Abstract
Humans show spontaneous synchronization of movements during social interactions; this coordination has been shown to facilitate smooth communication. Although human studies exploring spontaneous synchronization are increasing in number, little is known about this phenomenon in other species. In this study, we examined spontaneous behavioural synchronization between monkeys in a laboratory setting. Synchronization was quantified by changes in button-pressing behaviour while pairs of monkeys were facing one another. Synchronization between the monkeys was duly observed and it was participant-partner dependent. Further tests confirmed that the speed of button pressing changed to harmonic or sub-harmonic levels in relation to the partner's speed. In addition, the visual information from the partner induced a higher degree of synchronization than auditory information. This study establishes advanced tasks for testing social coordination in monkeys, and illustrates ways in which monkeys coordinate their actions to establish synchronization.
We observe coordinated activities between humans in social environments. Coordination refers to behavioural changes that occur continuously during the process of social interaction1. For example, when one helps a friend move a piece of furniture, one normally adjusts one's movements continuously, according to the friend's movements. Successful coordination can result in smooth cooperative action or interaction within the social bond1. There are two types of coordination: intentional and spontaneous. Intentional coordination requires recognition of an action-oriented goal and environmental constraints on the ability to perform adaptive actions. Using the above example, one needs to know where to move the furniture and to identify obstacles that must be avoided. In contrast, spontaneous coordination is achieved unconsciously, without engaging higher cognitive processes or a particular action goal. A typical example of spontaneous coordination is spontaneous synchronization in socially coupled dyads. Studies of spontaneous synchrony in humans have demonstrated synchronization of actions, including finger movement, walking, swinging, rocking, bodily gestures, and facial expressions in social environments2,3,4,5,6,7. A number of studies have further shown that spontaneous imitation or spontaneous synchronization apparently ‘smoothed' conversation and facilitated the building of social rapport8,9,10. The coordination in social situations commonly involves interaction between intentional and spontaneous processes2; however, the nature of this interaction remains unclear. Since spontaneous synchronization is triggered automatically, it could also occur during, or even serve as a basis for, higher-level intentional processes. Thus, it is crucial to extract and understand the mechanisms of spontaneous synchronization.
Non-human animals also construct social order, and socially adaptive interactive behaviours have been observed in numerous species11. Coordinated actions have been reported in non-human animals, and include behaviours such as flash synchronization in fireflies12, schooling in some fish species13, and flocking in birds14. However, these behaviours were observed in field studies in which the control of animal behaviour was not possible, and in which it could not be confirmed whether animals changed their behaviours intentionally or spontaneously to achieve synchronization. For further understanding the neural mechanisms of the synchronization, it is important to establish animal models and methods. Recently, studies in fowls and macaques have demonstrated that these animals can explicitly learn rhythmic synchronization to an external cue (sound and/or visual stimuli)15,16,17. Furthermore, a study in macaques has found that two groups of neurons are responsible to quantify the time for intentional synchronization15. Therefore, animal studies have been launched to explore intentional synchronization or voluntary movements. To date, however, there is no well-controlled experimental paradigm has provided a suitable animal model of spontaneous synchronization.
In this study, we developed an experimental paradigm for studying spontaneous synchronization in monkeys during the rhythmic behaviour of button pressing (BP). We studied the Japanese macaque (Macaca fuscata), a species for which a body of studies exploring socially adaptive behaviour and its neural mechanisms is accumulating18,19,20,21,22. Three monkeys (Monkeys B, C, and T) were individually trained to perform a BP task (see Methods), in which the monkey was seated in front of a button box, and was trained to press two buttons repeatedly and alternately by using one hand (Figure 1A, ‘Solo'). The behaviours of the monkeys and the experimental environment were recorded using a Multi-Dimensional Recording system, in which a motion capture device and conventional video cameras were integrated23.
Figure 1. Training and experimental settings.
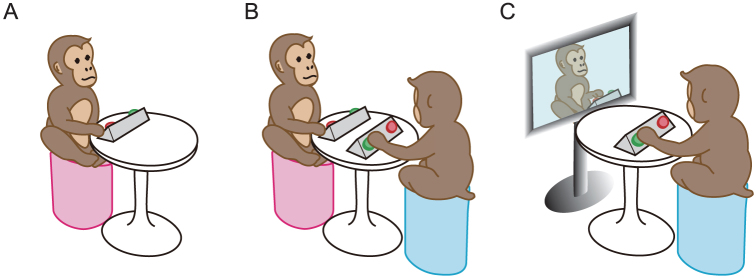
(A) Solo condition. Each monkey was individually trained on the BP task (‘Solo condition'). (B) Paired condition. The monkeys were tested (Test 1) in pairs immediately after learning the BP task. (C) Video play-back (vMonkey) condition. In order to manipulate the BP speed of a partner, and to control the auditory and visual characteristics of a partner, the partner was replaced by vMonkey (Tests 2 and 3).
