaging. She has a PhD in neuropsychology with a special interest in dyslexia. In the last 20 years, she has focused on functional neuroimaging methologies with the aim of understanding the neural systems that support language function in the healthy brain and following stroke.
Dr Mohamed Seghier is a Senior Lecturer in imaging neuroscience. He has a PhD in MRI physics and engineering with specialization in developing image analysis methodologies to understand the functional anatomy of language. His neuroscientific interests focus on the causes of individual variability in the neural networks that support language in healthy and brain damaged participants.
Dr Alex Leff is a consultant neurologist at the Institute of Neurology, UCL, with a PhD in the neural systems that support language. In his clinical role, he runs a cognitive rehabilitation clinic. In his research role, he is assessing the effect of pharmacological and behavioral therapy on language and brain function measured with fMRI and MEG. He is also pioneering the use of web-based language therapy interventions for patients to use in their own homes.
References
- 1.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 2.Caplan D, et al. A study of syntactic processing in aphasia II: neurological aspects. Brain Lang. 2007;101:151–177. doi: 10.1016/j.bandl.2006.06.226. [DOI] [PubMed] [Google Scholar]
- 3.Rijntjes M. Mechanisms of recovery in stroke patients with hemiparesis or aphasia: new insights, old questions and the meaning of therapies. Curr Opin Neurol. 2006;19:76–83. doi: 10.1097/01.wco.0000203886.28068.38. [DOI] [PubMed] [Google Scholar]
- 4.Lazar RM, Antoniello D. Variability in recovery from aphasia. Curr Neurol NeurosciRep. 2008;8:497–502. doi: 10.1007/s11910-008-0079-x. [DOI] [PubMed] [Google Scholar]
- 5.Crosson B, et al. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev. 2007;17:157–77. doi: 10.1007/s11065-007-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsh EB, Hillis AE. Recovery from aphasia following brain injury: the role of reorganization. Prog Brain Res. 2006;157:143–156. doi: 10.1016/S0079-6123(06)57009-8. [DOI] [PubMed] [Google Scholar]
- 7.Mesulam M, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- 9.Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- 10.Bright P, Moss HE, Stamatakis EA, Tyler LK. Longitudinal studies of semantic dementia: the relationship between structural and functional changes over time. Neuropsychologia. 2008;46:2177–2188. doi: 10.1016/j.neuropsychologia.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SM, et al. Automated MRI-based classification of primary progressive aphasia variants. Neuroimage. 2009;47:1558–1567. doi: 10.1016/j.neuroimage.2009.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen HJ, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67:1752–1756. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]
- 13.Broca P. Remarques sur le siége de la faculté du langage articulé; suivies d’une observation d’aphemie. Bull Soc. Anat. Paris. 1861;6:330–357. [Google Scholar]
- 14.Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130:1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- 15.Berker EA, Berker AH, Smith A. Translation of Broca’s 1865 report. Localization of speech in the third left frontal convolution. Arch Neurol. 1986;43:1065–1072. doi: 10.1001/archneur.1986.00520100069017. [DOI] [PubMed] [Google Scholar]
- 16.Wernicke C. Lehrbuch der gehirnkrankheiten fur aerzte und studirende. Kassel Theodor Fischer. 1881;2:229–242. [Google Scholar]
- 17.Bogen JE, Bogen GM. Wernicke’s region--Where is it? Ann N Y Acad Sci. 1976;280:834–843. doi: 10.1111/j.1749-6632.1976.tb25546.x. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker HA, Etlinger SC. Theodor Meynert’s contribution to classical 19th century aphasia studies. Brain Lang. 1993;45:560–471. doi: 10.1006/brln.1993.1060. [DOI] [PubMed] [Google Scholar]
- 19.Thomson AD, et al. Wernicke’s encephalopathy revisited. Translation of the case history section of the original manuscript by Carl Wernicke ‘Lehrbuch der Gehirnkrankheiten fur Aerzte and Studirende’ (1881) with a commentary. Alcohol Alcohol. 2008;43:174–179. doi: 10.1093/alcalc/agm144. [DOI] [PubMed] [Google Scholar]
