Abstract
The perception of external sensory information by the brain requires highly ordered synaptic connectivity between peripheral sensory neurons and their targets in the central nervous system. Since the discovery of the whisker-related barrel patterns in the mouse cortex, the trigeminal system has become a favorite model for study of how its connectivity and somatotopic maps are established during development. The trigeminal brainstem nuclei are the first CNS regions where whisker-specific neural patterns are set up by the trigeminal afferents that innervate the whiskers. In particular, barrelette patterns in the principal sensory nucleus of the trigeminal nerve provide the template for similar patterns in the face representation areas of the thalamus and subsequently in the primary somatosensory cortex. Here, we describe and review studies of neurotrophins, multiple axon guidance molecules, transcription factors, and glutamate receptors during early development of trigeminal connections between the whiskers and the brainstem that lead to emergence of patterned face maps. Studies from our laboratories and others’ showed that developing trigeminal ganglion cells and their axons depend on a variety of molecular signals that cooperatively direct them to proper peripheral and central targets and sculpt their synaptic terminal fields into patterns that replicate the organization of the whiskers on the muzzle. Similar mechanisms may also be used by trigeminothalamic and thalamocortical projections in establishing patterned neural modules upstream from the trigeminal brainstem.
Keywords: brainstem trigeminal complex, trigeminal ganglion, NGF, NT3, GDNF, Slits, transcription factors, NMDA receptors
The somatotopic map forms sequentially, beginning at the periphery and ending in the primary somatosensory cortex. In some species, such as rodents, neural modules form within the CNS body maps that correspond to patterned distribution of the sensory receptors in the periphery. In the mouse and rat muzzle, whiskers and sinus hairs are patterned in discrete arrays with constant numbers in each row of follicles (Yamakado and Yohro, 1979; Dörfl, 1985; Rice et al., 1986, 1993; Brecht et al., 1997). The infraobital nerve (ION) of the trigeminal ganglion (TG) innervates the whisker pad and relays the whisker-related information to the principal sensory nucleus (PrV) in the brainstem, which in turn conveys the signals to the thalamus and the cortex via ascending afferents. The trigeminal brainstem complex also contains the spinal trigeminal nucleus, including subnuclei oralis (SpVo), interpolaris (SpVi), and caudalis (SpVc) (Belford and Killackey, 1979a, 1979b; Waite and Tracey, 1995). Among them, the PrV-based lemniscal pathway and the SpVi-based paralemniscal pathway represent two parallel pathways that relay whisker-related information from the periphery to the cortex (Jacquin et al., 1988, 1989; Henderson and Jacquin, 1995). Trigeminal primary afferents segregate after they branch into the spinal nucleus and subsequently distinct patches (also called barrelettes) can be detected in the brainstem nuclei upon histochemical staining (Belford and Killackey, 1979a, 1979b; Ma and Woolsey, 1984; Ma, 1991, 1993). This discrete pattern is replicated sequentially in the PrV, the medial division of the ventroposterior nucleus of the thalamus (barreloids in VPM), and layer IV of the primary somatosensory cortex (barrels) (Woolsey and Van der Loos, 1970; Van der Loos, 1976; Belford and Killackey, 1980; Durham and Woolsey, 1984). In this way, the trigeminal system tells the brain not only what kind of sensory information it is receiving, but also where on the face this information is being sensed.
In rodents, the major peripheral target of TG axons is the whiskerpad, where they form patterned terminal arbors around the whisker follicles. Their central counterparts grow into the hindbrain and first lay down a highly restricted trigeminal tract. During tract formation, central TG axons grow unbranched for several days. Later, they emit collaterals into the brainstem trigeminal nuclei and form terminal axon clusters that replicate the patterned organization of the whisker follicles (Erzurumlu and Killackey, 1983; Erzurumlu and Jhaveri, 1992). These patterns are detected by select sets of postsynaptic neurons, and collectively pre- and postsynaptic processes form morphologically and functionally distinct modules that replicate the patterned display of whisker follicles on the muzzle.
The well-described somatotopic pattern in the BSTC and its convenient detection make it one of the most attractive systems for studying fundamental mechanisms that control neuronal connection and pattern formation. Insofar as the whisker-related patterns are clearest during early postnatal periods, much of our knowledge regarding the formation of whisker-related patterns has been obtained by surgical manipulation of the periphery in postnatal animals. To date, numerous studies indicated that the periphery plays an instructive role in somatotopic pattern formation (Belford and Killackey, 1980; Durham and Woolsey, 1984; Jacquin and Rhoades, 1985; Van der Loos et al., 1986; Welker and Van der Loos, 1986). Moreover, because lesions of the PrV lead to an absence of whisker-related patterns at higher levels of the brain, and lesions of the SpVi do not, the PrV has an indispensable role in thalamic and cortical patterning (Killackey and Fleming, 1985). Extrinsic factors, such as periphery-derived factors, as well as retrogradely transported factors, play a role in the development of the PrV (Henderson et al., 1994; Chiaia et al., 1996, 1997; Jhaveri et al., 1998). NMDA activity-dependent processes have also been proposed to account for whisker-related pattern formation in the PrV. Targeted gene deletion studies in mice suggest that NMDA-mediated activity plays a role in the consolidation of whisker-related pattern formation (Erzurumlu and Kind, 2001). In this review, we discuss the results of studies from our laboratories aimed at identifying molecular mechanisms that guide the formation of topographic projections, and patterning of trigeminal afferents in the brainstem, which set the stage for patterned topographic face maps in upstream trigeminal centers leading to the neocortex.
DEVELOPMENT OF RODENT TRIGEMINAL SYSTEM AND IN VITRO MODELS
TG cells are pseudounipolar neurons. Embryonic TG cells develop two axonal processes emerging from opposite ends of the cell body. One axon grows toward peripheral targets, navigating through nonneuronal cells, and the other enters the hindbrain and differentiates therein. The embryonic and postnatal development of the rodent trigeminal system has been studied in detail (Altman and Bayer, 1980, 1984; Erzurumlu and Killackey, 1983; Stainier and Gilbert, 1990, 1991; Rhoades et al., 1991; Erzurumlu and Jhaveri, 1992). A large body of information exists about various characteristics of TG and brainstem trigeminal cells. These include their electrophysiological properties, expression of cytoskeletal markers, neurotransmitters, receptors, and dependence on neurotrophins for survival (Davies et al., 1981, 1987; Jacquin et al., 1988, 1989; Wyatt et al., 1990; Davies, 1994, 1998; Ernfors et al., 1994; Snider, 1994; Silos-Santiago et al., 1995; Rice et al., 1998; Ho and Waite, 1999; Lo et al., 1999; Waite et al., 2000).
In both rats and mice, peripheral and central processes of TG cells show a distinct target-directed growth as soon as they emit axonal processes (Erzurumlu and Killackey, 1983; Stainier et al., 1990, 1991; Erzurumlu and Jhaveri, 1992). The development and differentiation of the rat and mouse trigeminal pathway are strikingly similar, with a slight shift in the timing of events. For trigeminal tract development, E13 in the mouse corresponds to E15 rat (trigeminal axon elongation), and E15 in the mouse to E17 in the rat (trigeminal axon branching/arborization) (Erzurumlu and Killackey, 1983; Stainier and Gilbert, 1990, 1991; Erzurumlu and Jhaveri, 1992; Waite et al., 2000). The three components of the peripheral projections, ophthalmic, maxillary, and mandibular nerves, follow specific routes separate from each other from the outset. The ION component of the maxillary division is a thick nerve and it invades the developing whiskerpad from a caudal-to-rostral direction. Tightly bundled IO axons fan out into fascicles as they enter the whiskerpad and segregate into row nerves as the follicular development proceeds. Axons gradually leave the row nerves and envelope developing follicles and form a branched terminal plexus. In contrast, the growth cones of central axons immediately bifurcate upon entry into the brainstem and elongate along a restricted pathway to form the ascending and descending components of the trigeminal tract (Erzurumlu and Jhaveri, 1992). The central axons grow in the elongation phase without branching for several days and then begin emitting radially oriented collaterals into the brainstem trigeminal nuclei and develop terminal synaptic arbors (Fig. 1).
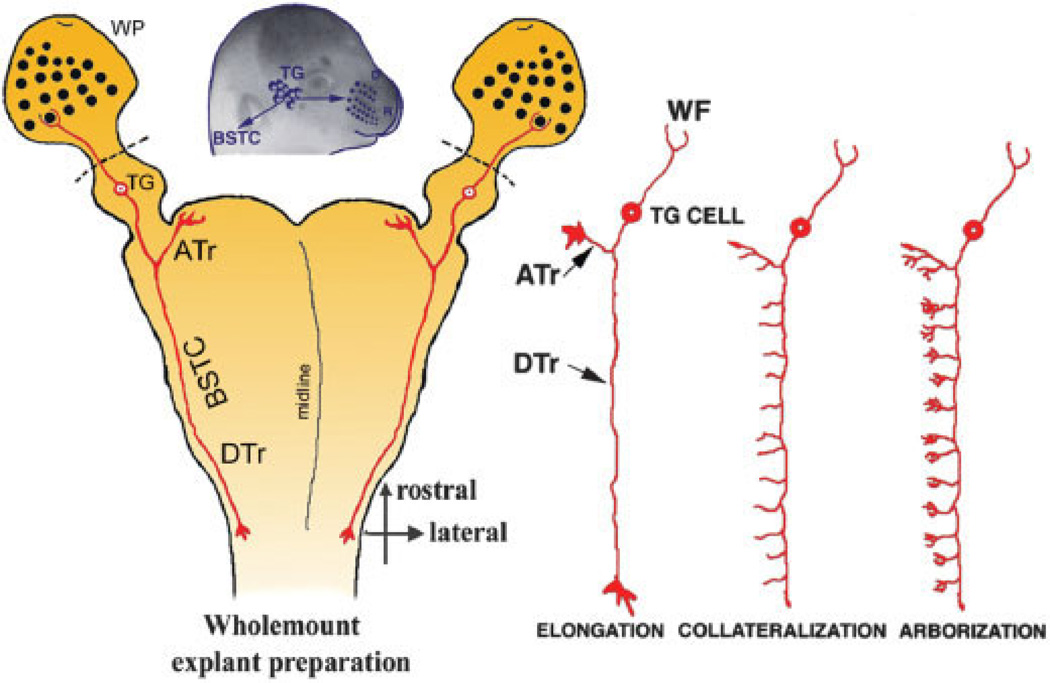
Fig. 1.
