Abstract
Background: Many pesticides in current use have recently been revealed as in vitro androgen receptor (AR) antagonists, but information about their combined effects is lacking.
Objective: We investigated the combined effects and the competitive AR antagonism of pesticide mixtures.
Methods: We used the MDA-kb2 assay to test a combination of eight AR antagonists that did not also possess AR agonist properties (“pure” antagonists; 8 mix: fludioxonil, fenhexamid, ortho-phenylphenol, imazalil, tebuconazole, dimethomorph, methiocarb, pirimiphos-methyl), a combination of five AR antagonists that also showed agonist activity (5 mix: cyprodinil, pyrimethanil, vinclozolin, chlorpropham, linuron), and all pesticides combined (13 mix). We used concentration addition (CA) and independent action (IA) to formulate additivity expectations, and Schild plot analyses to investigate competitive AR antagonism.
Results: A good agreement between the effects of the mixture of eight “pure” AR antagonists and the responses predicted by CA was observed. Schild plot analysis revealed that the 8 mix acted by competitive AR antagonism. However, the observed responses of the 5 mix and the 13 mix fell within the “prediction window” boundaries defined by the predicted regression curves of CA and IA. Schild plot analysis with these mixtures yielded anomalous responses incompatible with competitive receptor antagonism.
Conclusions: A mixture of widely used pesticides can, in a predictable manner, produce combined AR antagonist effects that exceed the responses elicited by the most potent component alone. Inasmuch as large populations are regularly exposed to mixtures of antiandrogenic pesticides, our results underline the need for considering combination effects for these substances in regulatory practice.
Keywords: antiandrogen, AR-antagonism, concentration addition, endocrine disruption, fungicide, mixture, pesticide
Certain pesticides are known to disrupt male sexual differentiation in vivo by antagonizing the androgen receptor (AR) (Gray et al. 1994; Lambright et al. 2000; Ostby et al. 1999) or by interfering with steroid-converting enzymes in fetal life (Blystone et al. 2007; Vinggaard et al. 2005). These pesticides can act together to produce combination effects (Christiansen et al. 2008; Vinggaard et al. 2005), which can also occur when combined with other chemicals known to disrupt androgen action (Rider et al. 2008, 2009). Data from food residues indicate that there is a potential for simultaneous human exposure to at least some of these pesticides.
We previously reported that a number of current-use pesticides are antiandrogenic (Orton et al. 2011). Using these data, we formulated mixtures based on the most common pesticides present in foods in Europe. Many of these pesticides are also commonly found in the United States (e.g., fludioxonil, in 26% of strawberries and 14% of grapes; fenhexamid, in 24% of strawberries; ortho-phenylphenol, in 34% of oranges; dimethomorph, in 28% of lettuces; cyprodinil, in 27% of grapes; pyrimethanil, in 31% of strawberries; chlorpropham, in 76% of potatoes) (U.S. Environmental Protection Agency 2011). Considering that risk assessment procedures do not currently account for mixture effects, it is possible that risks to male reproductive health by pesticides are being underestimated. Although antiandrogenic mixture effects have been described for certain pesticides, some of which are obsolete (Birkhoj et al. 2004; Kjærstad et al. 2010; Nellemann et al. 2003), similar data with more widely used pesticides are lacking. Because many current-use pesticides act as AR antagonists in vitro (Kojima et al. 2004; Orton et al. 2009, 2011), it is plausible to assume that these pesticides might also have mixture effects. However, empirical evidence to support this idea is lacking. Because none of the pesticides chosen for our mixture studies were tested in vivo, it was important to investigate whether these substances have the ability to act jointly at the receptor level in vitro. If that was found to be the case, it would create alerts for prioritization of in vivo testing.
This is all the more relevant because of indications of negative effects on male reproductive health from epidemiological studies of occupational pesticide exposures. For example, statistically significant associations between genital malformations or decreased penile length in boys with occupational maternal or paternal pesticide exposure have been observed in the Netherlands (Pierik et al. 2004), Denmark (Andersen et al. 2008; Wohlfahrt-Veje et al. 2012), and France (Gaspari et al. 2011) and also in a meta-analysis of hypospadias incidence in several countries (Rocheleau et al. 2009). However, these studies could not identify specific pesticides as being involved in the analyzed effects.
At present, there are 1,252 registered active ingredients in pesticide formulations in the United States (U.S. EPA, personal communication). There are 411 registered entities in Europe, with another 72 pending registration (European Commission 2011). With such a high number of registered active substances, it is practically impossible to test all possible combinations to arrive at robust conclusions about the nature of combination effects. Therefore, exploring the accurate predictability of mixture responses using modeling approaches is essential. Mixture modeling uses single compound testing data to describe the effects of simultaneous exposures to multiple chemicals, with the aim of replacing or significantly reducing testing for the prohibitively large number of chemicals and combinations present in the environment. In this context, modeling approaches work under the hypothesis that compounds elicit their effects without affecting the toxicity of other mixture components, i.e., the additivity assumption (reviewed by Kortenkamp 2007). Two concepts are commonly used to explore the additivity assumption: a) concentration addition (CA, also called dose addition), and b) independent action (IA, also called response addition). For CA, it is assumed that all compounds have a similar mechanism of action (e.g., binding the same receptor), whereas for IA, it is assumed that all mixture components affect the same end point via different sites or modes of action (i.e., using a dissimilar mechanism of action). Both additivity models assume no interaction between the compounds, neither on a physicochemical level nor in their toxicokinetics and toxicodynamics.
CA has consistently been shown to be a good model for predicting antiandrogenic effects [e.g., in vivo (Christiansen et al. 2008; Hass et al. 2007; Howdeshell et al. 2008) and in vitro (Ermler et al. 2011)]. To our knowledge, there are only two examples where CA has failed to predict the mixture effect. A significant deviation (synergism) was observed in response to five antiandrogenic parabens in vitro (Kjaerstad et al. 2010) and to four antiandrogenic contaminants in vivo [di(2-ethylhexyl) phthalate; two fungicides present in food, vinclozolin and prochloraz; and a pharmaceutical, finasteride] (Christiansen et al. 2009). To investigate the predictability of mixtures of AR antagonists using the MDA-kb2 cell assay, and considering the features of this assay, we hypothesized that CA and not IA would be the appropriate prediction concept (for an overview see Ermler et al. 2011).
