Abstract
In a neuronal overexpression screen focused on kinases and phosphatases, one “hit” was the dual specificity tyrosine phosphorylation-regulated kinase (Dyrk4), which increased the number of dendritic branches in hippocampal neurons. Overexpression of various Dyrk family members in primary neurons significantly changed neuronal morphology. Dyrk1A decreased axon growth, Dyrk3 and Dyrk4 increased dendritic branching, and Dyrk2 decreased both axon and dendrite growth and branching. Kinase-deficient mutants revealed that most of these effects depend on kinase activity. Because doublecortin (DCX), a microtubule-binding protein, regulates cytoskeletal dynamics and neuronal morphogenesis, we investigated the possibility that DCX is a target of Dyrks. We found that overexpression of Dyrk2 and Dyrk3, but not Dyrk1A or Dyrk4, can change DCX phosphorylation status. Mutation of a consensus phosphorylation site for Dyrk kinases at Serine 306 (Ser306) in DCX indicated that this is one target site for Dyrk2 and Dyrk3. Overexpression of Dyrk2 restored altered DCX distribution in the growth cones of dendrites and axons, and partially reversed the morphological effects of DCX overexpression; some of these effects were abrogated by mutation of Ser306 to alanine. These studies implicate Dyrks in the regulation of cytoskeletal organization and process outgrowth in neurons, and suggest that DCX is one relevant Dyrk target.
Keywords: actin, microtubules, dendrite branching, axon growth
Introduction
Central nervous system (CNS) injury results in the irreversible loss of connections owing to failure of axonal regeneration, among other challenges (Darian-Smith, 2009). Multiple extrinsic cues and intrinsic mechanisms have been implicated in regeneration failure, but a major point of convergence is the regulation and reorganization of the neuronal cytoskeleton (Dent et al., 2003, 2011; Ertürk et al., 2007; Usher et al., 2010; Hellal et al., 2011)
The organization and motility of axonal and dendritic growth cones is defined by the dynamics and interactions of actin filaments and microtubules (Dent et al., 2003, 2011). Among the critical regulators of the microtubule (MT) cytoskeleton is a large family of MT-associated proteins (MAPs). One MAP, doublecortin (DCX), can stabilize MTs in vitro and cause MT bundling in transfected cells (Moores et al., 2003). DCX contains two MT-binding domains within its N-terminus, and a serine and proline-rich domain at the C-terminus. Both the N- and C- termini of DCX can interact with MTs (Kim et al., 2003) and this interaction is regulated by DCX phosphorylation at multiple sites. Several kinases, including Cyclin-dependent kinase 5 (Cdk5), c-Jun N-terminal kinase (Jnk), protein kinase A (PKA) and Glycogen synthase kinase 3β (Gsk3β) have been shown to phosphorylate DCX (Graham et al., 2004; Reiner et al., 2004; Tanaka et al., 2004; Bilimoria et al., 2010; Jin et al., 2010).
DCX is well known for its role in neuronal migration (Kawauchi and Hoshino, 2008), and mutations of DCX cause developmental disorders such as X-linked lissencephaly and double cortex syndrome (Gleeson et al., 1998; des Portes et al., 1998). More recently, it has been appreciated that DCX is concentrated in neuronal growth cones and is important in axon growth (Deuel et al., 2006; Koizumi et al., 2006). DCX is highly enriched in the transition zone between MTs and actin filaments at the base of growth cones and it associates with MTs primarily in regions next to actin rich zones (Tint et al., 2009). DCX can associate with actin filaments through the spinophilin – protein phosphatase 1 (Spn-PP1) complex, which directly interacts with actin and mediates DCX dephosphorylation, promoting MT bundling (Bielas et al., 2007). Thus DCX may help coordinate the dynamic interactions of MTs and actin filaments in the growth cone.
In addition to neuronal migration and axon growth, DCX is implicated in neurite branching. Cortical neurons from DCX or Spn knockout mice exhibit excessively branched axons due to disrupted MT bundling (Bielas et al., 2007). It was proposed that the Spn/PP1complex is responsible for dephosphorylation of DCX on Ser297 and thus restores its ability to bundle MTs. Further, knockdown of DCX in cultured cerebellar neurons causes excessive axonal branching, and GSK3β-mediated phosphorylation of DCX on Ser327 inhibits this phenotype (Bilimoria et al., 2010). DCX has also been implicated in the regulation of dendrite growth and branching (Deuel et al., 2006; Cohen et al., 2008a; Kerjan et al., 2009). Thus, DCX is functionally involved in neuronal process outgrowth and branching, and these activities are regulated by phosphorylation. However, the exact roles of specific phosphorylation sites and the identities of the kinases responsible for DCX phosphorylation are not completely understood.
To begin to map out the phosphorylation events regulating neuronal morphology, we performed an overexpression screen of kinases and phosphatases in primary hippocampal neurons. In this screen we identified ~90 “hits” that affected neuronal process growth and/or branching, including PP1A, GSK3β, and others (Buchser et al., 2010). One hit was the Dual-specificity tyrosine (Y) phosphorylation-regulated kinase (Dyrk), Dyrk4, which had a clear effect on neurite branching.
Members of the Dyrk family are characterized by a unique kinase domain, which is highly conserved among eukaryotes (Aranda et al., 2010). The mammalian subfamily consists of 5 members: Dyrk1A, Dyrk1B/Mirk, Dyrk2, Dyrk3, and Dyrk4 (Aranda et al., 2010). Dyrks are activated in an autocatalytic process concurrent with translation (Lochhead et al., 2005). Regulation of Dyrk biological activities is likely to be complex, including control of subcellular localization and protein stability (Taira et al., 2010), transcriptional regulation (Lu et al., 2011), interaction with regulatory proteins (Alvarez et al., 2007), phosphorylation outside the activation loop (Lim et al., 2002), and microRNA binding (Martins et al., 2010).
Dyrks have diverse tissue distributions and participate in several signaling pathways critical for development and adult function (Aranda et al., 2010). Dyrk1A is ubiquitously expressed in the nervous system; it is localized to the “Down’s Syndrome critical region”, and is implicated in the pathology of this disease (Hammerle et al., 2003). Disruption of its Drosophila homolog, minibrain (MNB), causes abnormal neuronal development and reduction of brain size. In mammals, Dyrk1A is involved in various aspects of CNS development including neuronal proliferation, neuronal differentiation, cell death and synaptic plasticity (Tejedor and Hammerle, 2010). Little is known about the roles of other Dyrk family members in neuronal differentiation, although Dyrk2 has been implicated in the regulation of the cytoskeleton in other cells (Cole et al., 2006; Maddika and Chen, 2009). In the present work we find that several members of the Dyrk family can regulate the elongation and branching of axons and dendrites, and that some aspects of these activities are likely to be mediated by changes in the subcellular localization and phosphorylation status of DCX.
Results
Influence of Dyrk overexpression on neuronal morphology
In our initial neuronal overexpression screen we identified Dyrk4 as a regulator of neurite morphology, particularly neurite branching (Buchser et al., 2010). Because Dyrk4 was not thought to be widely expressed (Sacher et al., 2007), and is a member of a protein kinase family, we decided to investigate potential roles of other Dyrk family members in neuronal process outgrowth by overexpression in hippocampal neurons. These neurons are a useful model because of their stereotyped morphological development in culture (Dotti and Banker, 1987). Dyrk1A is thought to be mainly a nuclear protein (Becker and Joost, 1999), while Dyrks2–4 are thought to be mainly cytoplasmic (Taira et al., 2007). Since subcellular localization of other Dyrks is likely important in their function (Gwack et al., 2006; Hachet et al., 2011), but has not been examined in neurons, we examined the localization of labeled Dyrks in the hippocampal culture system. Dyrk1A was mainly localized to the nucleus, where it was concentrated in characteristic speckles (Fig. 1A, E), with a small amount in the perinuclear cytoplasm. A similar distribution of Dyrk1A was reported previously and attributed to a unique histidine stretch at the C-terminus (Alvarez et al., 2003). Dyrk2, Dyrk3, and Dyrk4 mostly localized in the cytosolic portion of cell bodies, with Dyrk2 seen in the dendrites and part of the axon, and Dyrk3 and Dyrk4 mainly confined to dendrites; nuclear localization was not apparent (Fig. 1B–D, F–H).
Fig. 1. Subcellular localization of Dyrks in transfected hippocampal neurons.
