Abstract
Glass-ceramics (GCs) are materials obtained from the crystallisation of functional phases in glass, and have a structure that the crystallised phase embedded in the glass matrix. Glass-forming oxides are commonly added to the functional phases to improve the stability of precursor glass; however, the issue of glass-ceramics permitting the presence of residual phases resulting from addition is required to be clarified. To elucidate this issue, we prepared ‘perfectly surface-crystallised’ GC consisting of fresnoite-type Sr2TiSi2O8 from a non-stoichiometric glass and performed texture/morphology observations. Numerous SiO2-rich binodal-like nanospheres (~10 nm) were parasitic on the fresnoite single-crystal domains. The parasitic texture is considered to form via the following process: (i) binodal-type phase separation into stoichiometric fresnoite (crystalline matrix) and SiO2-rich phases (amorphous nanoparticles) and (ii) single-domain formation by surface crystallisation in the matrix. Furthermore, in terms of texture, the resulting GC differs from the GCs reported to date, i.e., inverse GC.
Glass crystallisation is considered as the ordering process of a random structure that consists of ions or coordinated polyhedra in the supercooled liquid (SCL) regime. Because the glass phase is thermodynamically a non-equilibrium state, it could eventually convert to crystalline phases through structural relaxation by thermal/optical stimulation. The crystallised phases are mainly controlled by the composition of the glass and its short-/medium-range structure. Subsequently, glass-ceramics (GCs) characterised by dispersed crystalline nano or submicron particles, or highly oriented crystalline texture can be obtained1,2,3,4,5,6. Therefore, the production of crystalline materials with glass as the precursor, i.e. GC processing, has been recognised as an effective approach to fabricate functional materials and create nanostructures. Consequently the GC processing has been rigorously investigated with the aim of extending its use to the fabrication of sophisticated materials/devices in electric, photonic and energy fields7,8,9,10,11,12. Homogeneous-crystalline slab and line patterning for optical waveguides in glass in combination with special processing have also been realised13,14,15,16.
To synthesise stable precursor glass, the composition is often selected from systems in which typical glass-forming oxides are added to a functional (e.g. ferroelectric) phase, resulting in non-stoichiometric glass. On the other hand, non-stoichiometric precursors possess uniform crystalline domain textures, which is sufficient for light propagation via annealing/laser irradiation11,13,15. Although many functional optical-grade GCs have been prepared on the basis of the above procedure, the formation of a dense crystalline texture in non-stoichiometric glass is required to be clarified. If the precursor glass includes excess components such as glass-forming oxides, the residual phase should segregate from the SCL phase in the crystallisation regime, and consequently, the optical transmittance could considerably decrease owing to light scattering at the interface between the crystalline domain and the residual. To elucidate this issue, we prepared ‘perfectly surface crystallised (PSC)’ GC (PSC-GC), in which fresnoite-type Sr2TiSi2O8 crystal domains are observed17, and conducted micro and nanoscopic investigations of the GC texture.
Results
Preliminary studies of PSC-GC
Because fresnoite (Ba2TiSi2O8) and its derivatives (e.g. Sr2TiSi2O8 and Ba2TiGe2O8) are non-centrosymmetric phases with excellent dielectric and optical properties, many researchers devoted time and effort to fabricate fresnoite-type GC and apply them to photonic components11,17,18,19,20,21,22. Recently, Yamazaki et al. demonstrated the fabrication of a GC showing PSC, in which the crystalline domains are aligned along the crystallographic polar c-axis by isothermally heat treating a non-stoichiometric precursor glass with 35SrO−20TiO2−45SiO2 composition17. They also demonstrated that PSC-GC exhibit optical transmittance comparable to that of the precursor in the visible region. In this study, we followed the published procedures17 and were able to obtain a GC sample with good transparency via heat treatment [Fig. 1(a)]. We also confirmed the crystallisation of fresnoite-type Sr2TiSi2O8 by X-ray diffraction (XRD) analysis, and the results suggested that the non-stoichiometric precursor is compatible with the reported precursor.