Elongation and arborization phases of TG axons. The whiskerpad TG brainstem whole-mount explant is illustrated on the left and TG axon elongation, collateralization, and arborization phases are depicted on the right. ATr, ascending trigeminal tract; DTr, descending trigeminal tract; TG, trigeminal ganglion; BSTC, brainstem trigeminal complex; WF, whisker follicle; WP, whiskerpad.
The use of in vivo paradigms to determine trigeminal axon growth patterns and shifts from elongation to arborization remain technically challenging and often do not reach beyond descriptive chronicles. However, organotypic explant cultures and cocultures provide experimental access to biological phenomena that are difficult to address in vivo. During the past several years, we have demonstrated that many aspects of TG axon growth characteristics into peripheral and central targets can be recapitulated in organotypic explant cocultures of TG with peripheral and central targets. Furthermore, the entire pathway between the whiskerpad and the brainstem can be isolated as a whole and grown in culture. The three-dimensional morphology and the physiological characteristics of the explants are preserved to a remarkable degree under such culture conditions, and de novo functional connections form (Erzurumlu et al., 1993, 1994, 1997; Erzurumlu and Jhaveri, 1995; Ulupinar and Erzurumlu, 1998; Ulupinar et al., 2000). We have demonstrated that in cocultures of TG with whiskerpad explants, TG axons grow into this target (even in the absence of their central target, the brainstem) and form a patterned array of terminal arbors around developing whisker follicles within 3 days (Fig. 2). We have also demonstrated that peripheral and central processes of TG cells are highly plastic and both axonal processes can develop peripheral morphologies when cocultured in between two whiskerpad explants or develop central morphologies if cultured between two brainstem explants (Fig. 2) (Erzurumlu et al., 1994). In addition, elongating TG axons can be induced to form terminal arbors that are synaptically active when cocultured with a chronologically older brainstem explant (Fig. 2) (Erzurumlu and Jhaveri, 1995; Erzurumlu et al., 1997). On the other hand, older TG explants that have already developed terminal arbors in situ revert back to elongation phase when they are cocultured with chronologically younger brainstem explants (Fig. 2) (Erzurumlu and Jhaveri, 1995). Clearly, these results indicate the presence of target-derived molecular signals that regulate axonal differentiation. We also noted that such cues are preserved between species. We cocultured developmentally equivalent ages of chick TG with rat whiskerpad explants. We saw that chick TG axons invade this foreign target and form a circumfollicular pattern, whereas rat TG explants cocultured with chick maxillary process explants behave just like chick TG axons in this target, forming no patterns (Haeberle and Erzurumlu, 2001). We also observed that in cocultures of TG with whiskerpad explants, axon growth is preferentially from caudal to rostral in the whiskerpad explants. TG explants placed next to the rostral tip of the whiskerpad explants fail to grow in and they appear to be deflected by an invisible barrier (Gunhan-Agar et al., 2000). Collectively, these results from in vitro assays underscore the importance of target-derived signals in trafficking trigeminal axons and their terminal differentiation
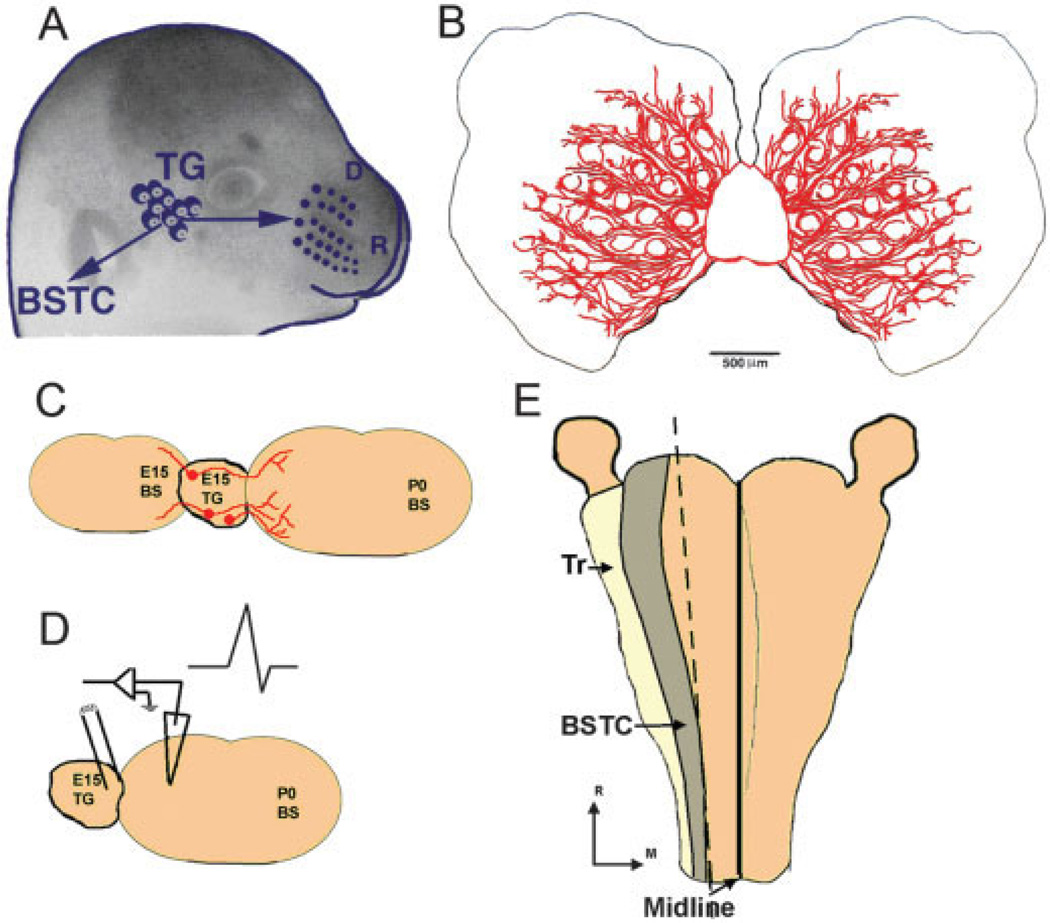
Fig. 2.
Trigeminal system coculture setups. A: TG and its projections in the embryo. D, dorsal; R, rostral; BSTC, brainstem trigeminal complex. B: TG axon growth when a single E15 TG is cocultured with two whiskerpads. C: Age-mismatched TG with brainstem targets. E15 TG axons elongate in E15 brainstem explant but arborize in P0 brainstem explants and form synaptic contacts. D: Following TG axon growth into older brainstem explants, synaptic activity can be recorded in the trigeminal nuclei after stimulation of the TG. E: Experimental setup for whole-mount brainstem explant cultures with TG intact on both sides. Tr, trigeminal tract; R, rostral; M, medial.
ROLE OF NEUROTROPHINS IN TRIGEMINAL DIFFERENTIATION
During embryonic development, primary sensory neurons compete for a limited supply of survival factors provided by their target tissues. NGF family polypeptides are major contributors to the regulation of neuronal numbers via cell survival (for reviews, see Barde, 1994; Snider, 1994; Lewin and Barde, 1996; Conover and Yancopoulos, 1997; Reichardt and Fariñas, 1997; Huang and Reichardt, 2001). Nerve growth factor (NGF) was the first identified neurotrophic factor (Levi-Montalcini, 1987). To date, four members of the NGF family of neurotrophic factors, NGF, brain-derived neurotrophic factor (BDNF) (Barde et al., 1982), neurotrophin3 (NT3) (Ernfors et al., 1990; Jones and Reichardt, 1990), and neurotrophin4/5 (NT4/5) (Berkemier et al., 1991; Ip et al., 1992), have been identified in mammals. These molecules exert their effects by binding specific receptor protein tyrosine kinases (the Trk family), and each neurotrophin also interacts with a low-affinity receptor, p75NTR. Internalized receptor-ligand complexes are retrogradely transported to the neuronal soma, where they activate a cascade of proto-oncogene signals involved in cell proliferation and differentiation (Bothwell, 1995; Segal and Greenberg, 1996). Numerous studies using in vitro assays and targeted gene deletions underscored a role for neurotrophins in directing several aspects of neuronal development such as dendritic and axonal differentiation and synaptic plasticity (Snider and Lichtman, 1996; McAllister et al., 1997; Horch et al., 1999; Schuman, 1999; Horch and Katz, 2002).
NEUROTROPHIN ACTIONS ON MORPHOLOGICAL DIFFERENTIATION OF AXONS
The NGF family of neurotrophins contributes to the differentiation of axonal processes of many types of neurons (Zhang et al., 1994; Cohen-Corey and Fraser, 1995; Castellani and Bolz, 1999). In dissociated primary sensory neuron cultures and in explant cultures of the embryonic trigeminal pathway, NGF promotes exuberant axon elongation outside the central trigeminal tract, whereas NT3 leads to precocious arborization of central tract axons (Ulupinar et al., 2000). Delineating neurotrophin effects on axonal differentiation has been difficult due to trophic effects on cellular viability. Two approaches have yielded significant information about neurotrophin effects on axonal differentiation. One is the use of compartmentalized chambers, whereby neurotrophins are applied selectively to axons or somata (Campenot, 1977, 1994). This in vitro approach revealed multiple aspects of neurotrophin signaling (Miller and Kaplan, 2001a, 2001b; Heerssen and Segal, 2002). The second approach is the analyses of neurons that survive independent of neurotrophins. Differential effects of NGF and NT3 on primary sensory axons were reported in mice with the null mutation of the preapoptotic gene Bax, in which sensory neurons survive independent of neurotrophins (Lentz et al., 1999). Thus, in vitro, sensory neurons that lack Bax or overexpress the survival-promoting gene Bcl2 show differential axonal growth when stimulated with neurotrophins (Lentz et al., 1999; Goldberg et al., 2002; Markus et al., 2002). Mice that lack the Bax gene and specific neurotrophin or Trk receptors have distinct phenotypes. In Bax and NGF or TrkA double knockout mice, central axons of NGF-dependent DRG neurons grow into the spinal cord but their peripheral counterparts fail to develop (Patel et al., 2000, 2003).