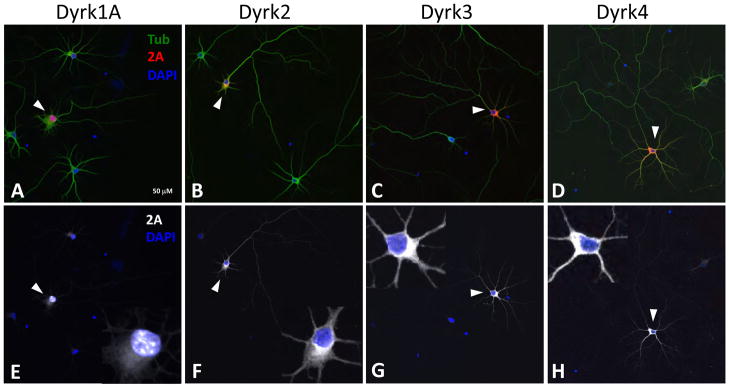
(A–D) E18 hippocampal neurons were transfected with 2A-tagged Dyrks, cultured for 5 days, and stained with anti-beta III tubulin (green) and anti-2A (red) antibodies. Arrows point to transfected cells. Scale bar is 50 μm (5μm in the inset). (E–H) 2A (white) and nuclear (DAPI, blue) staining only of the merged images. The insets show a magnified image of the cell body of the transfected cell. Dyrk1A (E) localizes in speckled structures of the nucleus. Dyrk2 (F), Dyrk3 (G), and Dyrk4 (H) are cytosolic, present in dendrites (Dyrk3 and 4), or both dendrites and axons (Dyrk2), and are not detected in the nucleus.
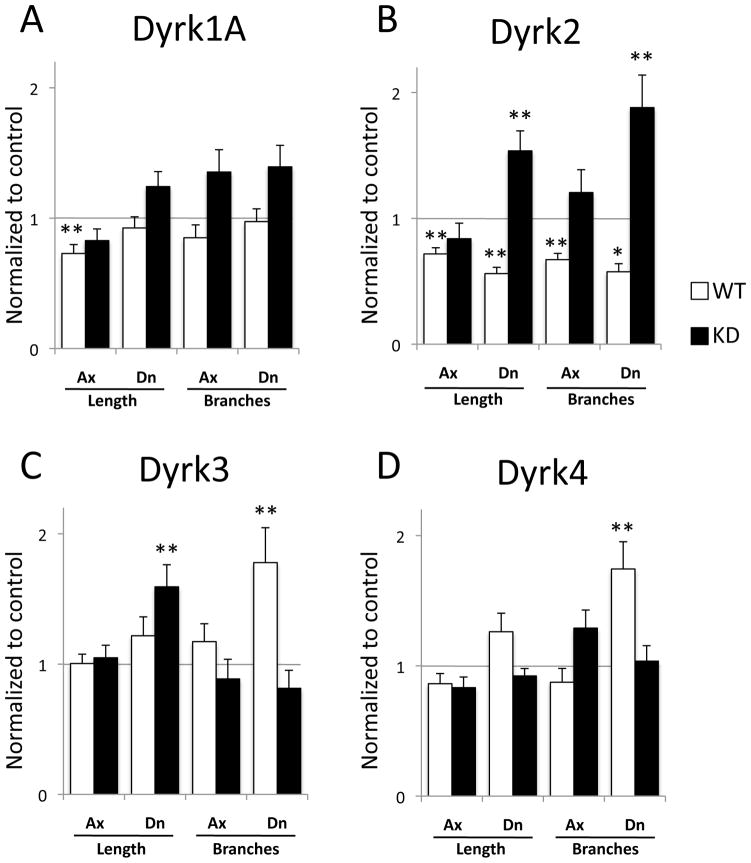
Interestingly, each of the 4 Dyrks affected neuronal process growth when overexpressed. Expression of Dyrk1A reduced overall axon growth by 27%, but did not affect overall dendrite growth or branching of axons or dendrites (Fig. 2A and Suppl. Data Table 1). Dyrk2 expression reduced overall axon growth by 28%, and reduced the number of axonal branches by 33% (Fig. 2B). Interestingly, Dyrk2 also shortened the total dendritic length by 44%, and reduced the number of dendritic branches by 42% (Fig. 2B). Neither Dyrk3 nor Dyrk4 affected axon growth in these experiments, but both increased dendritic branching more than 70% (Fig. 2C, D). Taken together, these results suggest that Dyrk family members regulate several aspects of neuronal process outgrowth, and that different family members have distinct roles.
Fig. 2. Effect of DYRKs on neuron morphology.
Primary neurons were transfected with Dyrk1A (A), Dyrk2 (B), Dyrk3 (C), or Dyrk4 (D) plasmids, either WT or kinase-deficient (KD), or with control mCherry plasmids. Transfected neurons (N ≥ 30 per condition) were manually traced (using the tubulin channel) to measure axonal (Ax) total length and branching, and dendritic (Dn) total length and branching. All measured parameters were normalized to the control. Dyrk1A overexpression significantly decreased axonal length (A). Dyrk2 overexpression decreased all 4 measured parameters, and the KD mutant increased dendritic length and branching (B). Dyrk3 overexpression increased dendritic branching, and the KD mutant tended to decrease this parameter. KD Dyrk3 also increased axonal length (C). Dyrk4 overexpression, but not the KD mutant, increased dendritic branching (D). Graphs show Mean ± SEM. *, p < 0.05; **, p< 0.01
Kinase-deficient Dyrk mutants have distinct and dominant-negative effects on process outgrowth
In addition to acting as protein kinases, some Dyrks have non-enzymatic functions; e.g., by acting as scaffolding proteins (Maddika and Chen, 2009). To test whether the effects of Dyrks on neuronal morphology could be attributed to their kinase activity, we compared the effects of overexpression of kinase-deficient (KD) mutants to those of their wild-type counterparts. In the KD mutants, a lysine residue crucial for kinase activation (Kannan and Neuwald, 2004; Lochhead et al., 2005) was mutated, either to an arginine (Dyrk 1A) or to a methionine (Dyrks 2–4); such mutants may be either inactive or dominant-negative (Varjosalo et al., 2008).
Although the KD mutant of Dyrk1A did not alter any morphological parameter significantly, axon lengths were reduced by this mutant to values similar to those seen with wt Dyrk1A, suggesting that this effect of Dyrk1A may be independent of kinase activity (Fig. 2A). The KD mutant of Dyrk2 mainly acted as a dominant negative, strongly increasing both dendrite length and dendrite branching, and showing a trend toward an increase in axon branching, all parameters that were reduced by expression of wt Dyrk2 (Fig. 2B). Thus effects of Dyrk2 on neuronal morphology mainly depend on its kinase activity. The KD mutants of Dyrk3 and Dyrk4, unlike the wt versions, failed to increase dendrite branching, consistent with these effects being due to kinase activity as well (Fig. 2C, D). In addition, the Dyrk3 KD mutant increased overall dendrite length, a new activity not predicted by effects of the wt protein (Fig. 2C). Whether this latter activity is a dominant negative effect or a novel gain of function is unknown. In summary, Dyrks regulate aspects of both axonal and dendritic morphology, but the most striking effects are on dendrites, and most of these effects depend on Dyrk kinase activity.
Dyrk1A and Dyrk2 influence subcellular localization of DCX
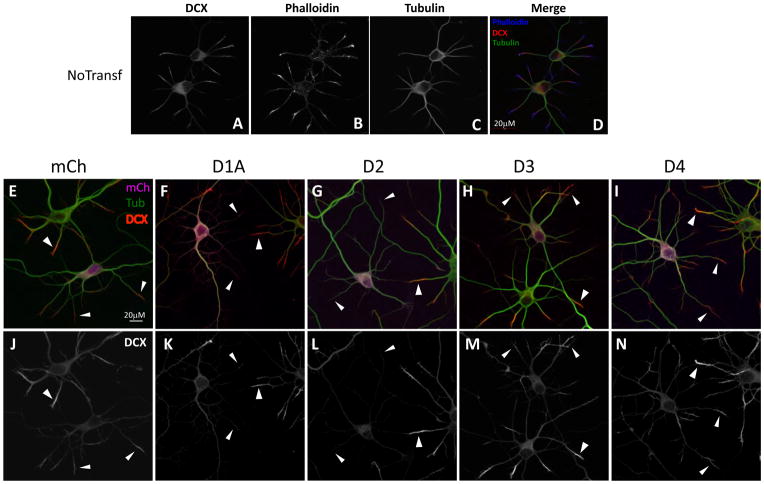
The morphological effects of Dyrk proteins in neurons suggest effects on the neuronal cytoskeleton, and Dyrks are known to regulate cytoskeletal dynamics (Pang et al., 2004; Cole et al., 2006; Tatebe et al., 2008; Maddika and Chen, 2009). We began to investigate this issue by examining the distribution of tubulin, F-actin, and DCX (a microtubule-binding protein enriched in the tips of growing neurites (Schaar et al., 2004)), in neurons expressing each of the four Dyrks. In untransfected hippocampal neurons, DCX immunoreactivity was mainly found in cell bodies and in growth cones. In growth cones, DCX (Fig. 3A) was enriched in the neurite tips between the ends of microtubule bundles in the neurite shaft (Fig 3C) and actin filaments found at the periphery (Fig 3B), consistent with earlier studies (Tint et al., 2009).
Fig. 3. Dyrk1A and Dyrk2 overexpression affect subcellular localization of DCX.