Results
Test 1: Behavioural synchronization between monkeys
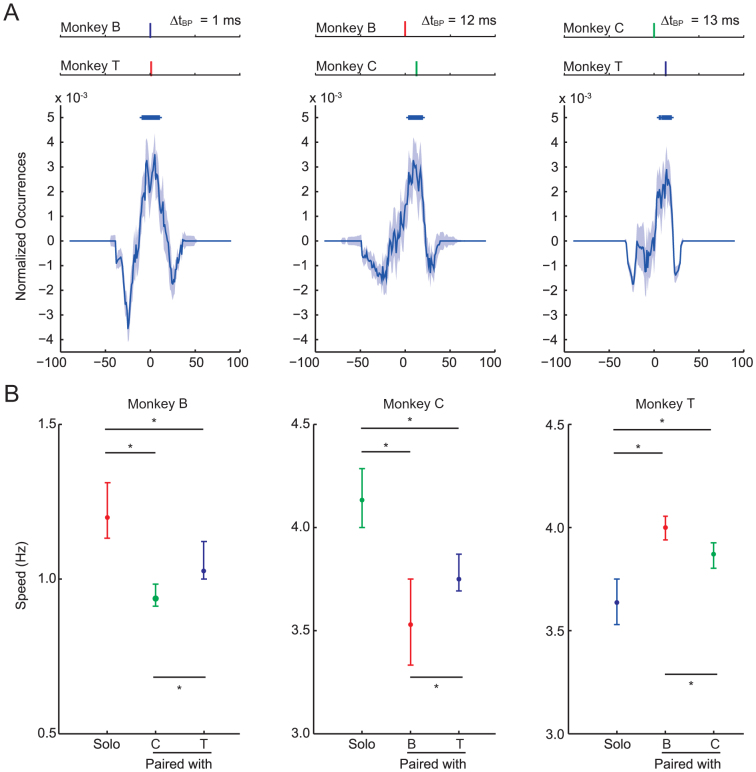
We first tested whether the monkeys showed synchronization in BP behaviour when they were paired with another monkey. In Test 1, two out of the three monkeys were paired and tested in each experimental session (yielding three pairs in total). The monkeys faced each other and pushed buttons placed in front of them, as they had learnt in training (Figure 1B, ‘Paired'; see Experimental Procedures and Supplemental Video S1). To evaluate behavioural synchronization between the paired monkeys, we focused on the timing, irrespective of the side, of BP. If two monkeys pressed buttons in synchrony, then the timing of their BP should occur more often with a time-locked structure with fixed delay (ΔtBP) (see Experimental Procedures). Time-locked structures were found in all pairs of monkeys (Figure 2A). The ΔtBP were 1, 12, and 13 ms for monkey pairs B-T, B-C, and C-T, respectively. These results indicated that synchronization in BP occurred when one monkey was paired with another; and the temporal dynamics of the synchronization were partner dependent, e.g. Monkey C pressed the buttons 1 ms later (or no lag) when paired with Monkey B, but 13 ms earlier when paired with Monkey T.
Figure 2. Synchrony, partner dependency, and harmonics in BP speed.
(A) Synchrony of BP in the Paired condition. Top panels show the time-locked structure with a fixed delay (ΔtBP) between paired monkeys for each pair. The monkey presented on top was assigned as the reference for identifying ΔtBP. ΔtBP was found at 1, 12, and 13 ms for pairs B-T, B-C, and C-T, respectively. Bottom panels show the difference of the distribution of ΔtBP calculated between real data and shuffled data for each pair. + indicates ΔtBP (p < 0.05, Wilcoxon signed-rank test with Bonferroni correction). The shaded areas show the 95% confidence interval of the median. (B) BP speeds for each monkey under Solo and Paired conditions. In the paired condition, BP speeds were partner dependent (p < 0.05, Wilcoxon signed rank test; see text for details). The BP speeds in paired monkeys were matched or close to the harmonics of the two animals (e.g., the BP speeds in Monkeys B and T were 1.0 and 4.0 Hz, respectively, when the monkeys were paired, see text for details). For all monkeys, significant differences were found between the Solo and each Paired condition (p < 0.05, Wilcoxon signed rank test see Supplementary information for details). Error bars represent the 95% confidence interval of the median.
Each monkey pressed the buttons with different speeds when paired with a different partner (p < 0.001, 95% confidence intervals of difference (CI) = [−0.16, −0.05]; p < 0.001, CI = [−0.34, −0.11]; and p < 0.001, CI = [−0.25, −0.12] for Monkeys B, C, and T, respectively; Wilcoxon signed rank test) (Figure 2B). These differences implied that the synchronizations that were established were participant and partner dependent. In addition, significant differences were found in the BP speed between the Solo and Paired conditions for all monkeys (p < 0.05; Wilcoxon signed rank tests; see Supplementary information S1 for details). The results suggested that the participant's own tempo of BP could be distracted by the information from the partner's action, and vice versa.
The monkeys' BP speeds were not always matched to those of a partner (Figure 2B). In monkey pair C-T, the BP speeds were matched at 3.8Hz; however, pair B-T showed speeds of 1.0 and 4.0 Hz (Monkeys B and T, respectively), and pair B-C showed speeds of 0.9 and 3.5 Hz (Monkeys B and C, respectively). These results indicated that the speeds of the pairs were matched (the pair C-T), or were close to (the pairs B-C and B-T) integer multiples, or harmonic to one another.