Some AR antagonists can stimulate the receptor, sometimes at concentrations higher than those required for antagonism and, in other cases, over the same concentration range (Ermler et al. 2011; Orton et al. 2011). Many AR antagonists are not capable of eliciting AR agonist effects, and these are referred to as “pure” antagonists. The antagonist/agonist activity of some antiandrogens is thought to be due to different actions on the AR receptor whereby the AR is simultaneously stimulated by binding to a distinct domain of the receptor (Tamura et al. 2006). However, it is not known how such effects might affect the predictability of mixture models and whether the “similarity” criterion of CA is fulfilled under these circumstances. Therefore, we investigated whether CA was a suitable prediction tool for mixtures regardless of mixture composition, or whether mixtures composed of antagonist/agonist antiandrogens produced responses that deviated from CA. We used a Schild plot analysis to distinguish the similarity requirements for both scenarios. This pharmacological method allowed us to assess whether the observed antiandrogenic activity was solely due to competitive antagonism of dihydrotestosterone (DHT) binding to the ligand binding domain of the AR (Kenakin 1993).
Methods
Test compound selection. We have previously shown that 24 current-use, environmentally relevant pesticides were AR antagonists (Orton et al. 2011), and our mixture selection for the present study was based on these data. For the 24 that were antiandrogenic, we ranked the pesticides by their environmental relevance ratio (ERR), a measure of combined potency and prevalence and excluded those with lapsed registration status (as of January 2010) and cytotoxicity at ≤ 10 µM. Twelve pesticides fulfilled these criteria, in order of ERR: dimethomorph (ERR 45.6; re-registration date September 2017), fludioxonil (31.2; October 2018), fenhexamid (11.9; December 2015), imazalil (9.9; December 2021), linuron (6.9; December 2013), ortho-phenylphenol (6.1; December 2019), pirimiphos-methyl (5.5; September 2017), tebuconazole (5.5; August 2019), chlorpropham (2.9; June 2015), methiocarb (2.5; September 2017), cyprodinil (2.2; April 2017), and pyrimethanil (1.0; May 2017). In addition, vinclozolin was included because of its high ERR (79.8), its known in vivo potency (e.g. Gray et al. 1994), and its continued detection in foodstuffs in Europe {0.38% in 2008 [(European Food Safety Authority (EFSA) 2010] and 0.2% in 2009 (EFSA 2011)}, despite its expired registration status of January 2007.
Chemicals. DHT (> 97% purity) was purchased from Steraloids Ltd. (Croydon, Surrey, UK); dimethomorph and methiocarb (> 98.7% purity were purchased from Greyhound Chromatography and Allied Chemicals; Birkenhead, Merseyside, UK); and all other pesticides (> 97% purity) were purchased from Sigma-Aldrich (Poole, Dorset, UK). All test compounds were dissolved in > 99.7%-purity ethanol to make stock solutions to be used in the assays.
MDA-kb2 assay. MDA-kb2 cells [catalog number CRL-2713; American Tissue Culture Collection (ATCC), Manassas, VA, USA] are human breast cancer cells stably transfected with a firefly luciferase reporter gene that is driven by an androgen–response element-containing promoter (Wilson et al. 2002). Details of the modified assay were published previously (Ermler et al. 2010). Briefly, cells were seeded at a concentration of 1 × 105 cells/mL in phenol red–free Leibowitz-15 medium (Invitrogen Ltd., Paisley, UK) containing 10% charcoal-stripped fetal calf serum (Invitrogen Ltd.) in white luminometer plates. After 28 hr, luciferase activity was determined with SteadyGlo assay reagent (Promega UK Ltd., Southampton, Hampshire, UK) and measured in a plate reader (FLUOstar Optima; BMG Labtech GmbH, Offenburg, Germany). For regression analysis, cells were exposed to eight serial dilutions of selected pesticides with or without DHT (0.25 nM). Subsequent to the initial testing range of 1.17 nM–150 µM, the mixtures’ concentrations were modified to reflect the potency and toxicity of each individual mixture. For Schild plot analysis, cells were coexposed with eight serial dilutions of DHT (0.009–20 nM) and fixed concentrations of pesticide mixtures (150–6.25 µM), which varied according to the individual activity/toxicity of each mixture. For all testing scenarios, the following controls were run on each plate: medium, ethanol (0.25%), DHT coexposure (0.25 nM), DHT serial dilutions (0.009–20 nM) with ethanol (0.25%), and procymidone (0.005–3.2 µM) with DHT (0.25 nM). All concentrations were tested in duplicate over two plates; each mixture stock was measured at least twice in separate experiments, and mixtures were independently tested at least three times (using new stock solutions, in separate experiments) by two experimenters. For comparative purposes, luminescence was normalized to DHT alone at the coexposure concentration (i.e., maximum response, 100%) and solvent-only (ethanol) controls (i.e., minimum response, 0%).
Cytotoxicity as a confounding factor. The MDA-kb2 assay measures decreases in luminescence of the DHT agonist that occur as a result of receptor antagonism. Because the luminescence signal can also be driven down by cytotoxicity, it is important to distinguish antagonism from interfering cytotoxicity, and we adopted well-established procedures (Ermler et al. 2010, 2011; Korner et al. 2004) to deal with this issue. Briefly, cytotoxicity was determined in treatments without DHT by a reduction in luminescence relative to the ethanol controls. Where agonism in the absence of DHT was observed, the comparison was with the maximal response.