(A–D) In non-transfected neurons DCX is in the cell body and is enriched at the neurite tips between actin filaments (B) and microtubules (C and E, J, arrowheads). (F–I) Merged images of neurons transfected with 2A-tagged mCherry control or Dyrks and stained with anti-2A (magenta), anti-beta III tubulin (green) and anti-DCX (red) antibodies. (K–N) The same images with DCX staining only. Overexpression of Dyrk1A (F, K) or Dyrk2 (G, L) alter DCX localization compared to control (E, J). Overexpression of Dyrk3 (H, M) or Dyrk4 (I, N) does not obviously affect DCX localization. Arrows show DCX staining in transfected neurons; arrows, in untransfected cells. Scale bar is 20 μm.
Unexpectedly, we found that overexpression of some Dyrk kinases altered the subcellular distribution of DCX. In particular, overexpression of Dyrk1A or Dyrk2 reduced DCX staining in dendritic growth cones (Fig. 3F, G, K, L); for Dyrk2 this effect appeared more robust, and was also detected in axons (Supl. Data Fig. 1). Neither Dyrk3 nor Dyrk4 influenced DCX distribution (Fig. 3H, I, M, N). Thus, overexpression of Dyrk1A and Dyrk2 in neurons causes redistribution of endogenous DCX from its normal location near the tips of dendrites. This effect is interesting in light of the effects of Dyrk2 on dendrite morphogenesis.
Ser306 of DCX is a potential target site for Dyrk2
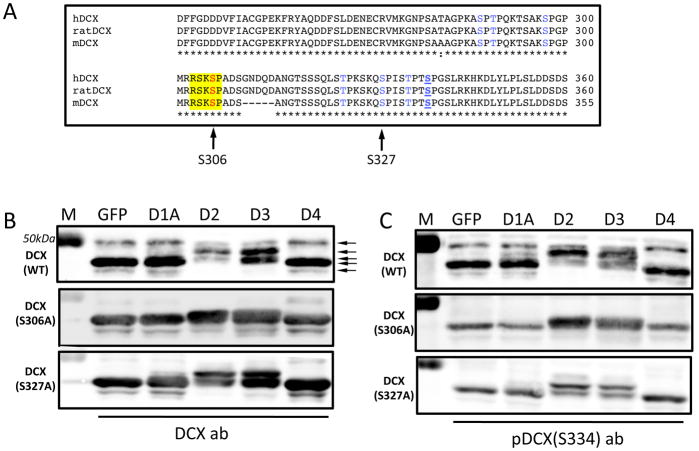
Analysis of the DCX peptide sequence revealed a stretch of amino acids (RSKSP) containing a Ser at position 306, located in the DCX C-terminus. This sequence matches the consensus site for Dyrk kinases (R(x)xxS/T(P/V)), and is located among other Ser and Thr residues known to be phosphorylated by other kinases ((Gdalyahu et al., 2004; Graham et al., 2004; Reiner et al., 2004; Schaar et al., 2004) Fig. 4A). We hypothesized that Dyrks may act through phosphorylation of DCX, and tested this hypothesis by co-transfecting DCX with Dyrks in HEK293 cells (Fig. 4B, C). When DCX was transfected with control plasmid (GFP) anti-DCX antibodies (Fig. 4B) recognized a major band at ~40 kDa and two minor bands at ~39 and 49 kDa, which were also recognized by antibodies raised against DCX phosphorylated on Ser334 (pDCX(S334); Fig 4C). The existence of multiple DCX bands on western blots has been noted (Bielas et al., 2007; Bilimoria et al., 2010), and is consistent with the existence of different phosphorylated forms of DCX.
Fig. 4. Dyrk2 and Dyrk3 affect DCX phosphorylation.
(A) Amino acid alignment of a C-terminal fragment of DCX from human (hDCX), rat, and mouse (mDCX). Highlighted in yellow is a putative consensus sequence for Dyrk phosphorylation containing Ser306 (left arrow). Phosphorylation sites in DCX targeted by known S-T kinases are shown in blue. Right arrow shows position of Ser327, a target site for GSK3beta (Bilimoria et al., 2010) (B, C) HEK293 cells were co-transfected with DCX(WT), DCX(S306A), or DCX(S327A) and either control (GFP) plasmid or plasmids expressing Dyrk1A (D1A), Dyrk2 (D2), Dyrk3 (D3), or Dyrk4 (D4). Western blots of cell lysates were performed with anti-pan DCX (DCX ab, B), or with a phospho-specific antibody raised against DCX(Ser334) (C, bolded and underlined in A). Arrows in B point to various phosphorylated bands of DCX. M, molecular weight markers.
Co-transfection with Dyrk1A or Dyrk4 did not substantially influence the DCX phosphorylation profile, but Dyrk1A expression produced an additional faint ~45 kDa band (Fig. 4B, C). In contrast, Dyrk2 and Dyrk3 caused major shifts in the DCX banding pattern. In cells co-expressing Dyrk2, DCX formed one major band at ~ 45 kDa and two minor bands of ~42 and 49 kDa (Fig 4C). A similar change occurred in the presence of Dyrk3, though the relative intensities of the different bands varied. Over the course of at least 5 transfection experiments, we observed some variation in relative intensities of the detected bands, but the overall pattern was consistent. We therefore conclude that Dyrk2 and Dyrk3, the two Dyrks most clearly associated with changes in dendritic arborization pattern, also affected the phosphorylation status of DCX when co-expressed in heterologous cells.
To test our hypothesis that Ser306 of DCX serves as a target site for Dyrk kinases, we mutated Ser306 to alanine and analyzed the behavior of this mutant in HEK293 cells co-expressing each of the 4 Dyrks. Mutation of Ser306 eliminated the 49 kDa minor band in control conditions, and largely abolished the changes in banding pattern induced by co-expression of Dyrk2 or Dyrk3 (Fig. 4B, C). However, the latter two Dyrks produced an apparent doublet at 45–46 kDa, suggesting that they are capable of affecting phosphorylation of the S306A DCX mutant, either directly or indirectly. Taken together, these results suggest that Dyrk2 and Dyrk3 can alter phosphorylation status of DCX in HEK293 cells, and that Ser306 of DCX is one relevant target site.
Dyrks can act as priming kinases, phosphorylating proteins so as to increase subsequent phosphorylation by other kinases, prominently including GSK3β (Gwack et al., 2006; Scales et al., 2009). The reported target site in DCX for GSK3β is Ser327 (Bilimoria et al., 2010). Although “classical” priming for GSK3β involves phosphorylation by Dyrks at the +4 position, priming at distant sites has also been reported (reviewed in Aranda et al., 2010), suggesting that Ser306 might be a priming site for GSK3β at Ser327. We mutated Ser327 to a non-phosphorylatable alanine residue to test this idea. However, co-expression of the various Dyrks with the DCX(S327A) mutant produced the same banding pattern as for wild type DCX (Fig. 4B, C), suggesting that Dyrks are not acting as priming kinases for GSK3β in this situation.
Dyrk2 reverses the effects of DCX overexpression on neuronal process outgrowth
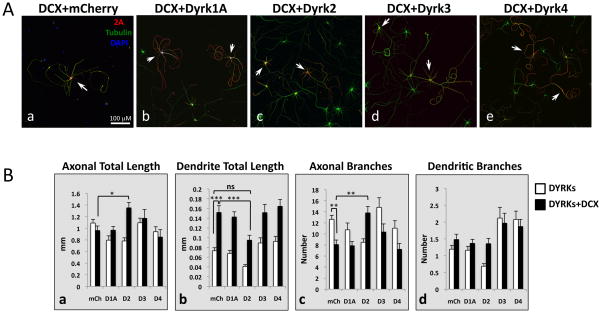
Our laboratory has shown that overexpression of DCX in postnatal cortical neurons produces a characteristic phenotype: increases in neurite lengths accompanied by striking curvature of individual neurites (Blackmore et al., 2010). In initial experiments we found a similar phenotype with DCX expression in the hippocampal neurons (e.g., Fig. 5Aa). Co-expression of DCX with Dyrk1A, Dyrk3 or Dyrk4 did not obviously change the morphology from that seen with DCX alone (Fig. 5Ab, d, and e). However, co-expression of Dyrk2 dramatically changed the DCX overexpression phenotype, such that processes of doubly transfected neurons were much less “curvy”, similar to untransfected cells (Fig. 5Ac).
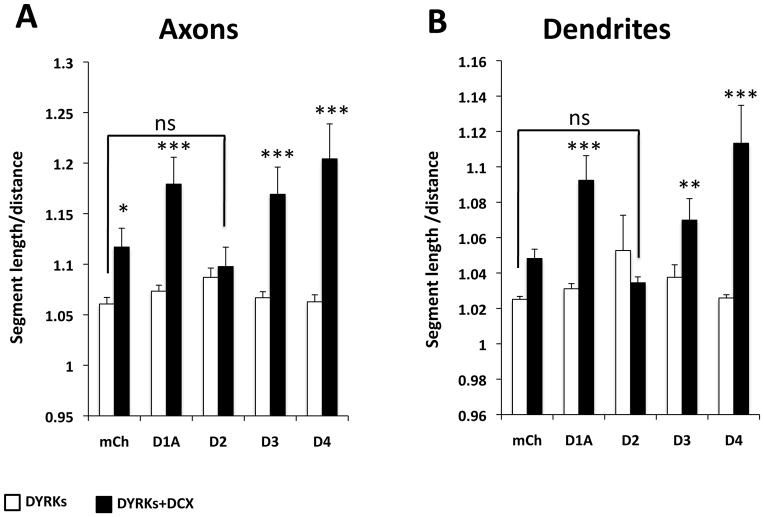
Fig. 5. Dyrk2 reverses the DCX overexpression phenotype.