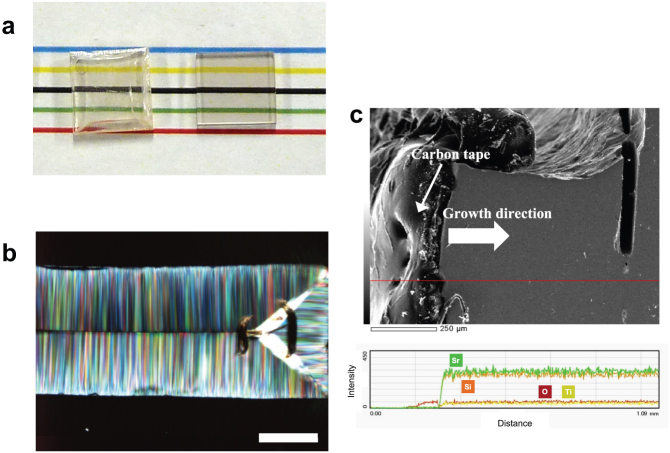
Figure 1. Macro and microscopic observations of GC samples.
(a) Appearance of GC (left) and its precursor glass (right). The GC sample was prepared on the basis of the report by Yamazaki et al. (b) Polarised light micrograph of the cross-section for the GC obtained at 940°C for 3 h. White bar corresponds to 0.5 mm. (c) SEM image (above) and EDX line analysis for Sr, Ti and Si (bottom) in the cross-section of PSC-GC. Red line corresponds to the scanned position.
To check the texture of the crystalline domain in the far-field view, we used polarised light microscopy (PLM); the observations revealed a uniform/dense crystalline texture in which fresnoite domains (width: ~5–10 μm) grow from the surface and eventually their growth fronts impinge on each other (one can imagine it as a cross-section of rectangular ice candy) [Fig. 1(b)]. At first, we speculated that the excess component, which could be excluded from the SCL phase, is present in the growth front of the fresnoite domains because of the difference in composition between the SCL and fresnoite phase. If so, the domain growth would cause an accumulation of the excess component, thus disrupting the domain growth. Nevertheless, field-emission (FE) scanning electron microscopy (SEM) with an energy dispersive X-ray fluorescence spectrometer (EDX) analysis revealed no significant variation in the composition along the domain growth [Fig. 1(c)]. Although the composition of the precursor glass is richer in SiO2 than the fresnoite phase, the residual glass phase could not been observed in the polarisation micrograph.
Nanoscopic observations of crystalline domains in PSC-GC
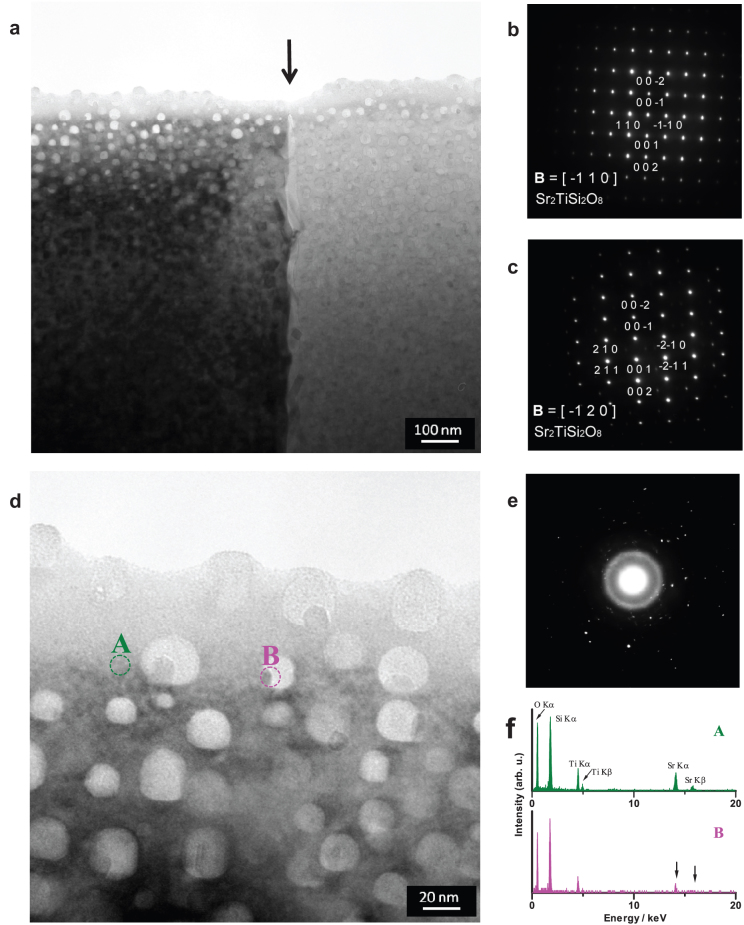
Next, we used field-emission transmission electron microscopy (FE-TEM) to study the texture or morphology within the crystalline domains and investigate the ‘missing’ residual phase in the domains. Figure 2 shows a typical TEM image of a PSC sample obtained by isothermal crystallisation. The observation geometry is the same as the microscopic observation (Fig. 1), i.e. cross section of the PSC sample. Distinct domain regions (i.e. left and right areas) [Fig. 2(a)] can be observed. From the selected area electron diffraction (SAED) patterns, it was found that the individual regions are fresnoite-type Sr2TiSi2O8 single-crystal domains with different crystal zone axes, i.e. 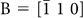 and
and 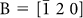 for the left and right domains, respectively, [Fig. 2(b) and (c)]; thus, the single-crystal domains grow along the polarisation c-axis. In addition, ‘white spots’ having a diameter of ~10–20 nm distributed over a single domain can be observed, which resembles binodal liquid−liquid phase separation. Furthermore, the SAED of such a spot exhibited a halo pattern, which is characteristic of amorphous phases [Fig. 2(d) and (e)]. Because the increase in the atomic number of the constituent elements decreased the intensity of the transmitting electrons, we deduced that the binodal-like nanoparticles mainly consist of lighter elements such as Si. From the TEM-EDX analysis, we detected Si, Ti and Sr (constituents of the fresnoite-type Sr2TiSi2O8) in the surrounding single-crystal region [Fig. 2(d), point A], while the signal intensity of Sr (Sr Kα and Sr Kβ) was hardly observed in the nanoparticle (point B) [Fig. 2(f)]. This means that the Sr abundance ratio of the particle is relatively less than that of the single domain. Both TEM and ED analyses strongly suggest that binodal-type phase separation, which provided the SiO2-enriched nanoparticles, occurred in the SCL regime before the crystallisation of the fresnoite phase.
for the left and right domains, respectively, [Fig. 2(b) and (c)]; thus, the single-crystal domains grow along the polarisation c-axis. In addition, ‘white spots’ having a diameter of ~10–20 nm distributed over a single domain can be observed, which resembles binodal liquid−liquid phase separation. Furthermore, the SAED of such a spot exhibited a halo pattern, which is characteristic of amorphous phases [Fig. 2(d) and (e)]. Because the increase in the atomic number of the constituent elements decreased the intensity of the transmitting electrons, we deduced that the binodal-like nanoparticles mainly consist of lighter elements such as Si. From the TEM-EDX analysis, we detected Si, Ti and Sr (constituents of the fresnoite-type Sr2TiSi2O8) in the surrounding single-crystal region [Fig. 2(d), point A], while the signal intensity of Sr (Sr Kα and Sr Kβ) was hardly observed in the nanoparticle (point B) [Fig. 2(f)]. This means that the Sr abundance ratio of the particle is relatively less than that of the single domain. Both TEM and ED analyses strongly suggest that binodal-type phase separation, which provided the SiO2-enriched nanoparticles, occurred in the SCL regime before the crystallisation of the fresnoite phase.
Figure 2. Nanoscopic observations of the crystalline domain of the PSC sample.
(a) TEM image of the PSC sample obtained at 1000°C for 1 h. Arrow indicates the crystalline domain boundary. The geometry is the same as that in the microscopic observation (cross-section). (b) SAED pattern of the left-side domain (dark region) and (c) the right-side domain (bright region). The SAED patterns revealed single-crystal features that were identified as fresnoite-type Sr2TiSi2O8 with different crystal zone axes. (d) Near-field TEM image. (e) SAED pattern of the binodal-like nanoparticles with the typical halo pattern, i.e. amorphous phase. (f) EDX spectra of the surrounding region (single-crystal domain, A) and nanoparticle (amorphous, B). At point B, the chemical abundance of Sr was relatively low (indicated by the arrows) compared with the single-domain region.