Earlier studies employed local applications of neurotrophins in dissociated cell cultures. For example, Gundersen and Barrett (1979) showed that dissociated chick dorsal root ganglion (DRG) cell axons grow toward a source of NGF. Collateralization of dissociated chick DRG neurites has also been noted in the presence of neurotrophin-coated beads (Gallo and Letourneau, 1998). Neurotrophin-coated beads most likely exert their effect on contact with the axonal processes. We investigated the effects of localized neurotrophin sources on the behavior of embryonic rat central trigeminal axons in the brainstem. We embedded neurotrophin-soaked beads along the central trigeminal tract in whole-mount cultures of the trigeminal pathway (Ozdinler et al., 2005). We showed that both NGF and NT3 attract and differentially affect developing central trigeminal axons. Surprisingly, in the presence of NGF, while many axons extend toward the neurotrophin source, several others grow long distances away from the NGF source. NT3-loaded beads, on the other hand, lead to dense arborization and axonal tangles close to the neurotrophin source (Fig. 3). Differential gradients of neurotrophins along with other signaling molecules most likely regulate the dynamics of axonal cytoskeletal elements via Rho GTPases and other intracellular signaling molecules. We showed that blocking Rac activity virtually eliminates neurotrophin-induced axonal growth outside the trigeminal tract, whereas blocking Rho activity attenuates this response (Ozdinler and Erzurumlu, 2001). Along with activation of Rho GTPases, Trk receptor signaling activates several small G-proteins (Ras, Rap1), MAP kinase, PI 3kinase, and phospholipase C pathways (Markus et al., 2002; Huang and Reichardt, 2003; Segal, 2003). Another neurotrophin receptor that plays a major role in affecting neurotrophin signaling by Trks is p75NTR, which modifies ligand-binding specificity and affinity (Huang and Reichardt, 2003). RhoA can be activated by p75NTR, and neurotrophin binding can abolish Rho activity (Yamashita et al., 1999). Thus, NGF could induce a variety of effects depending on its concentration and level of binding to Trks and p75NTR.
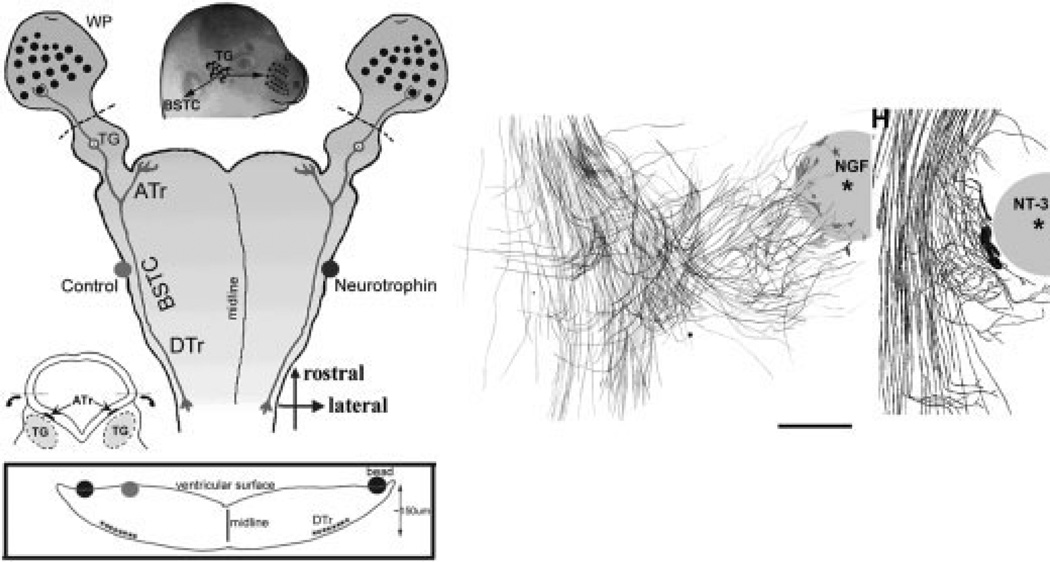
Fig. 3.
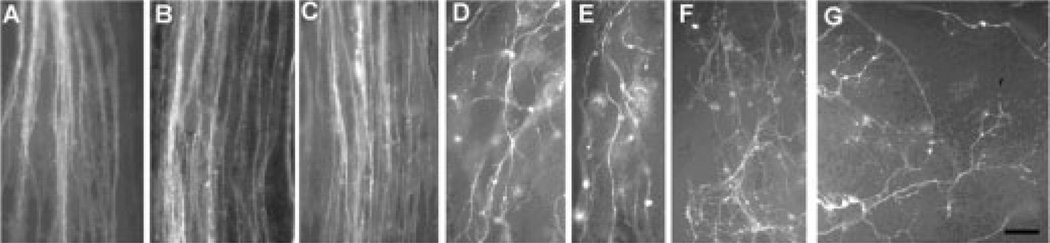
Left: Schematic diagram of trigeminal pathway whole-mount cultures and neurotrophin-loaded bead placement along the central trigeminal pathway. Cartoon diagrams illustrate the preparation of flattened whole-mounts and indicate the position of the neurotrophin-loaded beads with respect to the trigeminal tract and brainstem trigeminal nuclei. WP, whisker pad; BSTC, brainstem trigeminal complex; ATr, ascending trigeminal tract; TG, trigeminal ganglion; DTR, descending trigeminal tract. Right: Camera lucida drawings show the differential effects of NGF- and NT3-loaded beads on central trigeminal axons. Scale bar = 100 µm. Figure modified from Ozdinler et al. (2004).
NT3 AS A CHEMOTROPIC AXON GUIDANCE MOLECULE FOR SENSORY AXONS
Few studies have implicated NT3 as a chemotropic agent for sensory and motor axons. Chemotropic action of the embryonic mouse maxillary process on TG neurons was demonstrated in vitro (Lumsden and Davies, 1986), but the identity of the attractant, “Maxfactor,” remained unknown for a decade and is now known as a mixture of NT3 and BDNF (O’Connor et al., 1999). Tropic effects of neurotrophins on axon growth have emerged in recent years. Tucker et al. (2001) used slice cultures from embryonic transgenic mice that express GFP in their axons. They implanted neurotrophin-soaked beads in ectopic loci within the limb and examined axon growth. They showed that developing sensory and motor axons change their trajectories and preferentially grow toward neurotrophin-soaked beads. Conversely, beads soaked with neurotrophin function-blocking antibodies led to reduction of sensory and motor axon growth. In transgenic mice, which overexpress NT3 under the nestin promoter in the CNS, the course of the proprioceptive afferents is altered and directed toward the high levels of ectopic NT3 expression regions in the spinal cord (Ringstedt et al., 1997). Recently, Patel et al. (2003) reported on Bax/NT3 double knockout mice. They did not see any morphologically identifiable proprioceptive afferents in the periphery of the double knockouts and noted that central “proprioceptive” afferents terminate in the intermediate spinal cord without extending ventrally. Their observations are based on parvalbumin (PV) immunostaining and DiI labeling of the peripheral nerves. We have also bred Bax/NT3 double knockout mice and find that PV immunolabeling is diminished and that TrkC-immunopositive fibers are present within the peripheral nerves as they approach their target muscle (Genc et al., 2004). Incomplete labeling with DiI gives the impression that central proprioceptive axons stop in the intermediate laminae of the spinal cord (Patel et al., 2003). However, with complete dye diffusion, it is possible to trace these axons into the ventral midline and across to the contralateral side and visualize terminal boutons at their tips (Genc et al., 2004). Thus, our results showed that in Bax/NT3 double knockouts, proprioceptive afferents enter the ventral cord, but in the absence of NT3, they terminate in ectopic loci (Genc et al., 2004). In NT3/Bax double knockout mice, many TG neurons are rescued and both TrkA- and TrkC-positive cells are seen (Fig. 4). The development and phenotypes of NT3-dependent sensory axons in the trigeminal system are under investigation.
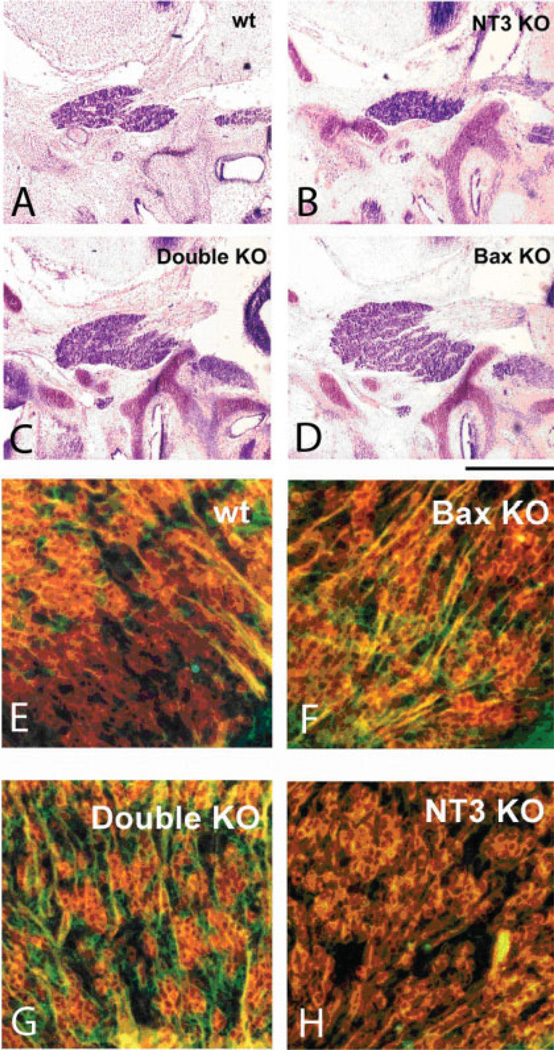
Fig. 4.
