To The Editor
Epithelial downgrowth is a rare but grave complication of intraocular surgery,1,2 that typically presents as epithelial sheets, cysts, or pearls1,2,3,4. Prognosis is poor as incursion of epithelial cells onto anterior chamber structures can result in corneal decompensation, refractory glaucoma, and visual deficits2. Treatment options include enucleation, surgical excision, irradiation, cryotherapy, cautery, laser coagulation, vitrectomy and injections of antimetabolites1,2,3,4,5. The present report describes a case in which epithelial downgrowth presented as an amorphous anterior chamber cellular aggregate, was diagnosed by anterior chamber tap and specular microscopy, and was treated successfully with 5-Fluorouracil.
Case Description
Clinical History
A 66-year-old female who had been receiving subconjunctival steroid injections for presumed graft rejection presented in December 2009 with decreased vision in her right eye. Her ocular history included Fuchs’ endothelial dystrophy and narrow angle glaucoma. Her right eye had undergone penetrating keratoplasty (PK), cataract extraction, and anterior chamber intraocular lens (ACIOL) implantation in November 2006 and pars plana vitrectomy and pars plana Ahmed tube shunt in October 2008.
Examination
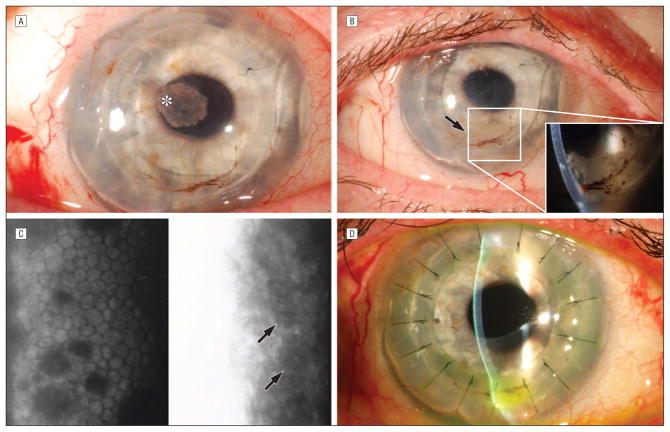
Examination of the right eye revealed a corrected vision of 20/150. Intraocular pressure measured <5 mmHg. Slitlamp examination revealed a resolving subconjunctival hemorrhage, and a pink, fibrous plaque on the anterior surface of the ACIOL (Figure 1A). The vitreous and retina were normal. Five days later, the plaque resolved without additional intervention. Subsequently, the patient developed a pupillary membrane and inflammatory, “fluffy,” white debris accumulated in the anterior chamber (Figure 1B).
Figure 1. Clinical Photographs.
A. Pink, fibrous plaque on the anterior surface of anterior chamber intraocular lens. B. Inflammatory, white, “fluffy” debris free-floating in the anterior chamber. C. Specular microscopy. Left panel demonstrates guttae characteristic of Fuchs’ endothelial dystrophy. Right panel demonstrates an amorphous pattern with larger cells and hyperreflective nuclei. Arrows = cells suggestive of epithelial cells. D. Complete resolution of inflammatory/epithelial anterior chamber debris.
Diagnostic testing
Review of systems and a systemic workup for anterior uveitis were negative. Anterior chamber taps were performed for stains, cultures, PCR, and pathology. Gram stains showed 10 to 25 mononuclear cells per low power field but no organisms. Fungal stains and bacterial and fungal cultures were negative. PCR was negative for Herpes Simplex virus, Herpes Zoster virus, and Cytomegalovirus. Cytology showed no morphologic evidence of lymphoid neoplasia. However, degenerating neutrophils and macrophages along with epithelioid non-hematopoietic cells consistent with epithelial downgrowth were identified (Figure 2A). An amorphous pattern of larger cells with hyperreflective nuclei suggestive of epithelial cells was seen on specular microscopy6 (Figure 1C).
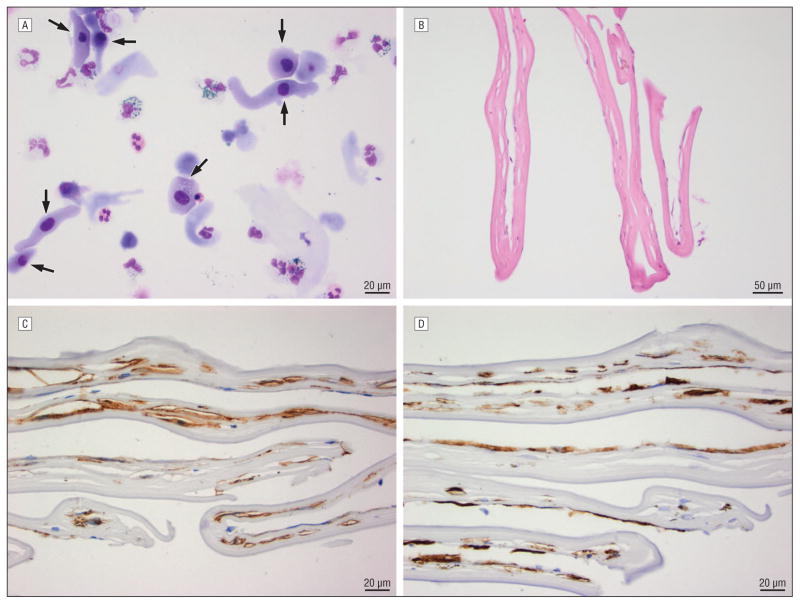
Figure 2. Pathology.