According to Baylor and Brown23, a large stable two-liquid region (i.e. opaque glass) is present in the ternary SrO−B2O3−SiO2 system between the stable immiscible regions of the SrO−SiO2 and SrO−B2O3 joins. In addition, in the vicinity of stable-immiscibility boundary, the transparent glass (i.e. one-liquid region) had dispersed SiO2-rich nanoparticles embedded in a SrO-rich glass matrix presumably because of metastable immiscibility. We find that the morphological features resemble those of PSC-GC in the SrO−TiO2−SiO2 system. Therefore, we examined the approximate immiscible and glass-forming regions and discussed the phase separation trend in the ternary system. We examined 55 compositions and found the following features (Fig. 3: i) for SiO2 content >30 mol%, the stable-immiscible region in the ternary system is considerably similar to that of the SrO−B2O3−SiO2 system, i.e. there is a large two-liquid region below the ~25 mol% SrO isopleth. Because the TiO2−SiO2 and B2O3−SiO2 joins have a large two-phase liquid region24, it is reasonable to assume that the ternary SrO−B2O3−SiO2 and SrO−TiO2−SiO2 systems exhibit immiscibility or phase-separation; and ii) for SiO2 <30 mol%, the immiscible region decreased towards the SrO−TiO2 join, probably due to the decrease in the glass former.
Figure 3. Glass-forming and approximate two-liquid regions in the SrO−TiO2−SiO2 system.
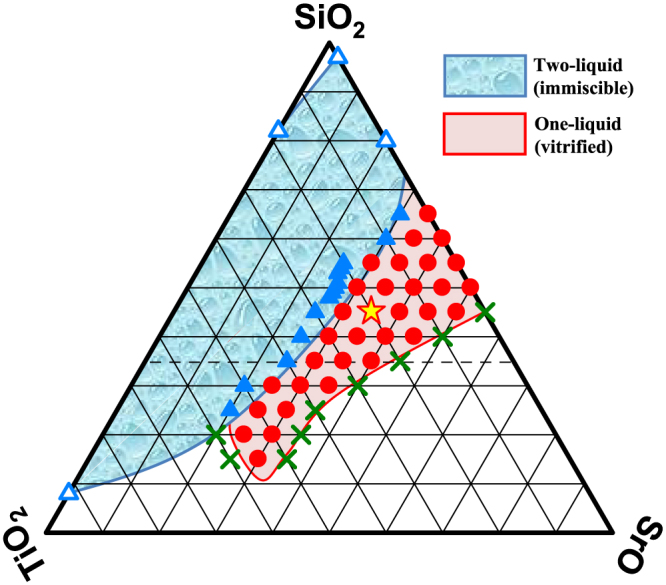
Closed and crossed symbols correspond to the examined compositions. Triangle: phase separated, circle: transparent glass and cross: devitrified. Star symbol shows the composition of PSC-GC, i.e. 35SrO−20TiO2−45SiO2. Open triangles in the TiO2−SiO2 and SrO−SiO2 joins correspond to the points where a two-liquid phase is reported by DeVries et al. and Fields, Jr. et al., respectively. Dashed line across the diagram represents the lower limit of the glass-forming region in terms of SiO2, which was reported by Imaoka and Yamazaki. We found a large glass-forming region that extends to the low-SiO2 compositions.
Discussion
In terms of the SrO−TiO2−SiO2 system, the analogy in the phase-separation morphology and immiscibility lead us to expect that an immiscible region also extends into the one-liquid region (from which the transparent samples were obtained) near the boundary below the liquidus temperature (metastable immiscibility). The composition of the precursor glass for PSC-GC is also situated near this boundary. Considering the phase-separation trend in the aforementioned one-liquid region23, and because the resulting PSC-GC crystallises single-phase fresnoite, the precursor glass in the SCL state could separate into a SrO-rich phase, which corresponds to the fresnoite stoichiometry (Sr2TiSi2O8) and a residual SiO2-rich phase. This may be expressed by the following formula,
35SrO−20TiO2−45SiO2 → 35SrO−17.5TiO2−35SiO2 (stoichiometric, surrounding matrix) + 2.5TiO2−10SiO2 (residual, nanoparticle).
Although the formula indicates an ideal case so that the phase-separation trend should be examined more in detail, the trend is supported by the TEM studies (EDX and ED analyses) in this study. Furthermore, we also showed that the glass-forming region is considerably extended to the high-TiO2 (or low-SiO2) region when compared with the region reported by Imaoka and Yamazaki25. In the glass-forming region in their study, the compositions with <30 mol% SiO2 did not vitrify25, while in the present study, we could obtain the 30SrO−55TiO2−15SiO2 glass. This indicates the necessity to reconsider the conventional glass-forming diagram, in which the intermediate oxide (TiO2 in this case) is largely included.
