Abstract
Stearoyl-CoA desaturase 1 (SCD1) is an essential regulator of fatty acid synthesis. We have previously shown that overexpression of SCD1 increases the growth of breast cancer cell lines. The purpose of this study was to determine the relationship between SCD1 expression level and clinical-pathologic characteristics and survival of patients with breast cancer. Fine-needle aspirates were collected from the primary tumors of 250 patients with stage I–III breast cancer. Demographic and clinical characteristics including patient age, ethnicity, and menopausal status and tumor clinical stage, grade, and subtype were reviewed. SCD1 expression was analyzed using reverse-phase protein arrays. Samples were divided into high or low SCD1 expression levels based on a cut-off determined from martingale residual plots and regression tree analysis. SCD1 levels were significantly higher in tumors from patients >50 years old compared to patients ≤50 years old and were lower in triple-negative (estrogen/progesterone receptor-negative and human epidermal growth factor receptor-2-negative) breast cancers than other tumor subtypes. After adjusting for patient age, tumor subtype, tumor grade, and clinical stage, we found that patients with primary breast cancers expressing high SCD1 levels had significantly shorter relapse-free survival (RFS) (P = 0.014) and overall survival (OS) (P = 0.039) in multivariable analysis. We conclude that SCD1 expression varies by breast cancer subtype and that high levels of SCD1 expression are associated with significantly shorter RFS and OS in multivariable analysis. Future studies are needed to define the role of SCD1 in the malignant phenotype of breast cancer and to evaluate the potential for SCD1 as a therapeutic target.
Keywords: Breast neoplasms, Fatty acid metabolism, Stearoyl-CoA desaturase, Survival, Protein array analysis
Introduction
Stearoyl-CoA desaturase 1 (SCD1) is a critical enzyme of fatty acid synthesis that catalyzes the conversion of saturated fatty acids into monounsaturated fatty acids [1]. The ratio of saturated fatty acids to monounsaturated fatty acids is tightly regulated by cells since alterations in this balance can affect cell membrane fluidity and cell signaling [2]. SCD1 is bound to the membrane of the endoplasmic reticulum, where it catalyzes the insertion of a cis-double bond at the Δ9 position, preferentially converting stearic acid to oleic acid and palmitic acid to palmitoleic acid [3]. Four isoforms of SCD have been described in mice, whereas only two (SCD1 and SCD5) have been described in humans. The ubiquitously expressed SCD1 is the nearest human ortholog of the murine enzymes [4].
The SCD1 promoter region is bound by a wide array of transcription factors, including SREBP-1 and PPAR-α. In previous work, we and others have shown that expression of SREBP-1 and SCD1 is upregulated by phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) signaling, a pathway known to play an important role in breast cancer pathophysiology [5–7]. Furthermore, SCD1 transcription is hormonally upregulated by insulin and growth factors but inhibited by estrogen and leptin [8–20]. Multiple studies, demonstrating SCD1 overexpression in lung cancer, esophageal cancer, colorectal cancer, ovarian cancer, and hepatocellular carcinoma [21–26], have added to the growing body of evidence implicating SCD1 in the development, maintenance, and progression of cancer [5,21,22,26–30].
SCD1 activity has been indirectly measured in the serum of breast cancer patients through the fatty acid saturation index (which determines the ratio of SCD1 substrate to product); a high saturation index (indicating low SCD1 activity) was found to be associated with a decreased risk of breast cancer [31–34]. However, to our knowledge, SCD1 expression has not been directly measured in primary breast cancers. Moreover, it is not known whether the level of SCD1 expression is associated with specific patient or tumor characteristics. Although we and others have hypothesized that SCD1 may play a key role in the development and maintenance of the malignant phenotype, there are no data indicating that SCD1 expression level is prognostic in patients with cancer. Thus, the purpose of this exploratory study was to measure levels of SCD1 expression in primary breast cancers and to determine the relationship between SCD1 expression and patient survival. We hypothesized that high SCD1 expression would correlate with poor prognosis. We used a reverse-phase protein array platform to measure SCD1 expression level and to determine the relationship among SCD1 expression, clinical-pathologic characteristics, and survival among tumor samples from patients with stage I–III primary breast cancer.
Materials and methods
Patients and Samples
This study followed the REMARK guidelines for reporting prognostic studies [35]. The frozen breast cancer set consisted of 250 fine-needle aspirate (FNA) samples from patients with breast cancer undergoing treatment at The University of Texas MD Anderson Cancer Center from 1994 to 2007. Patients with therapy naïve Stage I–III breast cancer were eligible for this prospective tissue collection protocol. Exclusion criteria for this sample set included patients with inflammatory breast cancer, with distant metastasis at the time of initial diagnosis, with axillary metastasis without an identifiable primary breast tumor, or with concurrent treatment for another primary cancer. In addition, patients were excluded who underwent partial or complete excisional biopsies of their primary breast tumor prior to initiation of chemotherapy or whose systemic therapy was managed by another institution [36]. Stratification and matching were not used in this study. This laboratory study was approved by the Institutional Review Board of MD Anderson. Complete clinical information was available for all 250 patients in an established prospectively collected database of patients and subsequently verified by retrospective review of each individual medical record. Follow-up time was a minimum of five years following diagnosis.
