Abstract
The serotonin 5-HT2A receptor (5-HT2AR) and dopamine D2 receptor (D2R) are high-affinity G protein-coupled receptor targets for two different classes of antipsychotic drugs used to treat schizophrenia. Interestingly, the antipsychotic effects are not based on the regulation of same signaling mediators since activation of the 5-HT2AR and of the D2R regulate Gq/11 protein and Gi/o protein, respectively. Here we use radioligand binding and second messenger production assays to provide evidence for a functional crosstalk between 5-HT2AR and D2R in brain and in HEK293 cells. D2R activation increases the hallucinogenic agonist affinity for 5-HT2AR and decreases the 5-HT2AR induced inositol phosphate production. In vivo, 5-HT2AR expression is necessary for the full effects of D2R antagonist on MK-801-induced locomotor activity. Co-immunoprecipitation studies show that the two receptors can physically interact in HEK293 cells and raise the possibility that a receptor heterocomplex mediates the crosstalk observed. The existence of this 5-HT2AR-D2R heteromer and crosstalk may have implications for diseases involving alterations of serotonin and dopamine systems and for the development of new classes of therapeutic drugs.
Keywords: Serotonin 5-HT2A receptor, Dopamine D2 receptor, Crosstalk, Schizophrenia, Hallucinogen, Antipsychotic
1. Introduction
The brain serotonin and dopamine systems are each strongly associated with mood regulation, cognition, memory, learning, motivation and pleasure (Adayev et al., 2005; Arias-Carrion and Poppel, 2007; Bromberg-Martin et al., 2010; Nichols and Nichols, 2008). Altered serotonin and dopamine neurotransmission has been implicated in various psychiatric disorders including anxiety, depression and schizophrenia (de Almeida et al., 2008; Kienast and Heinz, 2006; Mann et al., 2001; Wong and Licinio, 2001). Both dopamine and serotonin have each been proposed to play a fundamental role in the pathophysiology and/or symptoms of schizophrenia (Iqbal and van Praag, 1995; Joyce,1993; Laruelle et al., 2003). The dopamine hypothesis of schizophrenia was consistent with the observation that typical antipsychotic drugs, such as chlorpromazine and haloperidol, are antagonists of dopamine D2 receptor (D2R) signaling. Excessive dopamine activity in the mesolimbic system resulting from dysregulated dopamine neurotransmission has been hypothesized to contribute to the symptoms of the disease. The similarity to the symptoms of schizophrenia of some aspects of the responses to serotonergic hallucinogens implicated abnormalities of serotonergic signaling in the disease. LSD (lysergic acid diethylamide) and DOI ((+/−)-2,5-dimethoxy-4-iodoamphetamine), hallucinogenic serotonin receptor agonists, trigger effects comparable to positive symptoms (delusions, hallucinations) of schizophrenic patients (Costa, 1960; Nichols, 2004). The effects of these drugs in humans and animal models depend on the serotonin 5-HT2A receptor (5-HT2AR). Moreover, a second generation of drugs called atypical antipsychotics (e.g. clozapine, risperidone and olanzapine) has been developed. The antipsychotic effects of these dopamine–serotonin antagonists, which have higher affinity for 5-HT2AR, are believed to mediate the blockade of mesolimbic dopamine D2R and mesocortical serotonin 5-HT2AR (Kapur and Remington, 1996; Meltzer et al., 1989; Nichols, 2004).
However the relationship between classical and atypical anti-psychotic drugs remains to be elucidated. We speculated whether a serotonin 5-HT2AR-dopamine D2R complex might exist that could connect the effects of these drugs. Crosstalk among receptor systems can occur either at the level of downstream signaling or via direct functional interaction between the two receptor subtypes (Gurevich and Gurevich, 2008; Rives et al., 2009). Several studies have largely shown that dimerization of G protein-coupled receptors (GPCR) can contribute to regulation of receptor targeting/internalization, pharmacological or signaling properties (Bulenger et al., 2005; Jordan and Devi, 1999; Milligan, 2009; Terrillon and Bouvier, 2004). GPCR dimerization has been identified in native tissues, precluding an artifact due to artificial receptor aggregation (Albizu et al., 2010a). Moreover, its functional relevance in central nervous system and the relationship between altered receptor–receptor interactions and psychiatric diseases have been described (Albizu et al., 2010b; Ferre et al., 2007). Here we report functional crosstalk between serotonin 5-HT2AR and dopamine D2R, two major antipsychotic drug targets, and show a physical interaction between the two GPCR.
2. Experimental procedures
2.1. Cell culture and in vitro transfection
HEK293 cells were maintained in culture in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 100 units/ml penicillin and streptomycin in an atmosphere of 95% air and 5% CO2 at 37 °C. Cells were transiently transfected with serotonin 5-HT2AR and/or dopamine D2R by using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer’s instructions.
2.2. Membrane preparation
Membranes were prepared from mouse brain or HEK293 cell line. Mouse brain samples were obtained from adult (8–12 weeks old) male 129/SV mice. Mouse brain or culture dishes of HEK293 cells expressing serotonin 5-HT2AR or co-expressing 5-HT2AR and dopamine D2R were washed twice in PBS without calcium and magnesium, and cold lysis buffer (15 mM Tris–HCl, 2 mM MgCl2, 0.3 mM EDTA, pH 7.4) was added. HEK293 cells were scraped with a rubber. Then cells or brain samples were homogenized with a polytron and centrifuged at 100 g for 5 min at 4 °C. Supernatants were recovered and centrifuged at 44000 g for 30 min at 4 °C. Pellets were resuspended in a suspension medium (50 mM Tris–HCl, 5 mM MgCl2, pH 7.4) and centrifuged at 44000 g for 30 min at 4 °C. Pellets were resuspended in an appropriate volume of the same buffer. For each membrane preparation, protein content was evaluated and membranes were then aliquoted and frozen in liquid nitrogen.