Renilla assay. We constructed a Renilla luciferase plasmid with a mammalian selection marker and a constitutively active promoter [the herpes simplex virus–thymidine kinase (HSV-Tk) gene] in order to eliminate the possible interfering effects of cell proliferation. Briefly, 4 µg DNA was incubated with 6 µL TurboFect (Fermentas Gmbh, St. Leon-Rot, Germany) in 400 µL of serum-free Leibowitz L-15 medium (Invitrogen Ltd.) for 20 min. MDA-kb2 cells were transfected with the Renilla construct for 48 hr prior to following the normal procedure for the MDA-kb2 assay. In order to read both the luciferase and Renilla signals, after 28 hr of incubation, luciferase activity was determined with Dual-Glo Reporter assay reagent (Promega UK Ltd.), which employs the sequential addition of two reconstituted reagents with luminescence measurement after each reagent addition (FLUOstar Optima, BMG Labtech GmbH). The first reagent provides the necessary substrate for firefly luciferase, and the second reagent quenches this activity while at the same time activating Renilla luciferase. Cells transfected with the Renilla construct were exposed to the 5 mix IC10 only; for regression analysis, 5 mix (serial dilutions: 150–5.6 µM) was coexposed with a fixed concentration of DHT (0.25 nM), and for Schild plot analysis, serial concentrations of DHT (0.009–20 nM) with various fixed concentrations of 5 mix (110–13.75 µM).
Test mixtures. All mixtures were designed as fixed-ratio equipotent mixtures. We tested three distinct pesticide mixtures: an 8 mix, a 5 mix, and a 13 mix. The 8 mix comprised eight “pure” AR antagonists (fludioxonil, fenhexamid, ortho-phenylphenol, tebuconazole, dimethomorph, imazalil, methiocarb, pirimiphos-methyl); the 5 mix comprised five antagonists with additional agonist properties (cyprodinil, pyrimethanil, vinclozolin, chlorpropham, linuron); and the 13 mix comprised the eight “pure” antagonists together with the five “mixed” antagonists. Fixed-mixture ratios were calculated in proportion to the concentrations of the individual mixture components that led to a suppression of DHT effects by 1%, 10%, 20%, or 50% [here termed inhibitory concentrations (ICs) IC01, IC10, IC20, IC50]. The 13 mix was tested at four fixed mixture ratios (IC01, IC10, IC20, IC50), and the 8 mix and 5 mix were tested at two fixed mixture ratios (IC01, IC10) [see Supplemental Material, Table S1 (http://dx.doi.org/10.1289/ehp.1205391)]. The mathematical and statistical procedures used for calculating mixture effects according to CA and IA are described by Faust et al. (2001).
Schild plot calculations. To confirm applicability of the MDA-kb2 assay to Schild plot analysis, we first determined concentration–effect curves for the agonist DHT in the presence of various concentrations of flutamide. From the concentration–effect curves, we estimated a series of concentration ratios (i.e., the ratio of the DHT concentration to produce a specific effect in the presence of the antagonist to the concentration required in the absence of the antagonist) for a given effect. This was determined for several concentrations of the antagonists. To get the most accurate results, we used a 50% inhibition—the concentration ratio calculated as the IC50 in the presence of antagonist divided by the IC50 in the absence of antagonist. The Schild plot analysis was then based on the linear regression:
log(IC50DHT + A/IC50DHT – 1) = –log(KD) + θ × log(cA). [1]
Here, KD is the (unknown) dissociation constant of the antagonist, cA the concentration of the antagonist “A” held fixed in the experiments, and θ the slope parameter. The unknown parameters, θ and log(KD), were estimated by ordinary least squares. If the regression is linear with a slope of 1, the antagonism is competitive and, by definition, the agonist and antagonist act at the same sites (Kenakin 1993). The concentration–response curves recorded in the presence of a fixed concentration of the antagonist will shift to the right of the DHT curve, with the same maximum response and (generally) the same shape. Therefore, we always used the logit model for the data analysis and performed Schild regression analysis only when the assumption of similar maximum responses was justified. The same principles were applied to the pesticide mixtures.
Statistics. To analyze AR antagonist action, raw luminescence readings were normalized on a plate-by-plate basis to the means of the positive DHT controls (n = 8) and the solvent controls (n = 8), which were placed on the same plate. Luminescence readings from pesticides tested in the absence of DHT were divided by the mean of the solvent controls from the same plate and analyzed for negative and positive trends (suggestive of cytotoxic or androgenic action, respectively). All data from the same test compound were pooled and statistical concentration–response regression analyses were conducted by using the best-fit approach to derive ICs for androgenicity (Scholze et al. 2001). To control for variations between experiments, concentration–response data were analyzed by using a generalized nonlinear mixed modeling approach (Vonesh and Chinchilli 1996) with plate as a random effect modifier for individual effect data. If readings in the absence of DHT showed indications for cytotoxic or androgenic action, the nonmonotonic concentration–response relationship was modeled by nonparametric local regression methods (Cleveland et al. 1988). From this robust fitting method, we derived effect concentrations (ECs) for androgenicity, with a 10% increase over the mean solvent mean as the minimum effect criterion, and ECs for cytotoxicity (if present) as 10% reduction of the maximal observed androgenic action. Data points associated with cytotoxicity were not included in regression analysis for antiandrogenicity. Differences between predicted and observed effect doses were deemed statistically significant when the 95% confidence intervals (CIs) of the prediction did not overlap with those of the experimentally observed mixture effects. All statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
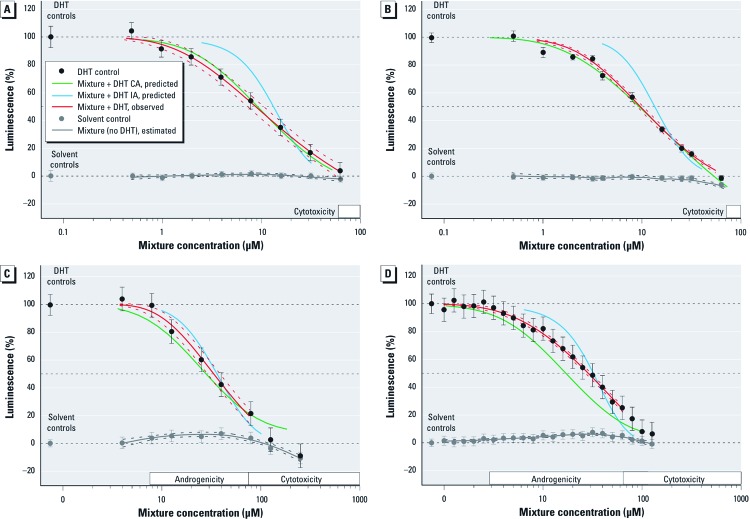
Low variation between experiments, good repeatability, and complete regression curves for all the selected individual pesticides meant that all compounds were suitable for mixture assessment. All mixtures showed AR antagonist activity in a clear dose-dependent way (Figure 1). The agreement between observed and predicted AR antagonistic activity for a given mixture is shown for two selected response levels in Table 1: IC50s for the mixtures were only once overestimated by both CA and IA (13 mix, IC01 mixture ratio, 10% inhibition) and in all other cases were never outside the range predicted by CA and IA. Cytotoxicity was only observed at high mixture concentrations (8 mix: EC10, 60–77 µM; 5 mix: EC10, 70–74 µM; 13 mix; EC10, 63–81 µM) [for more information, see Supplemental Material, Table S2 (http://dx.doi.org/10.1289/ehp.1205391)]. The overlap with antiandrogenic responses was negligible and did not interfere with the detection of AR antagonistic responses (Figure 1A–D). The model parameters, together with estimated AR antagonist concentrations and effect concentrations for androgenicity and cytotoxicity are listed in Supplemental Material, Table S2. Cytotoxicity data for all test mixtures are also shown in Supplemental Material, Figure S2.