Samples were obtained prior to patients’ receiving any systemic therapy. Most patients received sequential taxane and anthracycline-based chemotherapy followed by endocrine therapy if the tumor was hormone receptor positive, based on the standard of care and treatment selection by the patient’s medical oncologist. Biopsy samples were collected, flash-frozen and stored at −70°C prior to protein extraction. Receptor status was determined from diagnostic core biopsy samples. Immunohistochemistry was used to determine the estrogen receptor (ER) and progesterone receptor (PR) status and both immunohistochemistry and fluorescent in situ hybridization (FISH) were used to determine human epidermal growth factor receptor-2 (HER2) status, as appropriate.
Reverse phase protein array (RPPA)
Briefly, tumor FNA samples were lysed with RPPA lysis buffer and then sonicated. Protein quantification was performed using a bicinchoninic acid assay. Lysates were standardized to a 1 μg/μL concentration, serially diluted, and applied to FAST slides (Schleicher & Schuell BioScience, Keene, NH). The slides were then incubated with an antibody against SCD1 (sc-58420), obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and validated for the RPPA platform (data not shown). After secondary antibody application and signal amplification, slides were scanned and quantified to generate spot signal intensities using MicroVigene software version 5.0.0.0 (VigeneTech Inc., Carlisle, MA) and then analyzed by the R package SuperCurve (version1.01) as previously described [37,38].
Statistical analysis
All statistical analyses were performed in R version 2.12.0 (R Development Core Team [2010]. R: A language and environment for statistical computing [R Foundation for Statistical Computing, Vienna, Austria]. http://www.r-project.org). Boxplots, means, and standard deviations were generated for SCD1 expression levels categorized by tumor subtypes. Linear regression modeling was used to determine whether the mean SCD1 expression level significantly differed among breast cancer subtypes. Our survival analysis examined two endpoints, 5-year overall survival (OS) and 5-year relapse-free survival (RFS). Both survival times and relapse times were computed as months from diagnosis to death and relapse, respectively. As the martingale residual plots of SCD1 expression by breast cancer subtype suggested a non-linear relationship between SCD1 expression level and survival, we applied a binary decision tree with logistic regression modeling to divide SCD1 expression into high (>0) and low (≤0) categories. After separating samples into these two categories, we compared the patient demographic and clinical characteristics using X2 analysis. We estimated RFS and OS durations non-parametrically using Kaplan-Meier curves by SCD1 expression level and breast cancer subtype. Log-rank tests were conducted to compare survival (RFS and OS) between patients with high (>0) SCD1 expression versus low (≤0) SCD1 expression within the entire cohort and also within each tumor subtype. Combining univariable analyses (P-value <0.2) and exploratory decision tree analysis, we selected potential covariates of clinical-pathologic characteristics that induced significant differences in the survival endpoints for inclusion in the multivariable analysis. For multivariable survival analysis, we performed a Cox Proportional Hazards Model adjusting for patient age at diagnosis, tumor subtype, tumor grade, and clinical stage. We used the Student’s t-test to analyze the association between body mass index (BMI) and SCD1 expression level (high vs. low) and the Fisher’s exact test to assess the relationship between diabetes mellitus status at the time of breast cancer diagnosis and SCD1 expression level (high vs. low). All tests were two-sided, and P-values less than 0.05 were considered statistically significant.
Results
SCD1 expression level was higher in older patients and in HER2+ and HR+ breast cancers
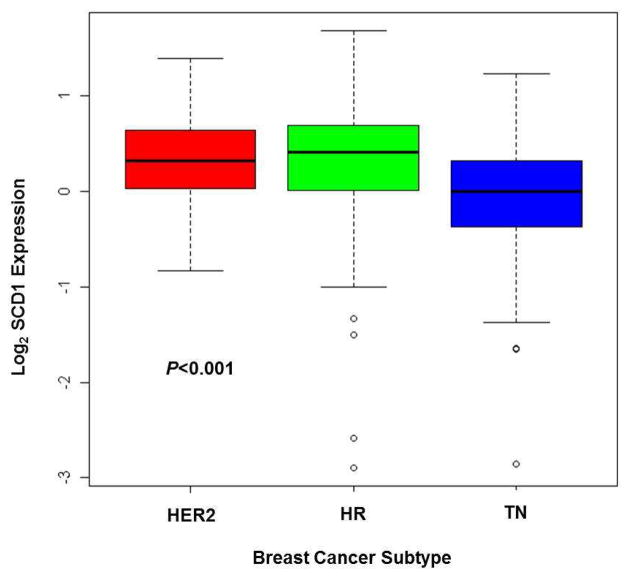
As measured by RPPA, SCD1 expression level (high vs. low) in FNA samples from primary breast cancers was assessed for association with the corresponding demographics of our patient cohort and the clinical characteristics of our samples (Table 1). Patients older than 50 years had higher levels of SCD1 expression than patients younger than 50 (P = 0.0061). Furthermore, SCD1 expression differed significantly among breast cancer subtypes (P = 0.0008): FNA samples from HER2+ or hormone receptor-positive (HR+, estrogen and/or progesterone positive) primary breast cancers had higher SCD1 expression levels than those from triple-negative (TN) primary breast cancers (Fig. 1). We did not find an association between the level of SCD1 expression measured in the primary breast tumor and patient ethnicity, menopausal status, clinical stage of tumor, or tumor grade. In addition, we did not find an association between the level of SCD1 expression and BMI or between the level of SCD1 expression and diabetes mellitus status (data not shown). Overall, we determined that SCD1 expression level was higher in breast cancers from patients older than 50 years and in HER2+ and HR+ breast cancers.