Nissl-stained sagittal sections shows the size of the trigeminal ganglion in wild-type (A), NT3 KO (B), NT3/Bax KO (C), and Bax KO (D) mice of equivalent ages. TrkA (red) and TrkC (green) immunostaining shows nonoverlapping groups of TrkA- and TrkC-positive TG cells in E13 wild-type (wt; E), Bax KO (F), Bax/NT3 double KO (G), and NT3 KO (H) mice. Scale bars = 2 mm (A–D); 100 µm (E–G). Figure provided by B. Genc.
GDNF FAMILY LIGANDS AND RECEPTORS IN TRIGEMINAL DEVELOPMENT
Glia-derived neurtrophic factor (GDNF) was first discovered from a glioma cell line that sustained embryonic midbrain neurons in culture (Lin et al., 1993). In vitro studies then revealed GDNF trophic support of other CNS neurons, including spinal motoneurons and peripheral ganglia (Buj-Bello et al., 1995; Ebendal et al., 1995; Trupp et al., 1995; Kotzbauer et al., 1996; Cacalano et al., 1998). Three more members of the GDNF family of neurotrophic factors were discovered. Neurturin displays a 44% homology to GDNF (Kotzbauer et al., 1996). GDNF and neurturin signal through a receptor complex consisting of a signaling subunit, the ret tyrosine kinase, and a binding subunit, the GFRα (GDNF family receptor alpha), which are glycosylphosphatidylinositol-linked cell surface proteins (Durbec et al., 1996; Jing et al., 1996; Treanor et al., 1996; Trupp et al., 1996; Creedon et al., 1997). Artemin and persephin are the other two GDNF family of ligands (GFLs) (Baloh et al., 1998a,b; Milbrandt et al., 1998). There are four members of the GFRα coreceptors: GFRα1, GFRα2, GFRα3, and GFRα4, which serve as preferential receptors for GDNF, neurturin, artemin, and persephin, respectively (for reviews, see Baloh et al., 2000; Enomoto et al., 2001). As ret itself cannot bind the GFLs, both GFRα and ret are required to form a functional GFL receptor. Because ret is the common signaling component for all of the GFLs, mice deficient in ret represent a complete pan-GFL knockout preparation. These mice die at birth due to absence of kidneys and enteric neurons distal to the stomach (Schuchardt et al., 1994). Many sensory neurons in the dorsal root and nodose ganglia require GDNF GFRα1 signaling for survival. In the dorsal root ganglia, one type of nociceptive neuron expresses ret and displays a postnatal shift from NGF to GDNF responsiveness (Molliver et al., 1997; Bennett et al., 1998). GDNF is also expressed in the developing vibrissa follicle-sinus complex, and GFRα1 and ret are expressed in the TG (Naveilhan et al., 1998; Fundin et al., 1999; Golden et al., 1999). The aforementioned studies have also shown that in GDNF knockout embryos, the TG is devoid of GFRα1-expressing cells, whereas in adult GDNF heterozygotes, the vibrissa follicle displays a significantly reduced number of thickly myelinated axons and their associated terminals.
Unlike GDNF and GFRα1 knockouts, mice lacking neurturin or GFRα2 are born and bred normally with no major organ deficiency, save for lacrimal dysfunction (Heuckeroth et al., 1999; Rossi et al., 1999). Yet this system acts later in development and appears to maintain the mature enteric and parasympathetic nervous systems. GFRα2 is also expressed in dorsal root ganglia, and neurturin is trophic for some dorsal root and TG cells in vitro (Kotzbauer et al., 1996; Bennett et al., 1998; Naveilhan et al., 1998), reflecting its presence in the whisker follicles. Numbers of dorsal root ganglion cells are, however, normal in neurturin or GFRα2 knockouts, whereas GFRα2-expressing cells are reduced in number in dorsal root and TG of neurturin knockout mice.
It is known that ret expression begins in the TG at E12, and that GDNF and neurturin knockout embryos lack GFRα1- and GFRα2-expressing neurons, respectively, though no data are available on TG cell survival. Moreover, the whisker follicle is a source of GDNF and neurturin that may be trophic for TG cells. Extrapolating from these data, it would appear that there are more ret-expressing cells in the TG than there are Trk-expressing cells there (Mosconi et al., 2001). It is therefore reasonable to hypothesize that the GFL family provides trophic support for TG cells. Data from other systems (Baloh et al., 2000) also suggest that the GDNF and NGF families account for the trophic support of almost all peripheral neurons at some point in development, and that these influences overlap and alternate.
While there is favorable evidence in support of GFLs as trophic factors for TG cells, there are no reports of growth-promoting actions of GFLs in trigeminal axon development. However, axon growth-promoting effects have been noted for motor and sympathetic neurons (Enomoto et al., 2001; Honma et al., 2002). GDNF has also been shown to have more robust axon growth-promoting effects than the NGF family of neurotrophins (Ho et al., 2000; Keller-Peck et al., 2001).
AXON GUIDANCE AND ARBORIZATION SIGNALS FOR DEVELOPING TRIGEMINAL AXONS
To date, numerous target-derived molecules have been identified that guide developing axons to correct addresses by attraction or repulsion. These are diffusible or membrane-bound molecules (e.g., netrins, semaphorins, ephrins, slits, neurotrophins, extracellular matrix, and cell adhesion molecules) that are functionally bidirectional (for reviews, see Tessier-Lavigne and Goodman, 1996; Mueller, 1999; Huber et al., 2003). Spatiotemporal expression patterns of these molecules and their receptors act in concert during target-directed path finding, collateralization, and arborizations; such is also the case for the trigeminal pathway.
Previous studies indicated that semaphorin 3A (Sema 3A) and neuropilin receptors play a major role in restricting trigeminal axons along specified routes. In neuropilin or Sema3A knockout mice, both peripheral and central TG axons expand out and invade territories they normally would not (Kitsukawa et al., 1997; Ulupinar et al., 1999). Studies in the chick also show spatiotemporal regulation of Sema 3A and neuropilin expression in the spinal cord and DRG (Pond et al., 2002). In vitro assays indicate that neurotrophins can differentially modify growth cone responses to Sema 3A (Tuttle and O’Leary, 1998). NT3-responsive early embryonic chick DRG neurons express neuropilin1 and show growth cone collapse in response to Sema 3A, but they no longer collapse at later ages when they lose neuropilin1 expression. In contrast, NGF-responsive neurons express progressively high levels of neuropilin1 and show a pronounced collapse response to Sema 3A (Pond et al., 2002). Growth cones of E7 chick DRG neurons that were previously conditioned with exogenous NGF become resistant to collapsing effects of Sema 3A (Dontchev and Letourneau, 2002). Furthermore, when an NGF-coated bead is placed adjacent to Sema 3A-secreting cells, these growth cones do not collapse; they steer toward the NGF-coated bead (Dontchev and Letourneau, 2003). Rodent TG cells and their axons express high levels of neuropilin1 as they form a highly restricted pathway in the brainstem (Ulupinar et al., 1999). At later stages, when TG axons leave the tract and invade the brainstem trigeminal nuclei (BSTC), neuropilin1 expression diminishes. In our experiments, we used E15 rat embryos at the time when the central trigeminal tract is in its elongation phase with high levels of neuropilin1 expression. We showed that ectopic neurotrophin sources during this stage can disrupt the streamlining of the trigeminal tract and induce axonal growth away from the tract into the BSTC (Ulupinar et al., 1999; Ozdinler and Erzurumlu, 2001; Ozdinler et al., 2004).
A number of in vitro studies indicated the presence of target-derived diffusible cues, which can induce axon branching, collateralization, and arborization. For example, studies on a major axonal pathway from layer V of neocortex to brainstem and spinal cord revealed that explants of pons tissue, at specific times in development, induce interstitial branching and attraction of layer V pyramidal cell axons (Heffner et al., 1990; O’Leary et al., 1991; Sato et al., 1994). Neurotrophins have also been implicated as arborization factors for primary sensory neurons and retinotectal axons (Diamond et al., 1992; Cohen-Cory and Fraser, 1995; Kennedy and Tessier-Lavigne, 1995; Gallo and Letourneau, 1998; Lentz et al., 1999; Ulupinar et al., 2000; Ozdinler and Erzurumlu, 2001). We recently reported that a member of the Slit family of proteins, Slit2, can induce arborization of central trigeminal axons at a time when they are in the elongation phase (Fig. 5) (Ozdinler and Erzurumlu, 2002). There are at least three members of the Slit family (Slit1, Slit2, Slit3) and three members of the Robo family, Robo1, Robo2, Robo3Rig1 (Rajagopalan et al., 2000; Simpson et al., 2000), which bind Slits. Slit proteins serve as “repellent” guidance cues for motor, olfactory, and retinal axons, hippocampal dentate gyrus axons, and muscle precursor cells in Drosophila, and postmitotic neurons from the subventricular zone in mammals (Brose et al., 1999; Hu, 1999; Kidd et al., 1999; Li et al., 1999; Nguyen Ba-Charvet et al., 1999; Wu et al., 1999; Erskine et al., 2000; Ringstedt et al., 2000). Slit proteins and Robo receptors show high levels of crosstalk. Slit-mediated repulsion of Robo expressing axons or cells and their function in development have been reviewed extensively (Chisholm and Tessier-Lavigne, 1999; Guthrie, 1999; Harris and Holt, 1999; Zinn and Sun, 1999; Brose and Tessier-Lavigne, 2000). Wang et al. (1999) found that E17 rat spinal cord extracts stimulate axon branching from dissociated E14 DRG cells. They also noted a similar branchinducing activity from calf brain extracts. Biochemical purification assays by fractionating the extract through several columns led to identification of a 140 kD protein, the N-terminal portion of Slit2, responsible for axon branching and arborization. The same group replicated this observation in DRG cells with membrane carpet assays (Nguyen Ba-Charvet et al., 2001a, 2001b). Trigeminal ganglion cells express high levels of Robo1 and -2 during development. Slit mRNAs are not present in the BSTC, but high levels of Slit2 mRNA appear when TG axons begin invading these nuclei and form arbors. Two reports documented the phenotypes of Slit2 and Slit1 double knockout mice (Bagri et al., 2002; Plump et al., 2002). These studies focused on forebrain fiber tracts. More recent studies noted that Slit receptors Rig1/Robo3 control midline crossing of precerebellar neurons and axons (Marillat et al., 2004) and in Slit triple knockout mice spinal commissural axons show defects in their midline crossing (Long et al., 2004). However, axon branching defects in these mice have not been reported.