2.3. Radioligand binding assay
Competition experiments were performed on membranes expressing serotonin 5-HT2AR or co-expressing 5-HT2AR and dopamine D2R. [3H]Ketanserin was used to label 5-HT2AR as previously reported (Gonzalez-Maeso et al., 2007). Membranes were incubated for 1 h at 30 °C with [3H]Ketanserin (1–5 nM) and with increasing concentrations of non radioactive ligand ranging from 1 pM to 100 μM. Non specific binding was determined with an excess of unlabeled ketanserin (1 μM). Bound tritiated ketanserin fractions were separated from the free tritiated ketanserin by filtration. We used Whatman GF-C filters preincubated with bovine serum albumin (10 mg/ml). Filtration was performed on a Tomtec harvester. Radioactivity was counted on a beta-counter Tri-carb 2100TR (PerkinElmer). Each assay was performed in triplicate. Data were analyzed with the program Graphpad Prism. The inhibition constants (Ki) for ligands were calculated by using the following relation: Ki = IC50/(1 + [*L]/Kd), where IC50 is the concentration of ligand leading to a decrease of 50% of the specific binding and [*L] and Kd are the concentration and dissociation constant of [3H] Ketanserin, respectively.
2.4. Inositol phosphate production
The accumulation of inositol phosphates was determined by measurement of [3H]inositol polyphosphate production. The inositol phosphate accumulation experiments on HEK293 cells-expressing serotonin 5-HT2AR and/or dopamine D2R were performed as previously described (Albizu et al., 2007). Briefly, experiments were performed in 12-well plates (7 × 105cells/well) after overnight labeling with [3H]-myo-inositol (1 μCi/ml/well) in serum-free medium at 37 °C. After washing cells three times with experimental medium (Hank’s buffer containing LiCl 20 mM and Hepes 20 mM), cells were preincubated in experimental medium with various concentrations of drugs for 10–30 min at 37 °C. The reaction was stopped by replacing the medium with 10 mM formic acid (4 °C). Plates were maintained at 4 °C for 30 min to extract the accumulated [3H] inositol polyphosphate from cells. Supernatants were recovered, and inositol phosphates were purified by ion exchange chromatography method using Dowex-1 resin X8 (Bio-Rad). Radioactivity was measured on a beta-counter Tri-carb 2100TR (PerkinElmer).
2.5. Behavioral studies
Experiments were performed on adult (8–12 weeks old) male 129S6/SvEv mice. 5-HT2AR-KO mice have been previously described (Gonzalez-Maeso et al., 2007). Animals were housed at 12 h light/dark cycle at 23 °C with food and water ad libitum. The Institutional Animal Use and Care Committee approved all experimental procedures at the Mount Sinai School of Medicine. Locomotor behavioral studies were performed as previously described (Gonzalez-Maeso et al., 2008).
Motor function was assessed with a computerized three-dimensional activity monitoring system (AccuScanInstruments). The activity monitor has 32 infrared sensor pairs, with 16 along each side spaced 2.5 cm apart. The system determines motor activity on the basis of the frequency of interruptions to infrared beams traversing the x, y and z planes. Total distance (cm) and horizontal activity were automatically determined from the interruptions of the beams in the horizontal plane.
2.6. Co-immunoprecipitation assays
Assays were performed in HEK293 cells using C-terminally RLuc-tagged D2R and GFP-tagged D2R, 5-HT2AR, metabotropic glutamate receptors mGluR2 and mGluR3. Proteins solubilized in lysis buffer (Hepes 50 mM, NaCl 100 mM, glycerol 10%, dodecylmaltoside 0.4% and a protease inhibitor mixture from Roche) were then centrifuged at 14,000 rpm for 30 min at 4 °C. Supernatants were incubated overnight with anti-GFP (Roche Diagnostics) or anti-RLuc (Millipore Corp) antibody at 4 °C on a rotating wheel. Protein G beads were added and incubated 2 h at 4 °C on a rotating wheel. Immunoprecipitated proteins were eluted in Laemli sample buffer, resolved by SDS-polyacrylamide gel electrophoresis, and detected by western blotting. Membranes were incubated overnight at 4 °C with the primary anti-RLuc, anti-GFP or anti-N-Cadherin antibody. Membranes were washed three times for 10 min each in blocking buffer and exposed for 1 h at room temperature with the secondary horseradish peroxidase labeled anti-mouse antibody (Amersham Life Sciences). Detection of proteins was conducted using ECL system.
2.7. Statistical analysis
All data were analyzed using GraphPad Prism 5.0 software. Data are expressed as mean ± SEM. Student’s t-test or F test were used, results were considered statistically significant when p < 0.05.
3. Results
3.1. D2R activation increases the serotonin agonist affinity for 5-HT2AR
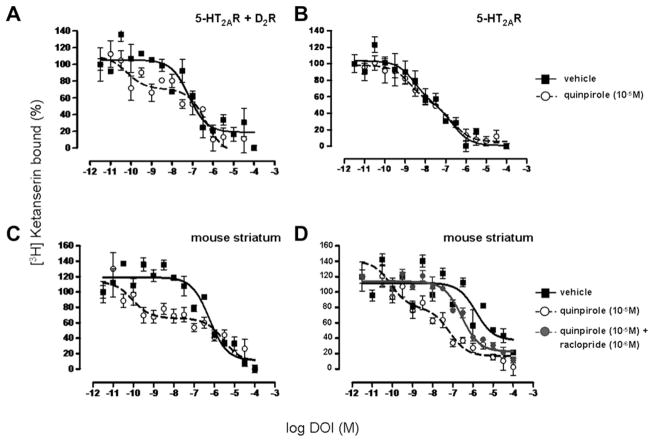
Competition binding assays can provide a sensitive measure of functional interactions within a receptor complex (Gonzalez-Maeso et al., 2008). The effect of receptor crosstalk is observed by determining whether the competition binding curve for one of the receptors is altered by a ligand for the other receptor. This assay can detect crosstalk of untagged receptors expressed in transfected cells and of endogenous receptors expressed in vivo. When appropriate specific controls are performed, the presence of cross-receptor binding modulation strongly supports the existence of a functional crosstalk between receptor subtypes. Therefore, we initially studied the effect of the D2R agonist quinpirole on the competition of the serotoninergic hallucinogen DOI for antagonist ketanserin binding sites on 5-HT2AR in membranes from HEK293 cells transfected with both receptors (Fig. 1A). Quinpirole caused a marked increase in the affinity of DOI for 5-HT2AR. Notably, the curve corresponding to the DOI displacement of [3H] Ketanserin fits to a one-site model while the curve corresponding to the DOI displacement of [3H] Ketanserin in the presence of quinpirole fits to a two-site model.