From the experimental results and the discussion above, the possible scenario for the precursive stage of PSC is as follows:
As the temperature increases above the glass-transition temperature at which the glass state changes to the SCL state, the constituent atoms acquire adequate fluidity for two-liquid phase separation caused by metastable immiscibility and results in binodal-like nanoparticles and the surrounding SCL phase.
The formation of the SiO2-rich nanoparticles causes the SCL matrix composition to approach that of stoichiometric Sr2TiSi2O8, thus establishing the conditions for the crystallisation of the fresnoite phase.
With further increase in the temperature at which inhomogeneous nucleation occurs, the SCL matrix is transformed into the fresnoite crystal domains due to surface crystallisation. During the domain growth, the already settled binodal-like nanoparticles are captured at the fresnoite growth front and consequently, the nanoparticles are frozen in the domains, result in ‘crystal domains parasitised by amorphous nanoparticles’. Surface crystallisation often exhibits a strongly oriented texture originating from competitive crystal growth of nuclei generated on the glass surface based on geometrical selection26. This mechanism can be adopted to explain the orientation of the c-axis in PSC-GC as well.
Wisniewski et al. recently reported the surface crystallisation of fresnoite-type Sr2TiSi2O8 in SiO2-enriched non-stoichiometric glass, in which the opaque GCs exhibited excellent piezoelectric properties owing to the strong orientation of the texture27,28. This research group has also attempted to explain residual SiO2 using electron backscatter diffraction and SEM and predicted the presence of a SiO2-rich structure at the nanoscale (i.e. nanoporous with channels similar to sponge)27. Our study validates this idea; however, the nanostructure in their study is apparently different from that in our study (i.e. binodal nanoparticle). This difference is presumably attributed to the difference in glass composition (i.e. 34.8SrO−17.4TiO2−47.8SiO2 by Wisniewski et al27. and 35SrO−20TiO2−45SiO2 by Yamazaki et al17.), thus suggesting that nanostructures are sensitive to variation in the composition.
Basically, the transparency of GC materials depends on two factors. According to Beall and Duke, the intensity of the light is described on the basis of Rayleigh scattering by the following equation,
where I is the intensity of the scattered light, θ is the scattering angle, r is the distance from the scattering source, λ is the wavelength of the light, a is the radius of the particle in the matrix, M is the ratio of the refractive index of the particle to that of the matrix and I0 is the intensity of the incident light29. From Eq. (1), either the smallness of the crystals in the glass matrix (i.e. nanocrystallisation: a ≪ λ) or the closeness of the refractive index of the crystals to that of the matrix (refractive matching; M ~ 1) is chosen. Nanocrystallisation can be used to reliably prepare precursor glass which would be transparent in the visible wavelength even if there is a significant difference in the refractive index between the glass matrix and the crystallised nanoparticles; GCs consisted of multi-component glass and Nb-ferroelectrics is a typical example30,31. On the other hand, the PSC-GC sample in this study is constructed by single-domain fresnoite in the matrix and parasitic nanoparticles (~10–20 nm), which correspond to the residual SiO2-rich amorphous phase. Phase separation typically makes glass opalescent because of the structure developed at the scale of the visible wavelength by spinodal or binodal decomposition. However, the size of the parasitic nanoparticles is significantly less than the visible wavelength, indicating that light scattering is minimised. Thus, we demonstrated a method to fabricate ‘inverse GC’, in which the texture is completely reversed. Furthermore, the study of inverse GC also provides significant insight into single-crystal line patterning using laser processing13,14,15,32 because this study clarifies the location of the residual phase after single-line formation.
Methods
Preparation of glass and PSC samples
The glass samples we dealt with belonged to the ternary SrO−TiO2−SiO2 system and were prepared by using the conventional melt-quenching technique. Commercial powders of reagent grade SrCO3 (99.9% purity), TiO2 (99.99%) and SiO2 (99.9%) were weighed and mixed. Then, the mixture was melted in a platinum crucible covered with a lid at 1350–1600°C (the temperature condition depends on the composition) for 1 h in air. For example, 35SrO−20TiO2−45SiO2 glass, which indicates PSC, was obtained at 1500°C. The melt was poured onto a steel plate (~200°C) and pressed with another steel plate. The as-quenched (amorphous) samples were annealed around the glass-transition temperature (Tg ~760°C) for 1 h to reduce internal stresses. The annealed samples were polished to get a mirror surface and then cut into several pieces with dimensions of ~10 mm × 10 mm × 1 mm. To fabricate PSC-GC, the precursor glass was subjected to isothermal heat treatment at appropriate temperatures (~900–1000°C) in an electric furnace in air.