Fig. 5.
Effects of Slit2N on central trigeminal tract axons (as visualized by DiI labeling) during the elongation phase (in brainstem whole-mount cultures). A: Normal E15 rat descending trigeminal tract (Dtr) in an explant culture maintained in SFM. B: E15 rat Dtr in the presence of control HEK cells. C: E15 rat Dtr in the presence of HEK cells secreting Slit2U. D: E15 rat Dtr in the presence of HEK cells secreting Slit2N. E: E15 Dtr in the presence of conditioned medium from Slit2N-secreting cells. Branching/arborization of E15 Dtr axons medial to the tract in the BSTC in the presence of concentrated Slit2N in the medium (F) and in the presence of cells secreting Slit2N (G). In all micrographs, anterior is up and medial is to the right. Scale bar = 20 µm. For orientation and location of the Dtr, refer to Figure 1.
ROLE OF TRANSCRIPTION FACTORS IN BRAINSTEM TRIGEMINAL DEVELOPMENT AND PATTERNING
In contrast to the wealth of knowledge pertaining to system aspects of whisker-related pattern formation in the CNS, very little is known of the transcriptional mechanisms that specify the embryonic development of the barrel neuraxis. This limitation in our knowledge is especially applicable to the PrV, the brainstem structure that is necessary for higher-order thalamic and cortical barrel formation. Indeed, only a handful of molecular studies have addressed PrV cell development. As such, little is known regarding PrV cell migration, differentiation, cell fate specification, cell death, and axonal guidance. The only transcription factor that has been shown to be necessary for the development of the PrV-based lemniscal pathway is Drg11 (Ding et al., 2003). The paucity of information on transcriptional regulators in the PrV likely reflects the fact that very few PrV-specific genes have been identified and functionally analyzed.
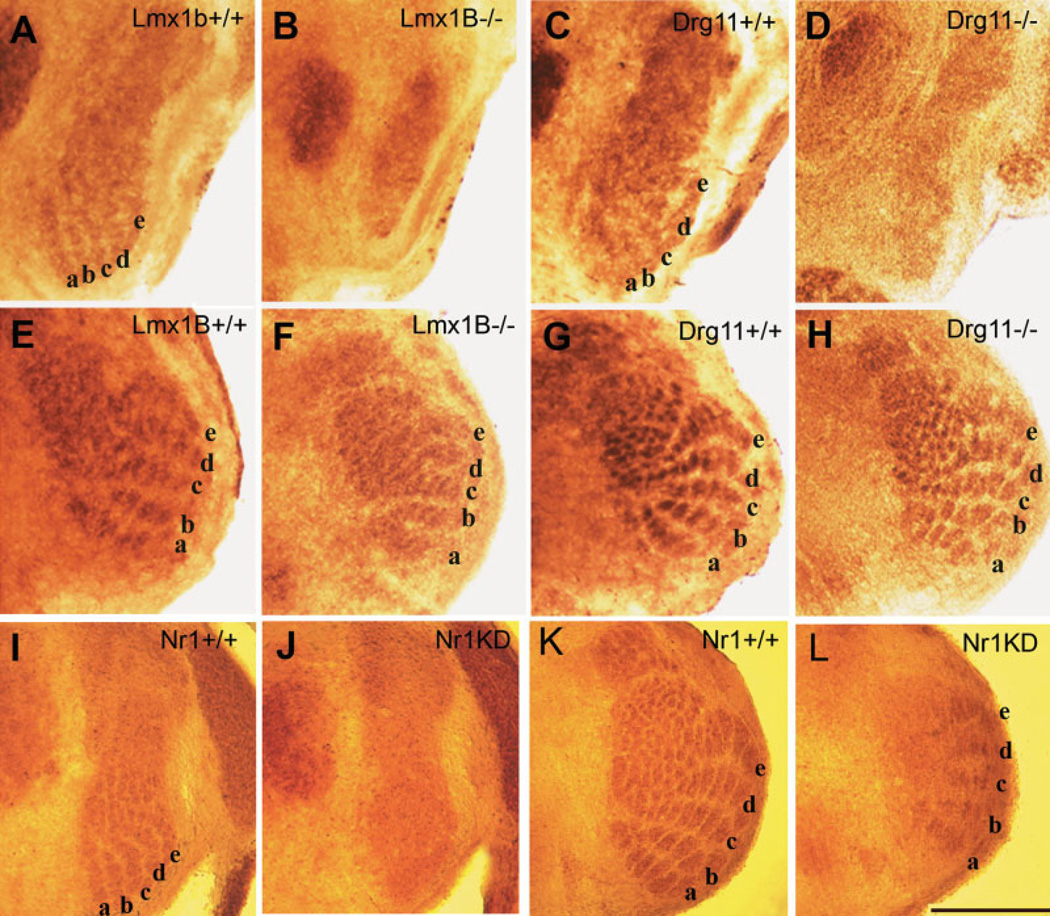
We have previously identified a critical role for Drg11, a paired homeodomain gene, in the development of the PrV (Ding et al., 2003). Drg11 null mutants exhibit several cellular defects within the PrV, unusual projections of primary afferents from the TG cells, and, subsequently, increased cell death. These early embryonic abnormalities in the PrV lead to the failure to develop whisker-related patterns in the PrV, VPM, and S1 cortex. By contrast, somatotopic patterns exist in the SpVi and SpVc and the dorsal column nucleus (DCN)-based lemniscal and cortical pathway. Thus, the deficits in the trigeminal system of Drg11 knockout mice are specific to the PrV (Fig. 6). In an effort to understand the transcriptional network that controls the development of the PrV, we have performed systematic screening to identify the genes that have important roles in the development of different aspects of PrV neurons. One of the genes we have identified is an LIM homeodomain transcription factor, Lmx1b. Interestingly, we found that Drg11 expression is absent in the PrV of Lmx1b mutants, suggesting that these two genes may reside in the same genetic pathway. However, there are marked differences between Lmx1b and Drg11. Drg11 is expressed in both TG and PrV, while it appears that Lmx1b is present only in the PrV. At birth, the PrV of Lmx1b mutant mice is much smaller than that of Drg11 mutants. Abnormally excessive PrV cell death in Lmx1b mutants occurs earlier than for Drg11 mutants. Expression of several axonal guidance molecules and transcription factors is downregulated or absent in the PrV of Lmx1b mutants, but their expression is unchanged in Drg11 mutants. Neurotransmitter phenotype is altered in the PrV of Lmx1b mutants, but not in Drg11 mutants. These results suggest that the Lmx1b-Drg11 pathway may control specific aspects of the development of the PrV-based primary afferent and lemniscal pathways. Importantly, however, Lmx1b appears to function in several Drg11-independent molecular and cellular processes.
Fig. 6.
CO staining in the PrV and SpVi of wild-type, Lmx1b, Drg11 knockout, and NR1 knockdown mice. Top row of micrographs (A–D) show the PrV of Lmx1b+/+, Lmx1b−/−, Drg11+/+, Drg11−/− mice at P0. Barrelette patterns in +/+ cases are indicated by letters a–e, corresponding to those rows of whiskers on the face. Middle row of micrographs (E–H) show whisker-related barrelette patterns in the SpVi of the same series of animals. Bottom row of micrographs show barrelette patterns in the PrV (I) and SpVi (K) of NR1+/+ mice and the PrV of NR1KD mice (J and L, respectively) at P14. Scale bars = 200 µm (A–H); 500 µm (I–L).
NEURAL ACTIVITY
Earlier studies noted that tetrodotoxin (TTX) blockade of ION afferents did not disrupt barrelette formation in the rat BSTC (Henderson et al., 1992). Later studies documented that TG cells have TTX-resistant Na+ channels and voltage-dependent Ca2+ channels that produce Ca2+ spikes (Kim and Chung, 1999; Cabanes et al., 2002). Spontaneous activity in the TG is also not blocked by TTX or bipuvicaine (Shoykhet et al., 2000, 2003; Minnery et al., 2003). Whisker-specific patterning in the brainstem emerges before birth in the rat (Chiaia et al., 1993; Waite et al., 2000) and at birth in mice (Ma, 1993). Central ION arbors at this stage are localized and densely branched, with synaptically active terminals (Leamey and Ho, 1998; Waite et al., 2000). The postsynaptic responses of PrV cells contain a predominant N-methyl-D-aspartate receptor (NMDAR) component, indicating that these receptors play a major role in the first relay station of the developing trigeminal pathway.