Fig. 1.
5-HT2AR radioligand competitive curves performed in HEK293 cells and mouse striatum. A, In HEK293 cells co-expressing 5-HT2AR and D2R, 5-HT2AR agonist DOI affinity is higher in the presence of D2R agonist quinpirole (10−5 M) (two-site model; Ki-high = 1.1 × 10−11 M and Ki-low = 2.8 × 10−8 M) (○) from the control (one-site model; Ki = 1.7 × 10−8 M) (■). B, In HEK293 cells expressing only 5-HT2AR, DOI affinity does not change in the presence of quinpirole (10−5 M) (two-site model; Ki-high = 2.9 × 10−10 M, Ki-low = 3.5 × 10−8 M) (○) from the control (two-site model; Ki-high = 5.8 × 10−10 M, Ki-low = 4.2 × 10−8 M) (■). C, In mouse striatum, DOI affinity is significantly higher in the presence of quinpirole (10−5 M) (two-site model; Ki-high = 1.5 × 10−11 M and Ki-low = 4.7 × 10−7 M) (○) from the control (one-site model; Ki = 9.5 × 10−8 M) (■). D, In mouse striatum, the increased DOI affinity in the presence of quinpirole (10−5 M) (two-site model; Ki-high = 8.5 × 10−11 M and Ki-low = 6.9 × 10−8 M) (○) from the control (one-site model; Ki = 3.3 × 10−6 M) (■) is decreased when D2R antagonist raclopride is added (one-site model; Ki = 2.7 × 10−7 M) (●). Data are means ± SEM for each experiment performed in triplicate. The Ki values shown are for this single experiment. Averages of Ki values are indicated in Table 1.
To confirm that the effect of quinpirole on 5-HT2AR agonist affinity resulted from its exclusive binding to the D2R, we repeated the experiment using membranes from HEK293 cells expressing only 5-HT2AR (Fig. 1B). We found that the dopamine agonist did not alter DOI affinity since the two competition curves in presence and absence of quinpirole are very close. We also observed an effect of receptor co-expression on DOI binding in the absence of D2R agonist. The competition curve seen with 5-HT2AR expressed alone fits to a two-site model (Fig. 1B, black curve). However, co-expression of 5-HT2AR and D2R caused one-site binding to be observed (Fig. 1A, black curve) suggesting that D2R expression is sufficient to alter the binding properties of DOI on 5-HT2AR. D2R expression decreases the affinity of the hallucinogenic drug for 5-HT2AR and this decrease is counteracted by D2R activation (in presence of D2R agonist).
We obtained similar results in membranes from the mouse striatum. D2R agonist induced a large increase in the affinity of DOI for 5-HT2AR (competition curve best fitted with a two-site model) (Fig. 1C). The specificity of this effect is demonstrated by using D2R antagonist raclopride (Fig. 1D). While DOI affinity in the presence of raclopride is not altered (data not shown), raclopride prevents the enhanced effect induced by quinpirole on DOI affinity. Thus dopamine D2R expression and activation alter the serotonin 5-HT2AR ligand binding properties (Table 1). This observation supports the presence of allosteric modulation across 5-HT2AR and D2R expressed in heterologous system and in native tissue.
Table 1.
5-HT2AR agonist DOI affinity calculated from [3H]Ketanserin competition curves in mouse striatum and HEK293 cells expressing 5-HT2AR and D2R or 5-HT2AR alone.
| Receptor system | Ligand |
Ki (M)
|
% High | |
|---|---|---|---|---|
| Ki-high | Ki-low | |||
| HEK-5HT2AR-D2R | Vehicle | NA | 7.7 × 10−9 ± 0.25 | NA |
| Quinpirole (10−5 M) | 6.2 × 10−11 ± 0.22 | 1.7 × 10−8 ± 0.32 | 46 ± 3 | |
| HEK-5HT2AR | Vehicle | 3.2 × 10−9 ± 0.18 | 6.1 × 10−7 ± 0.4 | 41 ± 5 |
| Quinpirole (10−5 M) | 1.5 × 10−9 ± 0.45 | 1.3 × 10−7 ± 0.33 | 48 | |
| Mouse striatum | Vehicle | NA | 2.5 × 10−7 ± 0.26 | NA |
| Quinpirole (10−5 M) | 6.5 × 10−11 ± 0.81 | 5.1 × 10−7 ± 0.89 | 44 ± 2 | |
| Raclopride (10−6 M) | NA | 6.6 × 10−7 ± 0.24 | NA | |
| Quinpirole (10−5 M) + raclopride (10−6 M) | NA | 6.9 × 10−7 ± 0.1 | NA | |
DOI competition of [3H]Ketanserin binding was performed in the absence (vehicle) or in the presence of D2R agonist quinpirole (10−5 M) and/or D2R antagonist raclopride (10−6 M). Competition curves were analyzed by nonlinear regression to derive dissociation constants for the high- (Ki-high) and low- (Ki-low) affinity states of the receptor. % High refers to the percentage of high-affinity binding sites as calculated from nonlinear fitting. One-site model or two-site model as a better description of the data was determined by F test. Two-site model, p < 0.05. NA, two-site model is not applicable (p > 0.05). Ki; averages are expressed as means ± SEM of at least three separate experiments.
