Abstract
On the way towards a sustainable low-carbon future, the design and construction of chemical or physical adsorbents for CO2 capture and clean energy storage are vital technology. The incorporation of accessible nitrogen-donor sites into the pore walls of porous adsorbents can dramatically affect the CO2 uptake capacity and selectivity on account of the dipole-quadrupole interactions between the polarizable CO2 molecule and the accessible nitrogen site. In the present work, a nitrogen-rich rth-type metal-organic framework (MOF) was constructed based on rational design and careful synthesis. The MOF presents exceptionally high uptake capacity not only for CO2 but also for H2, which is attributed to favorable interactions between the gas molecules and the nitrogen-rich triazole units of the MOF proved by both experimental measurements and theoretical molecular simulations.
Owing to their permanent porosity, high surface area, large pore volume, and adjustable pore size and shape, metal-organic frameworks (MOFs) have been extensively investigated in the past decades and have shown highly promising applications in gas storage, molecular recognition and separation, heterogeneous catalysis, and biomedical area1,2,3,4,5,6,7. Typically, MOFs are built by the self-assembly of metal ions or clusters and polytopic bridging ligands under solvothermal conditions6,7. In contrast to inorganic porous materials such as zeolites or activated porous carbons, numerous choices of organic linkers together with many coordination geometries indicate that MOFs could be tailored by judicious combinations of the building blocks for specific applications6,7,8,9. Theoretically, the incorporation of accessible nitrogen-donor groups, such as pyridine, imidazole, and tetrazole, into the pore walls of porous materials can dramatically affect the gas uptake capacity and selectivity of the materials, especially for CO2 capture on account of the dipole-quadrupole interactions between the polarizable CO2 molecule and the accessible nitrogen site10. Literature reports also indicated that the incorporation of accessible nitrogen-donor groups into the porous materials could enhance the CO2 uptake capacity and selectivity11,12,13,14,15,16. Such approach is strategically important for developing a low-carbon future by increasing the capacity of selective CO2 capture and by enhancing the storage capacity of clean energy source, such as H21,9,11,12,13,14,15,16,17,18. However, competitive coordination of these Lewis basic nitrogen sites with metal ions or clusters is a great challenge in direct synthesis of MOFs19,20.
Recently, rational design and synthesis of MOFs with target superstructures and desired physical properties have been significantly facilitated by the conceptual approach of reticular chemistry21,22, where in-situ generated metal-cluster secondary building units (SBUs) are connected by decorated organic linkers in a predetermined ordered network, as exemplified in a series of isoreticular IRMOF-n materials derived from prototypical MOF-5 superstructure. As an extension of the SBU approach, metal-organic polyhedra (MOPs) with large size as well as high symmetry and connectivity have recently been employed as supermolecular building blocks (SBBs) for the constructions of novel MOFs23,24,25. This strategy offers an improved control over the topology in the preparation of MOFs. One of the most investigated MOPs is rhombicuboctahedron with a formula [Cu2(BDC)2S2]12 (S = solvent, BDC = 1,3-benzenedicarboxylate) consisting of 12 dicopper paddlewheel SBUs jointed by 24 1,3-BDC linkers25,26,27,28. The 24 vertices from the 5-position of each BDC linker could be decorated to give rise to discrete polyhedra, chains, or networks29,30,31,32,33. Of particular interest among the networks derived from rhombicuboctahedron is (3,24)-connected rth-topology, where triangular metal clusters or triangular organic moieties are employed to rigidly cross-link three BDC ligands24,34,35,36,37,38,39,40,41. Such isoreticular MOFs with rth-topology possess a series of specific advantages, including extra-high surface area and pore volume with robust networks, the absence of framework interpenetration, and high concentration of open-metal sites upon the removal of axially coordinated solvent molecules. These salient features enable them to serve as promising porous materials for gas storage and separation.