In summary, Test 1 indicated that when monkeys were paired, (1) the monkeys' behaviour was synchronized (Figure 2A), (2) ΔtBP and the BP speed were partner dependent, and (3) the monkeys' BP speeds were harmonically related (Figure 2B). These results suggested that when the monkeys were paired, their BP speeds might be changed to harmonic, or close to harmonic accord with the BP speed of their partners, rather than to match the speed of their partners. In the next test, we examined how the monkeys adjusted the BP speed to synchronize with their partners.
Test 2: Monkeys' speed adjustment toward synchronization
In Test 1, we found that synchronization was established in the presence of a partner at speeds that were in harmony with one another. In Test 2, we further investigated how the partner's behaviour affected the BP speed. In order to precisely control the partner's behaviour, we introduced a video play-backed monkey (vMonkey; Figure 1C), in which BP speed could be easily manipulated. We then evaluated whether and how the monkey's behaviour changed according to the partner's behaviour, by measuring the monkey's BP speed when vMonkey's BP speed was altered in a controlled manner under two conditions. Each condition consisted of three phases; in one condition (Slow-Fast-Slow, or SFS), the BP speed of vMonkey was initiated at a speed of 1.9 Hz, increased to 3.7 Hz immediately following the 15th BP of the real monkey (1st switch), and then reduced back to 1.9 Hz immediately following the 30th BP of the real monkey (2nd switch). In the other condition (Fast-Slow-Fast, or FSF), the BP speed of vMonkey at the outset of a trial was 3.7 Hz, reduced to 1.9 Hz, and then increased back to 3.7 Hz (see Experimental Procedures for details).
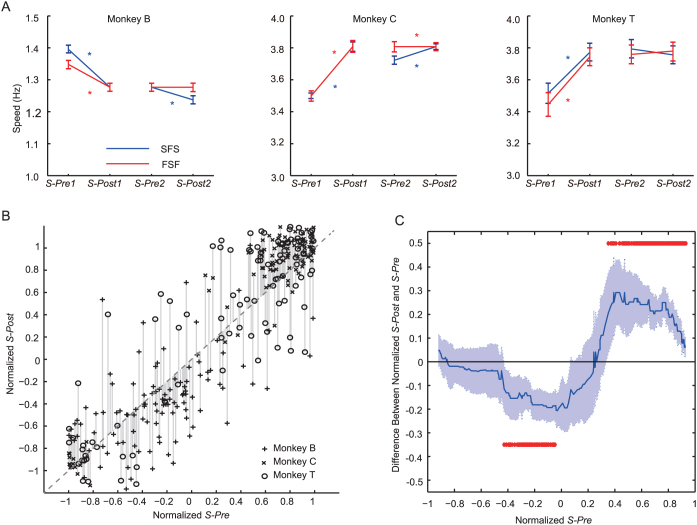
We compared the medians of BP speeds across speed switches: the last 10 BPs before the 1st switch (S-Pre1) vs. the first 10 BPs after the 1st switch (S-Post1), and the last 10 BPs before the 2nd switch (S-Pre2) vs. the first 10 BPs after the 2nd switch (S-Post2). All of the monkeys changed the BP speed at the first switch (S-Pre1 vs. S-Post1 in Figure 3A) under both SFS and FSF conditions (SFS: p = 0.004, CI = [0.02, 0.09]; p = 0.001, CI = [−0.15, −0.04]; and p < 0.001, CI = [−0.38, −0.18] for Monkeys B, C, and T, respectively; FSF: p < 0.001, CI = [0.05, 0.11]; p = 0.003, CI = [−0.17, −0.06]; and p < 0.001, CI = [−0.35, −0.09] for Monkeys B, C, and T, respectively; Wilcoxon rank-sum test). At the second switch (S-Pre2 vs. S-Post2 in Figure 3A), Monkeys B and C, under SFS conditions showed a significant change in BP speed at the 5% level (p = 0.002, CI = [0.02, 0.09]; and p = 0.003, CI = [−0.20, −0.06] for Monkeys B and C, respectively). Moreover, Monkey C under FSF condition showed a change in BP speed (p < 0.001, CI = [−0.30, −0.15]). No significant change in BP speed was observed in other cases; Monkey T in SFS condition (p = 0.09, CI = [−0.02, 0.33]), and Monkey B in FSF condition (p = 0.73, CI = [−0.03, 0.04]). The BP speeds in real monkeys were not matched to those in vMonkey; in some cases, the BP speed was unchanged. For example, Monkey T increased its BP speed after the BP speed of vMonkey was decreased at the 1st switch under FSF, whereas the same monkey did not change its BP speed after the 2nd switch under either condition (Figure 3A, right).
Figure 3. The change in BP speed in the monkeys before and after speed switching of vMonkey.