A. Cytologic preparation from anterior chamber aspirates. Arrows indicate mononuclear epithelioid cells with abundant cytoplasm. (Wright Giemsa stain at high power; white bar indicates 20 microns). B–D. Histopathological specimens from the retrocorneal membrane. B. H&E stain of the Descemet’s membrane and fibrous, retrocorneal membrane C. Smooth muscle actin and D. CK7 showed diffuse positive staining.
Treatment
The anterior chamber debris was removed with aspiration and the patient was treated twice with intracameral injections of 5-Fluorouracil (5-FU) 1000 mcg/0.1 mL in a dispersive viscoelastic4 with complete resolution of the anterior chamber findings. The patient required a repeat PK for graft failure that transpired over the course of her treatment. Three months post-operatively, uncorrected vision was 20/40. No recurrence of epithelial or inflammatory anterior chamber debris has occurred (Figure 1D).
Histopathological Evaluation
Descemet’s membrane separated from the overlying stroma intraoperatively. Histopathology did not reveal a migratory path of epithelial cells although the specimen lacked the complete graft/host junction. A fibrous retrocorneal membrane (Figure 2B) was identified and subsequently classified to be of metaplastic endothelial origin based on Jakobiec and Bhat’s established classification of retrocorneal membranes7(Figure 2C & 2D).
Comment
This case illustrates an unusual presentation of epithelial downgrowth as an amorphous cellular aggregate within the aqueous as opposed to more typical presentations of epithelial sheets, cysts, or pearls. Our diagnosis was based on the histopathological results of the aqueous tap and specular microscopy. The mechanism of entry of the ectopic epithelial cells remains unclear, but possibilities include entry through the corneal wound during the triple procedure or retrograde flow through the tube. Importantly, epithelial downgrowth should be considered in the differential diagnosis of chronic anterior chamber inflammation and cellular aggregates that are unresponsive to therapy.
In our case, 5-FU treatment achieved excellent anatomical and visual results. Two cases in 2002 documented the complete resolution of epithelial downgrowth in patients treated with 5-FU4, 5; however, neither case presented post-5FU treatment histopathological correlates. Our patient’s eye remains devoid of any ectopic epithelial cells; yet her initial graft failed after a retrocorneal membrane had developed.
The retrocorneal membrane may have resulted from metaplastic endothelial cell growth due to deposition of hematopoietic or epithelial cells in the anterior chamber, metaplasia of existing endothelial cells, or a reaction between the 5-FU treatment and epithelial cells7.
Acknowledgments
Financial Support: K23EY019353 and K23EY019353
The authors wish to thank the Flaum Eye Institute’s Diagnostic Imaging Service for obtaining the clinical images.
Holly B. Hindman: “I had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.”
Footnotes
Author Disclosures: None of the authors have any conflict of interest associated with this manuscript.
References
- 1.Vargas LG, Vroman DT, Soloman KD, et al. Epithelial downgrowth after clear cornea phacoemulsification: report of two cases and review of the literature. Ophthalmology. 2002;109:2331–2335. doi: 10.1016/s0161-6420(02)01274-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen SH, Pineda R., 2nd Epithelial and fibrous downgrowth: mechanisms of disease. Ophthalmol Clin North Am. 2002;15:41–48. doi: 10.1016/s0896-1549(01)00013-x. [DOI] [PubMed] [Google Scholar]
- 3.Haller JA, Stark WJ, Azab A, et al. Surgical management of anterior chamber epithelial cysts. Am J Ophthalmol. 2003;135:309–313. doi: 10.1016/s0002-9394(02)01960-8. [DOI] [PubMed] [Google Scholar]
- 4.Lai MM, Haller JA. Resolution of epithelial ingrowth in a patient treated with 5-fluorouracil. Am J Ophthalmol. 2002;133:562–564. doi: 10.1016/s0002-9394(01)01419-2. [DOI] [PubMed] [Google Scholar]
- 5.Shaikh AA, Damji KF, Mintsioulis G, et al. Bilateral epithelial downgrowth managed in one eye with intraocular 5-flourouracil. Arch Ophthalmol. 2002;120:1396–1398. [PubMed] [Google Scholar]
- 6.Chiou AG, Kaufman SC, Kaz K, et al. Characterization of epithelial downgrowth by confocal microscopy. J Cataract Refract Surg. 1999;25:1172–1174. doi: 10.1016/s0886-3350(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 7.Jakobiec FA, Bhat P. Retrocorneal membranes: A comparative immunohistochemical analysis of keratocytic, endothelial, and epithelial origins. Am J Ophthalmol. 2010;150:230–242. doi: 10.1016/j.ajo.2010.03.011. [DOI] [PubMed] [Google Scholar]