Hippocampal neurons were co-transfected with 2A-tagged DCX together with control plasmid (mCherry, Aa) or Dyrk1A (Ab), Dyrk2 (Ac), Dyrk3 (Ad), or Dyrk4 (Ae) and cultured for 5 days. (A) Transfected cells identified by staining with anti-2A antibody (red) and marked by arrows. Dyrk2, but not other Dyrks, reverted the DCX overexpression phenotype (Ac). Scale bar is 100 μm. (B) Graphs of axon total length (Ba), dendrite total length (Bb), axon branching (Bc), and dendrite branching (Bd). Means ± SEM; N ≥30 cells. White bars, cells transfected with Dyrks only; black bars, cells transfected with Dyrks + DCX. *, p <0.05; **, p< 0.01; ***; p< 0.001; ns, not significant.
Quantification demonstrated that axons of neurons overexpressing DCX alone had fewer branches compared to control, while the total axonal length was similar (Fig. 5Bc and a). Dyrk2 expression increased total axonal length by 40% compared to DCX alone, and increased the number of axonal branches by 30%, effectively reversing the axonal morphology changes in DCX-overexpressing neurons (Fig. 5Ba, c). Dendrites of DCX-overexpressing neurons were more than twice as long as control dendrites (Fig. 5Bb), while the number of dendritic branches was not significantly different (Fig. 5Bd). Co-expression of Dyrk2 shortened dendrites by 40% compared to DCX alone, making their lengths similar to those of control neurons (Fig. 5Bb). Neither Dyrk3 nor Dyrk4 significantly altered the effects of DCX on the length or number of branches in axons or dendrites. Thus Dyrk2, but not other Dyrks, interacts with DCX to reverse its effects on these parameters.
Examination of neurons doubly transfected with DCX and Dyrks revealed that neurons overexpressing Dyrk1A, Dyrk3, or Dyrk4 produce curvier neurites compared to those expressing DCX alone (with control plasmid; Fig. 5). To quantify this effect we measured tortuosity, defined as the ratio between the length of neurite segment between two nodes and the straight-line distance between these nodes. When these two parameters are equal (straight line) the tortuosity is 1; higher values of tortuosity correspond to more curviness. Overexpression of Dyrks alone did not affect neurite tortuosity (Fig. 6 white bars), while overexpression of DCX increased tortuosity of the axons (Fig. 6A) and possibly the dendrites (Fig. 6B). Co-expression of Dyrk1A, Dyrk3 or Dyrk4 substantially increased DCX-mediated increases in tortuosity of both axons and dendrites (Fig. 6A and B, black bars), while Dyrk2 completely reverted the tortuosity to the levels seen in control. Thus Dyrk2 also interacts with DCX to reverse its effect on curviness, while other Dyrks exacerbate the curviness due to DCX overexpression.
Fig. 6. Effect of Dyrks on the DCX-induced “curviness” phenotype.
Axon and dendrite curviness were assayed by measuring tortuosity (see text), the lowest possible value of which is 1 (straight line). Hippocampal neurons were transfected with plasmids expressing various Dyrks together with mCherry (mCh, control) or Dyrk1A (D1A), Dyrk2 (D2), Dyrk3 (D3), or Dyrk4 (D4). A and B show the average tortuosity (Mean ± SEM) for axons and dendrites, respectively. DCX overexpression significantly increased tortuosity of axons but not dendrites. Co-expression of Dyrk1A, Dyrk3, or Dyrk4 with DCX increases DCX-induced tortuosity in both axons and dendrites, whereas co-expression of Dyrk2 reduces the tortuosity to that of control. All neurites were measured for N≥30 cells. *, p <0.05; **, p< 0.01; ***; p< 0.001; ns, not significant.
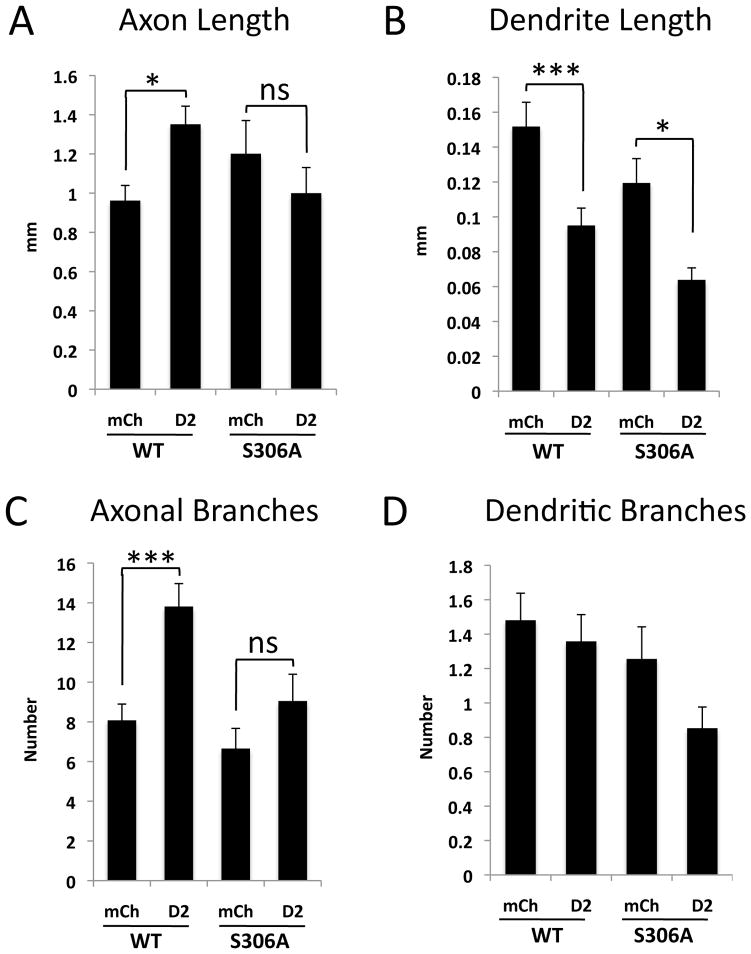
Since Ser306 of DCX appears to be one target site for Dyrk2 phosphorylation, we tested whether phosphorylation of this residue is required for the effect of Dyrk2 on DCX-transfected neurons. Interestingly, the S306A DCX mutant retained the ability to induce long and curved neurites; this effect appeared even more pronounced than with wt DCX (Fig 7C, H, M vs B, G, L and data not shown). Co-expression of Dyrk2 effectively reversed this curviness phenotype (Fig. 7E, J, O), and tortuosity measurements confirmed this finding (Supplemental Figure 2), suggesting that Dyrk2 phosphorylation of Ser306 is not responsible for overcoming neurite curviness. Similarly, the shortening effect of Dyrk2 on dendrites in DCX-overexpressing neurons was also evident when DCX S306A was used (Fig. 8B). However, unlike the situation with wt DCX, Dyrk2 overexpression was unable to reverse the effects of DCX S306A on axon length or axon branching (Fig. 8A, C). Thus the putative Dyrk2 phosphorylation site at Ser306 is required for the effects of Dyrk2 on the DCX-induced axonal elongation and branching, but not for other DCX-induced morphological changes.
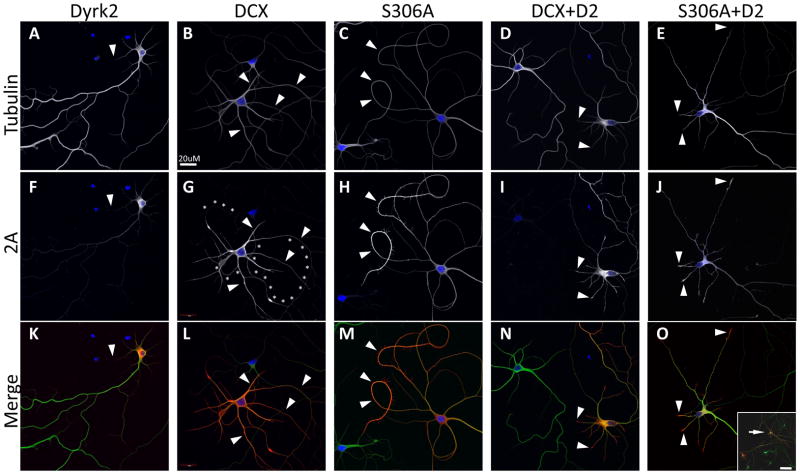
Fig. 7. Ser306 of DCX is not required for the Dyrk2 effect on the DCX phenotype.