The role of NMDAR-mediated activity in the development of patterns along the trigeminal pathway has been documented in genetically altered mice. Deletion of the NMDAR subunit genes NR1 or NR2B prevents the formation of barrelettes in the brainstem without affecting topographical projections of the trigeminal afferents (Li et al., 1994; Kutsuwada et al., 1996; Iwasato et al., 1997; Lee et al., 2005a). Thalamocortical patterning is also impaired and barrels fail to develop in cortex-specific NR1 knockout mice (Iwasato et al., 2000; Datwani et al., 2002; Lee et al., 2005b). Point mutations of NMDARs with impaired Mg2+ block and Ca2+ influx also lead to absence of barrelette patterns in the BSTC (Single et al., 2000; Rudhard et al., 2003). Based on analyses of single ION axon terminal development and dendritic differentiation of barrelette cells in NR1 knockout and NR1 knockdown mice, we concluded that NMDARs might act as stop-stabilization signals for presynaptic terminals and for the postsynaptic dendrites (Lee et al., 2005a). A similar role for NMDARs has been reported for the visual system (Wu and Cline, 1998; Zou and Cline, 1999) as well as in the barrel cortex of cortex-specific NR1 knockout mice (Datwani et al., 2002; Lee et al., 2005b). Collectively, these findings indicate that large NMDAR currents induced by correlated activity of afferents stabilize both pre- and postsynaptic elements. Thus, NMDARs are critical for the development and maintenance of neural patterns in the rodent trigeminal system. Curiously, the BSTC barrelette phenotype of NR1 knockdown mice is similar to that of Drg11 and Lmx1b null mice (Fig. 6). Possible relationships (if any) with NMDAR signaling remain to be determined.
CONCLUSIONS
The studies we describe here begin to reveal molecular mechanisms that are central to the development of the rodent trigeminal system (the whisker-barrel pathway). Since it was first recognized that the whiskers of mice, rats, and many other animals have plainly visible anatomical correlates (axons, dendrites, cell bodies, synapses, etc.) at every level of the trigeminal sensory pathway (brainstem, thalamus, and cerebral cortex), a great deal of work has been published on virtually every aspect of the structure and function of this pathway and its role in behavior. The early demonstration that the organization of the whisker-barrel pathway could be modified during development led many investigators to studies of development at all levels of this system. Until recently, our efforts have been directed to functional and anatomical development of all levels of this pathway from the sense organs (whiskers) to connections within the cerebral cortex. The timing of organizational changes in different locations along the sensory pathway is now known. Because of the “cookie cutter” whisker-related patterns in different individuals of the same species, data from a wide range of studies can be placed in a common framework. Furthermore, as each part of the whisker-barrel pathway develops at different times—whiskers, trigeminal ganglion, brainstem, thalamus and cortex—there are excellent opportunities to evaluate a wide range of fundamental issues, of which only two are information passed from one locus to another, and the degree to which mechanisms in one locus resemble those in another.
Shortly after the discovery of the whisker-barrel system, studies were undertaken to modify animals by selective breeding (e.g., the barrelless strain) to determine molecular mechanisms. However, these studies were at best difficult and costly because of prodigious breeding and husbandry requirements. The ability to modify selectively genes of particular interest directly has substantially reduced the effort and time necessary to generate animals with particular genotypes. This review outlines studies directed to understanding the roles of different classes of molecules in establishing the organization of the mammalian nervous system. These include neurotrophins and their receptors, axonal guidance molecules and their receptors, transcriptional regulators, and glutamate receptors. Understanding one class of molecular interaction could inform that of another class; understanding changes in one part of the pathway could inform that in another part. All of the studies included here address a general issue: what mechanisms establish a functioning central nervous system?
ACKNOWLEDGMENTS
The authors thank Dr. B. Genc for providing Figure 5, C. Xiang for help with Figure 6, and Z. Eryuksel for help with manuscript preparation. Supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke grant PO1 NS04904820A2 (to M.F.J., Z.-F.C., R.S.E.) and LSUHSC REF (to R.S.E.).
LITERATURE CITED
- Altman J, Bayer SA. Development of the brainstem in the rat: IV, thymidine-radiographic study of the time of origin of neurons in the pontine region. J Comp Neurol. 1980;194:905–929. doi: 10.1002/cne.901940411. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. The development of the rat spinal cord. Berlin: Springer; 1984. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger K, Leitner ML, Araki T, Johnson EM, Milbrandt J. Artemin, a novel member of the GDNF ligand family supports peripheral and central neurons and signals through the GFRα3-RET receptor complex. Neuron. 1998a;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Gorodinsky A, Golden JP, Tansey MG, Keck CL, Popescu NC, Johnson EM, Milbrandt J. GFRα3 is an orphan member of the GDNF/neurturin/persephin receptor family. Proc Natl Acad Sci USA. 1998b;95:5801–5806. doi: 10.1073/pnas.95.10.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RH, Enomoto H, Johnson EM, Milbrandt J. The GDNF family ligands and receptors: implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Belford GR, Killackey HP. Vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979a;183:305–321. doi: 10.1002/cne.901830207. [DOI] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. The development of vibrissae representation in subcortical trigeminal centers of the neonatal rat. J Comp Neurol. 1979b;188:63–74. doi: 10.1002/cne.901880106. [DOI] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. The sensitive period in the development of the trigeminal system of the neonatal rat. J Comp Neurol. 1980;193:335–350. doi: 10.1002/cne.901930203. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkemeier LR, Winslow JW, Kaplan DR, Nikoloics K, Goeddel DV, Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionary conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Brose K, Tessier-Lavigne M. Slit proteins: key regulators of axon guidance axonal branching and cell migration. Curr Opin Neurobiol. 2000;10:95–102. doi: 10.1016/s0959-4388(99)00066-5. [DOI] [PubMed] [Google Scholar]
- Buj-Bello A, Buchman VL, Horton A, Rosenthal A, Davies AM. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;5:821–828. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Cabanes C, de Armentia ML, Viana F, Belmonte C. Postnatal changes in membrane properties of mice trigeminal ganglion neurons. J Neurophysiol. 2002;87:2398–2407. doi: 10.1152/jn.2002.87.5.2398. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farina I, Wang L-C, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, et al. GFRα1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci USA. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. NGF and the local control of nerve terminal growth. J Neurobiol. 1994;25:599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- Castellani V, Bolz J. Opposing roles for neurotrophin-3 in targeting and collateral formation of distinct sets of developing cortical neurons. Development. 1999;126:3335–3345. doi: 10.1242/dev.126.15.3335. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Bauer WR, Rhoades RW. Prenatal development of the receptive fields of individual trigeminal ganglion cells in the rat. J Neurophysiol. 1993;69:1171–1180. doi: 10.1152/jn.1993.69.4.1171. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Bennett-Clarke CA, Crissman RS, Zheng L, Chen M, Rhoades RW. Effect of neonatal axoplasmic transport attenuation in the infraorbital nerve on vibrissae-related patterns in the rat’s brainstem, thalamus and cortex. Eur J Neurosci. 1996;8:1601–1612. doi: 10.1111/j.1460-9568.1996.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Bennett-Clarke CA, Crissman RS, Zhang S, Rhoades RW. Long-term effects of neonatal axoplasmic transport attenuation on the organization of the rat’s trigeminal system. J Comp Neurol. 1997;381:219–229. [PubMed] [Google Scholar]
- Chisholm A, Tessier-Lavigne M. Conservation and divergence of axon guidance mechanisms. Curr Opin Neurobiol. 1999;9:603–615. doi: 10.1016/S0959-4388(99)00021-5. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodeling in vivo. Nature. 1995;378:4663–4672. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Conover JC, Yancopoulos GD. Neurotrophin regulation of the developing nervous system: analyses of knockout mice. Annu Rev Neurosci. 1997;8:13–27. doi: 10.1515/revneuro.1997.8.1.13. [DOI] [PubMed] [Google Scholar]
- Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM, et al. Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic –neurons. Proc Natl Acad Sci USA. 1997;94:7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cell Neurosci. 2002;21:477–492. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Lumsden AGS, Slavkin HC, Burnstock G. Influence of nerve growth factor on the embryonic mouse trigeminal ganglion in culture. Dev Neurosci. 1981;4:150–156. doi: 10.1159/000112751. [DOI] [PubMed] [Google Scholar]
- Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- Davies AM. Intrinsic programmes of growth and survival in developing vertebrate neurons. Trends Neurosci. 1994;17:195–199. doi: 10.1016/0166-2236(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Davies AM. Developmental changes in the neurotrophic factor survival requirements of peripheral nervous system neurons. Prog Brain Res. 1998;117:47–56. doi: 10.1016/s0079-6123(08)64006-6. [DOI] [PubMed] [Google Scholar]
- Diamond J, Holmes M, Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J Neurosci. 1992;12:1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Yin J, Xu HM, Jacquin MF, Chen ZF. Formation of whisker-related principal sensory nucleus-based lemniscal pathway requires a paired homeodomain transcription factor, Drg11. J Neurosci. 2003;23:7246–7254. doi: 10.1523/JNEUROSCI.23-19-07246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontchev VD, Letourneau PC. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J Neurosci. 2002;22:6659–6669. doi: 10.1523/JNEUROSCI.22-15-06659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontchev VD, Letourneau PC. Growth cones integrate signaling from multiple guidance cues. J Histochem Cytochem. 2003;51:435–444. doi: 10.1177/002215540305100405. [DOI] [PubMed] [Google Scholar]