DOI displacement curve of [3H]Ketanserin with quinpirole (10−5 M) compared to the one with vehicle: F[2,43] = 3.42, *p < 0.05 in HEK-5-HT2AR-D2R; F[2,43] = 7, *p < 0.01 in HEK-5-HT2AR and F[2,43] = 12.88, **p < 0.001 in mouse striatum.
DOI displacement curve of [3H]Ketanserin with quinpirole (10−5 M) + raclopride (10−6 M) compared to the one with vehicle: F[2,40] = 3.2, *p < 0.05 in mouse striatum.
3.2. D2R alters the 5-HT2AR mediated inositol phosphate production
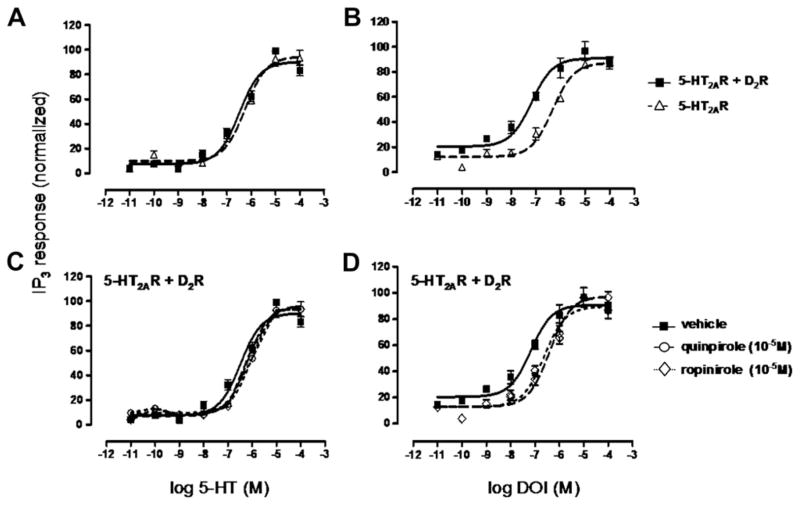
To test whether D2R expression modulates 5-HT2A mediated Gq/11 protein signaling, we measured inositol phosphate (IP) production induced by 5-HT2AR activation in the presence and absence of the D2R (Fig. 2A,B). The response to serotonin (5-HT) was not altered by the presence of D2R (Fig. 2A). However, efficacy of the hallucinogenic agonist DOI was significantly increased in the presence of the D2R since we observed a shift of the curve to the left (Fig. 2B). The apparent difference in 5-HT2AR signaling observed for DOI, but not for serotonin, is consonant with data from several studies suggesting that hallucinogenic serotonergic agonists stabilize a different 5-HT2AR conformation than do non-hallucinogenic ligands (Gonzalez-Maeso et al., 2007, 2003).
Fig. 2.
Inositol Phosphate (IP) production induced by 5-HT2AR activation with natural agonist 5-HT or hallucinogenic drug DOI. A, Serotonin (5-HT)-stimulated 5-HT2AR in HEK293 cells expressing 5-HT2AR (△) and HEK293 cells co-expressing 5-HT2AR and D2R (■). D2R expression does not affect 5-HT-induced 5-HT2AR-IP3 production (EC50 = 5.5 × 10−7 M). B, DOI-stimulated 5-HT2AR in HEK293 cells expressing 5-HT2AR (△) (EC50 = 3.8 × 10−6 M) and HEK293 cells co-expressing 5-HT2AR and D2R (■) (EC50 = 6.7 × 10−8 M). D2R expression increases DOI-induced 5-HT2AR-IP3 production. C, D2R agonists (10−5 M), quinpirole (○) (EC50 = 6.6 × 10−7 M) and ropinirole (◇) (EC50 = 8 × 10−7 M), have no effect on 5-HT-induced 5-HT2AR-IP3 production (vehicle; EC50 = 5.5 × 10−7 M). D, D2R agonists (10−5 M), quinpirole (○) (EC50 = 3.9 × 10−7 M) and ropinirole (◇) (EC50 = 2.4 × 10−7 M) decrease DOI-induced 5-HT2AR-IP3 production (vehicle; EC50 = 6.7 × 10−8 M). Data are means ± SEM for each experiment performed in triplicate. The EC50 values shown are for this single experiment. Averages of EC50 values over all experiments can be found in Table 2.
We also determined the consequences of D2R activation by two different agonists (quinpirole and ropinirole) on IP levels induced by 5-HT or DOI in cells co-expressing 5-HT2AR and D2R (Fig. 2C,D). No effect of either dopamine agonist was detected on serotonin induced IP production (Fig. 2C). In contrast, the promoting effect of D2R expression on DOI-induced IP production was abolished by D2R activation. 5-HT2AR activation is less efficient in presence of D2R agonists (the concentration–response curves shifted to the right) (Fig. 2D). This decrease of efficacy results strictly from 5-HT2AR stimulation since stimulation of D2R alone does not induce Gq/11 protein signaling pathway activation. Thus dopamine D2R expression and activation alter the hallucinogenic drug-stimulated 5-HT2AR second messenger production (Table 2).
Table 2.