(A) The change in BP speed for each monkey in trial phases under each condition. The median of 10 BPs before and after of each speed-switch were compared (S-Pre1 vs. S-Post1, and S-Pre2 vs. S-Post2). The blue and red lines represent the Slow-Fast-Slow (SFS) and Fast-Slow-Fast (FSF) conditions, respectively. Asterisks represent a significant difference between before and after speed switching (p < 0.05, Wilcoxon rank-sum test; see text for details). Error bars represent the 95% confidence interval of the median. (B) The normalized S-Pre (including S-Pre1 and S-Pre2) and S-Post (including S-Post1 and S-Post2) scores for all trials. The data from different monkeys are represented with different symbols. The vertical lines represent the distances between normalized S-Post and S-Pre. The diagonal dashed line represents normalized S-Post = normalized S-Pre. (C) The median distance between normalized S-Post and S-Pre. The red asterisks indicate significant differences of the medians from zero (p < 0.05, Wilcoxon signed-rank test). The shaded area represents the 95% confidence interval of the median.
We hypothesized that the monkeys' strategy of BP speed change would be as follows: if the monkey's pre-switch speed (S-Pre, including S-Pre1 and S-Pre2) was between two successive harmonics of vMonkey's post-switch speed (Sv-Post, including Sv-Post1 and Sv-Post2), and was closer to one of them (the ‘target' harmonic), then monkeys would alter its speed toward that target speed. As results, the monkeyxs post-switch speed (S-Post, including S-Post1 and S-Post2) would be closer to the target. In order to assess which harmonic S-Pre was closer to, S-Pre and S-Post were normalized within a range between −1 and 1 based on the corresponding Sv-Post, where −1 and 1 represented the two successive harmonics [e.g. the sub-harmonic (0.5 × Sv-Post) and the harmonic (Sv-Post), or the harmonic (Sv-Post) and the next harmonic (2 × Sv-Post)]. The normalized S-Pre vs. S-Post are plotted in Figure 3B, in which the vertical lines represent the speed change across the switches (normalized S-Post - normalized S-Pre). For example, the data points closer to the diagonal (dashed line) represent those with no significant speed changes, and the data points above the diagonal represent those with speed increases. The median distances were plotted and tested against zero (Figure 3C). The results indicated that: (1) if normalized S-Pre was close to −1 or 1 (the target), then the monkeys maintained a consistent BP speed across the switches (S-Post ≈ S-Pre); (2) if the normalized S-Pre ranged between 0.35 and 0.93 (closer to 1), then the monkeys increased the BP speed significantly (S-Post > S-Pre, with 5% level of Bonferroni correction, or αBonf = 0.05); and (3) if the normalized S-Pre ranged between −0.43 and −0.05 (closer to −1), then the monkeys decreased the BP speed significantly (S-Post < S-Pre, αBonf = 0.05). These results supported our hypothesis: the monkeys sped up, slowed down, or maintained the BP speed close to the harmonic or the sub-harmonic with the vMonkey. In addition, the curve showed an asymmetry biased toward to the right, implying that the monkeys ‘preferred' to decrease their BP speed. This slowing could be due to that the monkeys underestimated the vMonkey's speed, or that they simply conserved their effort.
Test 3: Effects of auditory and visual modalities on synchronization
In the final experiment (Test 3), we explored how auditory and/or visual properties of the partner contributed to the dynamics observed in Tests 1 and 2. vMonkey pressed the buttons at a constant speed of 2.8 Hz (see Experimental Procedures) and three experimental conditions were prepared for this test: auditory-only stimulus (A), visual-only stimulus (V), or both auditory and visual stimuli (AV) presented randomly.
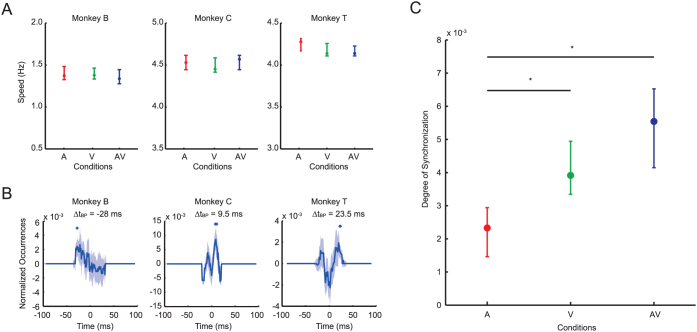
We first compared the BP speeds among experimental conditions (‘A', ‘V', and ‘AV') (Figure 4A). No significant differences were found among the three audio-visual conditions for each monkey (p = 0.14, p = 0.50, and p = 0.09 for Monkeys B, C, and T, respectively; Kruskal-Wallis test). This result indicated that the monkeys did not adapt their BP speeds when the auditory or visual information of partner's action was absent.
Figure 4. Effects of visual and auditory modalities of vMonkey on BP speed and synchrony.
(A) BP speeds for each monkey under conditions ‘A', ‘V', and ‘AV'. No significant difference was found in the BP speed among the A, V, and AV conditions for all monkeys (p > 0.05, Kruskal-Wallis test). Error bars represent the 95% confidence interval of the median. (B) Synchrony of BP for each monkey under the AV condition. vMonkey was assigned as the reference. ΔtBP was found at −28, 9.5, and 23.5 ms for Monkeys B, C, and T represented by + (p < 0.05, Wilcoxon signed-rank test with Bonferroni correction). The shaded areas show the 95% confidence interval of the median. (C) The degree of synchronization under each condition. Asterisks indicate significant differences (p < 0.05, repeated measure one-way analysis of variance and following multi-comparison tests). The V and AV conditions were significantly higher than the A condition (p < 0.05). No significant difference was found between the V and AV conditions (p > 0.05). Error bars represent the 95% confidence interval of the mean.