Table 1.
SCD1 expression by patient and tumor characteristics
| Characteristics | SCD1 Expression (No. of patients) | |||
|---|---|---|---|---|
| Overall (n = 250) | High SCD1 (n = 173) | Low SCD1 (n = 77) | P-value | |
|
| ||||
| No. (%) | No. (%) | No. (%) | ||
| Age (years) | 0.0061 | |||
| ≤ 50 | 125 (50.00) | 76 (43.93) | 49 (63.64) | |
| > 50 | 125 (50.00) | 97 (56.07) | 28 (36.36) | |
| Ethnicity | 0.2665 | |||
| Black | 45 (18.00) | 26 (15.03) | 19 (24.68) | |
| Hispanic | 47 (18.80) | 36 (20.81) | 11 (14.29) | |
| Non-Hispanic White | 144 (57.60) | 101 (58.38) | 43 (55.84) | |
| Other | 14 (5.60) | 10 (5.78) | 4 (5.19) | |
| Menopausal Statusa | 0.4004 | |||
| Pre/peri | 118 (47.20) | 78 (45.09) | 40 (52.63) | |
| Post | 128(52.20) | 92 (53.18) | 36 (47.37) | |
| Tumor Clinical stage | 0.6418 | |||
| I | 8 (3.20) | 7 (4.05) | 1 (1.30) | |
| II | 139 (55.60) | 95 (54.91) | 44 (57.14) | |
| III | 103 (41.20) | 71 (41.04) | 32 (41.56) | |
| Tumor Gradeb | 0.1067 | |||
| 1 | 12 (4.80) | 9 (5.20) | 3 (3.90) | |
| 2 | 72 (28.80) | 56 (32.37) | 16 (20.78) | |
| 3 | 163 (65.20) | 105 (60.69) | 58 (75.32) | |
| Subtype | 0.0008 | |||
| HER2 + | 51 (20.40) | 39 (22.54) | 12 (15.58) | |
| HR + | 137 (54.80) | 103 (59.54) | 34 (44.16) | |
| TN | 62 (24.80) | 31 (17.92) | 31 (40.26) | |
Menopausal status was not applicable for 3 male patients and unknown for 1 female patient
Tumor grade was unknown for 3 patients
HER2, human epidermal growth factor receptor-2; HR, hormone receptor (estrogen and/or progesterone receptor); TN, triple negative (estrogen, progesterone, and HER2 negative)
Fig. 1.