Neurons were transfected with 2A-tagged Dyrk2 (A, F, K), 2A-tagged wild type DCX (DCX) (B, G, L), or the DCX(S306A) mutant (C, H, M), or co-transfected with Dyrk2 + DCX (D, I, N) or Dyrk2 + DCX(S306A) (E, J, O). Cells were cultured for 5 days and stained with anti-β3 tubulin (Tubulin, A–E), and anti-doublecortin (DCX, F) or anti-2A (2A, G–J). Arrowheads point to localization of DCX and tubulin. Overexpression of Dyrk2 displaces endogenous DCX from neurites (A, F, K). Overexpressed WT DCX (B, G, L) or DCX(S306A) mutant (C, H, M) show co-localized staining with tubulin along the length of a neurite. The DCX(S306A) mutant produces even more curved neurites (C, H, M) compared to WT DCX (curved neurites traced by asterisks in G). Co-transfection of Dyrk2 with either WT or mutant DCX shortens dendrites and partially restores normal distribution of DCX in neurites where it is enriched at the tips (D, I, N and E, J, O). Inset in panel O shows transfected neuron (arrow) at the lower magnification to show similarity in overall morphology between cells co-transfected with DCX(S306A) and Dyrk2 (red) and untransfected cells (green). Scale bar, 20 μm; 100 μm, inset.
Fig. 8. Ser306 of DCX is required for Dyrk2 reversion of the axonal, but not the dendritic DCX overexpression phenotype.
Hippocampal neurons were co-transfected with wild type 2A-tagged DCX (WT) or DCX S306A together with control plasmid (mCherry) or Dyrk2 (D2). After 5 days, neurons were fixed, stained, and analyzed for axon length (A), dendrite length (B), axon branching (C), and dendrite branching (D). Dyrk2 decreased dendrite length, and increased axon length and branching, in wt DCX-expressing neurons (see also Fig. 5). Dyrk2 also decreased dendrite length in DCX S306A-expressing neurons, but did not affect axon length or axon branching. Means ± SEM; N≥30 cells. *, p <0.05; **, p< 0.01; ***; p< 0.001; ns, not significant.
The phenotype seen with DCX overexpression may be related to abnormal localization of DCX. In contrast to endogenous DCX (Fig. 3A), overexpressed DCX distributed evenly along the neurites, completely overlapping with tubulin staining (Fig. 7B, G, L). The DCX S306A mutant showed a similar distribution (Fig. 7C, H, M). However, co-expression of Dyrk2 with either wild type or mutant DCX caused exogenous DCX to become enriched at the neurite tips, similar to endogenous DCX (compare Fig. 3A–D with Fig. 7D, I, N, E, J, O). Thus Dyrk2 regulates both subcellular localization and the phenotypic effects of DCX, through a mechanism involving site(s) distinct from Ser306.
Discussion
Our studies implicate the Dyrk family of protein kinases in the regulation of neuronal process outgrowth, cytoskeletal organization, and DCX-induced changes in neurite morphology. Although Dyrk1A is well known for its role in neural development (Okui et al., 1999; Tejedor and Hammerle, 2010), the functional roles of other Dyrks in neurons, and even the extent of their expression in the nervous system, are not well understood. Dyrk4 expression in mice, for example, was thought to be restricted to testis at a specific stage of development (Sacher et al., 2007). However, Dyrk4 has several differentially expressed splice variants, one of which is expressed in the brain (Papadopoulos et al., 2010), and has been shown to be upregulated in human Ntera2 cells as they differentiate into neurons (Leypoldt et al., 2001). The expression patterns of Dyrk2 and Dyrk3 have not been well studied, but each gene is expressed in the developing nervous system (Kudo et al., 2007; Diez-Roux et al., 2011; Lerch et al., 2012).
We found that several Dyrk kinases, especially Dyrk2, regulate cytoskeletal organization in neuronal growth cones. Dyrk1A can affect cytoskeletal organization through its role as a priming kinase for GSK3β on MAP1b (Scales et al., 2009), but it is mainly the Dyrk2/3 family that has been implicated in MT dynamics. A yeast ortholog (Pom1) is involved in translation of MT-dependent polarity cues into actin filament formation (Tatebe et al., 2008), and an ortholog in C. elegans (Mbk-2) regulates an E3 ubiquitin ligase responsible for the degradation of the MT-severing protein MEI-1/katanin (Pang et al., 2004). In mammals, Dyrk2 is involved in a related E3 pathway for katanin degradation (Maddika and Chen, 2009), and also acts as a priming kinase for GSK3β in the phosphorylation and regulation of the MT binding protein CRMP4 (Cole et al., 2006). Our findings that Dyrk2 and Dyrk3 regulate dendritic branching as well as phosphorylation of DCX are consistent with a role for this subfamily in the regulation of MT dynamics.
Dyrks have differential effects on neurite morphology
Although Dyrk1A and Dyrk2 overexpression influenced axon growth, most effects of Dyrks in hippocampal neurons were on dendritic morphology, and different Dyrks appear to have distinct functions in these processes. For example, overexpression of Dyrk2 decreased both dendrite branching and overall dendrite lengths, while overexpression of Dyrk3 and Dyrk4 increased dendrite branching. Further studies will be required to elucidate the mechanisms underlying these effects, but phosphorylation and/or re-localization of DCX may be involved. It is unlikely that the differential effects of different Dyrks subtypes result from differential expression levels, as these seemed equivalent based on immunostaining of the 2A tag both in transfected neurons (Fig. 1) and in HEK293 cells (data not shown). Most overexpression phenotypes depended on kinase activity, as they were abolished by the KD mutations; effects on axons may be an exception to this rule. Notably, the KD mutant of Dyrk2 produced a loss of function phenotype suggesting that it acts as a dominant negative mutant; this mutant may be useful for future experiments. The gain of function phenotype on axon branching seen with the Dyrk3 KD mutant is interesting, but its mechanism is unknown.
Dyrks phosphorylate DCX and regulate its localization and activity
We have implicated Dyrk kinases, especially Dyrk2, in the regulation of DCX phosphorylation, localization, and function. First, overexpression of Dyrk2 and Dyrk3 substantially altered DCX phosphorylation. One site affected by Dyrks is Ser306, but other sites are also involved, since phosphorylation of a mutant lacking Ser306 could also be altered by Dyrks 2 and 3. Second, Dyrk1A, and especially Dyrk2, regulated the localization of DCX, abolishing its preferential distribution in the tips of growing axons and dendrites. Finally, and most striking, Dyrk2 was able to revert the DCX overexpression phenotype of long, curving neurites, and to “normalize”, at least partly, the distribution of overexpressed DCX. Overexpressed DCX, unlike the endogenous protein, was found throughout hippocampal neurites, and co-expression of Dyrk2 allowed its redistribution to neurite tips. These results, taken together, suggest that Dyrk2-regulated phosphorylation of DCX must be appropriately “balanced” for the proper development of neurite (especially dendrite) morphology. We speculate that a high concentration of DCX increases MT stability, resulting in elongation and curving of neurites, and that phosphorylation mediated by Dyrk2 restores normal MT dynamics. Expression of the Ser306A DCX mutant in neurons resulted in a stronger “curvy” phenotype compared to the WT DCX, suggesting that this site plays a role in the DCX phenotype. Interestingly, we found both Ser306-dependent and Ser306-independent effects of Dyrk2 on DCX. Reversion by Dyrk2 of the curviness phenotype, and of the dendritic shortening, did not require Ser306. However, Dyrk2 could not reverse the DCX effect on axon length or axon branching when Ser306 was mutated, suggesting that these effects require phosphorylation of Ser306 by Dyrk2. It is likely that other, unidentified, phosphorylation sites are important for effects of Dyrk2 on DCX dendritic phenotypes.
DCX is primarily known for its role in migration of cortical neurons during development. However, a growing body of evidence shows that DCX also plays a role in axon and dendrite growth and branching (Deuel et al., 2006; Cohen et al., 2008b; Kerjan et al., 2009). As a MT-associated protein, DCX is involved in MT polymerization, bundling and stabilization (Kim et al., 2003; Schaar et al., 2004; Bielas et al., 2007). DCX can be phosphorylated by at least 4 different kinases including Cdk5, PKA, Jnk, and GSK3β (Gdalyahu et al., 2004; Graham et al., 2004; Schaar et al., 2004; Tanaka et al., 2004; Bilimoria et al., 2010; Jin et al., 2010); our results add Dyrks to this list. Phosphorylation of DCX at specific sites can either increase or decrease its affinity to MTs and result in distinct functional effects (Reiner et al., 2004). We are unable to determine whether Dyrk2 phosphorylates DCX directly or exerts its action through an additional mechanism. It is possible that Dyrk2 can prime DCX for phosphorylation by another kinase, e.g. GSK3β (Gwack et al., 2006; Scales et al., 2009), but we have no evidence on this point. Dyrk2 can also act as a scaffolding protein (Maddika and Chen, 2009), but most of the Dyrk2 effects we have seen are abolished by loss of kinase activity.