To confirm whether real monkeys synchronized to vMonkey, we again evaluated ΔtBP between real monkeys and vMonkey in the AV condition, which was more natural to monkeys and more similar to the condition in Test 1. Time-locked structures in the timing of BP were found for all monkeys (p < 0.05 for all monkeys, Wilcoxon signed-rank test with αBonf = 0.05; Figure 4B). These results indicated that the monkeys synchronized to vMonkey when both auditory and visual properties of vMonkey were presented. In addition, the synchrony was formed differently for different monkeys: ΔtBP = −28, 9.5, and 23.5 ms for monkey pairs B-, C-, and T-vMonkey, respectively. Synchrony appeared to be established differently for each monkey, even when BP in the vMonkey was the same.
To quantify the degree of synchronization, we first measured the total of normalized occurrences at the time points in which ΔtBP(s) was found in the AV condition (crosses in Figure 4B), which represented the frequency of occurrence of time-locked structures. Since the temporal structure of stimuli (vMonkey's BP) was the main factor for the timing of synchronization, and the same temporal structure was used in all conditions, we measured the degree of synchronization for the A and V conditions by applying the same measure at the same ΔtBP identified in the AV condition. The degrees of synchronization for all three conditions are shown in Figure 4C.
The degree of synchronization was not equal among the conditions (F (2, 848) = 9.89, p <0.001, ηp2 = 0.02; repeated measures one-way analysis of variance), and the following post-hoc tests indicated that the degree of synchronization in the AV and V conditions was similar, and both were significantly different from that in the A condition (AV-V: p > 0.05, CI = [−94.4, 15.3]; AV-A: p < 0.05, CI = [−149.0, −39.3]; V-A: p < 0.05, CI = [−109.5, −0.2934], with αBonf = 0.05). These results indicated that each modality contributed differently to synchronization.
In summary, (1) the stimulus modalities of vMonkey did not affect the BP speeds of monkeys; (2) the monkeys' BP was synchronized with vMonkey when both auditory and visual modalities of vMonkey were combined; (3) at least the visual modality was required for engaging synchronization.
Discussion
To our knowledge, this is the first demonstration of social synchronization in non-human animals (here, Japanese macaques) under laboratory settings. The monkeys showed synchronization, and their behaviour was participant-partner dependent (Test 1). BP speed was determined by the relative speed of both self and partner (Test 2), and visual information from the partners facilitated synchronization (Test 3). These results indicate that non-human animals can establish coordination without explicit training.
To ensure the synchronization was induced by spontaneous rather than intentional factors, we trained each monkey separately. During training and paired testing, the monkeys were never differentially reinforced to change their BP speed. On paired trials, food rewards were presented to both monkeys at the same time regardless of synchronization, at the end of the trial (see Experimental Procedures). This procedure was unlikely to reinforce competition for food, changes in speed, or synchronization; thus, the monkeys should not have had the intention to synchronize their behaviour. Indeed, an additional analysis revealed that the monkeys exhibited synchronization from the first session and from succeeding sessions in Test 1 (mean ± SD of ΔtBP for 3 sessions = 1.0 ± 4.3, 7.0 ± 1.4, and 14.0 ± 1.4 ms, for Monkeys B-T, B-C, and C-T, respectively), suggesting that the synchronization was not a learned behaviour. In addition, intentional synchronization to external cues was very difficult for monkeys to learn, requiring extensive training for more than 1 year17. Therefore, the behavioural changes of the monkeys observed in the present study were unlikely to be due to an explicit learning process. This observation implies that the behavioural synchronization observed in our study may be qualitatively different from that described in studies involving explicitly trained animals15,16,17.
Behavioural synchronization among non-human animals has been reported, and has sometimes been interpreted as an adaptation to the natural environment12,13,14. However, the behavioural synchronization observed in this study cannot readily be attributed to a survival mechanism. Two critical questions remain unresolved.
First, the reasons why the monkeys showed behavioural synchronization are not clear. One possible explanation is that this behaviour may lack a direct benefit for an individual animal, but that the behaviour may be a vital aspect of other socially adaptive behaviours, such as automatic facial imitation in newborn macaques and apes24,25,26 and joint attention of a mother or caretaker to neonates and infants27,28,29. These socially adaptive behaviours are thought to be important for survival in wild animals. Because the social environment is always changing, animals are required to quickly/spontaneously adapt to the environment through certain inherited mechanisms. The monkeys' synchronized behaviour demonstrated in this study seems unlikely to be directly elicited by these inherited mechanisms. Thus, synchronization of an unnatural and learned behaviour, such as BP, could evidence the flexibility and generality of these mechanisms for adapting to the social environment. To determine which environmental aspects, especially factors such as the social hierarchy or the strength of the social bond, trigger synchronization could help elucidate whether spontaneous synchronization is a product of latent mechanisms of the ‘social brain'. Therefore, the simple BP task could be utilized for brief assessments of autistic children who show difficulty in responding to social cues and in maintaining social relationships27.