Figure 1.
Predicted and observed antiandrogenic activity (mean responses ± SDs) of mixtures with 8 pesticides composed in the ratio of their individual IC10 values (A) and IC01 values (B), with 5 pesticides mixed in the ratio of their individual IC01 values (C), and with 13 pesticides mixed in the ratio of their individual IC10 values (D). Observed mixture effects are from at least three independent mixture experiments and shown as mean ± SD, predicted effect curves were calculated using the model of CA and IA. Regression fit of the observed effects is shown as solid red line, with the dotted red lines indicating the corresponding 95% CI. Estimated mean effect (solid gray line) and 95% CI (dotted gray lines).
Table 1.
Statistical uncertainty of predicted and observed ICs [means (95% CIs)] for mixtures.
| Inhibition level x | Inhibition concentration ICX(mixture) [M] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Predicted by CA | Predicted by IA | ||||||||||
| 8 pesticides (IC01)a | ||||||||||||
| 10% | 1.65E-6 | (1.11E-6, 2.21E-6) | 2.12E-6 | (1.87E-6, 2.29E-6) | 5.47E-6 | (4.10E-6, 6.18E-6)* | ||||||
| 50% | 9.74E-6 | (7.91E-6, 1.07E-5) | 9.66E-6 | (9.00E-6, 1.03E-5) | 1.35E-5 | (1.20E-5, 1.46E-5)* | ||||||
| 8 pesticides (IC10)a | ||||||||||||
| 10% | 1.49E-6 | (1.23E-6, 1.85E-6) | 1.87E-6 | (1.64E-6, 2.03E-6) | 4.57E-6 | (3.46E-6, 5.26E-6)* | ||||||
| 50% | 8.79E-6 | (7.55E-6, 1.04E-5) | 9.13E-6 | (8.53E-6, 9.76E-6) | 1.32E-5 | (1.17E-5, 1.45E-5)* | ||||||
| 5 pesticides (IC01)a | ||||||||||||
| 10% | 1.03E-5 | (8.25E-6, 1.21E-5) | 6.73E-6 | (5.86E-6, 7.82E-6)* | 1.39E-5 | (1.13E-5, 1.66E-5) | ||||||
| 50% | 3.35E-5 | (2.95E-5, 3.78E-5) | 2.80E-5 | (2.58E-5, 3.09E-5) | 3.60E-5 | (3.28E-5, 4.10E-5) | ||||||
| 5 pesticides (IC10)a | ||||||||||||
| 10% | 8.97E-6 | (6.77E-6, 1.20E-5) | 6.38E-6 | (5.47E-6, 7.28E-6) | 1.33E-5 | (9.93E-6, 1.67E-5) | ||||||
| 50% | 3.54E-5 | (3.11E-5, 4.06E-5) | 2.89E-5 | (2.68E-5, 3.19E-5) | 3.77E-5 | (3.41E-5, 4.32E-5) | ||||||
| 13 pesticides (IC01)a | ||||||||||||
| 10% | 5.56E-6 | (4.38E-6, 7.39E-6) | 3.89E-6 | (3.55E-6, 4.16E-6)* | 1.38E-5 | (1.06E-5, 1.52E-5)* | ||||||
| 50% | 2.61E-5 | (2.38E-5, 2.95E-5) | 1.70E-5 | (1.61E-5, 1.80E-5)* | 3.11E-5 | (2.83E-5, 3.37E-5) | ||||||
| 13 pesticides (IC10)a | ||||||||||||
| 10% | 5.20E-6 | (3.47E-6, 7.28E-6) | 3.61E-6 | (3.24E-6, 3.88E-6) | 1.11E-5 | (8.24E-6, 1.27E-5)* | ||||||
| 50% | 2.86E-5 | (2.68E-5, 3.01E-5) | 1.71E-5 | (1.63E-5, 1.80E-5)* | 3.14E-5 | (2.81E-5, 2.81E-5) | ||||||
| 13 pesticides (IC20)a | ||||||||||||
| 10% | 3.25E-6 | (2.28E-6, 4.91E-6) | 3.48E-6 | (3.14E-6, 3.75E-6) | 1.01E-5 | (7.30E-6, 1.17E-5)* | ||||||
| 50% | 2.42E-5 | (2.09E-5, 2.89E-5) | 1.71E-5 | (1.63E-5, 1.80E-5)* | 3.06E-5 | (2.72E-5, 3.35E-5) | ||||||
| 13 pesticides (IC50)a | ||||||||||||
| 10% | 5.41E-6 | (3.70E-6, 7.44E-6) | 3.35E-6 | (2.99E-6, 3.64E-6)* | 9.14E-6 | (6.47E-6, 1.08E-5) | ||||||
| 50% | 3.24E-5 | (2.70E-5, 3.67E-5) | 1.75E-5 | (1.65E-5, 1.85E-5)* | 2.89E-5 | (2.57E-5, 3.20E-5) | ||||||
| aSee Supplemental Material, Table S1 (http://dx.doi.org/10.1289/ehp.1205391) for mixture ratios. *Statistically significant compared with observed ICs. Significance between predicted and observed ICX values was judged as a non-overlapping of their 95% percentile bootstrap CIs. | ||||||||||||
There was good agreement of the 8 mix responses with those predicted by CA over the entire concentration range and for both tested mixture ratios (Figure 1A,B, Table 1). This mixture was composed entirely of “pure” AR antagonists. However, CA consistently overestimated the combined effects of mixtures containing AR antagonists that also showed AR agonistic properties (5 mix, Figure 1C; 13 mix, Figure 1D). With these two mixtures, we observed androgenic activity at low concentrations when tested in the absence of DHT [see Supplemental Material, Table S2 (http://dx.doi.org/10.1289/ehp.1205391)]. When tested on their own, none of the individual pesticides in the mixtures showed AR agonistic effects at their concentration in the mixture. The androgenicity of 5 mix and 13 mix therefore appears to be a genuine combination effect. Indications for toxicity were detected only at high tested concentrations and are unlikely to interfere with the mixture assessment.