The modular nature of the rth-topology provides an ideal platform for the development of novel MOF materials with the same topology through rational combinations of such well-defined SBBs with suitable ligands that consist of three coplanar BDC units having an overall C3-symmetry (Figure S1 in the Supplementary Information (SI))34,35,36,37,38,39,40,41. In order to introduce nitrogen-rich units into highly porous MOFs by adopting the SBB strategy for enhancing the gas uptake capacity, a C3-symmetric triazole-containing dendritic hexacarboxylate linker, i.e., 5,5',5''-(4,4',4''-(benzene-1,3,5-triyl) tris(1H-1,2,3-triazole-4,1-diyl))triisophthalic acid (H6-1 in Figure 1), was designed and subsequently, a nitrogen-rich rth-MOF (NTU-105) incorporated with coordination-free triazole units was successfully synthesized. Herein, we present the successful fabrication of the nitrogen-rich rth-MOF, NTU-105, which shows high stability and large porosity and, as expected, exhibits significantly enhanced gas uptake capacity towards CO2 and H2 in comparison with its isoreticular analogues. The roles of the nitrogen-rich triazole units in the framework of NTU-105 for enhanced gas uptake capacity were demonstrated by both experimental measurements and theoretical molecular simulations.
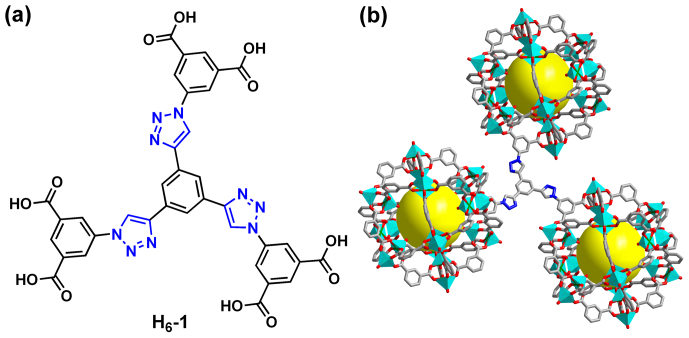
Figure 1.
(a) Chemical structure of ligand H6-1 and (b) crystal structure of (3,24)-connected rth-type framework of NTU-105. In the crystal structure, carbon atoms are colored in gray, nitrogen atoms are colored in blue, oxygen atoms are colored in red, copper atoms are colored in green, and hydrogen atoms and solvent molecules were omitted for sake of clarity.
Results
Synthesis of NTU-105
Based on rational design, the dendritic hexacarboxylate ligand H6-1 was conveniently synthesized in high yield by the reaction between 1,3,5-triethynylbenzene and pre-synthesized di-tert-butyl 5-azidoisophthalate through click chemistry, i.e. copper(I)-catalyzed azide-alkyne cycloaddition42, followed by deprotection in trifluoroacetic acid (see details in the SI). Here, tert-butyl group in di-tert-butyl 5-azidoisophthalate was introduced in order to increase the solubility of the precursor compound. For the construction of a MOF without the competitive coordination between these Lewis basic nitrogen sites and metal ions, drops of HNO3 were added in the reaction system. After solvothermal reaction of ligand H6-1 with Cu(NO3)2·3H2O in N,N-dimethylformamide (DMF) at 75°C for 3 days, blue crystals of NTU-105 were obtained and characterized (Figures S2 and S3 in the SI).
Structural characterization
Single-crystal X-ray diffraction investigations revealed that NTU-105 was crystallized in tetragonal system with space group I4/m, having the framework formula of [Cu3(1) (H2O)3]n (Table S1 in the SI). As expected, in the crystal structure, each organic linker 1 connects with three 24-connected SBBs while leaving the triazole moieties free of coordination (Figures 1 and S4–S6 in the SI). Each SBB consists of 12 dicopper paddlewheels jointed by 24 1,3-BDC linkers34,35,36,37,38,39,40,41. NTU-105 shares the same (3,24)-connected network as prototypical rht-type MOF and isoreticular NOTT-112 and PCN-6X34,35,36,37. In addition, there are three types of cages within NTU-105, namely, cuboctahedron (cub-Oh), truncated tetrahedron (T-Td), and truncated octahedron (T-Oh), with inner sphere diameters of about 12, 15, and 20 Å, respectively (Figures 2 and S5 in the SI). Powder X-ray diffraction (PXRD) measurements confirmed high phase purity and crystallinity of the bulk sample of as-synthesized NTU-105 (Figure S7 in the SI).