- 20.Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca’s area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Naeser M, Helm-Estabrooks N, Haas G. Relationship between lesionextent in Wernicke’s area on computed tomographic scan and predicting recovery of comprehension in Wernicke’s aphasia. Arch. Neurol. 1987;44:73–82. doi: 10.1001/archneur.1987.00520130057018. [DOI] [PubMed] [Google Scholar]
- 22.Wise RJ, et al. Separate neural subsystems within ‘Wernicke’s area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- 23.Tyler KL, Malessa R. The Goltz-Ferrier debates and the triumph of cerebral localizationalist theory. Neurology. 2000;55:1015–1024. doi: 10.1212/wnl.55.7.1015. [DOI] [PubMed] [Google Scholar]
- 24.Mohr JP, et al. Broca aphasia: pathologic and clinical. Neurology. 1978;28:311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- 25.Marie P. Revision de la question de l’aphasie: la troisième circonvolution frontale gauche ne joue aucun rôle spe’cial dans la fonction du langage. Brain Medicale. 1906;26:241–247. [Google Scholar]
- 26.Fridriksson J, Bonilha L, Rorden C. Severe Broca’s aphasia without Broca’s area damage. Behav Neurol. 2007;18:237–238. doi: 10.1155/2007/785280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage. 2006;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaffler L, Luders HO, Dinner DS, Lesser RP, Chelune GJ. Comprehension deficits elicited by electrical stimulation of Broca’s area. Brain. 1993;116:695–715. doi: 10.1093/brain/116.3.695. [DOI] [PubMed] [Google Scholar]
- 29.Davis C, et al. Speech and language functions that require a functioning Broca’s area. Brain Lang. 2008;105:50–58. doi: 10.1016/j.bandl.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Rogalsky C, Pitz E, Hillis AE, Hickok G. Auditory word comprehension impairment in acute stroke: relative contribution of phonemic versus semantic factors. Brain Lang. 2008;107:167–169. doi: 10.1016/j.bandl.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blank SC, Scott SK, Murphy K, Warburton E, Wise RJS. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- 32.Kreisler A, et al. The anatomy of aphasia revisited. Neurology. 2000;54:1117–1123. doi: 10.1212/wnl.54.5.1117. [DOI] [PubMed] [Google Scholar]
- 33.Willmes K, Poeck K. To what extent can aphasic syndromes be localized? Brain. 1993;116:1527–1540. doi: 10.1093/brain/116.6.1527. [DOI] [PubMed] [Google Scholar]
- 34.Signoret JL, Castaigne P, Lhermitte F, Abelanet R, Lavorel P. Rediscovery of Leborgne’s brain: anatomical description with CT scan. Brain Lang. 1984;22:303–319. doi: 10.1016/0093-934x(84)90096-8. [DOI] [PubMed] [Google Scholar]
- 35.Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- 36.Rorden C, Fridriksson J, Karnath HO. An evaluation of traditional and novel tools for lesion behavior mapping. Neuroimage. 2009;44:1355–1362. doi: 10.1016/j.neuroimage.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awad M, Warren JE, Scott SK, Turkheimer FE, Wise RJS. A common system for the comprehension and production of narrative speech. J Neurosci. 2007;27:11455–11464. doi: 10.1523/JNEUROSCI.5257-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newhart M, Ken L, Kleinman JT, Heidler-Gary J, Hillis AE. Neural networks essential for naming and word comprehension. Cogn Behav Neurol. 2007;20:25–30. doi: 10.1097/WNN.0b013e31802dc4a7. [DOI] [PubMed] [Google Scholar]
- 39.Hillis AE. Aphasia: progress in the last quarter of a century. Neurology. 2007;69:200–213. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- 40.Hillis AE, et al. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- 41.Vigneau M, et al. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. Neuroimage. 2008;42:1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kherif F, Josse G, Seghier ML, Price CJ. The main sources of inter-subject variability in neuronal activation for reading aloud. J Cogn Neurosci. 2009;21:654–668. doi: 10.1162/jocn.2009.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penfield W, Roberts L. Speech and Brain-Mechanisms. Princeton University Press; Princeton, N.J.: 1959. [Google Scholar]
- 45.Crosson B. Subcortical functions in language: a working model. Brain Lang. 1985;25:257–292. doi: 10.1016/0093-934x(85)90085-9. [DOI] [PubMed] [Google Scholar]
