Abstract
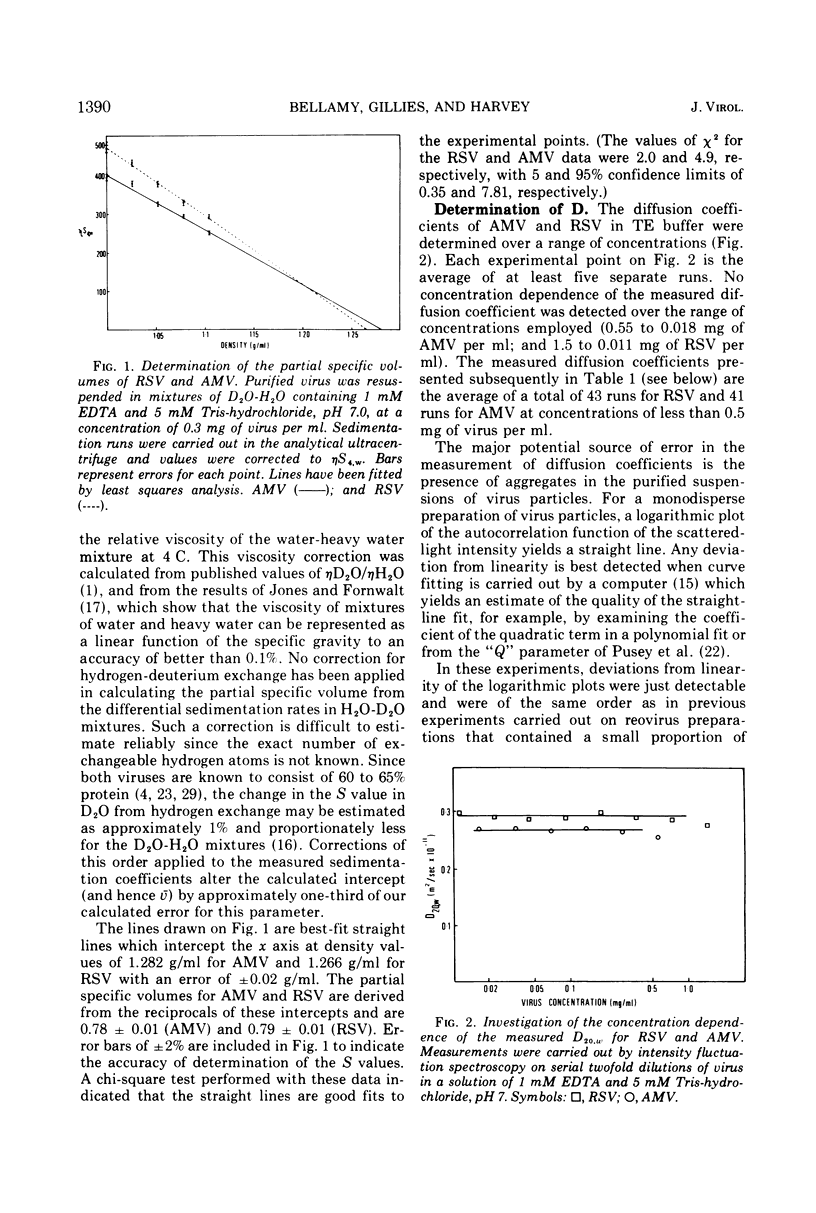
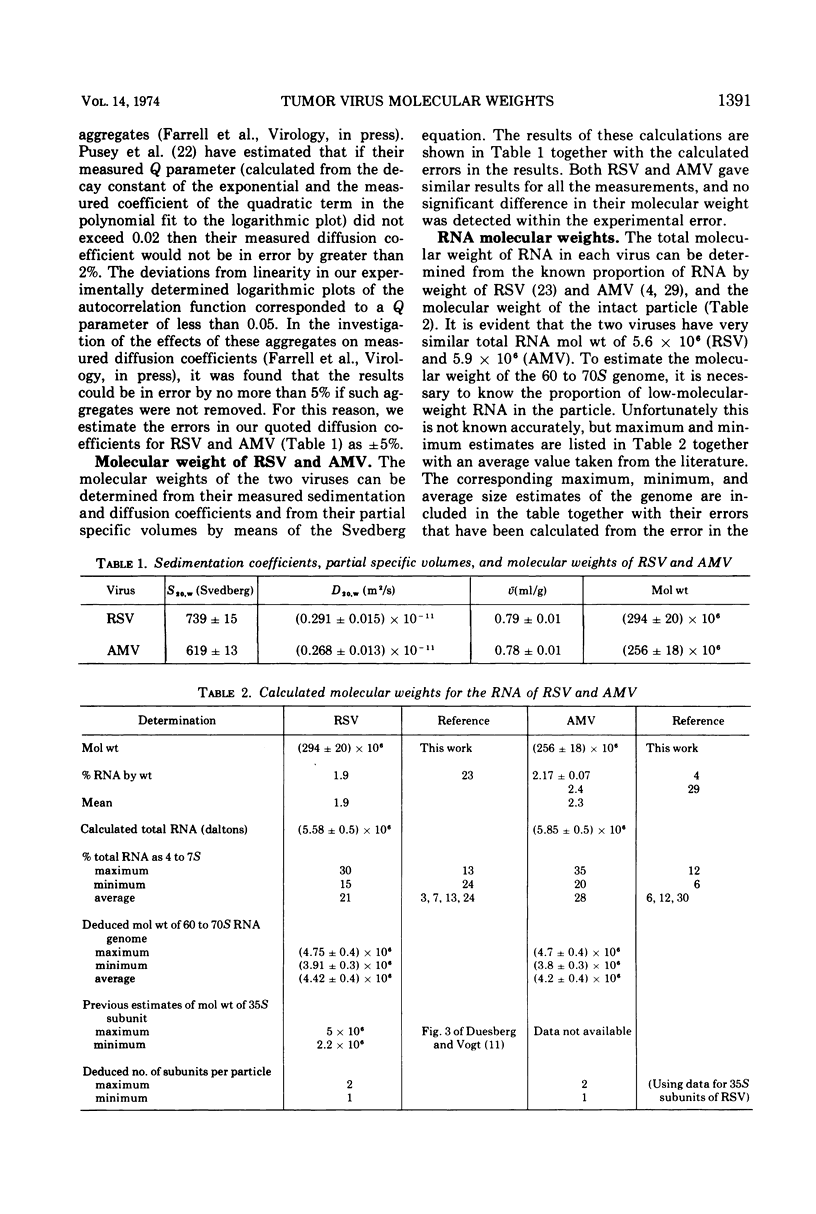
Sedimentation analysis and intensity fluctuation spectroscopy have been used in conjunction with the Svedberg equation to determine the particle molecular weights of Rous sarcoma virus (Prague strain) and avian myeloblastosis virus (BAI strain). The molecular weights of these two viruses are (294 ± 20) × 106 and (256 ± 18) × 106, respectively. Values for the molecular weight of the RNA contained in each particle have been calculated as (5.58 ± 0.5) × 106 and (5.88 ± 0.5) × 106. Since the proportion of the viral RNA represented by 4 to 7S low-molecular-weight material is known, the molecular weight of the 60 to 70S genomes may be calculated to lie in the range (3.8 ± 0.3 to 4.8 ± 0.4) × 106 for both particles. These estimates for the molecular weight of the 60 to 70S genome are much lower than previous estimates and fall within the range of current estimates of the size of a single 35S subunit. The implications of this finding are discussed in terms of current theories for the structure of the genome of RNA tumor viruses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONAR R. A., BEARD J. W. Virus of avian myeloblastosis. XII. Chemical constitution. J Natl Cancer Inst. 1959 Jul;23(1):183–197. [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Sullivan D., Fanshier L., Quintrell N., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. II. The 7 S RNA. Virology. 1970 Dec;42(4):927–937. doi: 10.1016/0042-6822(70)90341-7. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Pusey P. N., Koppel D. E., Schaefer D. W., Franklin R. M. Intensity fluctuation spectroscopy of laser light scattered by solutions of spherical viruses: R17, Q beta, BSV, PM2, and T7. II. Diffusion coefficients, molecular weights, solvation, and particle dimensions. Biochemistry. 1974 Feb 26;13(5):960–970. doi: 10.1021/bi00702a021. [DOI] [PubMed] [Google Scholar]