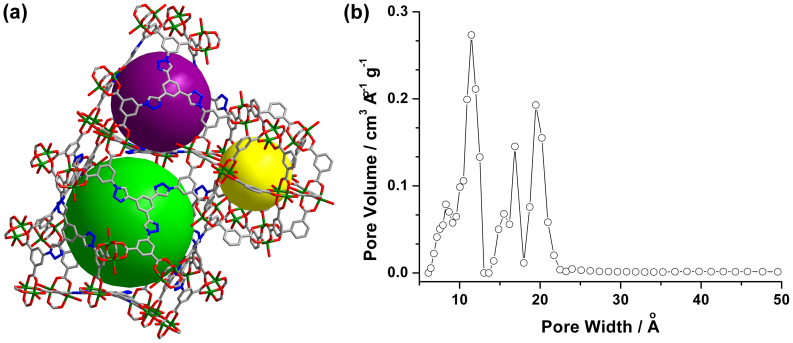
Figure 2.
(a) Three different cages, indicated as yellow, purple, and green balls, in the crystal structure of NTU-105, and (b) pore-size distribution plot calculated from experimental Ar adsorption isotherm using the nonlocal density functional theory (NLDFT).
Chemical and thermal stability
The as-synthesized crystals of NTU-105 are insoluble in common organic solvents including dichloromethane, chloroform, acetone, acetonitrile, toluene, DMF, and dimethyl sulfoxide (DMSO). The solvent molecules in NTU-105 can be easily exchanged by some organic solvents such as dichloromethane. Thermogravimetric analysis (TGA) was used to verify the thermal stability of NTU-105 (Figure S8 in the SI). The as-synthesized crystalline samples lost the solvent molecules below 260°C in N2 flow. When using the desolvated sample of NTU-105 instead of the as-synthesized NTU-105, only a plateau was observed from 80 to 260°C in the TGA profile, indicating there was nearly no weight loss. Both samples started to decompose rapidly to produce CuO above 260°C. The desolvated sample of NTU-105 is stable in dry environment, and the structural integrity of the framework is retained as verified by PXRD (Figure S7 in the SI).
Porosity
After removing the guest solvents and coordinated water molecules, the total solvent-accessible volume of desolvated NTU-105 is estimated to be 75.7% using the PLATON/VOID routine43, and the calculated density of the desolvated framework is 0.598 g cm−3. To confirm the porosity, NTU-105 was activated sequentially under vacuum at 60 and 120°C, after exchanging the solvent with dichloromethane. A color change from green-blue to deep purple-blue was observed, which is a typical phenomenon for dicopper-based paddlewheel frameworks where the open CuII sites were generated during the activation process. The PXRD pattern of the desolvated sample matches very well with the calculated pattern based on the crystal data (Figure S7 in the SI), indicating the retention of the framework after the activation. Then, nitrogen sorption at 77 K was carried out for the activated NTU-105, exhibiting a reversible pseudo-type I sorption isotherm with a quickly increased step prior to the plateau (Figure S9 in the SI)44. The observations indicate that NTU-105 possesses both micro- and mesopores24,34,35. The overall nitrogen uptake is 861 cm3 g−1 at 1 atm. The Brunauer-Emmett-Teller (BET) surface area was calculated to be 3543 m2 g−1 (Figure S9 in the SI). The total pore volume calculated from nitrogen isotherm is 1.33 cm3 g−1. The pore size distribution, calculated from the analysis of argon sorption isotherm at 87 K based on the non-local density functional theory, reveals peaks at 11, 17, and 19 Å respectively (Figures 2 and S10 in the SI), consistent well with the dimensions of the cages determined in the crystal structure of NTU-105, indicating that the desolvated NTU-105 presents high porosity.