Efficacy of 5-HT2AR agonists (5-HT and DOI) to induce inositol phosphate production in HEK293 cells expressing 5-HT2AR or 5-HT2AR and D2R.
| Receptor system | Ligands | 5-HT
|
DOI
|
||
|---|---|---|---|---|---|
| EC50 (M) | Emax (cpm) | EC50 (M) | Emax (cpm) | ||
| HEK-5HT2AR | Vehicle | 6.1 × 10−7 ± 0.27 | 647 ± 74 | 2.6 × 10−6 ± 0.33 | 329 ± 50 |
| HEK-5HT2AR-D2R | Vehicle | 6.1 × 10−7 ± 0.12 | 608 ± 12 | 8.9 × 10−8 ± 0.31 | 332 ± 9 |
| Quinpirole (10−5 M) | 7.2 × 10−7 ± 0.15 | 589 ± 22 | 2.3 × 10−7 ± 0.35 | 332 ± 20 | |
| Ropinirole (10−5 M) | 3.9 × 10−7 ± 0.42 | 564 ± 47 | 1.8 × 10−7 ± 0.1 | 338 ± 61 | |
Dose-responses: cells were stimulated by increasing concentrations (10−11 M–10−4 M) of serotonin 5-HT2AR agonist (serotonin 5-HT or hallucinogen DOI) in presence or not of dopamine D2R agonist (quinpirole or ropinirole) at 10−5 M. Efficient concentrations 50% (EC50) measured reflect the 5-HT/DOI efficacy to activate the 5-HT2AR mediated Gq/11 protein signaling pathway and inositol phosphate production. Emax represents the maximum possible effect for 5-HT/DOI. EC50 averages and Emax are expressed as means ± SEM of at least three separate experiments.
3.3. Role of the 5-HT2AR in behavioral effects of antipsychotic D2R antagonist
The identification of functional crosstalk between the 5-HT2AR and D2R in transfected cells and in mouse brain striatal membranes raised the question of whether evidence for crosstalk could be obtained in vivo. The potent and selective non-competitive NMDA receptor antagonist MK-801 (dizocilpine) is used as a pharmacological model for schizophrenia in mice (Reimherr et al., 1986). MK-801 increases the locomotor activity of mice, a behavior that is suppressed by the antipsychotic haloperidol which acting at D2R (Gattaz et al., 1994). In order to probe for 5-HT2AR and D2R crosstalk in vivo, we compared the effects of haloperidol on MK-801-stimulated locomotion in wild-type and 5-HT2AR-KO mice.
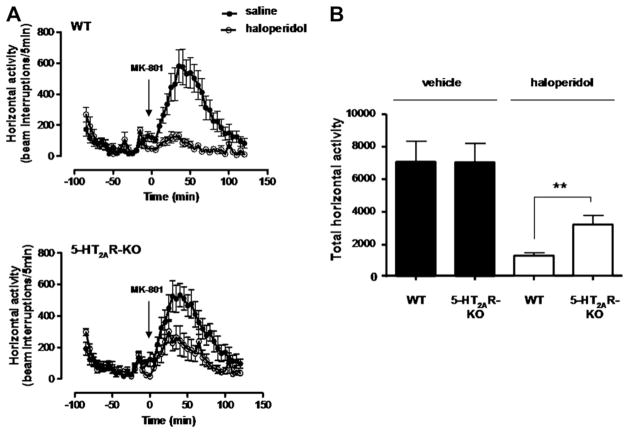
The increased locomotor activity elicited by MK-801 in wild-type and 5-HT2AR-KO mice was similar (Fig. 3A). In the wild-type mice, the effect of MK-801 was largely eliminated in animals pretreated with dopamine D2R antagonist haloperidol. Interestingly, the effect of haloperidol was significantly reduced in the 5-HT2AR-KO mice (p < 0.01) (Fig. 3B). These findings suggest that the haloperidol antipsychotic-like behavioral response requires the expression of 5-HT2AR.
Fig. 3.
5-HT2AR is necessary to induce D2R ligand dependent behavioral effects in mice. Expression of 5-HT2AR is necessary for the inhibition of MK-801-induced locomotor activity by D2R antagonist haloperidol. A, The time course of MK-801-induced locomotion is measured in 5 min blocks. Time of injections of MK-801 is indicated by arrows. Horizontal activity is measured in wild-type (WT) and 5-HT2AR-KO mice. B, Corresponding bar graph of the total MK-801-induced locomotion as a summation of horizontal activity from t = 10 min to t = 120 min. Mice were administered haloperidol (1 mg/kg) or vehicle followed by MK-801 (0.5 mg/kg; i.p.) (n = 6; **p < 0.01; error bars shows SEM).