- 46.Kuljic-Obradovic DC. Subcortical aphasia: three different language disorder syndromes? Eur J Neurol. 2003;10:445–448. doi: 10.1046/j.1468-1331.2003.00604.x. [DOI] [PubMed] [Google Scholar]
- 47.Radanovic M, Scaff M. Speech and language disturbances due to subcortical lesions. Brain Lang. 2003;84:337–352. doi: 10.1016/s0093-934x(02)00554-0. [DOI] [PubMed] [Google Scholar]
- 48.Alexander MP, Naeser MA, Palumbo CL. Correlations of subcortical CT lesion sites and aphasia profiles. Brain. 1987;110:961–991. doi: 10.1093/brain/110.4.961. [DOI] [PubMed] [Google Scholar]
- 49.Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30:100–108. doi: 10.1161/01.str.30.1.100. [DOI] [PubMed] [Google Scholar]
- 50.Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6:202–213. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- 51.Richter S, et al. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. J Neurol. 2007;254:1193–1203. doi: 10.1007/s00415-006-0500-9. [DOI] [PubMed] [Google Scholar]
- 52.Hofmann J, Kotz SA, Marschhauser A, Yves von Cramon D, Friederici AD. Lesion-site affects grammatical gender assignment in German: perception and production data. Neuropsychologia. 2007;45:954–965. doi: 10.1016/j.neuropsychologia.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 53.Amici S, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cogn Behav Neurol. 2007;20:203–11. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- 54.Amici S, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007;27:6282–90. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bates E, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 56.Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12:896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- 57.Baldo JV, Dronkers NF. Neural correlates of arithmetic and language comprehension: a common substrate? Neuropsychologia. 2007;45:229–35. doi: 10.1016/j.neuropsychologia.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Borovsky A, Saygin AP, Bates E, Dronkers NF. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45:2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dronkers NF, Wilkins DP, Van Valin RDJ, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–61. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- 61.Kinkingnehun S, et al. A novel approach to clinical-radiological correlations: Anatomo-Clinical Overlapping Maps (AnaCOM): method and validation. Neuroimage. 2007;37:1237–49. doi: 10.1016/j.neuroimage.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 62.Saygin AP, Wilson SM, Dronkers NF, Bates E. Action comprehension in aphasia: linguistic and non-linguistic deficits and their lesion correlates. Neuropsychologia. 2004;42:1788–804. doi: 10.1016/j.neuropsychologia.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Specht K, et al. Joint independent component analysis of structural and functional images reveals complex patterns of functional reorganisation in stroke aphasia. Neuroimage. 2009;47:2057–2063. doi: 10.1016/j.neuroimage.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Wilson SM, Saygin AP. Grammaticality judgment in aphasia: deficits are not specific to syntactic structures, aphasic syndromes, or lesion sites. J Cogn Neurosci. 2004;16:238–252. doi: 10.1162/089892904322984535. [DOI] [PubMed] [Google Scholar]
- 65.Broca P. Sur le siège de la faculté du langage articulé. Bull Soc Anthropol. 1865;6:337–393. [Google Scholar]
- 66.Naeser MA, Hayward RW. Lesion localization in aphasia with cranial computed tomography and the Boston Diagnostic Aphasia Examination. Neurology. 1978;28:545–551. doi: 10.1212/wnl.28.6.545. [DOI] [PubMed] [Google Scholar]
- 67.Naeser MA, Palumbo CL, Helm-Estabrooks N, Stiassny-Eder D, Albert ML. Severe non-fluency in aphasia: Role of the medial subcallosal fasciculus plus other white matter pathways in recovery of spontaneous language. Brain. 1989;112:1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- 68.Basso A, Lecours AR, Moraschini S, Vanier M. Anatomoclinical correlations of the aphasias as defined through computerized tomography: Exceptions. Brain Lang. 1985;26:201–229. doi: 10.1016/0093-934x(85)90039-2. [DOI] [PubMed] [Google Scholar]