The second question relates to which brain areas are responsible for behavioural synchronization. In monkey studies, the ‘mirror system', including the ventral premotor cortex and the rostral region of the inferior parietal lobule, was activated when a monkey was performing an action by himself and also when the same monkey was observing a similar action sharing the same intention of the action28,29. Therefore, it is likely that these areas of the brain are involved in the coordination of actions. Damage to the anterior inferior part of the frontal lobe causes severe behavioural dysfunction (echopraxia or echolalia), triggering automatic gestural or vocal imitation of another person30,31. These inappropriate imitations, which can also be initiated by the mirror system, might occur because of the lack of a suppression mechanism in the middle and inferior frontal cortex in these patients32. Consequently, several brain areas might be responsible for the synchronization that we observed. Our future goal is to record neural activity in multiple brain areas in monkeys performing the task described in this study, in order to identify the dynamic functional connections that initiate and maintain synchronization. The present task is clearly suitable for investigating the nervous system of social synchronization by its precise control of the stimuli and the animals' behaviours. In order to better understand coordination mechanisms of the social brain, integration between the neural substrates of spontaneous and intentional interactive behaviour should be addressed.
Methods
Subjects and materials
Three male Japanese macaques (Macaca fuscata; B, C, and T) participated in this study (see supplemental information S2 for details). During the task, each monkey was seated in a primate chair with his head, chest, and arms free. Each monkey wore its own custom jacket, and 6 infrared-reflective markers were placed at the shoulders, elbows, and wrists on the jacket. The markers were recorded using a motion capture system (Vicon Motion Systems, CA, USA) with a 120-Hz sampling rate. A button box (33 cm wide, 8 cm high, and 6 cm deep) was placed on a round table (60 cm diameter) approximately 30–35 cm from the monkey. The box contained two buttons (4 cm diameter) with internal LEDs, aligned horizontally and set 27 cm apart. One button box was used for training, and for Tests 2 and 3, whereas both boxes were used for Test 1. In Test 1, two monkeys were seated facing each other at opposite ends of the table. In Tests 2 and 3, a 24-inch LCD display was placed 60 cm in front of the monkey. Video clips (vMonkeys, see below) showing a life-sized monkey alternately pressing two buttons were played on the display. The behaviours of the monkeys and the experimental environment were recorded with a Multi-Dimensional Recording system that employed a motion capture device and several conventional video cameras23. The experimental session was controlled by an iMac computer (Apple Inc., Cupertino, CA, USA) and was programmed with MATLAB (MathWorks Inc., Natick, MA, USA) using the Psychophysics Toolbox extensions33,34,35. All experimental protocols were approved by the RIKEN Ethical Committee (No. H22-2-202 (4)).
Training
Each monkey was trained solely for a button-pressing (BP) task. Monkeys were trained to press two buttons alternately, with a preferred hand: the right hand in Monkeys C and T, and the left hand in Monkey B. At the outset of each trial, both buttons were illuminated and the monkey could begin pressing either the left or right button. When an illuminated button was pressed, a short sound was played for the monkey to ensure the BP and the LED was turned off, after which the monkey was expected to press the button that remained illuminated. A trial was considered to be successful when a randomly assigned number of button presses (30 to 36) was achieved without any pause greater than 5 s (BP criterion). At the end of each trial, both LEDs were turned off. After each successful trial, a chime was presented, and the monkey was rewarded with nuts or a piece of apple. The training was finished when a monkey performed 10 consecutive successful trials for two consecutive days. The data in the last two sessions were used as the “Solo” condition for the following analyses.
Experimental procedures
Test 1: Behavioural Synchronization between Real Monkeys
Two of the three monkeys were seated facing one another while performing the BP task. Test 1 followed the same protocol as the training procedure, except that the trial was considered successful when: (1) each monkey satisfied the BP criterion, and (2) the monkey that met its criterion first continued to press buttons until the second monkey met its criterion. Food rewards were presented at the same time for both monkeys. In a daily session, three test blocks were conducted. A total of 90 trials over 3 days were obtained for each monkey pair.
Test 2: Effects of speed manipulation on synchronization
The procedures for Test 2 were the same as those for Test 1, except that one monkey from the pair was replaced by video playback of a recorded monkey (vMonkey). vMonkey was prepared by editing the recorded clip of Monkey T. Two experimental conditions were created, in which the BP speed of the vMonkey was manipulated. In the first treatment, a Slow-Fast-Slow (SFS) condition was presented in which the BP speed of the vMonkey was initiated at 1.9 Hz, and increased to 3.7 Hz immediately after the 15th BP event. After the 30th BP event, the BP speed of the vMonkey was immediately reduced to 1.9 Hz and maintained at that level until the real monkey attained the criterion (47 BPs). In the second treatment, a Fast-Slow-Fast (FSF) scenario was presented in which the BP speed of vMonkey was initially presented at 3.7 Hz, reduced to 1.9 Hz, and then increased again to 3.7 Hz at the same BP intervals as used for the SFS condition. Monkeys' head movements were gently restricted and large movements of the head were prevented by using a custom-made helmet, which was fixed to a pole that extended from the primate chair. A daily session consisted of 10 trials of each condition (SFS or FSF) per monkey, carried out in random order. Eighty trials were conducted with each monkey over 4 days.