Experimental and theoretical CO2 uptake capability
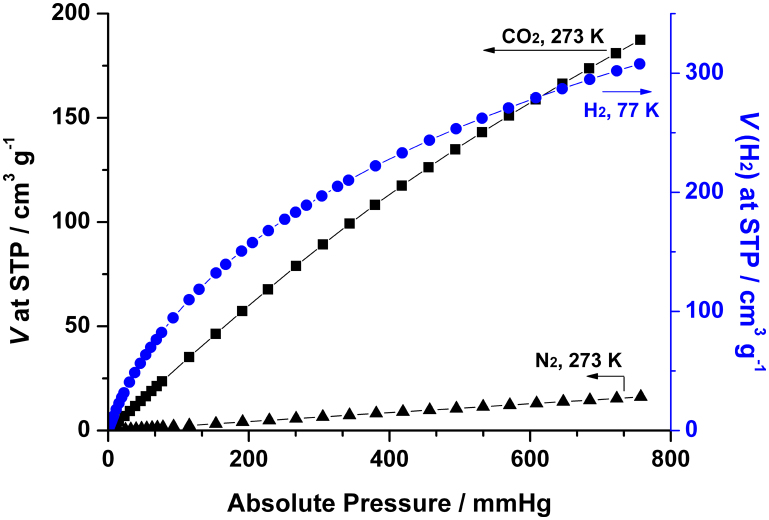
The high porosity and stability together with the coordination-free triazole moieties and open metal sites are favorable to the interactions between gas molecules and the framework of NTU-105, which inspired us to investigate its gas uptake capacity and selectivity towards CO2. As shown in Figure 3, CO2 sorption isotherms reveal an uptake capability of NTU-105 as high as 187 cm3 g−1 (36.7 wt%) at 1 atm and 273 K, which makes NTU-105 a top MOF material for CO2 uptake reported to date17,18, including its isoreticular MOFs containing acylamide groups, amine units, or triazine moieties (i.e., 42.6% for Cu-TPBTM39 and 44.5% for Cu-TDPAT41). For comparison, the N2 uptake of NTU-105 at 273 K and 1 atm was measured to be only 16 cm3 g−1. The high adsorption selectivity of NTU-105 towards CO2 over N2 under the same conditions (Figure 3) indicates that NTU-105 is highly applicable in the separation of CO2 over N2. Then, the isosteric heat of adsorption (Qst) for CO2 was calculated based on the adsorption isotherms at different temperatures (273 and 298 K, Figure S11 in the SI) through Clausius-Clapeyron equation17. It was found that Qst is ~35 kJ mol−1 at low loading range, followed by the convergence into a pseudo-plateau (~24 kJ mol−1) with relatively high uptake, the observations which are indicative of strong CO2-framework interactions. The Qst value at low loading range is the second highest one among the reported rth-MOFs, while Cu-TDPAT presents the highest value (42.2 kJ mol−1) so far41.
Figure 3. Gas adsorption isotherms of activated NTU-105 for CO2 and N2 measured at 273 K, and H2 measured at 77 K.
The remarkable CO2-framework interactions were further supported by molecular simulation studies (Figure 4 and S12–S14 in the SI). In order to estimate the charges of the atoms in NTU-105, density functional theory (DFT) calculation was performed in a fragmental cluster shown in Figure 4, and the Lee-Yang-Parr correlation function was employed with Gaussian 0345. To investigate the interactions between the adsorbate and the framework, radial distribution functions g(r) were calculated by:
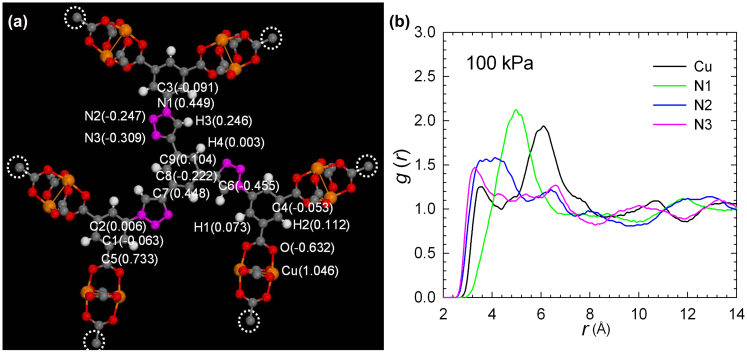
where r is the distance between atoms i and j, Nij(r, r + Δr) is the number of atom j around i within a shell from r to r + Δr, V is the system volume, and Ni and Nj are the numbers of atoms i and j, respectively. Figure 4 and Figure S14 show the radial distribution functions of CO2 around Cu and N1, N2 and N3 atoms on the triazole ring at 1 kPa, 10 kPa and 100 kPa, respectively. Under the pressure range from low to high values (e.g. from 1–100 kPa), all of the three N atoms from the coordination-free triazole unit show high affinities with CO2. In particular, the N1 atom that is closer to metal cluster shows the strongest interaction with CO2 than those of the N2 and N3 atoms. From the simulation studies, not only the open CuII sites within NTU-105 as in other isoreticular rth-MOFs, but also the introduced nitrogen-rich triazole units were identified as the preferential interaction sites for CO2. Thus, the obtained results clearly demonstrate that the coordination-free nitrogen-rich triazole moieties and open metal sites within the framework serve as strong interaction sites, mainly responsible for the high and selective CO2 uptake.