- Dörfl J. The innervation of the mystacial region of the white mouse: a topographical study. J Anat. 1985;142:173–184. [PMC free article] [PubMed] [Google Scholar]
- Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Constantini F, Saarma M, et al. GDNF signaling through the Ret receptor tyrosine kinase. Nature. 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Effects of neonatal whisker lesions on mouse central trigeminal pathways. J Comp Neurol. 1984;223:424–447. doi: 10.1002/cne.902230308. [DOI] [PubMed] [Google Scholar]
- Ebendal T, Tomac A, Hoffer BJ, Olson L. Glial cell line-derived neurotrophic factor stimulates fiber formation and survival in cultured neurons from peripheral autonomic ganglia. J Neurosci Res. 1995;40:276–284. doi: 10.1002/jnr.490400217. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Ibanez CF, Ebendal T, Olson L, Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Nat Acad Sci USA. 1990;87:5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and los of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Erskine L, Williams SE, Brose K, Kidd T, Rachel RA, Goodman CS, Tessier-Lavigne M, Mason CA. Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of robos and slits. J Neurosci. 2000;20:4975–4982. doi: 10.1523/JNEUROSCI.20-13-04975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Killackey HP. Development of order in the rat trigeminal system. J Comp Neurol. 1983;213:365–380. doi: 10.1002/cne.902130402. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Trigeminal ganglion cell processes are spatially ordered prior to the differentiation of the vibrissa pad. J Neurosci. 1992;12:3946–3955. doi: 10.1523/JNEUROSCI.12-10-03946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S, Takahashi H, McKay RD. Target-derived influences on axon growth modes in cultures of trigeminal neurons. Proc Natl Acad Sci USA. 1993;90:7235–7239. doi: 10.1073/pnas.90.15.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, McKay RD, Jhaveri S. Morphological specification of trigeminal neurites depends on target fields. Dev Brain Res. 1994;83:132–137. doi: 10.1016/0165-3806(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Target influences on the morphology of trigeminal axons. Exp Neurol. 1995;135:1–16. doi: 10.1006/exnr.1995.1061. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Lo FS, Gunhan-Agar E, Guido W. Functional connectivity in the rodent trigeminal pathway grown in vitro. Dev Brain Res. 1997;101:37–47. doi: 10.1016/s0165-3806(97)00046-1. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of “barrels” in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundin BT, Mikaels L, Westphal H, Ernfors P. A rapid and dynamic regulation of GDNF-family ligands and receptors correlate with the developmental dependency of cutaneous sensory innervation. Development. 1999;126:2597–2610. doi: 10.1242/dev.126.12.2597. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc B, Ozdinler PH, Mendoza A, Erzurumlu RS. A chemoattractant role for NT-3 in proprioceptive axon guidance. PLoS Biol. 2004;2:2112–2121. doi: 10.1371/journal.pbio.0020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM. Expression of Neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Gundersen LW, Barrett JN. Neuronal chemotaxis: chick dorsal root axons turn towards high concentrations of nerve growth factor. Science. 1979;206:1079–1080. doi: 10.1126/science.493992. [DOI] [PubMed] [Google Scholar]
- Gunhan-Agar E, Haeberle A, Erzurumlu RS. Directional specificity and patterning of sensory axons in trigeminal ganglion-whiskerpad cocultures. Dev Brain Res. 2000;119:277–281. doi: 10.1016/s0165-3806(99)00107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie S. Axon guidance: starting and stopping with slit. Curr Biol. 1999;9:R432–R435. doi: 10.1016/s0960-9822(99)80274-7. [DOI] [PubMed] [Google Scholar]
- Haeberle AS, Erzurumlu RS. Target specific differentiation of peripheral trigeminal axons in rat-chick chimeric explant cocultures. Dev Brain Res. 2001;131:1–8. doi: 10.1016/s0165-3806(01)00235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WA, Holt CE. Slit the midline repellent. Nature. 1999;398:462–463. doi: 10.1038/18970. [DOI] [PubMed] [Google Scholar]
- Heerssen HM, Segal RA. Location, location, location: a spatial view of neurotrophin signal transduction. Trends Neurosci. 2002;25:160–165. doi: 10.1016/s0166-2236(02)02144-6. [DOI] [PubMed] [Google Scholar]
- Heffner CD, Lumsden AG, O’Leary DD. Target control of collateral extension and directional axon growth in the mammalian brain. Science. 1990;247:217–220. doi: 10.1126/science.2294603. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Woolsey TA, Jacquin MF. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Dev Brain Res. 1992;66:146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Johnson EM, Osborne PA, Jacquin MF. Fetal NGF augmentation preserves excess trigeminal ganglion cells and interrupts whisker-related pattern formation. J Neurosci. 1994;14:3389–3403. doi: 10.1523/JNEUROSCI.14-05-03389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TA, Jacquin MF. What makes subcortical barrels? requisite trigeminal circuitry and developmental mechanisms. Barrel Cortex Cerebral Cortex. 1995;11:123–187. [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM, et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Ho SM, Waite PM. Spontaneous activity in the perinatal trigeminal nucleus of the rat. Neuroreport. 1999;10:659–664. doi: 10.1097/00001756-199902250-00039. [DOI] [PubMed] [Google Scholar]
- Ho TW, Bristol LA, Coccia C, Li Y, Milbrandt J, Johnson EM, Jin J, Bar-Peled O, Griffin JW, Rothstein JD. TGF-beta trophic factors differentially modulate motor axon outgrowth and protection from neurotoxicity. Exp Neurol. 2000;161:664–675. doi: 10.1006/exnr.1999.7290. [DOI] [PubMed] [Google Scholar]
- Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth RO, Johnson EM, Milbrandt J. Artemin is a vascular-derived neurotrophic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999;23:703–711. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Ip NY, Ibanez CF, Nye SH, McClain J, Jones PF, Gies DR, Belluscio L, Le Beau MM, Espinosa R, III, Squinto SP, Persson H, Yancopoulos GD. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Nat Acad Sci USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin MF, Rhoades RW. Effects of neonatal infraorbital lesions upon central trigeminal primary afferent projections in rat and hamster. J Comp Neurol. 1985;235:129–143. doi: 10.1002/cne.902350110. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Golden J, Panneton WM. Structure and function of barrel “precursor” cells in trigeminal nucleus principalis. Brain Res. 1988;471:309–314. doi: 10.1016/0165-3806(88)90109-5. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Barcia M, Rhoades RW. Structure-function relationships in rat brainstem subnucleus interpolaris: IV, projection neurons. J Comp Neurol. 1989;282:45–62. doi: 10.1002/cne.902820105. [DOI] [PubMed] [Google Scholar]
- Jhaveri S, Erzurumlu RS, Chiaia N, Kumar TR, Matzuk MM. Defective whisker follicles and altered brainstem patterns in activin and follistatin knockout mice. Mol Cell Neurosci. 1998;12:206–219. doi: 10.1006/mcne.1998.0710. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Jones KR, Reichardt LF. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Nat Acad Sci USA. 1990;87:8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TE, Tessier-Lavigne M. Guidance and induction of branch formation in developing axons by target-derived difusible factors. Cur Opin Neurobiol. 1995;5:83–90. doi: 10.1016/0959-4388(95)80091-3. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Fleming K. The role of the principal sensory nucleus in central trigeminal pattern formation. Brain Res. 1985;354:141–145. doi: 10.1016/0165-3806(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Kim H-C, Chung M-Y. Voltage-dependent sodium and calcium currents in acutely isolated adult rat trigeminal root ganglion neurons. J Neurophysiol. 1999;81:1123–1134. doi: 10.1152/jn.1999.81.3.1123. [DOI] [PubMed] [Google Scholar]
- Kitsukawa TM, Shimizu M, Sanbo T, Hirata M, Taniguchi Y, Bekku T, Yagi H, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Milbrandt JD. Neurturin: a relative of glial cell line-derived neurotrophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Leamey CA, Ho SM. Afferent arrival and onset of functional activity in the trigeminothalamic pathway of the rat. Brain Res Dev Brain Res. 1998;105:195–207. doi: 10.1016/s0165-3806(97)00170-3. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Lo FS, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci. 2005a;25:2304–2311. doi: 10.1523/JNEUROSCI.4902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol. 2005b;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz SI, Knudson CM, Korsmeyer SJ, Snider WD. Neurotrophins support the development of diverse sensory axon morphologies. J Neurosci. 1999;19:1038–1048. doi: 10.1523/JNEUROSCI.19-03-01038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Li H, Chen J, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang Z, Nash W, Gick C, Ornitz DM, Wu J, Rao Y. Vertebrate Slit, a secreted ligand for the transmembrane protein Rundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lo FS, Guido W, Erzurumlu RS. Electrophysiological properties and synaptic responses of cells in the trigeminal principal sensory nucleus of postnatal rats. J Neurophysiol. 1999;82:2765–2775. doi: 10.1152/jn.1999.82.5.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Lumsden AG, Davies AM. Chemotropic effect of specific target epithelium in the developing mammalian nervous system. Nature. 1986;323:538–539. doi: 10.1038/323538a0. [DOI] [PubMed] [Google Scholar]
- Ma PM, Woolsey TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Res. 1984;306:374–379. doi: 10.1016/0006-8993(84)90390-1. [DOI] [PubMed] [Google Scholar]
- Ma PM. The barrelettes: architectonic vibrissal representations in the brainstem trigeminal complex of the mouse: I, normal structural organization. J Comp Neurol. 1991;309:161–199. doi: 10.1002/cne.903090202. [DOI] [PubMed] [Google Scholar]
- Ma PM. Barrelettes: architectonic vibrissal representations in the brainstem trigeminal complex of the mouse: II, normal postnatal development. J Comp Neurol. 1993;327:376–397. doi: 10.1002/cne.903270306. [DOI] [PubMed] [Google Scholar]