By performing Schild plot analysis with the pure antiandrogen flutamide and DHT, we were able to confirm competitive receptor antagonism. Increasing concentrations of this antagonist shifted the dose–response curve of the agonist DHT to the left [see Supplemental Material, Figure S1A,B (http://dx.doi.org/10.1289/ehp.1205391)], indicating that agonist and antagonist acted in a competitive manner at the same receptor site (i.e., the ligand-binding domain of the AR).
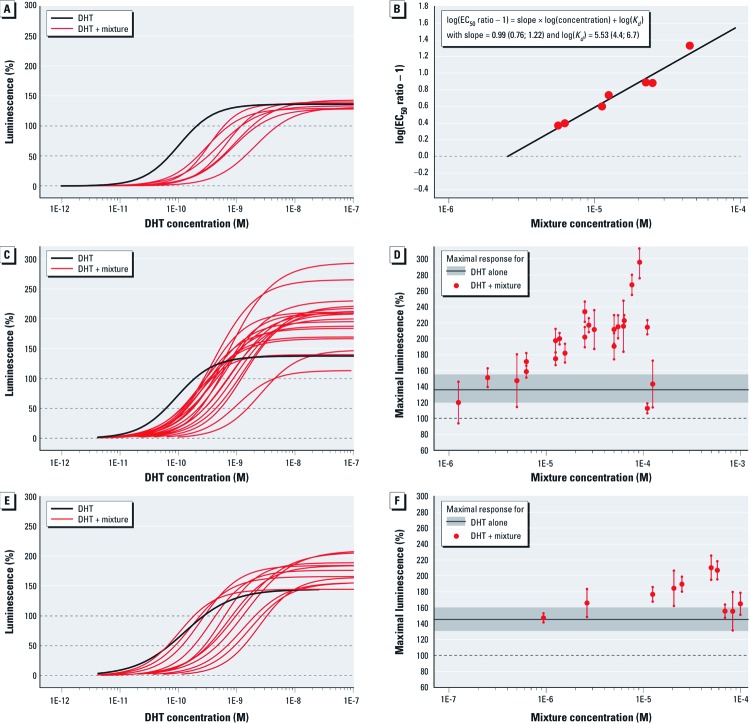
We obtained similar results with a Schild plot analysis of the 8 mix, which was composed of “pure” AR antagonists. Increasing concentrations of the 8 mix shifted the DHT curve progressively towards lower concentrations, without affecting the maximal response of the agonist. The resulting Schild plot was linear, suggesting that the observed AR antagonistic effect of the mixture was indeed due to competitive AR antagonism without being confounded by multiple binding sites or pharmacokinetic interactions (Figure 2A,B).
Figure 2.
Antiandrogenic activity of DHT in the presence of fixed mixture concentrations of 8 pesticides (IC10 mixture ratio, A); 5 pesticides (IC01 mixture ratio, C); and 13 pesticides (IC01 mixture ratio, E). (B) Schild regression plot for the mixture of 8 pesticides. (C,F) Estimated maximal effect levels (mean and 95% CI) in response to the 5‑ and 13-component mixtures, respectively; gray shading indicates the average maximal effect level (and 95% empirical confidence belt) of DHT alone on the basis of all experiments.
However, in the presence of the 5 mix and 13 mix, the maximal effects observed at saturating DHT concentrations rose far above the levels normally seen with the agonist on its own (i.e., “supramaximal” effects) (Figure 2C,E). These supramaximal DHT responses increased with rising mixture concentrations until 100 µM (5 mix) and 70 µM (13 mix). Beyond these concentrations, a downturn of responses was observed (Figure 2D,F). This downturn corresponded with the cytotoxicity values obtained by analysis of the test mixture in the absence of DHT and thus can be explained in terms of this mechanism. Supramaximal effects violate one basic assumption of the Schild plot analysis, namely that an antagonist should not influence the maximal response of the agonist. Therefore, Schild plots could not be constructed for the 5 mix and 13 mix. These results show that the suppression of DHT effects seen with these two mixtures are not solely due to competitive receptor antagonism and suggest that more complex processes are operational at the receptor.
To investigate whether stimulation of the maximal response with the 5 mix and DHT was the result of cell proliferation, we used a Renilla luciferase construct in our assay. This construct produces luminescence in proportion to cell number, independent of AR activation. There was no dose–response relationship between rising concentrations of the 5 mix and Renilla luminescence of the MDA-kb2 cells and no differences in luminescence between ethanol only (mean ± SD, 3,335 ± 896) and the DHT background concentration only (3,036 ± 756). The same applied to DHT (3,059 ± 689), the positive control procymidone (4,115 ± 820), and to any concentration of the 5 mix with DHT (110 µM: 2,198 ± 418; 55.5 µM: 1,909 ± 399; 27.5 µM: 2,080 ± 359; 13.75 µM: 2,340 ± 379) and the 5 mix on its own (150–5.6 µM: 2,134 ± 322) (data not shown). This indicates that cell proliferation was not the cause of the increased luminescence observed with this mixture and DHT, and that the supramaximal responses were the consequence of phenomena at the receptor.