Boxplot of SCD1 expression by breast cancer subtype demonstrating that HER2+ and HR+ primary breast cancers had higher SCD1 expression than TN primary breast cancers. The median for each subtype is indicated by the heavy line within each box. The first and third quartiles are represented by the upper and lower edges of each box, respectively. The circle icons represent the extreme values, which are potential outliers. The dashed line includes the most extreme data points, which are no more than 1.5 times the interquartile range from the box. HER2, human epidermal growth factor receptor-2; HR, hormone receptor (estrogen and/or progesterone); TN, triple negative (estrogen, progesterone, and HER2 negative)
High SCD1 expression was associated with shorter survival
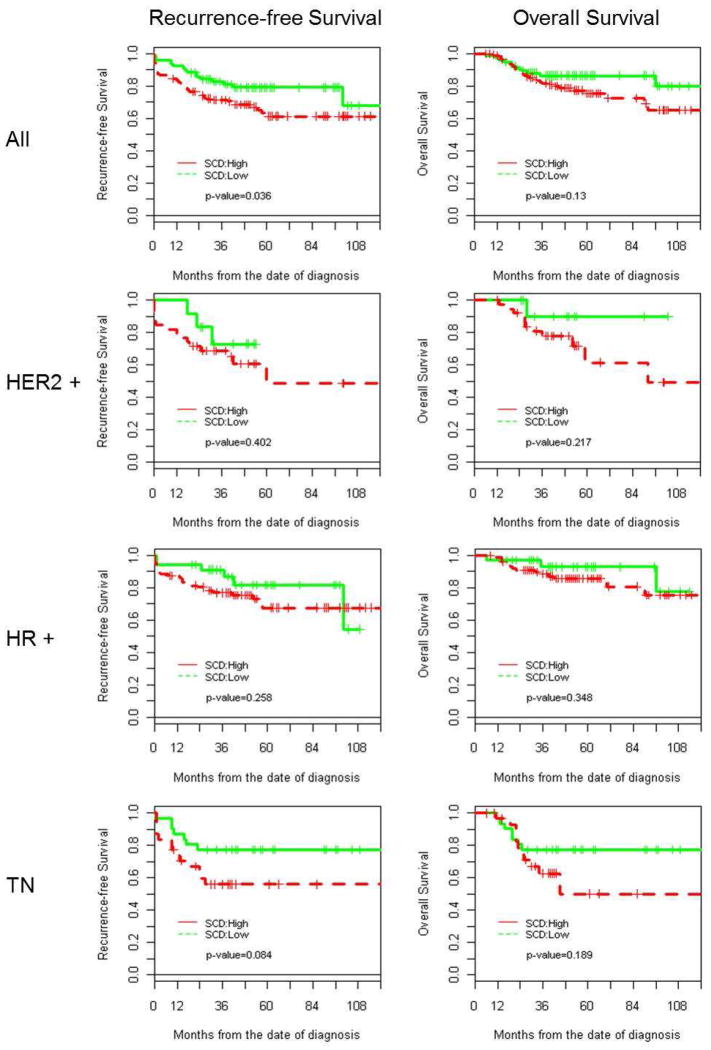
For all patients, the estimated 5-year RFS rate was 67.2% (95% CI: 60.5–74.6%). However, our univariable analysis determined that the 5-year RFS was significantly lower (61.2%; 95% CI: 52.5–71.4%) among patients with cancers expressing high levels of SCD1 than the RFS among patients with cancers expressing low SCD1 levels (79.1%; 95% CI: 70.1–89.3%), (P = 0.036), as shown in Fig. 2. The estimated hazard ratio of RFS for patients with cancers expressing high levels of SCD1 compared with low levels was 1.802 (95% CI: 1.033–3.142, P = 0.038). We did not find statistically significant differences in RFS within each breast cancer subtype by SCD1 level (high v. low).
Fig. 2.
Kaplan-Meier curves demonstrating that 5-year RFS was significantly lower in tumors with low SCD1 expression and in TN tumors; HER2, human epidermal growth factor receptor-2-positive; HR, hormone receptor (estrogen and/or progesterone receptor); TN, triple negative (estrogen, progesterone, and HER2 negative)
For all patients, the estimated 5-year OS rate was 79%. Although a trend was noted toward shorter 5-year OS for patients with tumors expressing high levels of SCD1, our univariable analysis found no significant differences between patients with tumors having high SCD1 expression and patients with tumors having low SCD1 expression (P = 0.13).
The multivariable analysis indicated that after adjusting for patient age at diagnosis and for tumor subtype, grade, and clinical stage (Table 2), high SCD1 expression was associated with lower RFS (P = 0.014) and OS (HR(95% CI)=2.092(1.166–3.754), P = 0.039). Not surprisingly, a more advanced clinical stage at the time of diagnosis correlated with significantly lower RFS and OS (P < 0.001 for both). Similarly, OS estimated for patients with TN breast cancers was significantly lower than that estimated for HR-positive breast cancers (Hazard Ratio (95% CI) =2.410 (1.171–4.959), P = 0.018). Taken as a whole, high SCD1expression in FNAs from primary breast cancers was associated with shorter RFS in univariable and multivariable analyses and shorter OS by multivariable analysis.
Table 2.
Multivariable analysis of relapse-free survival and overall survival correlated with SCD1 expression, patient age at diagnosis, and tumor subtype, grade, and stage
| Relapse-free survival
|
Overall survival
|
|||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value | |
|
|
|
|||||
| SCD1 expression: High vs. low | 2.093 | 1.164–3.762 | 0.0140 | 2.136 | 1.038–4.397 | 0.039 |
| Patient Age at diagnosis | 1.004 | 0.983–10.024 | 0.7290 | 1.014 | 0.990–1.039 | 0.264 |
| Tumor Subtype | ||||||
| HER2 + vs. HR + | 1.379 | 0.779–2.442 | 0.2700 | 1.541 | 0.744–3.19 | 0.244 |
| TN vs. HR + | 1.323 | 0.728–2.423 | 0.3640 | 2.410 | 1.171–4.959 | 0.017 |
| Tumor Grade | ||||||
| 1 & 2 vs. 3 | 0.806 | 0.457–1.422 | 0.4560 | 0.645 | 0.306–1.361 | 0.249 |
| Tumor Clinical stage | ||||||
| III vs. I & II | 3.350 | 2.042–5.483 | <0.0001 | 2.770 | 1.518–5.072 | <0.001 |
SCD1 Log2 expression: high > 0, low ≤ 0; HER2, human epidermal growth factor receptor-2; HR, hormone receptor (estrogen and/or progesterone), TN, triple negative (estrogen, progesterone, and HER2 negative)
Discussion
In this study, we found that SCD1 expression in FNA samples of primary breast cancers varied by breast cancer subtype and patient age. Although several studies previously reported that estrogen negatively regulates SCD1 [16–18], we observed the highest SCD1 levels in HR+ breast tumors. This finding is paradoxical since ER+ tumors tend to have the best prognosis with current treatment yet had the highest SCD1 expression levels in this cohort. We also observed higher SCD1 levels in older (> 50 years) patients, possibly owing to the higher frequency of HR+ tumors in older patients.
