Abstract
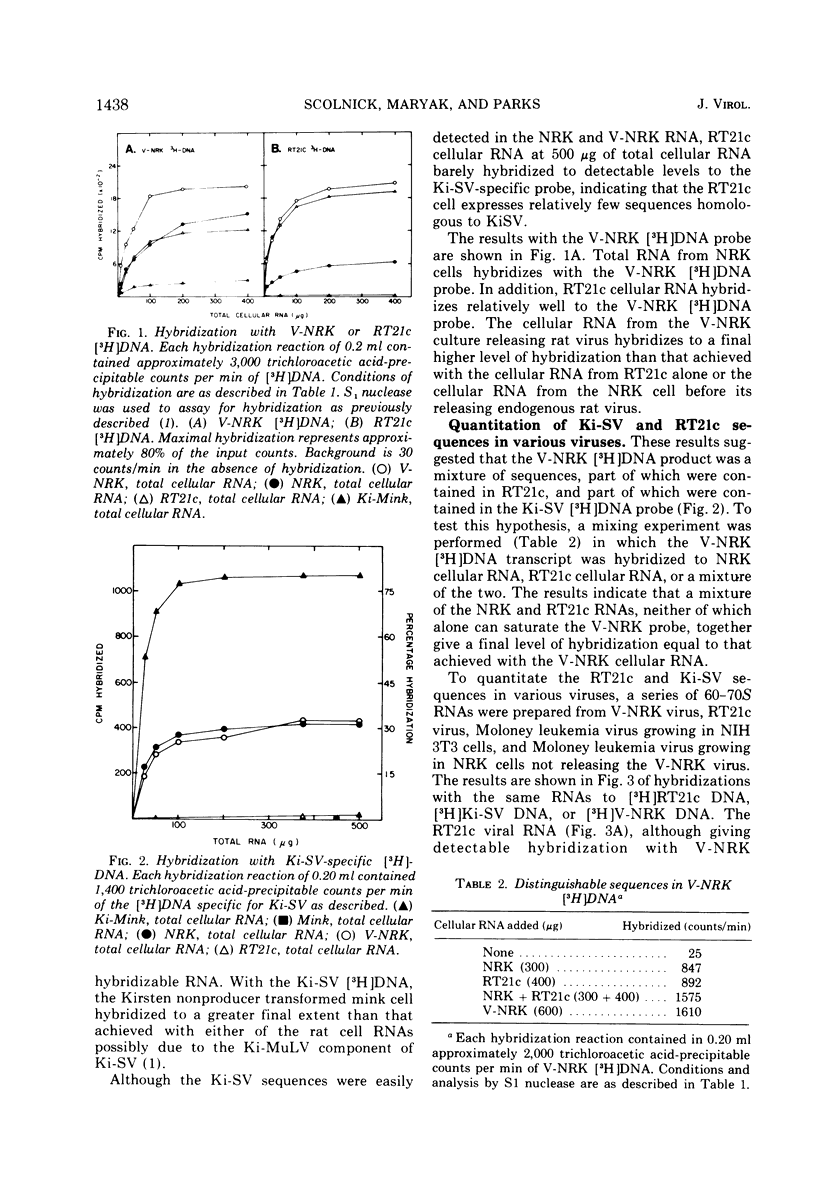
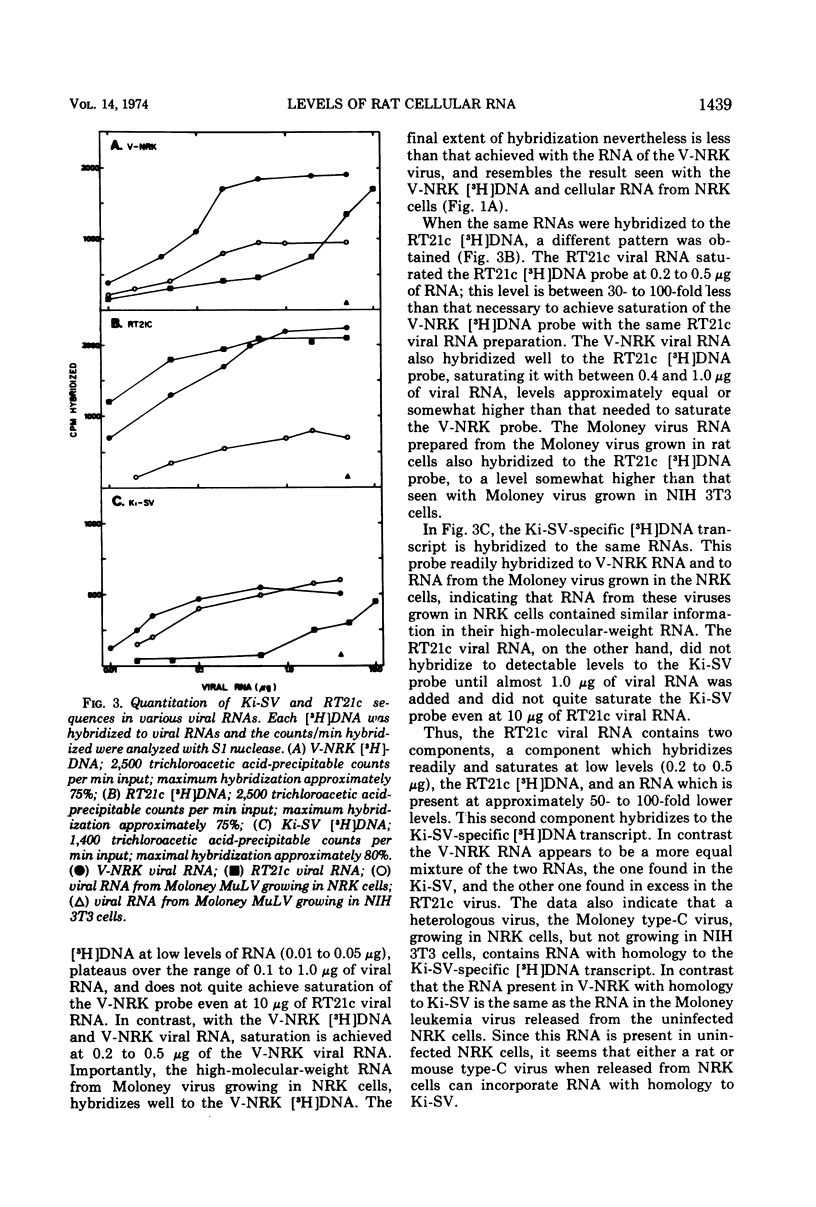
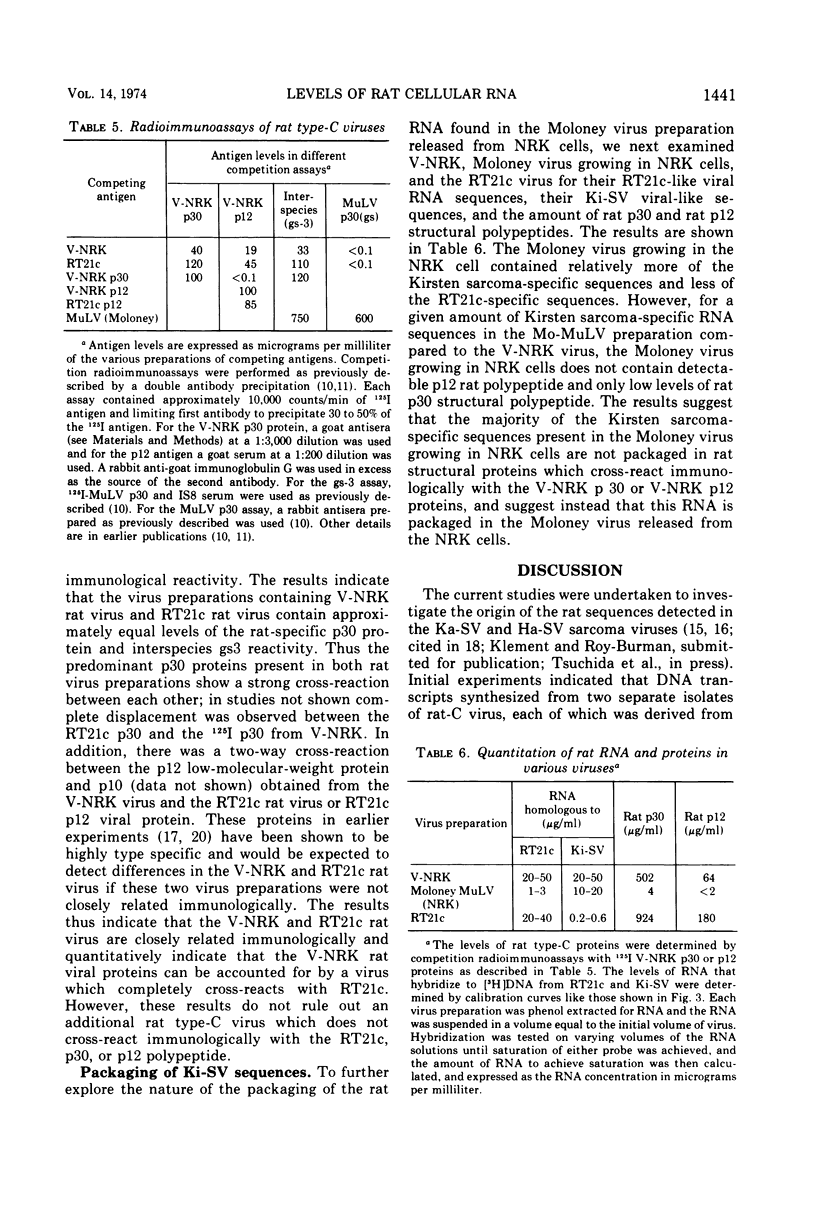
DNA transcripts from V-NRK and RT21c rat type-C viruses were found to differ in their sequence homology to Kirsten and Harvey sarcoma viruses. V-NRK DNA transcripts consistently had homology to Kirsten and Harvey sarcoma virus, whereas RT21c DNA transcripts did not. To explain the differences, the nucleic acids and structural proteins of the two type-C viruses, released from each of two cell lines derived from Osborne-Mendel rats, were analyzed by molecular hybridization and competition radioimmunoassays. The p30 and p12 structural proteins of the two viruses were found to be highly related immunologically. In the V-NRK virus preparation, two sets of distinct RNA sequences were found in approximately equal amounts. One set is homologous to Ki-SV, and the other homologous to RT21c. In contrast, the RT21c virus preparation was found to contain a different ratio of these sequences. In this case the RT21c-like RNA sequences are present in 100-fold excess as compared to the additional Ki-SV specific sequences. Both NRK and RT21c cells contain in their DNA the full complement of Ki-SV homologous sequences, but NRK cells express much higher levels of these Ki-SV sequences in their RNA. These additional sequences, not homologous to RT21c, which are detected in uninfected NRK cellular RNA or V-NRK rat virus, could