In contrast to the other Dyrks we examined, Dyrk1A was mostly expressed in the nucleus in hippocampal neurons. This localization is consistent with the recent report that Dyrk1A can influence splicing of the MT-associated protein tau through phosphorylation of the nuclear alternative splicing factor (Wegiel et al., 2011). Dyrks 2–4 were expressed in the cytosol and in the growing neurites, where they would be in a position to influence cytoskeletal organization in the growth cones. Indeed, effects of Dyrk2 on DCX function were correlated with changes in DCX localization in growth cones. Subcellular localization of DCX is known to be critical for its neuronal functions. For example, phosphorylation of perinuclear DCX by Cdk5 increases its affinity to MTs, facilitating neuronal migration during cortical development (Tanaka et al., 2004). Phosphorylation of DCX in growth cones by Jnk, mediated by the scaffolding protein JIP (Jnk-interacting protein) promotes neurite outgrowth and changes the velocity of migrating neurons (Gdalyahu et al., 2004), and phosphorylation of DCX in axons by GSK3β, which reduces axonal branching, is mediated by the JIP3 scaffolding protein (Bilimoria et al., 2010).
In summary, our results demonstrate that Dyrks, especially Dyrk2, are novel regulators of the neuronal cytoskeleton, dendrite branching, and DCX function. This work was initially enabled by phenotypic screens in primary neurons, which implicated Dyrks and DCX in neurite development (Blackmore et al., 2010; Buchser et al., 2010). In addition to Dyrk4, our overexpression screen of kinases and phosphatases implicated a number of other novel genes in neuronal morphology (Buchser et al., 2010). The insights into Dyrk function that are discussed here provide further evidence of the utility of such screening approaches for the generation of novel hypotheses.
Materials and methods
Antibodies
The following antibodies were used in these study: anti-beta-tubulin 3 (TUJ1; Aves Labs, Tigard, OR), anti-2A peptide (Millipore, Temecula, CA), anti-DCX (Abcam, Cambrige, MA), anti-phospho-DCX (Ser334) (Cell Signaling, Danvers, MA), anti-cMyc (Clontech, Mountain View, CA), and anti-tubulin (mouse monoclonal antibody E7, produced in house from a clone provided by Dr. Itzhak Fischer).
Plasmids and constructs
Human DCX cDNA and mouse cDNAs of Dyrk family kinases were obtained from the NIH Mammalian Genome Collection (Tang et al., 2009), which was purchased from Open Biosystems (Thermofisher, Huntsville, Alabama). The library included human (IRAT) and mouse (IRAV) clones in a pCMV-SPORT6 backbone. To obtain 2A-tagged or Myc-tagged proteins, coding sequences of DCX and Dyrks were PCR-amplified and recloned into pAAV-MCS (Stratagene, La Jolla, CA) containing 2A-mCherry or pCMV-Myc (Clontech, Mountain View, CA) expression plasmids. All cDNAs cloned into vectors containing the 2A peptide produce 2A-tagged proteins at their C-terminus, while the Myc tag was at the N-terminus. To introduce mutations for DCX(S306A), DCX(S327A) and Dyrk1A-KD (K188R substitution) by 2-step PCR we used the following primers:
5′-S306A CCTATGCGCCGAAGCAAGGCTCCAGCTGACTCAGC
3′-S306A GCTGAGTCAGCTGGAGCCTTGCTTCGGCGCATAGG
5′-S327A AGTCTAAGCAGGCTCCCATCTCTACGCCCA
3′-S327A GAGATGGGAGCCTGCTTAGACTTGGGGG
5′-D1A-K188R AAGAATGGGTCGCCATTAGAATCATCAAGAACAA
3′-D1A-K188R TTGTTCTTGATGATTCTAATGGCGACCCATTCTT
The nucleotides changed from the WT sequence are underlined.
Kinase-deficient mutants of human Dyrk2 (K251M), Dyrk3 (K238M), and Dyrk4 (K133M) were purchased from OriGene (Rockville, MD).
Preparation and culture of primary hippocampal neurons
All animal work and procedures were done in accordance with the animal care and use policies of the University of Miami, an AALAC-accredited institution, and are approved by the University of Miami IACUC. Hippocampal neurons from E18 rat embryos were prepared and cultured as described (Buchser et al., 2010) with minor modifications. Briefly, E18 rat hippocampi were isolated and dissociated in Hibernate E (BrainBits, Springfield, IL) using 0.25% trypsin (Invitrogen, Carlsbad, CA) and DNAse, 30ug/ml (Sigma-Aldrich, St. Louis, MO). The neurons were cultured in plastic dishes or glass cover slips coated with PDL (0.1 mg/ml) (Sigma-Aldrich, St. Louis, MO) and laminin (0.01 mg/ml) (Trevigen, Helgerman, CT) containing equilibrated NbActiv4 (BrainBits, Springfield, IL) growth media. The plating density of cultured cells was dependent on the goal of the experiment. For manual tracing, cells were plated at a low density of 5.5×103 cells/cm2 and cultured for 5 days. For western blot analysis neurons were plated at high density of 2×105 cells/cm2.
Transfection and immunostaining of neurons
Freshly prepared primary hippocampal neurons were transfected using either Amaxa Rat Neurons or Amaxa P3 Primary Cells 96-well Nucleofector kits (Lonza, Basel, Switzerland). Cells were electroporated with either Nucleofector II or 96-well Shuttle electroporation devises (Lonza, Basel, Switzerland) following manufacturer’s guidelines. Equilibrated growth media was added to neurons immediately following electroporation to recover them from electric shock, and cells were plated into prepared culture dishes. Neurons were cultured at 37°C, 5% CO2 at 100% humidity for 5 days unless indicated otherwise. Before staining with antibodies cells were fixed with 4% paraformaldehyde (PFA) for 20 minutes at RT and blocked in phosphate-buffered saline (PBS) containing 5% normal goat serum (NGS), 1% BSA, 0.2 % fish gelatin, and 0.05% Triton X-100. Cells were exposed to primary antibodies diluted in the same blocking buffer for 1 hr followed by washing and 1 hr incubation with the secondary antibody. Cells grown on glass cover slips were mounted on slides with ProLong Gold anti-fade reagent (Invitrogen, Carlsbad, CA) for microscopy.
Transfection of HEK293 cells and Western Blot
HEK293T cells were plated in 12-well culture dishes and transfected using FuGENE 6 transfection reagent (Roche, Indianapolis, IN) according to manufacturer’s guidelines. Cells were collected 36 hrs post-transfection with 150 ul of loading buffer containing 100 mM Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 5% beta-mercaptoethanol, phosphatase (Clontech, Mountain View, CA) and protease (Roche, Indianapolis, IN) inhibitors and 0.2% bromophenol blue. Lysates were boiled for 5 minutes and 1/10 of the volume was loaded on a 10% polyacrylamide gel. Resolved proteins were transferred to a nitrocellulose membrane. The membrane was blocked in Odyssey blocking buffer (BB) (LiCore, Lincoln, NE), following by 1 hr incubation at RT with primary antibodies diluted in BB supplemented with 0.1% Tween-20. After washing 4 times with 1xPBS supplemented with 0.1% Tween-20 the blots were incubated for 1 hr at room temperature with secondary antibody conjugated with either 700 or 800 IrDye (Rockland, Gilbertsville, PA) in BB plus 0.1% Tween-20 and 0.01% SDS. Stained proteins were detected on an Odyssey infrared scanner (LiCore, Lincoln, NE) and images were analyzed using Odyssey software.
Imaging and quantification
Confocal images of immunostained cells were taken with an Olympus IV81 FV1000 inverted microscope and processed on the FluoView browser. For manual tracing of neurites XYZ multichannel mosaic images were collected using a macro for Multi Area time lapse. The montages of multiple fields were saved as multi-TIFF files for each channel and loaded into Neurolucida (MBF Bioscience) for manual tracing. We selected 20–30 2A-positive or GFP-positive cells for manual tracing. Since the DCX phenotype appeared to vary with the expression level of the protein (more brightly-stained cells had curvier neurites), we chose equal numbers of weakly- and strongly-stained cells (based on somal intensities) for quantification to avoid bias of the phenotype. The software was calibrated for each image with corresponding micron size of each pixel. The number of axonal and dendritic branches was counted as a sum of branch nodes. If more than two branches originated in a node all of them (e.g. 3) were added. To quantify the “curviness” we used Neurolucida to analyze tortuosity, defined as the ratio of the segment length between two nodes of a neurite and the distance between these two nodes. A tortuosity of 1 represents a straight line, while any curvature of the segment will result in tortuosity >1. Average tortuosity was based on combined measurements for all segments, from all neurites, for ≥ 20 neurons. Data were exported and processed by Neuroexplorer browser (MBF Bioscience) and results were statistically analyzed using InStat 3 (GraphPad Software, Inc).