Characterisation of the glass and GC samples
The precursor glass was characterised by differential thermal analysis (bulk state, 10°C/min heating rate) and XRD. In addition, XRD was used to identify the crystalline phase in the PSC-GC samples. We also used PLM, FE-SEM with EDX (JEOL, JSM-6500F) and FE-TEM with EDX (Hitachi, HF-2000) to characterise the crystallised fresnoite-phase. To determine the glass-forming region in the ternary system, the as-quenched samples were examined (i) by powder X-ray diffraction (XRD) using Cu Kα radiation to determine whether the samples were amorphous and (ii) optically to clarify two-liquid phase (opaque/white) or one-liquid phase (transparent) (Fig. 3).
Author Contributions
Y. Y. and R. I. performed the fabrication and characterisation of materials. Y. T. and T. F. designed the study, analyzed the data and prepared the paper.
Acknowledgments
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government. We would like to thank Dr. Takamichi Miyazaki of Department of Instrumental Analysis, School of Engineering, Tohoku University for his significant contributions to this study.
References
- Borrelli N. F., Herczog A. & Maurer R. D. Electro-optic effect of ferroelectric microcrystals in a glass matrix. Appl. Phys. Lett. 7, 117–118 (1965). [Google Scholar]
- Majumdar A. J., Nurse R. W., Chatterji S. & Jeffrey J. W. Three-dimensional view of phase separation in glass ceramics. Nature 211, 622–624 (1966). [Google Scholar]
- Pinckney L. R. & Beall G. H. Nanocrystalline non-alkali glass-ceramics. J. Non-Cryst. Solids 219, 219–227 (1997). [Google Scholar]
- Rüssel C. Oriented crystallization of glass. A review. J. Non-Cryst. Solids 219, 212–218 (1997). [Google Scholar]
- Jain H. Transparent ferroelectric glass-ceramics. Ferroelectr 306, 111–127 (2004). [Google Scholar]
- Cabral A. A., Fokin V. M. & Zanotto E. D. Nanocrystallization of fresnoite glass. II. Analysis of homogeneous nucleation kinetics. J. Non-Cryst. Solids 343, 85–90 (2004). [Google Scholar]
- Halliyal A., Bhalla A. S., Newnham R. E. & Cross L. E. Piezoelectric properties of lithium borosilicate glass ceramics. J. Appl. Phys. 53, 2871–2874 (1982). [Google Scholar]
- Ding Y. et al. Lithium disilicate crystalline slab waveguides from surface crystallized glass. Electron. Lett. 35, 504–505 (1999). [Google Scholar]
- Rosenflanz A. et al. Bulk glasses and ultrahard nanoceramics based on alumina and rare-earth oxides. Nature 430, 761–764 (1999). [DOI] [PubMed] [Google Scholar]
- Murugan G. S. & Varma K. B. R. Dielectric, linear and non-linear optical properties of lithium borate-bismuth tungstate glasses and glass-ceramics. J. Non-Cryst. Solids 279, 1–13 (2001). [Google Scholar]
- Iwafuchi N., Hirokazu M., Takahashi Y. & Fujiwara T. Electro-optic measurement in glass ceramics with highly oriented crystalline layers. Electron. Lett. 46, 69–71 (2010). [Google Scholar]
- Hayashi A., Noi K., Sakuda A. & Tatsumisago M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 3, 856, doi: 10.1038/ncomms1843 (2012). [DOI] [PubMed] [Google Scholar]
- Himei Y., Ding Y., Ikebe M. & Miura Y. Ca2Al2SiO7 crystalline slab waveguides from surface crystallized glass. J. Ceram. Soc. Japan 109, 474–476 (2001). [Google Scholar]
- Komatsu T. & Honma T. Laser-induced line patterning of nonlinear optical crystals in glass. IEEE J. Select. Top. Quantum Electron. 14, 1289–1297 (2008). [Google Scholar]