- 69.Murdoch BE, Afford RJ, Ling AR, ganguley B. Acute computerized tomographic scans: their value in the localization of lesions and as prognostic indicators in aphasia. J Commun Disord. 1986;19:311–345. doi: 10.1016/0021-9924(86)90025-0. [DOI] [PubMed] [Google Scholar]
- 70.Kertesz A, Harlock W, Coates R. Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain Lang. 1979;8:34–50. doi: 10.1016/0093-934x(79)90038-5. [DOI] [PubMed] [Google Scholar]
- 71.Schiff HB, Alexander MP, Naeser MA, Galaburda AM. Aphemia. Clinical-anatomic correlations. Arch Neurol. 1983;40:720–727. doi: 10.1001/archneur.1983.04050110038005. [DOI] [PubMed] [Google Scholar]
- 72.Alexander MP, Naeser MA, Palumbo CL. Broca’s area aphasias: aphasia after lesions including the frontal operculum. Neurology. 1990;40:353–362. doi: 10.1212/wnl.40.2.353. [DOI] [PubMed] [Google Scholar]
- 73.Grodzinsky Y. The neurology of syntax: language use without Broca’s area. 2000;23:1. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- 74.Godefroy O, et al. Brain-behaviour relationships. Some models and related statistical procedures for the study of brain-damaged patients. Brain. 1998;121:1545–1556. doi: 10.1093/brain/121.8.1545. [DOI] [PubMed] [Google Scholar]
- 75.Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends Cogn Sci. 2002;6:416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 76.Ruppin E, Reggia JA. Patterns of functional damage in neural network models of associative memory. Neural Comput. 1995;7:1105–1127. doi: 10.1162/neco.1995.7.5.1105. [DOI] [PubMed] [Google Scholar]
- 77.Chen R, Hillis AE, Pawlak M, Herskovits EH. Voxelwise Bayesian lesion-deficit analysis. Neuroimage. 2008;40:1633–1642. doi: 10.1016/j.neuroimage.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herskovits EH, Gerring JP. Application of a data-mining method based on Bayesian networks to lesion-deficit analysis. Neuroimage. 2003;19:1664–1673. doi: 10.1016/s1053-8119(03)00231-3. [DOI] [PubMed] [Google Scholar]
- 79.Mummery CJ, et al. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 80.Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- 81.Butler CR, Brambati SM, Miller BL, Gorno-Tempini M. The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cogn Behav Neurol. 2009;22:73–80. doi: 10.1097/WNN.0b013e318197925d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bates E, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–50. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 83.Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- 84.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 85.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 86.Kimberg DY, Coslett HB, Schwartz MF. Power in Voxel-based lesion-symptom mapping. J Cogn Neurosci. 2007;19:1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- 87.Naeser MA, Borod JC. Aphasia in left-handers: lesion site, lesion side, and hemispheric asymmetries on CT. Neurology. 1986;36:471–488. doi: 10.1212/wnl.36.4.471. [DOI] [PubMed] [Google Scholar]
- 88.Naugle RI, Raymond MJ. Neuropsychological sequelae of stroke as a function of handedness. Percept Mot Skills. 1991;73:555–562. doi: 10.2466/pms.1991.73.2.555. [DOI] [PubMed] [Google Scholar]
- 89.Arnold M, et al. Age-dependent differences in demographics, risk factors, co-morbidity, etiology, management, and clinical outcome of acute ischemic stroke. J Neurol. 2008;255:1503–1507. doi: 10.1007/s00415-008-0949-9. [DOI] [PubMed] [Google Scholar]
- 90.Basso A, Bracchi M, Capitani E, Laiacona M, Zanobio ME. Age and evolution of language area functions. A study on adult stroke patients. Cortex. 1987;23:475–483. doi: 10.1016/s0010-9452(87)80008-4. [DOI] [PubMed] [Google Scholar]
- 91.Thomalla G, et al. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–1774. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 92.Young MP, Hilgetaq CC, Scannell JW. On imputing function to structure from the behavioural effects of brain lesions. Philos Trans R Soc Lond B Biol Sci. 2000;355:147–161. doi: 10.1098/rstb.2000.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keinan A, Kaufman A, Sachs N, Hilgetaq CC, Ruppin E. Fair localization of function via multi-lesion analysis. Neuroinformatics. 2004;2:163–168. doi: 10.1385/NI:2:2:163. [DOI] [PubMed] [Google Scholar]