Although high SCD1 levels were associated with shorter OS and RFS in our cohort, SCD1 expression levels were lower in TN cancers. This finding is unexpected since TN breast cancer is typically associated with a worse prognosis than other tumor subtypes, and indeed, in our study set, the TN subtype was independently associated with a worse prognosis. The lower SCD1 levels noted in TN tumors are also interesting since PI3K/AKT signaling, activated in TN breast cancers owing to loss of PTEN and INPP4B, is an important regulator of SREBP1 expression [7,39]. As SCD1 maintains the balance between saturated fatty acids and monounsaturated fatty acids and prevents the toxic intracellular accumulation of saturated fatty acids [1,2], our findings suggest that the signaling mechanism allowing TN breast cancers to survive lipotoxic stress could be independent of SCD1 and its downstream effects. A recent study did not demonstrate antitumor effects resulting from SCD1 knockdown in the TN breast cancer cell line MDA-MB-468 [28], thus supporting the theory that TN breast cancers may use other mechanisms to tolerate the lipotoxic consequences of SCD1 knockdown and possibly lower SCD1 expression levels.
However, in our previous work, we demonstrated that overexpression of SCD1 in the TN breast cancer cell line MDA-MB-231 enhanced growth and SCD1 knockdown in the TN breast cancer cell line MDA-MB-468 decreased growth, suggesting that SCD1 is indeed important in regulating growth in TN breast cancers [5]. SCD1 knockdown in the HR+ breast cancer cell line MCF7 and the HER2+ breast cancer cell line BT474 also inhibited growth, indicating that SCD1 plays an important role in regulating growth in all three breast cancer subtypes. In this current study, we noted a trend toward worse RFS and OS with high SCD1 expression in all three subtypes, consistent with our previous in vitro findings that decreased SCD1 expression inhibited growth in HR+, HER2+, and TN breast cancer cell lines [5].
SCD1 overexpression has been associated with a malignant phenotype in several models [21,27,40]. However, no study has yet demonstrated that SCD1 overexpression definitively triggers or sustains a malignant phenotype. Even still, considerable evidence for an association between SCD1 overexpression and neoplastic behavior supports our findings that high SCD1 expression in primary breast cancers is independently associated with shorter survival in breast cancer. Although our study is novel in its assessment of SCD1 expression in primary breast tumors, our data are consistent with those of another group that investigated the prognostic significance of fatty acids in breast cancer and found that low stearic acid levels in patients with primary breast tumors were associated with metastasis, relapse, and death [41]. Our results add to the accumulating evidence favoring SCD1 as an essential contributor to the malignant phenotype. However, one limitation of our study is that we compared SCD1 expression across tumor subtypes but did not compare SCD1 expression in the tumor with expression in normal breast epithelium. Thus, our experimental design did not allow us to determine whether SCD1 is overexpressed in breast cancer.
The association of SCD1 with obesity, metabolic syndrome, and type 2 diabetes mellitus suggests that therapies for these diseases may also be applicable to breast cancer. For example, inhibiting SCD1 in mice decreases body fat and prevents obesity [42,43], thus current research is targeting SCD1 for treatment of obesity and metabolic syndrome. Toward this end, several groups recently identified inhibitors for SCD1 enzyme activity [42–46]. These inhibitors could also be pursued as novel therapies for breast cancer. However, it is important to note that we did not find any association between SCD1 expression in primary breast cancers and BMI or diabetes mellitus status. As patients with diabetes mellitus in our study were on insulin and/or oral anti-hyperglycemic agents, further clinical studies are needed to determine whether drugs for treating obesity, metabolic syndrome, and diabetes mellitus may have any benefit for patients with primary breast cancer and whether baseline SCD1 expression in the primary tumor correlates with clinical benefit from these agents.