- Carnegie J. W., Deeney A. O., Olson K. C., Beaudreau G. S. An RNA fraction from myeloblastosis virus having properties similar to transfer RNA. Biochim Biophys Acta. 1969 Oct 22;190(2):274–284. doi: 10.1016/0005-2787(69)90079-3. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin S. B., Benedek G. B., Bancroft F. C., Freifelder D. Molecular weights of coliphages and colip- hage DNA. II. Measurement of diffusion coefficients using optical mixing spectroscopy, and measurement of sedimentation coefficients. J Mol Biol. 1970 Dec 28;54(3):547–556. doi: 10.1016/0022-2836(70)90125-7. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Isolation of amino acid acceptor RNA from purified avian myeloblastosis virus. J Mol Biol. 1970 Sep 14;52(2):387–390. doi: 10.1016/0022-2836(70)90038-0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granboulan N., Huppert J., Lacour F. Examen au microscope electronique du RNA du virus de la myeloblastose aviaire. J Mol Biol. 1966 Apr;16(2):571–575. doi: 10.1016/s0022-2836(66)80196-1. [DOI] [PubMed] [Google Scholar]
- Harvey J. D. Diffusion coefficients and hydrodynamic radii of three spherical RNA viruses by laser light scattering. Virology. 1973 Nov;56(1):365–368. doi: 10.1016/0042-6822(73)90313-9. [DOI] [PubMed] [Google Scholar]
- Hvidt A., Nielsen S. O. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386. doi: 10.1016/s0065-3233(08)60129-1. [DOI] [PubMed] [Google Scholar]
- Kakefuda T., Bader J. P. Electron microscopic observations on the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):460–474. doi: 10.1128/jvi.4.4.460-474.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luborsky S. W. Sedimentation equilibrium analysis of the molecular weight of a tumor virus RNA. Virology. 1971 Sep;45(3):782–787. doi: 10.1016/0042-6822(71)90195-4. [DOI] [PubMed] [Google Scholar]