Discussion
This is the first time that a mixture of widely used pesticides has been shown to produce combined AR antagonist effects that exceed the responses elicited by the most potent component alone. Furthermore, these mixture effects occurred in a quite predictable manner. The responses of the 8 mix composed of “pure” AR antagonists agreed very well with the combined effects predicted by CA. However, the combined effects seen with the two mixtures containing AR antagonists that also showed AR agonist properties (5 mix and 13 mix) were somewhat lower than anticipated by CA and fell between the “prediction window” boundaries defined by the predicted regression curves of CA and IA. These deviations are highly unlikely to be due to experimental artefacts because the mixtures were tested independently by different experimenters who prepared several independent mixture stock solutions.
By conducting Schild plot analysis, we were able to pinpoint competitive AR antagonism as a key factor that influenced agreement of the experimentally observed responses with the CA predictions. The combination of 8 “pure” AR antagonists (8 mix), well predicted by CA, produced Schild plots typical of competitive receptor antagonism. In contrast, anomalous supramaximal effects were observed with the 5 mix and 13 mix in experiments where increasing concentrations of these two mixtures were combined with DHT. These anomalies suggest that the 5 mix and 13 mix displaced DHT from the AR in ways not compatible with competitive antagonism. This allows us to infer that the lack of competitive AR antagonism is the likely cause of the observed deviations from CA. Chemicals that display mixed androgenic/antiandrogenic activity interact in ways with the amino acid residues in the AR binding domain that are distinct from those of “pure” AR antagonists (Tamura et al. 2006). Chemicals containing pyrimidine domains such as cyprodinil and pyrimethanil can cause AR antagonism via a non-ligand binding domain of the AR (Gunther et al. 2009). Pesticides of this kind formed a large proportion of the 5 mix and 13 mix, but were not present in the 8 mix. Although further mechanistic studies would be necessary to substantiate these ideas, we suggest that these modalities may play a role in the deviations from expected concentration additivity that we observed with the 5 mix and 13 mix. However, other explanations, such as stabilization of the AR-DNA binding complex or downstream effects of the signaling pathway may also be valid. In addition, there is some evidence that estrogenic supramaximal effects may be assay specific (Montaño et al. 2010), and although similar data are not available for AR antagonist assays, this is also a possible explanation for observed effects.
Deviations from expected additivity are interesting from a mechanistic viewpoint, but their relevance in relation to the application of CA or DA in risk assessment practice is not well defined. While it is reasonable that similarly acting pollutants should be assessed together, it is a matter for debate how chemicals should be grouped that do not match very narrowly defined criteria for similarity. Specifically, the question raised by our results is whether only “pure” AR antagonists that displace DHT in a competitive manner qualify for inclusion in groupings conforming to CA and whether, therefore, AR antagonists with AR agonistic properties should be excluded from joint assessments under CA principles. In resolving this issue, it is helpful to consider how combinations of AR antagonists behave in vivo. The applicability of CA for mixtures composed of AR antagonists was tested in a rat developmental toxicity model with flutamide, procymidone (both “pure” antagonists) and vinclozolin (which liberates metabolites that possess mixed AR antagonistic and agonistic properties) (Hass et al. 2007; Metzdorff et al. 2007). In these studies, the observed responses, including anogenital distance, reproductive organ weights, and prostate gene expression [PBP C3 (the prostate-specific binding protein polypeptide C3)] did not differ significantly from CA. For nipple retention, the observed response slightly exceeded the predicted mixture effects (Hass et al. 2007). Although it is not possible to arrive at firm conclusions based on these studies, it appears that the AR antagonist and agonist properties observed in vitro do have negligible impacts on the effects that are observed in vivo. A recent report has recommended that, despite the small deviations from CA that have sometimes been observed in vitro and in vivo, the evidence overwhelmingly suggests that it is a more accurate prediction model than IA (Kortenkamp et al. 2012). Furthermore, CA is a more conservative estimate of effect than IA, and thus CA would be protective for mixtures that fall in the “prediction window” (Kortenkamp et al. 2012). Therefore, we propose that CA is a suitable model for mixtures that contain AR antagonists with agonist properties, and that these chemicals should be grouped together with “pure” AR antagonists.
Early-life exposure is thought to be crucial for the development of abnormalities in male reproductive health (Skakkebaek et al. 2001). Fresh fruit and vegetables are consumed in high amounts by women and young children (Claeys et al. 2010), but these food items contain both the highest concentrations of single pesticides and the highest percentage with multiple residues (EFSA 2010; Inigo-Nunez et al. 2011, 87% of foodstuffs; Schiliro et al. 2011, 90% of foodstuffs). It is also interesting to note that a strong association between hypospadias and consumption of market fruit (odds ratio 5.10; CI: 1.31, 19.82) was reported for an agricultural population of Italy (Giordano et al. 2008). Fungicides were the most common pesticides detected in fruits and vegetables in several studies (Claeys et al. 2010; Inigo-Nunez et al. 2011; Schiliro et al. 2011), and they make up 9 of the 13 pesticides selected for testing in the present study. Although fungicides have broadly comparable use, as indicated by their global market share (22%) compared to herbicides (40%) and insecticides (29%) (Grube et al. 2011), fungicides are often applied post-harvest. As a result, their contribution to human exposures may well be higher than that of other pesticides. Furthermore, because of the rapidly evolving resistance of target organisms to fungicides, fungicides are recommended to be applied in mixtures for maximum effectiveness (Fungicide Resistance Action Committee 2010). For example, the commercial formulation “Switch” contains cyprodinil and fludioxonil (Syngenta 2011), “Forum” is composed of dithianon and dimethomorph (BASF 2010), “Justmeet” of fenhexamid and fludioxonil, and “Teldor Combi” contains fenhexamid and tebuconazole (Bayer 2011). There is a clear potential for human exposures via residues on foodstuffs, but to our knowledge, human biomonitoring data for the fungicides tested in this study are not available. There is also a lack of in vivo data for pesticides tested in this mixture (see Orton et al. 2011). Thus, it is currently not possible to ascertain the relationship between in vitro potency, in vivo effects, and exposure with adequate certainty, but such information is required if the risks to human health are to be properly assessed.