Figure 4.
(a) Atomic charges in a fragmental cluster of NTU-105. The dangling bonds, indicated by white circles, were terminated by hydrogen atoms. Color code: Cu, orange; O, red; N, pink; C, grey; H, white. (b) Radial distribution functions of CO2 around Cu, N1, N2 and N3 atoms at 100 kPa.
H2 uptake capacity
A further application of NTU-105 for the uptake of clean energy, H2, was investigated. The desolvated NTU-105 exhibits an exceptionally high H2 uptake capacity up to 308 cm3 g−1 (2.75 wt%) at 77 K and 1 atm (Figure 3), which is the highest value at low pressures in comparison with other isoreticular rth-MOFs (Table S2 in the SI)46,47. The isosteric heat of adsorption (Qst) for H2 was calculated from the isotherms at 77 and 87 K by using Clausius-Clapeyron equation (Figure S15 in the SI)46,47. The Qst value at low coverage was estimated to be 6.61 kJ mol−1, and it was gradually reduced to 5.07 kJ mol−1 upon increasing the coverage. The present average Qst value is similar to the average isosteric heat (~6.1 kJ mol−1) of H2 uptake in the case of HKUST-148. In the low-pressure region, the H2 uptake capacity is mainly controlled by its affinity with the framework. Taking into account of the structural similarity and differences with other rth-MOFs, the remarkable H2 uptake capacity of NTU-105 should be attributed to the synergistic effect of the coordination-free nitrogen-rich triazole units and opening metal sites towards H2 molecules. In addition, the H2 uptake isotherms predicted from computational simulations match fairly well with the experimental measurements (Figure S13 in the SI).
Discussion
Based on the understanding of isoreticular MOFs along with the concept of supermolecular building blocks, a dendritic nitrogen-rich hexacarboxylate ligand has been designed and synthesized by utilizing click chemistry, which has been employed to construct a (3,24)-connected MOF (NTU-105) with the rth-type topology. As compared with reported amino-functionalized MOFs in literature11,12,13,14,15,16,39,41, this is the first time that nitrogen-rich triazole units are successfully introduced onto the struts of an rth-type MOF targeted for CO2 capture and storage. On account of the easy and efficient approach of using click chemistry in the preparation of triazole-containing MOF for selective gas adsorption, one can envisage that more delicate nitrogen-rich MOFs as efficient adsorbents could be developed.
Comparing with some of reported isoreticular MOFs34,35,36,37,38,39,40,41, the present nitrogen-rich NTU-105 possesses the BET surface area of only 3543 m2 g−1, but exhibits exceptionally high CO2 (36.7 wt% at 273 K and 1 atm) and H2 (2.75 wt% at 77 K and 1 atm) uptake capacities. The simulation studies have confirmed that the coordination-free triazole moieties and the open metal sites within the framework provide strong interactions with the gas molecules, thus leading to significantly enhanced gas update capacity and selectivity. Since the gas uptake capacity of porous materials is not always proportional to their surface areas, engineering nitrogen-rich organic units within porous materials to enhance the adsorption affinity towards specific gas molecules, especially CO2, would be one of the ideal and convenient choices for gas adsorption and selective separation on the way towards a sustainable future.
In summary, we have designed and synthesized a (3,24)-connected nitrogen-rich rth-MOF. The nitrogen-rich MOF exhibits high thermal stability and large porosity along with exceptionally high CO2 and H2 uptake capacities. Both the experimental measurements and theoretical molecular simulations have indicated that, although the BET surface area of nitrogen-rich MOF is not significantly high as compared with some of its isoreticular MOFs34,35,36,37,38,39,40,41, the incorporation of accessible nitrogen-donor sites into the framework dramatically enhances its gas uptake capacity and selectivity.