- Pusey P. N., Koppel D. E., Schaefer D. W., Camerini-Otero R. D., Koenig S. H. Intensity fluctuation spectroscopy of laser light scattered by solutions of spherical viruses: R17, Q beta, BSV, PM2, and T7. I. Light-scattering technique. Biochemistry. 1974 Feb 26;13(5):952–960. doi: 10.1021/bi00702a020. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Reich E. Phospholipid composition of Rous sarcoma virus, host cell membranes and other enveloped RNA viruses. Virology. 1971 Oct;46(1):106–116. doi: 10.1016/0042-6822(71)90010-9. [DOI] [PubMed] [Google Scholar]
- SHARP D. G., BEARD J. W. Virus of avian erythromyeloblastic leukosis. IV. Sedimentation, density and hydration. Biochim Biophys Acta. 1954 May;14(1):12–17. doi: 10.1016/0006-3002(54)90124-9. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg K., Gantt R., Wilson S. H. Structural studies on avian myeloblastosis virus: conditions for isolation and biochemical characteristics of the core component. Biochim Biophys Acta. 1973 Mar 30;304(1):1–11. doi: 10.1016/0304-4165(73)90109-8. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Hurley N. E., Davis N. L., Rueckert R. R., Fleissner E. Structural studies of avian myeloblastosis virus: comparison of polypeptides in virion and core component by dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):513–528. doi: 10.1128/jvi.13.2.513-528.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware B. R., Raj T., Flygare W. H., Lesnaw J. A., Reichmann M. E. Molecular weights of vesicular stomatitis virus and its defective particles by laser light-scattering spectroscopy. J Virol. 1973 Jan;11(1):141–145. doi: 10.1128/jvi.11.1.141-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