Supplementary Material
Neurons were co-transfected with Dyrk2 and a GFP expressing plasmid, and stained for DCX (red) and β3 tubulin (white). A cell expressing Dyrk2 is marked by the arrow on panel A. Magnified images of the axon (inset a) and dendrites (inset b) from the transfected neuron are shown on the right (B, C and D, E, respectively). The arrows point to the axon and dendrites in the transfected neuron, and the arrowheads to the untransfected neuron.
A. Hippocampal neurons were transfected with plasmids expressing 2A-tagged DCX S306A together with mCherry (mCh, control) or Dyrk2 (D2). Cells were stained with anti-2A and anti-β3 tubulin antibodies. Arrows mark transfected cells. The mutant DCX produces long and curvy neurites, and expression of Dyrk2 strongly reduces this curviness. B. Average tortuosity was measured as in Figure 6 (Mean ± SEM) for axons (black bars) and dendrites (white bars), respectively. DCX S306A overexpression significantly increased tortuosity of axons but not dendrites. Co-expression of Dyrk2 reduced the tortuosity to that of control. All neurites were measured for N≥20 cells. ***; p< 0.0001.
Axon total length (Ax Lngth), dendrite total length (Dn Lngth), number of axonal branches (Ax Br), and number of dendritic branches (Dn Br) were each normalized to values for control transfected cells and expressed as a percentage (Mean ± SEM). Data were collected from 2 independent experiments (N ≥30 cells) and analyzed using one-way Analysis of Variance (ANOVA), Dunnett’s Multiple Comparison Test.
Acknowledgments
We thank Dr. Itzhak Fischer for his kind gift of E7 hybridoma cells, Beata Frydel for assistance with imaging and tracing software, and Drs. Murray Blackmore and Vladlen Slepak for helpful discussions. This work was supported by grants from the NIH (NS059866, JLB; HD057632, VPL), U.S. Army (W81XWH-05-1-0061), and the Buoniconti Foundation. VPL holds the Walter G. Ross Distinguished Chair in Developmental Neuroscience.
References
- Alvarez M, Altafaj X, Aranda S, de la Luna S. DYRK1A autophosphorylation on serine residue 520 modulates its kinase activity via 14-3-3 binding. Molecular biology of the cell. 2007;18:1167–1178. doi: 10.1091/mbc.E06-08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M, Estivill X, de la Luna S. DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. Journal of cell science. 2003;116:3099–3107. doi: 10.1242/jcs.00618. [DOI] [PubMed] [Google Scholar]
- Aranda S, Laguna A, de la Luna S. DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010 doi: 10.1096/fj.10-165837. [DOI] [PubMed] [Google Scholar]
- Becker W, Joost HG. Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. [Accessed October 29, 2011];Progress in nucleic acid research and molecular biology. 1999 62:1–17. doi: 10.1016/s0079-6603(08)60503-6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9932450. [DOI] [PubMed] [Google Scholar]
- Bielas SL, Serneo FF, Chechlacz M, Deerinck TJ, Perkins GA, Allen PB, Ellisman MH, Gleeson JG. Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell. 2007;129:579–591. doi: 10.1016/j.cell.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilimoria PM, de la Torre-Ubieta L, Ikeuchi Y, Becker EB, Reiner O, Bonni A. A JIP3-regulated GSK3beta/DCX signaling pathway restricts axon branching. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:16766–16776. doi: 10.1523/JNEUROSCI.1362-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore MG, Moore DL, Smith RP, Goldberg JL, Bixby JL, Lemmon VP. High content screening of cortical neurons identifies novel regulators of axon growth. Molecular and cellular neurosciences. 2010;44:43–54. doi: 10.1016/j.mcn.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchser WJ, Slepak TI, Gutierrez-Arenas O, Bixby JL, Lemmon VP. Kinase/phosphatase overexpression reveals pathways regulating hippocampal neuron morphology. Molecular systems biology. 2010;6:391. doi: 10.1038/msb.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Segal M, Reiner O. Doublecortin supports the development of dendritic arbors in primary hippocampal neurons. [Accessed August 2, 2011];Developmental neuroscience. 2008a 30:187–199. doi: 10.1159/000109862. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18075265. [DOI] [PubMed] [Google Scholar]
- Cohen D, Segal M, Reiner O. Doublecortin supports the development of dendritic arbors in primary hippocampal neurons. Developmental neuroscience. 2008b;30:187–199. doi: 10.1159/000109862. [DOI] [PubMed] [Google Scholar]
- Cole AR, Causeret F, Yadirgi G, Hastie CJ, McLauchlan H, McManus EJ, Hernandez F, Eickholt BJ, Nikolic M, Sutherland C. Distinct priming kinases contribute to differential regulation of collapsin response mediator proteins by glycogen synthase kinase-3 in vivo. The Journal of biological chemistry. 2006;281:16591–16598. doi: 10.1074/jbc.M513344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2009;15:149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Merriam EB, Hu X. The dynamic cytoskeleton: backbone of dendritic spine plasticity. Current opinion in neurobiology. 2011;21:175–181. doi: 10.1016/j.conb.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Tang F, Kalil K. Axon guidance by growth cones and branches: common cytoskeletal and signaling mechanisms. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2003;9:343–353. doi: 10.1177/1073858403252683. [DOI] [PubMed] [Google Scholar]
- Deuel TAS, Liu JS, Corbo JC, Yoo S-Y, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. [Accessed July 8, 2011];Neuron. 2006 49:41–53. doi: 10.1016/j.neuron.2005.10.038. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16387638. [DOI] [PubMed] [Google Scholar]
- Diez-Roux G, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. [Accessed July 23, 2011];PLoS biology. 2011 9:e1000582. doi: 10.1371/journal.pbio.1000582. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3022534&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. [Accessed October 31, 2011];Nature. 1987 330:254–256. doi: 10.1038/330254a0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3313064. [DOI] [PubMed] [Google Scholar]
- Ertürk A, Hellal F, Enes J, Bradke F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. [Accessed October 11, 2011];The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007 27:9169–9180. doi: 10.1523/JNEUROSCI.0612-07.2007. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17715353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdalyahu A, Ghosh I, Levy T, Sapir T, Sapoznik S, Fishler Y, Azoulai D, Reiner O. DCX, a new mediator of the JNK pathway. The EMBO journal. 2004;23:823–832. doi: 10.1038/sj.emboj.7600079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Graham ME, Ruma-Haynes P, Capes-Davis AG, Dunn JM, Tan TC, Valova VA, Robinson PJ, Jeffrey PL. Multisite phosphorylation of doublecortin by cyclin-dependent kinase 5. The Biochemical journal. 2004;381:471–481. doi: 10.1042/BJ20040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, Rao A. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- Hachet O, Berthelot-Grosjean M, Kokkoris K, Vincenzetti V, Moosbrugger J, Martin SG. A phosphorylation cycle shapes gradients of the DYRK family kinase Pom1 at the plasma membrane. Cell. 2011;145:1116–1128. doi: 10.1016/j.cell.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Hammerle B, Carnicero A, Elizalde C, Ceron J, Martinez S, Tejedor FJ. Expression patterns and subcellular localization of the Down syndrome candidate protein MNB/DYRK1A suggest a role in late neuronal differentiation. The European journal of neuroscience. 2003;17:2277–2286. doi: 10.1046/j.1460-9568.2003.02665.x. [DOI] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. [Accessed July 21, 2011];Science (New York, N Y) 2011 331:928–931. doi: 10.1126/science.1201148. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21273450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Suzuki H, Hirai S, Mikoshiba K, Ohshima T. JNK phosphorylates Ser332 of doublecortin and regulates its function in neurite extension and neuronal migration. Developmental neurobiology. 2010;70:929–942. doi: 10.1002/dneu.20833. [DOI] [PubMed] [Google Scholar]
- Kannan N, Neuwald AF. Evolutionary constraints associated with functional specificity of the CMGC protein kinases MAPK, CDK, GSK, SRPK, DYRK, and CK2alpha. Protein science: a publication of the Protein Society. 2004;13:2059–2077. doi: 10.1110/ps.04637904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Hoshino M. Molecular pathways regulating cytoskeletal organization and morphological changes in migrating neurons. [Accessed July 26, 2011];Developmental neuroscience. 2008 30:36–46. doi: 10.1159/000109850. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18075253. [DOI] [PubMed] [Google Scholar]