Conclusions
Widely used pesticides act additively in vitro as AR antagonists. The less accurate predictability of mixtures containing antagonists that also have agonist activity may due to distinct action at the ligand-binding domain of the AR. Despite the unknown pharmacological cause of deviation from CA, it should still be used for risk assessment because of the minimal deviation observed and the protective (worst-case) nature of CA. It is well known that people are exposed to mixtures of pesticides, and therefore the additive nature of these pollutants is a cause for concern.
Supplemental Material
Footnotes
This work was funded by the European Commission, FP7 programme (CONTAMED, grant 212502).
The authors declare they have no actual or potential competing financial interests.
References
- Andersen HR, Schmidt IM, Grandjean P, Jensen TK, Budtz-Jorgensen E, Kjaerstad MB, et al. Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environ Health Perspect. 2008;116:566–572. doi: 10.1289/ehp.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASF. BASF Launches Forum® Gold Fungicide for Grapes in Germany. 2010. Available: http://www.agro.basf.com/agr/AP-Internet/en/content/news_room/news/basf-launches-forum-gold-fungicide [accessed 30 September 2011]
- Bayer. Why Fungicides Matter to Us. 2011. Available: http://www.bayercropscience.com/bcsweb/cropprotection.nsf/id/Fungicides-products [accessed 30 September 2011]
- Birkhoj M, Nellemann C, Jarfelt K, Jacobsen H, Andersen HR, Dalgaard M, et al. The combined antiandrogenic effects of five commonly used pesticides. Toxicol Appl Pharmacol. 2004;201(1):10–20. doi: 10.1016/j.taap.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Blystone CR, Lambright CS, Furr J, Wilson VS, Gray LE. Iprodione delays male rat pubertal development, reduces serum testosterone levels, and decreases ex vivo testicular testosterone production. Toxicol Lett. 2007;174:74–81. doi: 10.1016/j.toxlet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Scholze M, Axelstad M, Boberg J, Kortenkamp A, Hass U. Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat. Int J Androl. 2008;31(2):241–247. doi: 10.1111/j.1365-2605.2008.00866.x. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, et al. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ Health Perspect. 2009;117:1839–1846. doi: 10.1289/ehp.0900689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys WL, Schmit J-F, Bragard C, Maghuin-Rogister G, Pussemier L, Schiffers B. Exposure of several Belgian consumer groups to pesticide residues through fresh fruit and vegetable consumption. Food Control. 2010;22(3–4):508–516. [Google Scholar]
- Cleveland WS, Devlin SJ, Grosse E. Regression by local fitting: methods, properties, and computational algorithms. J Econom. 1988;37(1):87–114. [Google Scholar]
- EFSA (European Food Safety Authority) 2008 Annual report on pesticide residues according to Article 32 of Regulation (EC) No 396/2005. EFSA J. 2010;8(7):1646–2088. [Google Scholar]
- EFSA (European Food Safety Authority) The 2009 European Union report on pesticide residues in food. EFSA J. 2011;9(11):2430–2655. [Google Scholar]
- Ermler S, Scholze M, Kortenkamp A. The sensitivity of the MDA-kb2 cell in vitro assay in detecting anti-androgenic chemicals—Identification of sources of variability and estimation of statistical power. Toxicol In vitro. 2010;24(6):1845–1853. doi: 10.1016/j.tiv.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Ermler S, Scholze M, Kortenkamp A. The suitability of concentration addition for predicting the effects of multi-component mixtures of up to 17 anti-androgens with varied structural features in an in vitro AR antagonist assay. Toxicol Appl Pharmacol. 2011;257(2):189–197. doi: 10.1016/j.taap.2011.09.005. [DOI] [PubMed] [Google Scholar]
- European Commission. Pesticide Index. 2011. Available: http://ec.europa.eu/sanco_pesticides/public/index.cfm [accessed 30 September 2011]
- Faust M, Altenburger R, Backhaus T, Blanck H, Boedeker W, Gramatica P, et al. Predicting the joint algal toxicity of multi-component s-triazine mixtures at low-effect concentrations of individual toxicants. Aqu Toxicol. 2001;56(1):13–32. doi: 10.1016/s0166-445x(01)00187-4. [DOI] [PubMed] [Google Scholar]
- Fungicide Resistance Action Committee. FRAC Recommendations for Fungicide Mixtures Designed to Delay Resistance Evolution. 2010. Available: http://www.frac.info/frac/publication/anhang/Resistance%20and%20Mixtures%20Jan2010_ff.pdf [accessed 19 May 2010]
- Gaspari L, Paris Fo, Jandel C, Kalfa N, Orsini M, Daurès JP, et al. 2011Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: a nested case–control study. Hum Reprod 26113155–3162. [DOI] [PubMed] [Google Scholar]
- Giordano F, Carbone P, Nori F, Mantovani A, Taruscio D, Figa-Talamancaa I. Maternal diet and the risk of hypospadias and cryptorchidism in the offspring. Paediatr Perinat Epidemiol. 2008;22(3):249–260. doi: 10.1111/j.1365-3016.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Pharmacol. 1994;129(1):46–52. doi: 10.1006/taap.1994.1227. [DOI] [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L. Pesticide Industry Sales and Usage: 2006 and 2007 Market Estimates. Washington DC:U.S. Environmental Protection Agency. 2011 Available: http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf [accessed 18 September 2012] [Google Scholar]
- Gunther JR, Parent AA, Katzenellenbogen JA. Alternative inhibition of androgen receptor signaling: peptidomimetic pyrimidines as direct androgen receptor/coactivator disruptors. ACS Chem Biol. 2009;4(6):435–440. doi: 10.1021/cb900043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, et al. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ Health Perspect. 2007;115(suppl 1):122–128. doi: 10.1289/ehp.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105(1):153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Inigo-Nunez S, Herreros MA, Encinas T, Gonzalez-Bulnes A. Estimated daily intake of pesticides and xenoestrogenic exposure by fruit consumption in the female population from a Mediterranean country (Spain). Food Control. 2011;21(4):471–477. [Google Scholar]