Test 3: Effects of auditory and/or visual modality on synchronization
In Test 3, the BP speed of the vMonkey was maintained at a constant rate of 2.8 Hz, and the vMonkey was shut off when the real monkey reached the criterion. Three experimental conditions were prepared for presenting vMonkey: auditory-only stimulus (A), visual-only stimulus (V), or both auditory and visual stimuli (AV). Auditory and visual stimuli presented by vMonkey were those recorded from real Monkey T. A daily session consisted of 10 trials under each condition (A, V, or AV) conducted in random order. A total of 300 trials were conducted with each monkey over 10 days.
Quantification of behavioural synchronization (ΔtBP)
We defined behavioural synchronization as occurring when the timing of BPs between paired monkeys were time-locked. We assumed that the series of BP timings for Monkeys B and C in a single trial were B = {b1, b2, … bN} and C = {c1, c2, … cM}, respectively, where bN and cM represent the total number of BPs for Monkeys B and C, respectively. Assuming that Monkey C pushed buttons more slowly than Monkey B, i.e. M < N, we then used Monkey C as the reference. For each timing in C, cm, we identified the timing in B, bn, so that the absolute difference between bn and cm was the smallest (bn could be immediately before or after cm). Thus, the relative delays between Monkeys C and B could be estimated as:
In order to identify delays that occurred more frequently than by chance, we compared the calculated value of DelaysB-C to the value obtained using a shuffling procedure. To perform shuffling that destroyed the temporal structure between B and C, each bn in B was replaced by B′n, a randomly selected value within the interval of (bn−1 + bn)/2 and (bn + bn+1)/2. This method is equivalent to adding jitter, a value between (bn−1 − bn)/2 and (bn+1 − bn)/2, to bn. By repeating this process for all elements in B and C, the shuffled sets B′ = {b′1, b′2, … b′N} and C′ = {c′1, c′2, … c′M} were created, and the corresponding delays, DelaysB′−C′, were measured. This process was repeated 500 times. A histogram of DelaysB−C (Horg), and histograms of 500 DelaysB′−C′ (Hsh1, Hsh2, … Hsh500) were then created using a 100-ms window with 20-ms steps and normalized by dividing by the total count of all windows. Significant differences between histograms, i.e. Horgvs. {Hsh1, Hsh2, …, Hsh500} (α = 0.05, one-tailed Wilcoxon signed-rank test with Bonferroni correction), indicated delays (ΔtBP) that occurred more frequently than by chance (time-locked), which further implied that synchronization had occurred.
Author Contributions
Y.N. designed the experiments, constructed the apparatus, developed the computer programs, analyzed the data, and wrote the paper. Z.C. analyzed the data and wrote the paper. N.H. and T.N assisted in taking care of the monkeys and conducting the experiments. N.F. provided ideas and supervised the project.
Supplementary Material
Movie-S1: Representative example of a trial in Test 1.
Supplementary Information
Acknowledgments
We thank Edward A. Wasserman for his help to edit the text of the manuscript, as well as Nobuhito Hanashima for his help with the illustrations. This study was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas ‘Neural creativity for communication' (22120522 and 24120720) of MEXT, Japan.
References
- Sebanz N., Bekkering H. & Knoblich G. Joint action: bodies and minds moving together. Trends Cogn Sci 10, 70–76 (2006). [DOI] [PubMed] [Google Scholar]
- Chartrand T. L. & Bargh J. A. The chameleon effect: the perception-behavior link and social interaction. J Pers Soc Psychol 76, 893–910 (1999). [DOI] [PubMed] [Google Scholar]
- Oullier O., de Guzman G. C., Jantzen K. J., Lagarde J. & Kelso J. A. Social coordination dynamics: measuring human bonding. Soc Neurosci 3, 178–192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M. J., Marsh K. L. & Schmidt R. C. Effects of visual and verbal interaction on unintentional interpersonal coordination. J Exp Psychol Hum Percept Perform 31, 62–79 (2005). [DOI] [PubMed] [Google Scholar]
- Honma M., Kumada T., Osada Y. & Nagai M. The synchronized stepping -Automatic imitation behavior between persons-. The Japanese Journal of Psychonomic Science 27, 127–128 (2008). [Google Scholar]
- Richardson M. J., Marsh K. L., Isenhower R. W., Goodman J. R. & Schmidt R. C. Rocking together: dynamics of intentional and unintentional interpersonal coordination. Hum Mov Sci 26, 867–891 (2007). [DOI] [PubMed] [Google Scholar]
- Sato W. & Yoshikawa S. Spontaneous facial mimicry in response to dynamic facial expressions. Cognition 104, 1–18 (2007). [DOI] [PubMed] [Google Scholar]
- Bernieri F. J. Coordinated movement and rapport in teacher-student interactions. Journal of Nonverbal Behavior 12, 120–138 (1988). [Google Scholar]