Methods
Synthesis of H6-1
In a degassed THF/H2O (100 mL/40 mL) solution containing 1,3,5-triethynylbenzene (0.21 g, 1.40 mmol) and di-tert-butyl 5-azidoisophthalate (1.55 g, 4.85 mmol), CuSO4 (0.11 g, 0.44 mmol) and sodium ascorbate (0.17 g, 0.86 mmol) were added and the mixture solution was stirred at 60°C under Ar for 3 days. Ester tBu6-1 (1.10 g, 0.99 mmol, yield: 71%) was obtained after purifying the crude product through silica gel flash column chromatography (CH2Cl2/EtOAc, 100/1). Then, trifluoroacetic acid (TFA, 10 mL) was added to a CH2Cl2 solution (20 mL) of tBu6-1 (1.23 g, 1.11 mmol), and mixture solution was stirred at room temperature over 5 h. The suspension was filtered and washed with CH2Cl2, and the obtained solid was dried under vacuum to afford the compound H6-1 (0.73 g, 0.95 mmol, yield: 86%) as white solid. 1H NMR (500 MHz, DMSO) δ 9.64 (s, 3H), 8.68 (s, 6H), 8.53 (s, 3H), 8.48 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.6, 147.0, 136.6, 132.8, 130.8, 129.1, 122.9, 121.6, 119.0. ESI-TOF-HRMS: m/z calcd for C36H22N9O12: 772.1388, found: 772.1384 [M+H]+.
Synthesis of NTU-105
Compound H6-1 (21 mg, 0.03 mmol) and Cu(NO3)2·3H2O (22 mg, 0.09 mmol) were dissolved in DMF (12 mL). Then, drops of HNO3 (1.5 mol L−1, 60 drops) were added into the solution. The mixture solution was placed in a tightly capped 20 mL vial, which was heated in an oven at 75°C for 3 days. The light blue block crystals of NTU-105 were collected after cooling the sample down to room temperature. These crystals were washed by fresh DMF for 3 times, and dried under vacuum. The obtained crystals were subjected to elemental analysis and TGA analysis in order to estimate the empirical formula as Cu3(1)(H2O)3·11DMF·8H2O (44.8 mg, yield: 76% based on H6-1). Elemental analysis calcd (%) for Cu3C69H120N20O34: C 42.19, H 6.16, N 14.26; found: C 42.17, H 6.48, N 14.33. IR (KBr) (cm−1): 1652 (s), 1583 (m), 1428 (m), 1379 (s), 1104 (m), 1063 (m), 777 (m).
Author Contributions
X.J.W. and P.Z.L. designed and carried out the experiments. Y.C. and J.J. carried out the molecular simulations. R.G. and Y.L. performed the major part of single crystal X-ray diffraction analysis. Q.Z., H.Z. and X.X. Chan joined in the experiments and discussions. X.J.W., P.Z.L. and Y.Z. discussed and analyzed the data, interpreted the results and jointly wrote the paper.
Supplementary Material
Supplementary Information
Acknowledgments
We thank the financial support of the Singapore National Research Foundation Fellowship (NRF2009NRF-RF001-015), Singapore National Research Foundation CREATE program – Singapore Peking University Research Centre for a Sustainable Low-Carbon Future, and Nanyang Technological University.