- Marillat V, Sabatier C, Failli V, Matsunaga E, Sotelo C, Tessier-Lavigne M, Chedotal A. The slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron. 2004;43:69–79. doi: 10.1016/j.neuron.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Markus A, Patel TD, Snider WD. Neurotrophic factors and axonal growth. Curr Opin Neurobiol. 2002;12:523–531. doi: 10.1016/s0959-4388(02)00372-0. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MF, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, et al. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron. 1998;20:245–253. doi: 10.1016/s0896-6273(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. Neurotrophin signaling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001a;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001b;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- Minnery BS, Simons DJ. Response properties of whisker-associated trigeminothalamic neurons in rat nucleus principalis. J Neurophysiol. 2003;89:40–56. doi: 10.1152/jn.00272.2002. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Mosconi T, Snider WD, Jacquin MF. Neurotrophin receptor expression in retrogradely labeled trigeminal nociceptors: comparisons with spinal nociceptors. Somatosens Mot Res. 2001;18:312–321. doi: 10.1080/01421590120089695. [DOI] [PubMed] [Google Scholar]
- Mueller BK. Growth cone guidance: first steps towards a deeper understanding. Annu Rev Neurosci. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Baudet C, Mikaels A, Shen L, Westphal H, Ernfors P. Expression and regulation of GFRα3, a glial cell line-derived neurotrophic factor family receptor. Proc Natl Acad Sci USA. 1998;95:1295–1300. doi: 10.1073/pnas.95.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chedotal A. A Slit-2 mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22:463–473. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001a;21:4281–4289. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Sensory axon response to substrate-bound Slit2 is modulated by laminin and cyclic GMP. Mol Cell Neurosci. 2001b;17:1048–1058. doi: 10.1006/mcne.2001.0994. [DOI] [PubMed] [Google Scholar]
- O’Connor R, Tessier-Lavigne M. Identification of maxillary factor, a maxillary process-derived chemoattractant for developing trigeminal sensory axons. Neuron. 1999;24:165–178. doi: 10.1016/s0896-6273(00)80830-2. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Heffner CD, Kutka L, Lopez-Mascaraque L, Missias A, Reinoso BS. A target-derived chemoattractant controls the development of the corticopontine projection by a novel mechanism of axon targeting. Development. 1991;1991;2(Suppl):123–130. [PubMed] [Google Scholar]
- Ozdinler PH, Erzurumlu RS. Regulation of neurotrophin-induced axonal responses via Rho GTPases. J Comp Neurol. 2001;438:377–387. doi: 10.1002/cne.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Erzurumlu RS. Slit2, a branching-arborization factor for sensory axons in the mammalian CNS. J Neurosci. 2002;22:4540–4549. doi: 10.1523/JNEUROSCI.22-11-04540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Ulupinar E, Erzurumlu RS. Local neurotrophin effects on central trigeminal axon growth patterns. Dev Brain Res. 2004;151:55–66. doi: 10.1016/j.devbrainres.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdinler PH, Ulupinar E, Erzurumlu RS. Dose- and age-dependent axonal responses of embryonic trigeminal axons to localized NGF via p75NTR receptor. J Neurobiol. 2005;62:189–206. doi: 10.1002/neu.20074. [DOI] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Pond A, Roche FK, Letourneau PC. Temporal regulation of neuropilin-1 expression and sensitivity to semaphorin 3A in NGF-and NT3-responsive chick sensory neurons. J Neurobiol. 2002;51:43–53. doi: 10.1002/neu.10041. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Reichardt LF, Fariñas I. Neurotrophic factors and their receptors. In: Cowan WM, et al., editors. Molecular and cellular approaches to neural development. New York: Oxford University Press; 1997. pp. 220–263. [Google Scholar]
- Rhoades RW, Enfiejian HL, Chiaia NL, MacDonald GJ, Miller MW, McCann P, Goddard CM. Birthdays of trigeminal ganglion cells contributing to the infraorbital nerve and specific vibrassa follicles in the rat. J Comp Neurol. 1991;307:163–175. doi: 10.1002/cne.903070114. [DOI] [PubMed] [Google Scholar]
- Rice FL, Manse A, Munger BL. A comparative light microscopic analysis of sensory innervation of the mystacial pad: I, innervation of vibrissal follicle-sinus complexes. J Comp Neurol. 1986;252:154–174. doi: 10.1002/cne.902520203. [DOI] [PubMed] [Google Scholar]
- Rice FL, Kinnman E, Aldskogius H, Johansson O, Arvidsson J. The innervation of the mystacial pad as revealed by PGP 9.5 immunofluorescence. J Comp Neurol. 1993;337:366–385. doi: 10.1002/cne.903370303. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, Barbacid M, DeChiara TM, Yancopoulos GD, Dunne CE, Fundin BT. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Dev Biol. 1998;198:57–81. [PubMed] [Google Scholar]
- Ringstedt T, Kucera J, Lendahl U, Ernfors P, Ibanez CF. Limb proprioceptive deficits without neuronal loss in transgenic mice overexpressing neurotrophin-3 in the developing nervous system. Development. 1997;124:2603–2613. doi: 10.1242/dev.124.13.2603. [DOI] [PubMed] [Google Scholar]
- Ringstedt T, Braisted JE, Brose K, Kidd T, Goodman C, Tessier-Lavigne M, O’Leary DDM. Slit inhibition of retinal axon growth and its role in retinal axon path finding and innervation patterns in the diencephalon. J Neurosci. 2000;20:4983–4991. doi: 10.1523/JNEUROSCI.20-13-04983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H, et al. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFRα2, a functional neurturin receptor. Neuron. 1999;22:243–252. doi: 10.1016/s0896-6273(00)81086-7. [DOI] [PubMed] [Google Scholar]
- Rudhard Y, Kneussel M, Nassar MA, Rast GF, Annala AJ, Chen PE, Tigaret CM, Dean I, Roes J, Gibb AJ, Hunt SP, Schoepfer R. Absence of whisker-related pattern formation in mice with NMDA receptors lacking coincidence detection properties and calcium signaling. J Neurosci. 2003;23:2323–2332. doi: 10.1523/JNEUROSCI.23-06-02323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Lopez-Mascaraque L, Heffner CD, O’Leary DD. Action of a diffusible target-derived chemoattractant on cortical axon branch induction and directed growth. Neuron. 1994;13:791–803. doi: 10.1016/0896-6273(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Constantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor. Ret Nat. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Schuman EM. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shoykhet M, Doherty D, Simons DJ. Coding of deflection velocity and amplitude by whisker primary afferent neurons: implications for higher level processing. Somatosens Mot Res. 2000;17:171–180. doi: 10.1080/08990220050020580. [DOI] [PubMed] [Google Scholar]
- Silos-Santiago I, Molliver DC, Ozaki S, Smeyne RJ, Fagan AM, Barbacid M, Snider WD. Non-TrkA-expressing small DRG neurons are lost in TrkA deficient mice. J Neurosci. 1995;15:5929–5942. doi: 10.1523/JNEUROSCI.15-09-05929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Single FN, Rozov A, Burnashev N, Zimmermann F, Hanley DF, Forrest D, Curan T, Jensen V, Hvalby O, Sprengel R, Seeburg PH. Dysfunctions in mice by NMDA receptor point mutations NR1(N598Q) and NR1(N598R) J Neurosci. 2000;20:2558–2566. doi: 10.1523/JNEUROSCI.20-07-02558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors: Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Snider WD, Lichtman JW. Are neurotrophins synaptotrophins? Mol Cell Neurosci. 1996;7:433–442. doi: 10.1006/mcne.1996.0031. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc Natl Acad Sci USA. 1990;87:923–927. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY, Gilbert W. Neuronal differentiation and maturation in the mouse trigeminal sensory system, in vivo and in vitro. J Comp Neurol. 1991;311:300–312. doi: 10.1002/cne.903110210. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Treanor JJS, Goodman L, Sauvage F, Stone DM, Poulson KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumae U, et al. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Tuttle R, O’Leary DD. Neurotrophins rapidly modulate growth cone response to the axon guidance molecule, collapsin-1. Mol Cell Neurosci. 1998;11:1–8. doi: 10.1006/mcne.1998.0671. [DOI] [PubMed] [Google Scholar]
- Ulupinar E, Erzurumlu RS. Peripheral target-specific influences on embryonic neurite growth vigor and patterns. J Comp Neurol. 1998;399:427–439. doi: 10.1002/(sici)1096-9861(19981005)399:4<427::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ulupinar E, Datwani A, Behar O, Fujisawa H, Erzurumlu RS. Role of semaphorin III in the developing rodent trigeminal system. Mol Cell Neurosci. 1999;13:281–292. doi: 10.1006/mcne.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulupinar E, Jacquin MF, Erzurumlu RS. Differential effects of NGF and NT-3 on embryonic trigeminal axon growth patterns. J Comp Neurol. 2000;425:202–218. doi: 10.1002/1096-9861(20000918)425:2<202::aid-cne4>3.0.co;2-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 1976;2:1–6. doi: 10.1016/0304-3940(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Welker E, Dorfl J, Rumo G. Selective breeding for variations in patterns of mystacial vibrissae of mice: bilaterally symmetrical strains derived from ICR stock. J Hered. 1986;77:66–82. doi: 10.1093/oxfordjournals.jhered.a110201. [DOI] [PubMed] [Google Scholar]
- Waite PME, Tracey DJ. Trigeminal sensory system. In: Paxinos G, editor. The rat nervous system. 2nd ed. New York: Academic Press; 1995. pp. 705–724. [Google Scholar]
- Waite PM, Ho SM, Henderson TA. Afferent ingrowth and onset of activity in the rat trigeminal nucleus. Eur J Neurosci. 2000;12:2781–2792. doi: 10.1046/j.1460-9568.2000.00161.x. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: a comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J Neurosci. 1986;6:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex: the description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- Wu WWK, Chen JH, Jiang ZH, Dupuis S, Wu JY, Rao Y. Directional guidance by neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt S, Shooter EM, Davies AM. Expression of the NGF receptor gene in sensory neurons and their cutaneous targets prior to and during innervation. Neuron. 1990;4:421–427. doi: 10.1016/0896-6273(90)90054-j. [DOI] [PubMed] [Google Scholar]
- Yamakado M, Yohro T. Subdivision of mouse vibrissae on an embryological basis, with descriptions of variations in the number and arrangement of sinus hairs and cortical barrels in BALB/c (nu/+; nude, nu/nu) and hairless (hr/hr) strains. Am J Anat. 1979;155:153–173. doi: 10.1002/aja.1001550202. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Schmidt RE, Yan Q, Snider WD. NGF and NT-3 have different effects on the growth of dorsal root axons in the developing mammalian spinal cord. J Neurosci. 1994;14:5187–5201. doi: 10.1523/JNEUROSCI.14-09-05187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K, Sun Q. Slit branches out: a secreted protein mediates both attractive and repulsive axon guidance. Cell. 1999;97:1–4. doi: 10.1016/s0092-8674(00)80707-2. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Cline HT. Postsynaptic calcium/calmodulin-dependent protein kinase II is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. J Neurosci. 1999;19:8909–8918. doi: 10.1523/JNEUROSCI.19-20-08909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]