- 94.Seghier ML, Ramlackhansingh A, Crinion J, Leff A, Price CJ. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. Neuroimage. 2008;41:1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warburton E, Price CJ, Swinburn K, Wise RJS. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66:155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hillis AE, et al. Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Ann Neurol. 2001;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- 97.Breier JI, et al. Spatiotemporal patterns of language-specific brain activity in patients with chronic aphasia after stroke using magnetoencephalography. Neuroimage. 2004;23:1308–1316. doi: 10.1016/j.neuroimage.2004.07.069. [DOI] [PubMed] [Google Scholar]
- 98.Meinzer M, et al. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39:2038–2046. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 99.Saur D, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 100.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 101.van der Zwan A, Hillen B. Review of the variability of the territories of the major cerebral arteries. Stroke. 1991;22:1078–1084. doi: 10.1161/01.str.22.8.1078. [DOI] [PubMed] [Google Scholar]
- 102.Yang ZH, Zhao XQ, Wang CX, Chen HY, Zhang YM. Neuroanatomic correlation of the post-stroke aphasias studied with imaging. Neurol Res. 2008;30:356–360. doi: 10.1179/174313208X300332. [DOI] [PubMed] [Google Scholar]
- 103.Meindl JD. Beyond Moore’s law: the interconnect era. Comput Sci Engineer. 2003;5:20–24. [Google Scholar]
- 104.Pozamantir A, Lee H, Chapman J, Prohovnik I. Web-based Multi-center Data Management System for Clinical Neuroscience Research. J Med Syst. 2009 doi: 10.1007/s10916-008-9212-2. (in press) [DOI] [PubMed] [Google Scholar]
- 105.Bandettini PA. What’s new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crinion J, et al. Spatial normalization of lesioned brains: Performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37:866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.The Governing Council of the Organization for Human Brain Mapping. Neuroimaging databases. Science. 2001;292:1673–1676. doi: 10.1126/science.1061041. [DOI] [PubMed] [Google Scholar]
- 108.Amari S, et al. Neuroinformatics: the integration of shared databases and tools towards integrative neuroscience. J Integr Neurosci. 2002;1:117–28. doi: 10.1142/s0219635202000128. [DOI] [PubMed] [Google Scholar]
- 109.Van Horn JD, Toga AW. Is it time to re-prioritize neuroimaging databases and digital repositories? Neuroimage. 2009;47:1720–1734. doi: 10.1016/j.neuroimage.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. [accessed 06.01.2010];The Traumatic Brain Injury Model Systems National Data and Statistical Center. http://www.tbindsc.org/
- 111.Mueller SG, et al. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brain Development Cooperative Group. Evans AC. The NIH MRI study of normal brain developmentstar, open. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 113.Price CJ, Mummery CJ, Moore CJ, Frackowiak RS, Friston KJ. Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J Cogn Neurosci. 1999;11:371–382. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- 114.Price CJ, Friston KJ. Functional imaging studies of neuropsychological patients: applications and limitations. Neurocase. 2002;8:345–354. doi: 10.1076/neur.8.4.345.16186. [DOI] [PubMed] [Google Scholar]
- 115.Dick F, et al. What is involved and what is necessary for complex linguistic and nonlinguistic auditory processing: evidence from functional magnetic resonance imaging and lesion data. J Cogn Neurosci. 2007;19:799–816. doi: 10.1162/jocn.2007.19.5.799. [DOI] [PubMed] [Google Scholar]