- Kerjan G, Koizumi H, Han EB, Dubé CM, Djakovic SN, Patrick GN, Baram TZ, Heinemann SF, Gleeson JG. Mice lacking doublecortin and doublecortin-like kinase 2 display altered hippocampal neuronal maturation and spontaneous seizures. [Accessed October 27, 2011];Proceedings of the National Academy of Sciences of the United States of America. 2009 106:6766–6771. doi: 10.1073/pnas.0812687106. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2672532&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Cierpicki T, Derewenda U, Krowarsch D, Feng Y, Devedjiev Y, Dauter Z, Walsh CA, Otlewski J, Bushweller JH, Derewenda ZS. The DCX-domain tandems of doublecortin and doublecortin-like kinase. Nature structural biology. 2003;10:324–333. doi: 10.1038/nsb918. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Higginbotham H, Poon T, Tanaka T, Brinkman BC, Gleeson JG. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. [Accessed September 28, 2011];Nature neuroscience. 2006 9:779–786. doi: 10.1038/nn1704. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16699506. [DOI] [PubMed] [Google Scholar]
- Kudo LC, Karsten SL, Chen J, Levitt P, Geschwind DH. Genetic analysis of anterior posterior expression gradients in the developing mammalian forebrain. [Accessed June 18, 2011];Cerebral cortex (New York, N Y: 1991) 2007 17:2108–2122. doi: 10.1093/cercor/bhl118. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17150988. [DOI] [PubMed] [Google Scholar]
- Lerch JK, Kuo F, Motti D, Morris R, Bixby JL, Lemmon VP. Isoform diversity and regulation in peripheral and central neurons revealed through RNA-Seq. PLoS One. 2012;7:e30417. doi: 10.1371/journal.pone.0030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypoldt F, Lewerenz J, Methner A. Identification of genes up-regulated by retinoic-acid-induced differentiation of the human neuronal precursor cell line NTERA-2 cl.D1. [Accessed October 29, 2011];Journal of neurochemistry. 2001 76:806–814. doi: 10.1046/j.1471-4159.2001.00079.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11158252. [DOI] [PubMed] [Google Scholar]
- Lim S, Jin K, Friedman E. Mirk protein kinase is activated by MKK3 and functions as a transcriptional activator of HNF1alpha. The Journal of biological chemistry. 2002;277:25040–25046. doi: 10.1074/jbc.M203257200. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Sibbet G, Morrice N, Cleghon V. Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell. 2005;121:925–936. doi: 10.1016/j.cell.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Lu M, Zheng L, Han B, Wang L, Wang P, Liu H, Sun X. REST regulates DYRK1A transcription in a negative feedback loop. The Journal of biological chemistry. 2011;286:10755–10763. doi: 10.1074/jbc.M110.174540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddika S, Chen J. Protein kinase DYRK2 is a scaffold that facilitates assembly of an E3 ligase. Nature cell biology. 2009;11:409–419. doi: 10.1038/ncb1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins PA, Salic K, Gladka MM, Armand AS, Leptidis S, el Azzouzi H, Hansen A, de Roo CJC, Bierhuizen MF, van der Nagel R, van Kuik J, de Weger R, de Bruin A, Condorelli G, Arbones ML, Eschenhagen T, Windt LJD. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nature cell biology. 2010;12:1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- Moores CA, Francis F, Perderiset M, Houdusse A. A double-take on MAPs. Nature structural biology. 2003;10:314–316. doi: 10.1038/nsb0503-314. [DOI] [PubMed] [Google Scholar]
- Okui M, Ide T, Morita K, Funakoshi E, Ito F, Ogita K, Yoneda Y, Kudoh J, Shimizu N. High-level expression of the Mnb/Dyrk1A gene in brain and heart during rat early development. Genomics. 1999;62:165–171. doi: 10.1006/geno.1999.5998. [DOI] [PubMed] [Google Scholar]
- Pang KM, Ishidate T, Nakamura K, Shirayama M, Trzepacz C, Schubert CM, Priess JR, Mello CC. The minibrain kinase homolog, mbk-2, is required for spindle positioning and asymmetric cell division in early C. elegans embryos. [Accessed October 29, 2011];Developmental biology. 2004 265:127–139. doi: 10.1016/j.ydbio.2003.09.024. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14697358. [DOI] [PubMed] [Google Scholar]
- Papadopoulos C, Arato K, Lilienthal E, Zerweck J, Schutkowski M, Chatain N, Muller-Newen G, Becker W, de la Luna S. Splice variants of the dual-specificity tyrosine phosphorylation-regulated kinase 4 (DYRK4) differ in their subcellular localization and catalytic activity. The Journal of biological chemistry. 2010 doi: 10.1074/jbc.M110.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Reiner O, Gdalyahu A, Ghosh I, Levy T, Sapoznik S, Nir R, Sapir T. DCX’s phosphorylation by not just another kinase (JNK) Cell cycle (Georgetown, Tex) 2004;3:747–751. [PubMed] [Google Scholar]
- Sacher F, Moller C, Bone W, Gottwald U, Fritsch M. The expression of the testis-specific Dyrk4 kinase is highly restricted to step 8 spermatids but is not required for male fertility in mice. Molecular and cellular endocrinology. 2007;267:80–88. doi: 10.1016/j.mce.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Scales TM, Lin S, Kraus M, Goold RG, Gordon-Weeks PR. Nonprimed and DYRK1A-primed GSK3 beta-phosphorylation sites on MAP1B regulate microtubule dynamics in growing axons. Journal of cell science. 2009;122:2424–2435. doi: 10.1242/jcs.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar BT, Kinoshita K, McConnell SK. Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron. 2004;41:203–213. doi: 10.1016/s0896-6273(03)00843-2. [DOI] [PubMed] [Google Scholar]
- Taira N, Nihira K, Yamaguchi T, Miki Y, Yoshida K. DYRK2 is targeted to the nucleus and controls p53 via Ser46 phosphorylation in the apoptotic response to DNA damage. Molecular cell. 2007;25:725–738. doi: 10.1016/j.molcel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Taira N, Yamamoto H, Yamaguchi T, Miki Y, Yoshida K. ATM augments nuclear stabilization of DYRK2 by inhibiting MDM2 in the apoptotic response to DNA damage. The Journal of biological chemistry. 2010;285:4909–4919. doi: 10.1074/jbc.M109.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, Tsai LH, Gleeson JG. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron. 2004;41:215–227. doi: 10.1016/s0896-6273(03)00852-3. [DOI] [PubMed] [Google Scholar]
- Tatebe H, Nakano K, Maximo R, Shiozaki K. Pom1 DYRK regulates localization of the Rga4 GAP to ensure bipolar activation of Cdc42 in fission yeast. [Accessed August 19, 2011];Current biology: CB. 2008 18:322–330. doi: 10.1016/j.cub.2008.02.005. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2277499&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor FJ, Hammerle B. MNB/DYRK1A as a multiple regulator of neuronal development. The FEBS journal. 2010 doi: 10.1111/j.1742-4658.2010.07954.x. [DOI] [PubMed] [Google Scholar]
- Tint I, Jean D, Baas PW, Black MM. Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:10995–11010. doi: 10.1523/JNEUROSCI.3399-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher LC, Johnstone A, Ertürk A, Hu Y, Strikis D, Wanner IB, Moorman S, Lee J-W, Min J, Ha H-H, Duan Y, Hoffman S, Goldberg JL, Bradke F, Chang Y-T, Lemmon VP, Bixby JL. A chemical screen identifies novel compounds that overcome glial-mediated inhibition of neuronal regeneration. [Accessed October 12, 2011];The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010 30:4693–4706. doi: 10.1523/JNEUROSCI.0302-10.2010. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2855497&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M, Björklund M, Cheng F, Syvänen H, Kivioja T, Kilpinen S, Sun Z, Kallioniemi O, Stunnenberg HG, He W-W, Ojala P, Taipale J. Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. [Accessed July 16, 2011];Cell. 2008 133:537–548. doi: 10.1016/j.cell.2008.02.047. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18455992. [DOI] [PubMed] [Google Scholar]
- Wegiel J, et al. Link between DYRK1A overexpression and several-fold enhancement of neurofibrillary degeneration with 3-repeat tau protein in Down syndrome. Journal of neuropathology and experimental neurology. 2011;70:36–50. doi: 10.1097/NEN.0b013e318202bfa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neurons were co-transfected with Dyrk2 and a GFP expressing plasmid, and stained for DCX (red) and β3 tubulin (white). A cell expressing Dyrk2 is marked by the arrow on panel A. Magnified images of the axon (inset a) and dendrites (inset b) from the transfected neuron are shown on the right (B, C and D, E, respectively). The arrows point to the axon and dendrites in the transfected neuron, and the arrowheads to the untransfected neuron.
A. Hippocampal neurons were transfected with plasmids expressing 2A-tagged DCX S306A together with mCherry (mCh, control) or Dyrk2 (D2). Cells were stained with anti-2A and anti-β3 tubulin antibodies. Arrows mark transfected cells. The mutant DCX produces long and curvy neurites, and expression of Dyrk2 strongly reduces this curviness. B. Average tortuosity was measured as in Figure 6 (Mean ± SEM) for axons (black bars) and dendrites (white bars), respectively. DCX S306A overexpression significantly increased tortuosity of axons but not dendrites. Co-expression of Dyrk2 reduced the tortuosity to that of control. All neurites were measured for N≥20 cells. ***; p< 0.0001.
Axon total length (Ax Lngth), dendrite total length (Dn Lngth), number of axonal branches (Ax Br), and number of dendritic branches (Dn Br) were each normalized to values for control transfected cells and expressed as a percentage (Mean ± SEM). Data were collected from 2 independent experiments (N ≥30 cells) and analyzed using one-way Analysis of Variance (ANOVA), Dunnett’s Multiple Comparison Test.