Taken together with our previous work demonstrating that siRNA knockdown of SCD1 inhibited cell growth in breast cancer cells, our cumulative data suggest that SCD1 is critical for malignant progression and has potential as a therapeutic target. However, as we found SCD1 expression to be significantly lower in TN breast cancers, the utility of SCD1 as a target may be limited to HR+ and HER2+ breast cancers. In light of this study’s findings and recent studies demonstrating that SCD1 inhibitors diminish tumor growth in a model of prostate cancer and reduce cell proliferation in prostate and lung cancer cells [29,30], investigating the effect of SCD1 inhibitors in breast cancer is a crucial next step that has the potential to improve survival in patients with breast cancers having high SCD1 expression, such as HR+ and HER2+ breast cancer.
Acknowledgments
This research was supported in part by the National Cancer Institute T32 CA009599-23 (AH, FMB), the Elsa Pardee Foundation (FMB), Susan G. Komen for the Cure SAC10006 (FMB, KAD) and KG 081694 (AMG, GBM) Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209) (FMB, AA, AMG, GBM), Society of Surgical Oncology Clinical Investigator Award in Breast Cancer Research (FMB), the Kleberg Center for Molecular Markers at The University of Texas MD Anderson Cancer Center, National Cancer Institute 1K23CA121994-01 (AMG),, the Cancer Center Support Grant CCSG P30 CA016672 (KAD), and the National Center for Research Resources Grants 3UL1RR024148 (FMB, KAD) and UL1TR000371 (FMB, AA and KAD).
Abbreviations
- SCD1
Stearoyl-CoA desaturase 1
- RFS
Relapse-free survival
- OS
Overall survival
- mTOR
Mammalian target of rapamycin
- TN
Triple negative
- HR+
Hormone receptor-positive
- HER2+
Human epidermal growth factor receptor-2-positive
- FNA
Fine needle aspirate
- FISH
Fluorescent in situ hybridization
- ER
Estrogen receptor
- PR
Progesterone receptor
- RPPA
Reverse phase protein array
- siRNA
Small interfering RNA
- PI3K
Phosphatidylinositol 3-kinase
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40(9):1549–1558. [PubMed] [Google Scholar]
- 2.Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31(9):1509–1515. doi: 10.1093/carcin/bgq131. [DOI] [PubMed] [Google Scholar]
- 3.Enoch HG, Catala A, Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J Biol Chem. 1976;251(16):5095–5103. [PubMed] [Google Scholar]
- 4.Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM. Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem J. 1999;340 ( Pt 1):255–264. [PMC free article] [PubMed] [Google Scholar]
- 5.Luyimbazi D, Akcakanat A, McAuliffe PF, Zhang L, Singh G, Gonzalez-Angulo AM, Chen H, Do KA, Zheng Y, Hung MC, Mills GB, Meric-Bernstam F. Rapamycin regulates stearoyl CoA desaturase 1 expression in breast cancer. Mol Cancer Ther. 2010;9(10):2770–2784. doi: 10.1158/1535-7163.MCT-09-0980. 1535-7163.MCT-09-0980 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146(3):408–420. doi: 10.1016/j.cell.2011.06.034. S0092-8674(11)00709-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–183. doi: 10.1016/j.molcel.2010.06.022. S1097-2765(10)00463-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Q, Mak KM, Lieber CS. Leptin enhances alpha1(I) collagen gene expression in LX-2 human hepatic stellate cells through JAK-mediated H2O2-dependent MAPK pathways. J Cell Biochem. 2006;97(1):188–197. doi: 10.1002/jcb.20622. [DOI] [PubMed] [Google Scholar]
- 9.Mauvoisin D, Prevost M, Ducheix S, Arnaud MP, Mounier C. Key role of the ERK1/2 MAPK pathway in the transcriptional regulation of the Stearoyl-CoA Desaturase (SCD1) gene expression in response to leptin. Mol Cell Endocrinol. 2010;319(1–2):116–128. doi: 10.1016/j.mce.2010.01.027. S0303-7207(10)00044-4 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Tabor DE, Kim JB, Spiegelman BM, Edwards PA. Identification of conserved cis-elements and transcription factors required for sterol-regulated transcription of stearoyl-CoA desaturase 1 and 2. J Biol Chem. 1999;274(29):20603–20610. doi: 10.1074/jbc.274.29.20603. [DOI] [PubMed] [Google Scholar]