also be detected in the 60-70S RNA from a Moloney mouse type-C virus released from the NRK cells infected with the Moloney type-C virus. The results suggest that type-C viruses released from NRK cells incorporate species of RNA present in NRK cells which are homologous to Kirsten and Harvey sarcoma viruses. Either these sequences are of cellular origin, or rat cells contain two endogenous viruses with completely distinct nucleic acid sequences.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J., Scolnick E. M., Parks W. P. Partial transcription of murine type C viral genomes in BALB c cell lines. J Virol. 1973 Oct;12(4):711–720. doi: 10.1128/jvi.12.4.711-720.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer N. E., Taylor D. O., Oshiro L. S., Teitz Y. Transformation and virus production in normal rat thymus cells and those infected with Moloney leukemia virus. J Natl Cancer Inst. 1970 Jul;45(1):37–48. [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J., Klement V., Lai M. M., Ostertag W., Duesberg P. Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3536–3540. doi: 10.1073/pnas.70.12.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Bova D., Huebner R. J., Gilden R. V. Major group-specific protein of rat type C viruses. J Virol. 1972 Oct;10(4):746–750. doi: 10.1128/jvi.10.4.746-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Murine mammary tumor cell clones with varying degrees of virus expression. Virology. 1973 Sep;55(1):163–173. doi: 10.1016/s0042-6822(73)81018-9. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Tronick S. R., Scolnick E. M. Polyadenylate rich RNA in the 70 S RNA of murine leukemia-sarcoma virus. Virology. 1972 Jul;49(1):230–235. doi: 10.1016/s0042-6822(72)80025-4. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. J Virol. 1974 Jun;13(6):1211–1219. doi: 10.1128/jvi.13.6.1211-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Anderson G. R., Tronick S. R., Aaronson S. A. Evidence for genetic recombination between endogenous and exogenous mouse RNA type C viruses. Cell. 1974 Jun;2(2):87–94. doi: 10.1016/0092-8674(74)90096-8. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Tronick S. R., Aaronson S. A. Analysis of type specific antigenic determinants of two structural polypeptides of mouse RNA C-Type viruses. Virology. 1974 Mar;58(1):1–8. doi: 10.1016/0042-6822(74)90135-4. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The protovirus hypothesis: speculations on the significance of RNA-directed DNA synthesis for normal development and for carcinogenesis. J Natl Cancer Inst. 1971 Feb;46(2):3–7. [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Aaronson S. A. Comparative immunological studies of RNA C-type viruses: radioimmunoassay for a low molecular weight polypeptide of woolly monkey leukemia virus. Virology. 1974 Feb;57(2):347–356. doi: 10.1016/0042-6822(74)90174-3. [DOI] [PubMed] [Google Scholar]