- Kenakin TP. Pharmacologic Analysis of Drug-Receptor Interaction. 2nd ed. New York:Raven Press 1993 [Google Scholar]
- Kjærstad MB, Taxvig C, Andersen HR, Nellemann C. Mixture effects of endocrine disrupting compounds in vitro. Int J Androl. 2010;33(2):425–433. doi: 10.1111/j.1365-2605.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect. 2004;112:524–531. doi: 10.1289/ehp.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner W, Vinggaard AM, Terouanne B, Ma R, Wieloch C, Shlumpf M, et al. Interlaboratory comparison of four in vitro assays for assessing androgenic and antiandrogenic activity of environmental chemicals. Environ Health Perspect. 2004;112:695–702. doi: 10.1289/ehp.112-1241964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect. 2007;115(Suppl 1):98–105. doi: 10.1289/ehp.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A, Evans R, Faust M, Kalberlah F, Scholze M, Schuhmacher-Wolz U. Investigation of the State of the Science on Combined Actions of Chemicals in Food through Dissimilar Modes of Action and Proposal for Science-based Approach for Performing Related Cumulative Risk Assessment. Supporting Publications 2012:EN-232. 2012 Available: http://www.efsa.europa.eu/en/supporting/doc/232e.pdf [accessed 18 September 2012] [Google Scholar]
- Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC, et al. Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol Sci. 2000;56(2):389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- Metzdorff SB, Dalgaard M, Christiansen S, Axelstad M, Hass U, Kiersgaard MK, et al. Dysgenesis and histological changes of genitals and perturbations of gene expression in male rats after in utero exposure to antiandrogen mixtures. Toxicol Sci. 2007;98(1):87–98. doi: 10.1093/toxsci/kfm079. [DOI] [PubMed] [Google Scholar]
- Montaño M, Bakker EJ, Murk AJ. Meta-analysis of supramaximal effect in in vitro estrogenicity assays. Tox Sci. 2010;115(2):462–474. doi: 10.1093/toxsci/kfq056. [DOI] [PubMed] [Google Scholar]
- Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicol Sci. 2003;71(2):251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- Orton F, Lutz I, Kloas W, Routledge EJ. Endocrine disrupting effects of herbicides and pentachlorophenol: in vitro and in vivo evidence. Environ Sci Technol. 2009;43(6):2144–2150. doi: 10.1021/es8028928. [DOI] [PubMed] [Google Scholar]
- Orton F, Rosivatz E, Scholze M, Kortenkamp A. Widely used pesticides with previously unknown endocrine activity revealed as in vitro antiandrogens. Environ Health Perspect. 2011;119:794–800. doi: 10.1289/ehp.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE. The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol Ind Health. 1999;15(1–2):80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RFA. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case–control study in newborn boys. Environ Health Perspect. 2004;112:1570–1576. doi: 10.1289/ehp.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CV, Furr J, Wilson VS, Gray LE. A mixture of seven antiandrogens induces reproductive malformations in rats. Int J Androl. 2008;31(2):249–262. doi: 10.1111/j.1365-2605.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR, et al. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicol Pathol. 2009;37(1):100–113. doi: 10.1177/0192623308329478. [DOI] [PubMed] [Google Scholar]
- Rocheleau CM, Romitti PA, Dennis LK. Pesticides and hypospadias: a meta-analysis. J Pediatr Urol. 2009;5(1):17–24. doi: 10.1016/j.jpurol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiliro T, Gorrasi I, Longo A, Coluccia S, Gilli G. Endocrine disrupting activity in fruits and vegetables evaluated with the E-screen assay in relation to pesticide residues. J Steroid Biochem Mol Biol. 2011;127(1–2):139–146. doi: 10.1016/j.jsbmb.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Scholze M, Boedeker W, Faust M, Backhaus T, Altenburger R, Grimme LH. A general best-fit method for concentration-response curves and the estimation of low-effect concentrations. Environ Toxicol Chem. 2001;20(2):448–457. [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM.2001Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. APMIS 109: S22–S28. [DOI] [PubMed] [Google Scholar]
- Syngenta. Switch®. 2011. Available: http://www.syngentacropprotection.com/prodrender/index.aspx?prodid = 757&ProdNM=Switch 62.5WG [accessed 30 September 2011]
- Tamura H, Ishimoto Y, Fujikawa T, Aoyama H, Yoshikawa H, Akamatsu M. Structural basis for androgen receptor agonists and antagonists: Interaction of SPEED 98-listed chemicals and related compounds with the androgen receptor based on an in vitro reporter gene assay and 3D-QSAR. Bioorg Med Chem. 2006;14(21):7160–7174. doi: 10.1016/j.bmc.2006.06.064. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Pesticide Product Information System (PPIS). 2011. Available: http://www.epa.gov/pesticides/PPISdata/index.html [accessed 1 November 2011]
- Vinggaard AM, Christiansen S, Laier P, Poulsen ME, Breinholt V, Jarfelt K, et al. Perinatal exposure to the fungicide prochloraz feminizes the male rat offspring. Toxicol Sciences. 2005;85(2):886–897. doi: 10.1093/toxsci/kfi150. [DOI] [PubMed] [Google Scholar]
- Vonesh E, Chinchilli VM. NewYork: Marcel Dekker; 1996. Linear and Nonlinear Models for the Analysis of Repeated Measurements. [Google Scholar]
- Wilson VS, Bobseine K, Lambright CR, Gray LE. A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci. 2002;66(1):69–81. doi: 10.1093/toxsci/66.1.69. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, Andersen HR, Jensen TK, Grandjean P, Skakkebæk NE, Main KM. Smaller genitals at school age in boys whose mothers were exposed to non-persistent pesticides in early pregnancy. Int J Androl. 2012;35:265–272. doi: 10.1111/j.1365-2605.2012.01252.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.