3.4. Interaction between serotonin 5-HT2AR and dopamine D2R
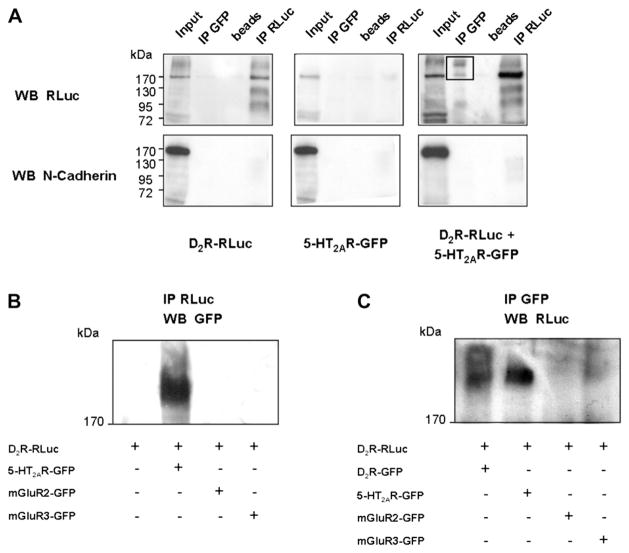
We then examined the possible existence of a physical interaction between serotonin 5-HT2AR and dopamine D2R which could contribute to the receptor crosstalk observed. We performed co-immunoprecipitation assays to test whether 5-HT2AR and D2R could form heteromeric complexes. Receptor co-immunoprecipitation was performed in HEK293 cells co-expressing RLuciferase (RLuc)-tagged D2R and green fluorescent protein (GFP)-tagged 5-HT2AR (Fig. 4A). In control cells expressing D2R-RLuc alone, the RLuc form was immunoprecipitated with the anti-RLuc antibody and D2R-RLuc homomers were detected. No GFP immunoreactivity was detected. In control cells expressing 5-HT2AR-GFP alone, no bands were detected by probing with the anti-RLuc antibody, whether immunoprecipitation was done with the anti-GFP antibody or the anti-RLuc antibody, demonstrating the selectivity of the anti-RLuc antibody used. In cells co-expressing both receptors, immunoprecipitation with the anti-GFP antibody led to immunoreactivity detected by the anti-RLuc antibody, supporting the presence of heteroreceptor complexes. Signal was detected at ~170 kDa, which are consistent with the presence of heteromers. Larger receptor heterocomplexes were also observed. As a negative control, membranes were probed for N-Cadherin immunoreactivity, a protein which is highly expressed in the plasma membrane. The absence of N-Cadherin signal in the immunoprecipitated samples indicates the specificity of the assay and supports a physical association between 5-HT2AR and D2R in cotransfected cells.
Fig. 4.
5-HT2AR and D2R co-immunoprecipitate. A, Specific co-immunoprecipitation of 5-HT2AR and D2R in HEK293 cells. RLuc-tagged D2R and GFP-tagged 5-HT2AR were immunoprecipitated using anti-GFP or RLuc antibodies. Membranes were probed with either anti-RLuc antibody or anti-N-Cadherin antibody. The co-immunoprecipitated receptor complex is indicated by the box in the upper right panel. B, RLuc-tagged D2R and GFP-tagged 5-HT2AR, mGluR2 or mGluR3 were immunoprecipitated using anti-RLuc antibody and membranes were probed with anti-GFP antibody. A co-immunoprecipitated receptor is detected only for D2R with 5-HT2AR. C, RLuc-tagged D2R and GFP-tagged D2R, 5-HT2AR, mGluR2 or mGluR3 were immunoprecipitated using anti-GFP antibody and membranes were probed with anti-RLuc antibody. Complex formation was observed only for D2R homomers and D2R-5-HT2AR heteromers. Panels are representative of three independent experiments. WB, western blot. IP, immunoprecipitation.
To confirm the specificity of the 5-HT2AR-D2R interaction, D2R-RLuc was co-expressed with metabotropic glutamate receptors mGluR2-GFP or with mGluR3-GFP. No co-immunoprecipitation was detected in either case (Fig. 4B,C).
4. Discussion
The related effects of serotonin 5-HT2AR and dopamine D2R antagonists on the symptoms of psychosis in humans and animal models and the colocalization of the two receptors in dopaminergic neurons motivated the study of their heteromerization. Studies showed 5-HT2AR and D2R are both expressed in dopaminergic cells in the ventral tegmental area of the midbrain, the substantia nigra pars compacta and the arcuate nucleus of the hypothalamus (Doherty and Pickel, 2000; Nocjar et al., 2002; Pazos et al., 1985). 5-HT2AR activation by serotonin leads to the dopaminergic neuron activation (Bortolozzi et al., 2005). Radioligand assays confirmed that both receptors are co-expressed in mouse striatal membranes (data not shown). In this study, we found evidence for functional crosstalk between 5-HT2AR and D2R involving allosteric modulation, receptor signaling and drug-induced behavior. Moreover, we provided first evidence supporting the existence of 5-HT2AR-D2R heteromers expressed in transfected cells and in brain.
5-HT2AR is mainly coupled to the Gq/11 protein and activates inositol phosphate production (Conn and Sanders-Bush, 1984) while D2R is mainly coupled to the Gi/o protein and inhibits cAMP production (Montmayeur et al., 1993). We found that D2R expression increases the efficacy of DOI to activate the phosphoinositol Gq/11 signaling pathway downstream of the 5-HT2AR. Addition of D2R agonists eliminates the effect of D2R expression on 5-HT2AR signaling. This effect on 5-HT2AR signaling is seen only for the hallucinogenic partial agonist DOI and not for serotonin. Notably, some studies suggest that hallucinogenic 5-HT2AR agonists stabilize a different receptor conformation than do non-hallucinogenic serotonin agonists (Gonzalez-Maeso et al., 2007, 2003). Thus our results suggest that the allosteric crosstalk observed between the 5-HT2AR and D2R may be influenced by the conformational selectivity of the ligands complexing with the receptors. It is also interesting that the effects of D2R expression and activation on 5-HT2AR signaling properties appear opposite to the effects on 5-HT2AR ligand affinity. D2R expression eliminates the high-affinity DOI binding site and D2R agonist restores it. Finally, the 5-HT2AR-D2R crosstalk might be associated to a decrease in the 5-HT2AR agonist binding potency and an increase in the 5-HT2AR agonist functional potency. When D2R is activated, opposite effects are observed (Fig. 5). It is difficult to compare directly the allosteric effects obtained by antagonist-displacement competition binding in membrane preparations and by intact cell signaling assays. However, the two effects can be reconciled if we assume that the high-affinity site in the membrane binding assay corresponds to a receptor that is uncoupled from Gq/11 protein. In addition, the involvement of other interacting proteins cannot be excluded.
Fig. 5.