- Honma T. & Komatsu T. Patterning of two-dimensional planar lithium niobate architectures on glass surface by laser scanning. Opt. Express 18, 8019–8024 (2010). [DOI] [PubMed] [Google Scholar]
- Stone A. et al. Formation of ferroelectric single-crystal architectures in LaBGeO5 glass by femtosecond vs. continuous-wave lasers. J. Non-Cryst. Solids 356, 3059–3065 (2010). [Google Scholar]
- Yamazaki Y., Takahashi Y., Ihara R. & Fujiwara T. Surface crystallization of fresnoite-type crystallized glasses with large thickness. J. Ceram. Soc. Japan 119, 757–762 (2011). [Google Scholar]
- Takahashi Y., Benino Y., Fujiwara T. & Komatsu T. Optical second order nonlinearity of transparent Ba2TiGe2O8 crystallized glasses. Appl. Phys. Lett. 81, 223–225 (2002). [Google Scholar]
- Takahashi Y., Benino Y., Fujiwara T. & Komatsu T. Large second-order optical nonlinearities of fresnoite-type crystals in transparent surface-crystallized glasses. J. Appl. Phys. 95, 3503–3508 (2004). [Google Scholar]
- Takahashi Y., Kitamura K., Benino Y., Fujiwara T. & Komatsu T. Second-order optical nonlinear and luminescent properties of Ba2TiSi2O8 nanocrystallized glass. Appl. Phys. Lett. 86, 091110 (2005). [Google Scholar]
- Zhu B. et al. Greatly enhanced effect of silver on femtosecond laser-induced precipitation of nonlinear optical crystals in glasses. Opt. Lett. 34, 1666–1668 (2009). [DOI] [PubMed] [Google Scholar]
- Müller A. et al. Fresnoite thin films grown by pulsed laser deposition: photoluminescence and laser crystallization. Cryst. Eng. Comm. 13, 6377–6385 (2011). [Google Scholar]
- Baylor R. Jr & Brown J. Jr Phase separation of glasses in the system SrO−B2O3−SiO2. J. Am. Ceram. Soc. 59, 131–136 (1976). [Google Scholar]
- DeVries R. C., Roy R. & Osborn E. F. The system TiO2−SiO2. Trans. Bri. Ceram. Soc. 53, 525–540 (1954). [Google Scholar]
- Imaoka M. & Yamazaki T. Glass-formation ranges of ternary systems (Part 1) silicates of a−group elements. Rep. Inst. Ind. Sci. Univ. Tokyo. 18, 240–273 (1968). [Google Scholar]
- Takahashi Y., Saitoh K., Benino Y., Fujiwara T. & Komatsu T. Formation of Ba2TiGe2O8 phase in BaO−TiO2−GeO2 glasses and their optical nonlinearities. J. Non-Cryst. Solids 345, 412–416 (2004). [Google Scholar]
- Wisniewski W., Patschger M. & Rüssel C. Sr-fresnoite surface crystallisation in a 2SrO·TiO2·2.75SiO2 glass studies by EBSD. Cryst. Eng. Comm. 14, 5425–5433 (2012). [Google Scholar]
- Patschger M., Wisniewski W. & Rüssel C. Piezoelectric glass-ceramics produced via oriented growth of Sr2TiSi2O8 fresnoite: thermal annealing of surface modified quenched glasses. Cryst. Eng. Comm. 14, 7368–7373 (2012). [Google Scholar]
- Beall G. H. & Duke D. A. Transparent glass-ceramics. J. Mater. Sci. 4, 340–352 (1969). [Google Scholar]
- Ahamad M. N. & Varma K. B. R. Structural and optical properties of (100-x)Li2B4O7·x(Ba5Li2Ti2Nb8O30) glasses and glass nanocrystal composites. Dalton Trans. 39, 4624–4630 (2010). [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Fujie N. & Fujiwara T. Nano-sized Ba2NaNb5O15−NaNbO3 co-crystallized glass-ceramics in phosphoniobate system. Appl. Phys. Lett. 100, 201907 (2012). [Google Scholar]
- Honma T., Benino Y., Fujiwara T., Komatsu T. & Sato R. Technique for writing of nonlinear optical single-crystal lines in glass. Appl. Phys. Lett. 83, 2796–2798 (2003). [Google Scholar]