- 11.Mauvoisin D, Rocque G, Arfa O, Radenne A, Boissier P, Mounier C. Role of the PI3-kinase/mTor pathway in the regulation of the stearoyl CoA desaturase (SCD1) gene expression by insulin in liver. J Cell Commun Signal. 2007;1(2):113–125. doi: 10.1007/s12079-007-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheneval D, Christy RJ, Geiman D, Cornelius P, Lane MD. Cell-free transcription directed by the 422 adipose P2 gene promoter: activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1991;88(19):8465–8469. doi: 10.1073/pnas.88.19.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christy RJ, Yang VW, Ntambi JM, Geiman DE, Landschulz WH, Friedman AD, Nakabeppu Y, Kelly TJ, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989;3(9):1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- 14.Singh MV, Ntambi JM. Nuclear factor 1 is essential for the expression of stearoyl-CoA desaturase 1 gene during preadipocyte differentiation. Biochim Biophys Acta. 1998;1398(2):148–156. doi: 10.1016/s0167-4781(98)00037-2. S0167-4781(98)00037-2 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68(2):72–82. doi: 10.1159/000100426. 000100426 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49(3):588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 17.Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, Dahlman-Wright K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20(6):1287–1299. doi: 10.1210/me.2006-0012. me.2006-0012 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, Dahlman-Wright K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295(4):E904–912. doi: 10.1152/ajpendo.90248.2008. 90248.2008 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science. 1996;274(5290):1185–1188. doi: 10.1126/science.274.5290.1185. [DOI] [PubMed] [Google Scholar]
- 20.Elam MB, Yellaturu C, Howell GE, Deng X, Cowan GS, Kumar P, Park EA, Hiler ML, Wilcox HG, Hughes TA, Cook GA, Raghow R. Dysregulation of sterol regulatory element binding protein-1c in livers of morbidly obese women is associated with altered suppressor of cytokine signaling-3 and signal transducer and activator of transcription-1 signaling. Metabolism. 2009;59(4):587–598. doi: 10.1016/j.metabol.2009.09.001. S0026-0495(09)00369-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaglia N, Caviglia JM, Igal RA. High stearoyl-CoA desaturase protein and activity levels in simian virus 40 transformed-human lung fibroblasts. Biochim Biophys Acta. 2005;1687(1–3):141–151. doi: 10.1016/j.bbalip.2004.11.015. S1388-1981(04)00199-4 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Scaglia N, Chisholm JW, Igal RA. Inhibition of StearoylCoA Desaturase-1 Inactivates Acetyl-CoA Carboxylase and Impairs Proliferation in Cancer Cells: Role of AMPK. PLoS One. 2009;4(8):e6812. doi: 10.1371/journal.pone.0006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Ding SF, Habib NA, Fermor BF, Wood CB, Gilmour RS. Partial characterization of a cDNA for human stearoyl-CoA desaturase and changes in its mRNA expression in some normal and malignant tissues. Int J Cancer. 1994;57(3):348–352. doi: 10.1002/ijc.2910570310. [DOI] [PubMed] [Google Scholar]
- 24.Yahagi N, Shimano H, Hasegawa K, Ohashi K, Matsuzaka T, Najima Y, Sekiya M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Nagai R, Ishibashi S, Kadowaki T, Makuuchi M, Ohnishi S, Osuga J, Yamada N. Coordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005;41(9):1316–1322. doi: 10.1016/j.ejca.2004.12.037. S0959-8049(05)00204-2 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Thai SF, Allen JW, DeAngelo AB, George MH, Fuscoe JC. Detection of early gene expression changes by differential display in the livers of mice exposed to dichloroacetic acid. Carcinogenesis. 2001;22(8):1317–1322. doi: 10.1093/carcin/22.8.1317. [DOI] [PubMed] [Google Scholar]
- 26.Roongta UV, Pabalan JG, Wang X, Ryseck R-P, Fargnoli J, Henley BJ, Yang W-P, Zhu J, Madireddi MT, Lawrence RM, Wong TW, Rupnow BA. Cancer Cell Dependence on Unsaturated Fatty Acids Implicates Stearoyl-CoA Desaturase as a Target for Cancer Therapy. Molecular Cancer Research. 9(11):1551–1561. doi: 10.1158/1541-7786.mcr-11-0126. [DOI] [PubMed] [Google Scholar]
- 27.Scaglia N, Igal RA. Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol. 2008;33(4):839–850. [PubMed] [Google Scholar]
- 28.Morgan-Lappe SE, Tucker LA, Huang X, Zhang Q, Sarthy AV, Zakula D, Vernetti L, Schurdak M, Wang J, Fesik SW. Identification of Ras-related nuclear protein, targeting protein for xenopus kinesin-like protein 2, and stearoyl-CoA desaturase 1 as promising cancer targets from an RNAi-based screen. Cancer Res. 2007;67(9):4390–4398. doi: 10.1158/0008-5472.CAN-06-4132. 67/9/4390 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Fritz V, Benfodda Z, Rodier Gv, Henriquet C, Iborra Fo, Avancès C, Allory Y, de la Taille A, Culine Sp, Blancou H, Cristol JP, Michel Fo, Sardet C, Fajas L. Abrogation of De novo Lipogenesis by Stearoyl-CoA Desaturase 1 Inhibition Interferes with Oncogenic Signaling and Blocks Prostate Cancer Progression in Mice. Mol Cancer Ther. 2010;9(6):1740–1754. doi: 10.1158/1535-7163.mct-09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess D, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS One. 2010;5(6):e11394. doi: 10.1371/journal.pone.0011394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pala V, Krogh V, Muti P, Chajes V, Riboli E, Micheli A, Saadatian M, Sieri S, Berrino F. Erythrocyte membrane fatty acids and subsequent breast cancer: a prospective Italian study. J Natl Cancer Inst. 2001;93(14):1088–1095. doi: 10.1093/jnci/93.14.1088. [DOI] [PubMed] [Google Scholar]