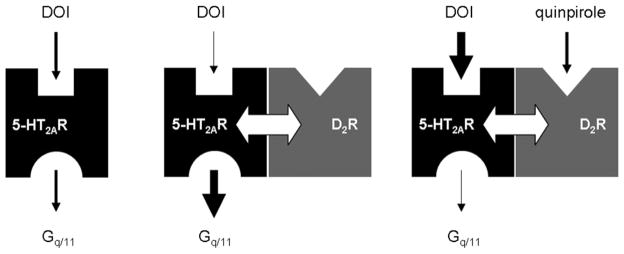
5-HT2AR-D2R heteromer crosstalk hypothesis: effects of D2R expression and activation on 5-HT2AR binding and signaling properties. On the left, serotonin 5-HT2AR is illustrated as binding its agonist DOI and being coupled to Gq/11 protein. In the middle, dopamine D2R expression might have an allosteric effect (illustrated with a white arrow) on 5-HT2AR by decreasing DOI agonist affinity to 5-HT2AR and increasing 5-HT2AR coupling to Gq/11 protein. In contrast, on the right, D2R activation (induced by its agonist quinpirole) leads to an increased affinity of 5-HT2AR for DOI and a decreased Gq/11 protein coupling to 5-HT2AR.
We also found that functional 5-HT2AR-D2R crosstalk occurs at the level of the animal behavior. 5-HT2AR expression is necessary for the full antipsychotic effect of drug acting at the D2R. The haloperidol induced inhibitory effect on animal locomotion is reduced in 5-HT2AR-KO mice. This raises the possibility that the effects of classical antipsychotics are mediated, at least in part, through a 5-HT2AR-D2R interaction. Notably, the 5-HT2AR is expressed in midbrain dopaminergic neurons that express D2R and that project to striatal and limbic areas. It will be interesting to determine if both 5-HT2AR and D2R co-localize presynaptically on dopaminergic neurons where they would significant influence the patterns of dopamine release in these behaviorally important circuits.
The functional crosstalk observed could result either from the direct physical interactions of the two receptors in a heterocomplex or from interactions of pathways downstream of each receptors. Our results do not distinguish between these two possibilities, which are not mutually exclusive. Both the 5-HT2AR and D2R have been identified as forming heteromers with other GPCR with implications in neurological disorders. 5-HT2AR forms a functional interaction in cortical neurons with mGluR2 (Gonzalez-Maeso et al., 2008) while D2R has been reported to form heteromers with dopamine D1R, D3R, adenosine A2AR, cannabinoid CB1R, histamine H3Rs and metabotropic glutamate receptor 5 (mGluR5) (Albizu et al., 2010b). D2R homomers have been identified in living cells (Armstrong and Strange, 2001). Recently, 5-HT2AR homomers and 5-HT2AR-D2R heteromers have been detected by biophysical approaches in HEK293 cells cotransfected with tagged receptors (Borroto-Escuela et al., 2010; Lukasiewicz et al., 2010). While many receptors have been reported to form heteromers in transfected cells, the evidence for functional interactions and for complex formation in vivo is more limited (Albizu et al., 2010a; Barki-Harrington et al., 2003; Damak et al., 2003; Zhao et al., 2003). Here we found that the D2R itself has an allosteric effect on the binding of an agonist to the 5-HT2AR. Moreover, a high concentration of D2R agonist increases hallucinogenic agonist DOI binding on 5-HT2AR. The monophasic competition curve of [3H] ketanserin by DOI changed to become biphasic when D2R is activated by quinpirole. The detection of a high-affinity binding site revealed the positive cooperative effect between the serotoninergic and dopaminergic binding sites. Several studies showed the link between ligand dependent allosteric modulations and GPCR oligomer existence (Durroux, 2005; Giraldo, 2008; Mattera et al., 1985; Springael et al., 2006; Urizar et al., 2005; Wreggett and Wells, 1995). In agreement with previous work (Albizu et al., 2006), the positive cooperative binding can be explained by considering a multivalent complex in which the binding of a ligand on one protomer enhances the binding of a second ligand on the second binding site. Associated with co-immunoprecipitation data, these results provide evidence showing that 5-HT2AR and D2R form functional heterocomplexes both in cell lines and in brain.
5. Conclusion
Understanding the mechanism by which hallucinogens induce their effects and antipsychotics block them is essential in the treatment of psychosis. Such mechanism helps to explain communication between two different Gq/11 and Gi/o protein-coupled receptors involved in the altered brain processes of schizophrenia. Because schizophrenia can be treated by medications that act on the 5-HT2AR and/or the D2R, mutual influences of their signaling pathways would improve the understanding of antipsychotic drug interactions and the development of better therapeutics (Rozenfeld and Devi, 2010). The 5-HT2AR-D2R functional crosstalk provides a new window into novel mechanisms for signal integration across serotonin and dopamine systems with relevance for normal brain physiology and for psychopharmacology. The discovery of a 5-HT2AR-D2R heterocomplex represents a potential opportunity as a new target for antipsychotics. Future investigations are necessary to confirm the role of the 5-HT2AR-D2R heteromer in mediating the receptor crosstalk observed.
Acknowledgments
We thank Yuuya Okawa for technical assistance and Fernand Hayot for the critical reading of the manuscript. This work was supported by NIH PO1 DA012923 (S.C.S.), R01 MH084894 (J.G.M.) and a NARSAD Distinguished Investigator Award (S.C.S.).