- Lakin J. L. & Chartrand T. L. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol Sci 14, 334–339 (2003). [DOI] [PubMed] [Google Scholar]
- Lakin J. L., Chartrand T. L. & Arkin R. M. I am too just like you: nonconscious mimicry as an automatic behavioral response to social exclusion. Psychol Sci 19, 816–822 (2008). [DOI] [PubMed] [Google Scholar]
- Emery N., Clayton N. & Frith C. Social Intelligence: From Brain to Culture (Oxford University Press, USA, 2008). [Google Scholar]
- Copeland J. & Moiseff A. The occurrence of synchrony in the North American firefly Photinus carolinus (Coleoptera: Lampyridae). Journal of Insect Behavior 8, 381–394 (1994). [Google Scholar]
- Pitcher T. J. & Parrish J. K. in Behaviour of Teleost Fishes (2nd ed.) , ed. 363–439 (Springer-Verlag, London, 1993). [Google Scholar]
- Iztok L. B. & Frank H. H. Organized flight in birds. Animal Behaviour 78, 777–789 (2009). [Google Scholar]
- Merchant H., Zarco W., Perez O., Prado L. & Bartolo R. Measuring time with different neural chronometers during a synchronization-continuation task. Proc Natl Acad Sci U S A 108, 19784–19789 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A., Okanoya K., Hasegawa T. & Seki Y. Rhythmic synchronization tapping to an audio–isual metronome in budgerigars. Scientific Reports 1, 120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarco W., Merchant H., Prado L. & Mendez J. C. Subsecond timing in primates: comparison of interval production between human subjects and rhesus monkeys. J Neurophysiol 102, 3191–3202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N., Hihara S. & Iriki A. Dynamic social adaptation of motion-related neurons in primate parietal cortex. PLoS ONE 2, e397 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N., Hihara S. & Iriki A. Social cognition in premotor and parietal cortex. Soc Neurosci 3, 250–260 (2008). [DOI] [PubMed] [Google Scholar]
- Fujii N., Hihara S., Nagasaka Y. & Iriki A. Social state representation in prefrontal cortex. Soc Neurosci 4, 73–84 (2008). [DOI] [PubMed] [Google Scholar]
- Yoshida K., Saito N., Iriki A. & Isoda M. Representation of others' action by neurons in monkey medial frontal cortex. Curr Biol 21, 249–253 (2011). [DOI] [PubMed] [Google Scholar]
- Santos G. S., Nagasaka Y., Fujii N. & Nakahara H. Encoding of social state information by neuronal activities in the macaque caudate nucleus. Soc Neurosci 7, 42–58 (2012). [DOI] [PubMed] [Google Scholar]
- Nagasaka Y., Shimoda K., Fujii N. Multidimensional Recording (MDR) and Data Sharing: An Ecological Open Research and Educational Platform for Neuroscience. PLoS ONE 6, e22561 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P. F. et al. Neonatal Imitation in Rhesus Macaques. PLoS Biol 4, e302. (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myowa-Yamakoshi M., Tomonaga M., Tanaka M. & Matsuzawa T. Imitation in neonatal chimpanzees (Pan troglodytes). Dev Sci 7, 437–442 (2004). [DOI] [PubMed] [Google Scholar]
- Bard K. A. Neonatal imitation in chimpanzees (Pan troglodytes) tested with two paradigms. Anim Cogn 10, 233–242 (2007). [DOI] [PubMed] [Google Scholar]
- Trevarthen C. & Daniel S. Disorganized rhythm and synchrony: early signs of autism and Rett syndrome. Brain Dev 27 Suppl 1, S25–S34 (2005). [DOI] [PubMed] [Google Scholar]
- Gallese V., Fadiga L., Fogassi L. & Rizzolatti G. Action recognition in the premotor cortex. Brain 119, 593–609 (1996). [DOI] [PubMed] [Google Scholar]
- Rizzolatti G. & Craighero L. The mirror-neuron system. Annu Rev Neurosci 27, 169–192 (2004). [DOI] [PubMed] [Google Scholar]
- Lhermitte F., Pillon B. & Serdaru M. Human autonomy and the frontal lobes. Part I: Imitation and utilization behavior: a neuropsychological study of 75 patients. Ann Neurol 19, 326–334 (1986). [DOI] [PubMed] [Google Scholar]
- Cho Y. J., Han S. D., Song S. K., Lee B. I. & Heo K. Palilalia, echolalia, and echopraxia-palipraxia as ictal manifestations in a patient with left frontal lobe epilepsy. Epilepsia 50, 1616–1619 (2009). [DOI] [PubMed] [Google Scholar]
- Bien N., Roebroeck A., Goebel R. & Sack A. T. The Brain's Intention to Imitate: The Neurobiology of Intentional versus Automatic Imitation. Cereb Cortex 19, 2338–2351 (2009). [DOI] [PubMed] [Google Scholar]
- Brainard D. H. The Psychophysics Toolbox. Spat Vis 10, 433–436 (1997). [PubMed] [Google Scholar]
- Pelli D. G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10, 437–442 (1997). [PubMed] [Google Scholar]
- Kleiner M., Brainard D. & Pelli D. What's new in Psychtoolbox-3? Perception 36, ECVP Abstract Supplement (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie-S1: Representative example of a trial in Test 1.
Supplementary Information