- 116.Vandenberghe R, Gillebert CR. Parcellation of parietal cortex: convergence between lesion-symptom mapping and mapping of the intact functioning brain. Behav Brain Res. 2009;199:171–182. doi: 10.1016/j.bbr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 117.Schnur TT, et al. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proc Natl Acad Sci U S A. 2009;106:322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Molenberghs P, Gillebert CR, Peeters R, Vandenberghe R. Convergence between lesion-symptom mapping and functional magnetic resonance imaging of spatially selective attention in the intact brain. J Neurosci. 2008;28:3359–3373. doi: 10.1523/JNEUROSCI.5247-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rudrauf D, Mehta SG, T J. Disconnection’s renaissance takes shape: Formal incorporation in group-level lesion studies. Cortex. 2008;44:1048–1096. doi: 10.1016/j.cortex.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 120.Price CJ, Friston KJ. Functional ontologies for cognition : The systematic definition of structure and function. Cogn Neuropsychol. 2005;22:262–275. doi: 10.1080/02643290442000095. [DOI] [PubMed] [Google Scholar]
- 121.Honey CJ, Sporns O. Dynamical consequences of lesions in cortical networks. Hum Brain Mapp. 2008;29:802–809. doi: 10.1002/hbm.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O. Modeling the impact of lesions in the human brain. PLoS Comput Biol. 2009;5:e1000408. doi: 10.1371/journal.pcbi.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 124.Basso A. How intensive/prolonged should an intensive/prolonged treatment be? Aphasiology. 2005;19:975–984. [Google Scholar]
- 125.Basso A, Capitani E, Vignolo LA. Influence of rehabilitation on language skills in aphasic patients. A controlled study. Arch Neurol. 1979;36:190–6. doi: 10.1001/archneur.1979.00500400044005. [DOI] [PubMed] [Google Scholar]
- 126.Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke. 2003;34:987–93. doi: 10.1161/01.STR.0000062343.64383.D0. [DOI] [PubMed] [Google Scholar]
- 127.Hinckley JJ, Carr TH. Comparing the outcomes of intensive and non-intensive contextbased aphasia treatment. Aphasiology. 2005;19:965–974. [Google Scholar]
- 128.Holland AL, Fromm DS, DeRuyter F, Stein M. Treatment efficacy: aphasia. J Speech Hear Res. 1996;39:S27–36. doi: 10.1044/jshr.3905.s27. [DOI] [PubMed] [Google Scholar]
- 129.Pulvermuller F, Berthier ML. Aphasia therapy on a neuroscience basis. Aphasiology. 2008;22:563–599. doi: 10.1080/02687030701612213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Raymer AM, Kohen FP, Saffell D. Computerised training for impairments of word comprehension and retrieval in aphasia. Aphasiology. 2006;20:257–268. [Google Scholar]
- 131.Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. Journal of Speech, Language, and Hearing Research. 1998;41:172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- 132.Cherney LR, Patterson JP, Raymer A, Frymark T, Schooling T. Evidence-based systematic review: effects of intensity of treatment and constraint-induced language therapy for individuals with stroke-induced aphasia. J Speech Lang Hear Res. 2008;51:1282–99. doi: 10.1044/1092-4388(2008/07-0206). [DOI] [PubMed] [Google Scholar]
- 133.Swinburn K, Porter G, Howard D. Comprehensive Aphasia Test. Psychology Press; 2004. [Google Scholar]
- 134.Moss A, Nicholas M. Language rehabilitation in chronic aphasia and time postonset: a review of single-subject data. Stroke. 2006;37:3043–51. doi: 10.1161/01.STR.0000249427.74970.15. [DOI] [PubMed] [Google Scholar]
- 135.Meinzer M, Elbert T, Djundja D, Taub E, Rockstroh B. Extending the Constraint-Induced Movement Therapy (CIMT) approach to cognitive functions: Constraint-Induced Aphasia Therapy (CIAT) of chronic aphasia. NeuroRehabilitation. 2007;22:311–8. [PubMed] [Google Scholar]
- 136.Pulvermuller F, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–6. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 137.Berthier ML, et al. A randomized, placebo-controlled study of donepezil in poststroke aphasia. Neurology. 2006;67:1687–9. doi: 10.1212/01.wnl.0000242626.69666.e2. [DOI] [PubMed] [Google Scholar]
- 138.Berthier ML, et al. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Ann Neurol. 2009;65:577–85. doi: 10.1002/ana.21597. [DOI] [PubMed] [Google Scholar]
- 139.Seniow J, Litwin M, Litwin T, Lesniak M, Czlonkowska A. New approach to the rehabilitation of post-stroke focal cognitive syndrome: effect of levodopa combined with speech and language therapy on functional recovery from aphasia. J Neurol Sci. 2009;283:214–8. doi: 10.1016/j.jns.2009.02.336. [DOI] [PubMed] [Google Scholar]
- 140.Crinion J, Price CJ. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain. 2005;128:2858–2871. doi: 10.1093/brain/awh659. [DOI] [PubMed] [Google Scholar]