References
- Adayev T, Ranasinghe B, Banerjee P. Transmembrane signaling in the brain by serotonin, a key regulator of physiology and emotion. Biosci Rep. 2005;25:363–385. doi: 10.1007/s10540-005-2896-3. [DOI] [PubMed] [Google Scholar]
- Albizu L, Balestre MN, Breton C, Pin JP, Manning M, Mouillac B, et al. Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol Pharmacol. 2006;70:1783–1791. doi: 10.1124/mol.106.025684. [DOI] [PubMed] [Google Scholar]
- Albizu L, Teppaz G, Seyer R, Bazin H, Ansanay H, Manning M, et al. Toward efficient drug screening by homogeneous assays based on the development of new fluorescent vasopressin and oxytocin receptor ligands. J Med Chem. 2007;50:4976–4985. doi: 10.1021/jm061404q. [DOI] [PubMed] [Google Scholar]
- Albizu L, Cottet M, Kralikova M, Stoev S, Seyer R, Brabet I, et al. Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat Chem Biol. 2010a;6:587–594. doi: 10.1038/nchembio.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizu L, Moreno JL, Gonzalez-Maeso J, Sealfon SC. Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neuro-therapeutics. CNS Neurol Disord Drug Targets. 2010b;9:636–650. doi: 10.2174/187152710793361586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Carrion O, Poppel E. Dopamine, learning, and reward-seeking behavior. Acta Neurobiol Exp (Wars) 2007;67:481–488. doi: 10.55782/ane-2007-1664. [DOI] [PubMed] [Google Scholar]
- Armstrong D, Strange PG. Dopamine D2 receptor dimer formation: evidence from ligand binding. J Biol Chem. 2001;276:22621–22629. doi: 10.1074/jbc.M006936200. [DOI] [PubMed] [Google Scholar]
- Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Circulation. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Tarakanov AO, Marcellino D, Ciruela F, Agnati LF, et al. Dopamine D2 and 5-hydroxytryptamine 5-HT(A) receptors assemble into functionally interacting heteromers. Biochem Biophys Res Commun. 2010;401:605–610. doi: 10.1016/j.bbrc.2010.09.110. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Diaz-Mataix L, Scorza MC, Celada P, Artigas F. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem. 2005;95:1597–1607. doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and hetero-dimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. Selective 5HT-2 antagonists inhibit serotonin stimulated phosphatidylinositol metabolism in cerebral cortex. Neuropharmacology. 1984;23:993–996. doi: 10.1016/0028-3908(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Costa E. The role of serotonin in neurobiology. Int Rev Neurobiol. 1960;2:175–227. doi: 10.1016/s0074-7742(08)60123-3. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- de Almeida J, Palacios JM, Mengod G. Distribution of 5-HT and DA receptors in primate prefrontal cortex: implications for pathophysiology and treatment. Prog Brain Res. 2008;172:101–115. doi: 10.1016/S0079-6123(08)00905-9. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Pickel VM. Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res. 2000;864:176–185. doi: 10.1016/s0006-8993(00)02062-x. [DOI] [PubMed] [Google Scholar]
- Durroux T. Principles: a model for the allosteric interactions between ligand binding sites within a dimeric GPCR. Trends Pharmacol Sci. 2005;26:376–384. doi: 10.1016/j.tips.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Woods AS, Lluis C, Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007;30:440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Gattaz WF, Schummer B, Behrens S. Effects of zotepine, haloperidol and clozapine on MK-801-induced stereotypy and locomotion in rats. J Neural Transm Gen Sect. 1994;96:227–232. doi: 10.1007/BF01294789. [DOI] [PubMed] [Google Scholar]
- Giraldo J. On the fitting of binding data when receptor dimerization is suspected. Br J Pharmacol. 2008;155:17–23. doi: 10.1038/bjp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and non-hallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. GPCR monomers and oligomers: it takes all kinds. Trends Neurosci. 2008;31:74–81. doi: 10.1016/j.tins.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N, van Praag HM. The role of serotonin in schizophrenia. Eur Neuropsychopharmacol. 1995;(Suppl 5):11–23. doi: 10.1016/0924-977x(95)00027-m. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN. The dopamine hypothesis of schizophrenia: limbic interactions with serotonin and norepinephrine. Psychopharmacology (Berl) 1993;112:S16–S34. doi: 10.1007/BF02245004. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin–dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Kienast T, Heinz A. Dopamine and the diseased brain. CNS Neurol Disord Drug Targets. 2006;5:109–131. doi: 10.2174/187152706784111560. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158. doi: 10.1196/annals.1300.063. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz S, Polit A, Kedracka-Krok S, Wedzony K, Mackowiak M, Dziedzicka-Wasylewska M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim Biophys Acta. 2010;1803:1347–1358. doi: 10.1016/j.bbamcr.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Brent DA, Arango V. The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacology. 2001;24:467–477. doi: 10.1016/S0893-133X(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Mattera R, Pitts BJ, Entman ML, Birnbaumer L. Guanine nucleotide regulation of a mammalian myocardial muscarinic receptor system. Evidence for homo- and heterotropic cooperativity in ligand binding analyzed by computer-assisted curve fitting. J Biol Chem. 1985;260:7410–7421. [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- Milligan GG. Protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Guiramand J, Borrelli E. Preferential coupling between dopamine D2 receptors and G-proteins. Mol Endocrinol. 1993;7:161–170. doi: 10.1210/mend.7.2.7682286. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Roth BL, Pehek EA. Localization of 5-HT(2A) receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience. 2002;111:163–176. doi: 10.1016/s0306-4522(01)00593-0. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Reimherr FW, Wood DR, Wender PH. The use of MK-801, a novel sympathomimetic, in adults with attention deficit disorder, residual type. Psychopharmacol Bull. 1986;22:237–242. [PubMed] [Google Scholar]
- Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, et al. Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. Embo J. 2009;28:2195–2208. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heteromerization and drug discovery. Trends Pharmacol Sci. 2010;31:124–130. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springael JY, Le Minh PN, Urizar E, Costagliola S, Vassart G, Parmentier M. Allosteric modulation of binding properties between units of chemokine receptor homo- and hetero-oligomers. Mol Pharmacol. 2006;69:1652–1661. doi: 10.1124/mol.105.019414. [DOI] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. Embo Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, et al. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. Embo J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Wreggett KA, Wells JW. Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J Biol Chem. 1995;270:22488–22499. doi: 10.1074/jbc.270.38.22488. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]