- 32.Chajes V, Hulten K, Van Kappel AL, Winkvist A, Kaaks R, Hallmans G, Lenner P, Riboli E. Fatty-acid composition in serum phospholipids and risk of breast cancer: an incident case-control study in Sweden. Int J Cancer. 1999;83(5):585–590. doi: 10.1002/(SICI)1097-0215(19991126)83:5<585::AID-IJC2>3.0.CO;2-Z. [pii] [DOI] [PubMed] [Google Scholar]
- 33.Chajes V, Thiebaut AC, Rotival M, Gauthier E, Maillard V, Boutron-Ruault MC, Joulin V, Lenoir GM, Clavel-Chapelon F. Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC Study. Am J Epidemiol. 2008;167(11):1312–1320. doi: 10.1093/aje/kwn069. kwn069 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shannon J, King IB, Moshofsky R, Lampe JW, Gao DL, Ray RM, Thomas DB. Erythrocyte fatty acids and breast cancer risk: a case-control study in Shanghai, China. Am J Clin Nutr. 2007;85(4):1090–1097. doi: 10.1093/ajcn/85.4.1090. 85/4/1090 [pii] [DOI] [PubMed] [Google Scholar]
- 35.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100(2):229–235. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 36.Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF, Kuerer HM, Mittendorf EA, Hortobagyi GN, Meric-Bernstam F. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(11):1821–1828. doi: 10.1200/JCO.2009.25.3286. JCO.2009.25.3286 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Angulo AM, Hennessy BT, Meric-Bernstam F, Sahin A, Liu W, Ju Z, Carey MS, Myhre S, Speers C, Deng L, Broaddus R, Lluch A, Aparicio S, Brown P, Pusztai L, Symmans WF, Alsner J, Overgaard J, Borresen-Dale AL, Hortobagyi GN, Coombes KR, Mills GB. Functional proteomics can define prognosis and predict pathologic complete response in patients with breast cancer. Clin Proteomics. 2011;8(1):11. doi: 10.1186/1559-0275-8-11. 1559-0275-8-11 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meric-Bernstam F, Akcakanat A, Chen H, Do KA, Sangai T, Adkins F, Gonzalez-Angulo AM, Rashid A, Crosby K, Dong M, Phan AT, Wolff RA, Gupta S, Mills GB, Yao J. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clin Cancer Res. 2012;18(6):1777–1789. doi: 10.1158/1078-0432.CCR-11-2123. 18/6/1777 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, Barretina J, Lin WM, Rameh L, Salmena L, Pandolfi PP, Cantley LC. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16(2):115–125. doi: 10.1016/j.ccr.2009.06.006. S1535-6108(09)00180-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falvella FS, Pascale RM, Gariboldi M, Manenti G, De Miglio MR, Simile MM, Dragani TA, Feo F. Stearoyl-CoA desaturase 1 (Scd1) gene overexpression is associated with genetic predisposition to hepatocarcinogenesis in mice and rats. Carcinogenesis. 2002;23(11):1933–1936. doi: 10.1093/carcin/23.11.1933. [DOI] [PubMed] [Google Scholar]
- 41.Bougnoux P, Chajes V, Lanson M, Hacene K, Body G, Couet C, Le Floch O. Prognostic significance of tumor phosphatidylcholine stearic acid level in breast carcinoma. Breast Cancer Res Treat. 1992;20(3):185–194. doi: 10.1007/BF01834624. [DOI] [PubMed] [Google Scholar]
- 42.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. The Journal of clinical investigation. 2005;115(4):1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dillon R, Greig MJ, Bhat BG. Development of a novel LC/MS method to quantitate cellular stearoyl-CoA desaturase activity. Anal Chim Acta. 2008;627(1):99–104. doi: 10.1016/j.aca.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Xin Z, Zhao H, Serby MD, Liu B, Liu M, Szczepankiewicz BG, Nelson LT, Smith HT, Suhar TS, Janis RS, Cao N, Camp HS, Collins CA, Sham HL, Surowy TK, Liu G. Discovery of piperidine-aryl urea-based stearoyl-CoA desaturase 1 inhibitors. Bioorg Med Chem Lett. 2008;18(15):4298–4302. doi: 10.1016/j.bmcl.2008.06.088. [DOI] [PubMed] [Google Scholar]
- 46.Soulard P, McLaughlin M, Stevens J, Connolly B, Coli R, Wang L, Moore J, Kuo MS, LaMarr WA, Ozbal CC, Bhat BG. Development of a high-throughput screening assay for stearoyl-CoA desaturase using rat liver microsomes, deuterium labeled stearoyl-CoA and mass spectrometry. Anal Chim Acta. 2008;627(1):105–111. doi: 10.1016/j.aca.2008.04.017. [DOI] [PubMed] [Google Scholar]