References
- Rosi N. L. et al. Hydrogen storage in microporous metal-organic frameworks. Science 300, 1127–1129 (2003). [DOI] [PubMed] [Google Scholar]
- Bloch E. D. et al. Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science 335, 1606–1610 (2012). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Docking in metal-organic frameworks. Science 325, 855–859 (2009). [DOI] [PubMed] [Google Scholar]
- Ma L., Abney C. & Lin W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 38, 1248–1256 (2009). [DOI] [PubMed] [Google Scholar]
- Keskin S. & Kızılel S. Biomedical applications of metal-organic frameworks. Ind. Eng. Chem. Res. 50, 1799–1812 (2011). [Google Scholar]
- Kitagawa S., Kitaura R. & Noro S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004). [DOI] [PubMed] [Google Scholar]
- O'Keeffe M. & Yaghi O. M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets. Chem. Rev. 112, 675–702 (2012). [DOI] [PubMed] [Google Scholar]
- Tang L. et al. A zeolite family with chiral and achiral structures built from the same building layer. Nat. Mater. 7, 381–385 (2008). [DOI] [PubMed] [Google Scholar]
- Kuchta B. et al. Hypothetical high-surface-area carbons with exceptional hydrogen storage capacities: Open carbon frameworks. J. Am. Chem. Soc. 134, 15130–15137 (2012). [DOI] [PubMed] [Google Scholar]
- Vogiatzis K. D., Mavrandonakis A., Klopper W. & Froudakis G. E. Ab initio study of the interactions between CO2 and N-containing organic heterocycles. ChemPhysChem 10, 374–383 (2009). [DOI] [PubMed] [Google Scholar]
- Vaidhyanathan R. et al. Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid. Science 330, 650–653 (2010). [DOI] [PubMed] [Google Scholar]
- Vaidhyanathan R., Iremonger S. S., Dawson K. W. & Shimizu G. K. H. An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 5230–5232 (2009). [DOI] [PubMed] [Google Scholar]
- Couck S. et al. An amine-functionalized MIL-53 metal-organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 131, 6326–6327 (2009). [DOI] [PubMed] [Google Scholar]
- Wang F., Tan Y.-X., Yang H., Kang Y. & Zhang J. Open diamondoid amino-functionalized MOFs for CO2 capture. Chem. Commun. 48, 4842–4844 (2012). [DOI] [PubMed] [Google Scholar]
- Du N. et al. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 10, 372–375 (2011). [DOI] [PubMed] [Google Scholar]
- Wang X.-J. et al. Significant gas uptake enhancement by post-exchange of zinc(II) with copper(II) within a metal-organic framework. Chem. Commun. 48, 10286–10288 (2012). [DOI] [PubMed] [Google Scholar]
- Sumida K. et al. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 112, 724–781 (2012). [DOI] [PubMed] [Google Scholar]
- Li J.-R. et al. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 255, 1791–1823 (2011). [Google Scholar]
- Zhang J.-P., Zhang Y.-B., Lin J.-B. & Chen X.-M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 112, 1001–1033 (2012). [DOI] [PubMed] [Google Scholar]
- Aromí G., Barrios L. A., Roubeau O. & Gamez P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 255, 485–546 (2011). [Google Scholar]
- Ockwig N. W., Delgado-Friedrichs O., O'Keeffe M. & Yaghi O. M. Reticular chemistry: Occurrence and taxonomy of nets and grammar for the design of frameworks. Acc. Chem. Res. 38, 176–182 (2005). [DOI] [PubMed] [Google Scholar]
- Yaghi O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003). [DOI] [PubMed] [Google Scholar]
- Perry IV J. J., Perman J. A. & Zaworotko M. J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 38, 1400–1417 (2009). [DOI] [PubMed] [Google Scholar]
- Cairns A. J. et al. Supermolecular building blocks (SBBs) and crystal design: 12-Connected open frameworks based on a molecular cubohemioctahedron. J. Am. Chem. Soc. 130, 1560–1561 (2008). [DOI] [PubMed] [Google Scholar]
- Nouar F. et al. Supermolecular building blocks (SBBs) for the design and dynthesis of highly porous metal-organic frameworks. J. Am. Chem. Soc. 130, 1833–1835 (2008). [DOI] [PubMed] [Google Scholar]
- Eddaoudi M. et al. Porous metal-organic polyhedra: 25 Å Cuboctahedron constructed from 12 Cu2(CO2)4 paddle-wheel building blocks. J. Am. Chem. Soc. 123, 4368–4369 (2001). [DOI] [PubMed] [Google Scholar]
- Moulton B., Lu J., Mondal A. & Zaworotko M. J. Nanoballs: Nanoscale faceted polyhedra with large windows and cavities. Chem. Commun. 863–864 (2001). [Google Scholar]
- Tranchemontagne D. J., Ni Z., O'Keeffe M. & Yaghi O. M. Reticular chemistry of metal-organic polyhedra. Angew. Chem. Int. Ed. 47, 5136–5147 (2008). [DOI] [PubMed] [Google Scholar]
- McManus G. J., Wang Z. & Zaworotko M. J. Suprasupermolecular chemistry: Infinite networks from nanoscale metal-organic building blocks. Cryst. Growth Des. 4, 11–13 (2004). [Google Scholar]
- Furukawa H., Kim J., Plass K. E. & Yaghi O. M. Crystal structure, dissolution, and deposition of a 5 nm functionalized metal-organic great rhombicuboctahedron. J. Am. Chem. Soc. 128, 8398–8399 (2006). [DOI] [PubMed] [Google Scholar]
- Perry IV J. J., Kravtsov V. C., McManus G. J. & Zaworotko M. J. Bottom up synthesis that does not start at the bottom: Quadruple covalent cross-linking of nanoscale faceted polyhedra. J. Am. Chem. Soc. 129, 10076–10077 (2007). [DOI] [PubMed] [Google Scholar]
- Wang X.-S. et al. Enhancing H2 uptake by “close-packing” alignment of open copper sites in metal-organic frameworks. Angew. Chem. Int. Ed. 47, 7263–7266 (2008). [DOI] [PubMed] [Google Scholar]
- Li J.-R. & Zhou H.-C. Bridging-ligand-substitution strategy for the preparation of metal-organic polyhedra. Nat. Chem. 2, 893–898 (2010). [DOI] [PubMed] [Google Scholar]
- Yan Y. et al. Exceptionally high H2 storage by a metal-organic polyhedral framework. Chem. Commun. 1025–1027 (2009). [DOI] [PubMed] [Google Scholar]
- Zhao D., Yuan D., Sun D. & Zhou H.-C. Stabilization of metal-organic frameworks with high surface areas by the incorporation of mesocavities with microwindows. J. Am. Chem. Soc. 131, 9186–9188 (2009). [DOI] [PubMed] [Google Scholar]
- Yan Y. et al. Metal-organic polyhedral frameworks: High H2 adsorption capacities and neutron powder diffraction studies. J. Am. Chem. Soc. 132, 4092–4094 (2010). [DOI] [PubMed] [Google Scholar]
- Yuan D., Zhao D., Sun D. & Zhou H.-C. An isoreticular series of metal-organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew. Chem. Int. Ed. 49, 5357–5361 (2010). [DOI] [PubMed] [Google Scholar]
- Farha O. K. et al. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nature Chem. 2, 944–948 (2010). [DOI] [PubMed] [Google Scholar]
- Zheng B., Bai J., Duan J., Wojtas L. & Zaworotko M. J. Enhanced CO2 binding affinity of a high-uptake rht-type metal-organic framework decorated with acylamide groups. J. Am. Chem. Soc. 133, 748–751 (2011). [DOI] [PubMed] [Google Scholar]
- Farha O. K. et al. Designing higher surface area metal-organic frameworks: Are triple bonds better than phenyls? J. Am. Chem. Soc. 134, 9860–9863 (2012). [DOI] [PubMed] [Google Scholar]
- Li B. et al. Enhanced binding affinity, remarkable selectivity, and high capacity of CO2 by dual functionalization of a rht-type metal-organic framework. Angew. Chem. Int. Ed. 51, 1412–1415 (2012). [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V., Green L. G., Fokin V. V. & Sharpless K. B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation”of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002). [DOI] [PubMed] [Google Scholar]
- Spek A. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 65, 148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing K. S. W. et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985). [Google Scholar]
- Frisch M. J. et al. Gaussian 03, Revision D.01 ed.; Gaussian, Inc., Wallingford CT, 2004. [Google Scholar]
- Suh M. P., Park H. J., Prasad T. K. & Lim D.-W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 112, 782–835 (2012). [DOI] [PubMed] [Google Scholar]
- Murray L. J., Dincă M. & Long J. R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 38, 1294–1314 (2009). [DOI] [PubMed] [Google Scholar]
- Krawiec P. et al. Improved hydrogen storage in the metal-organic framework Cu3(BTC)2. Adv. Eng. Mater. 8, 193–196 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information