Abstract
Background: Coula edulis Bail (Olacaceae), is an evergreen tree growing to a height of 25. This study aimed at evaluating the antidermatophytic and toxicological properties of the stem bark of C. edulis extract as well as fractions and compounds isolated from it.
Methods: The plant extract was prepared by maceration in CH2Cl2-MeOH (1:1 v/v). The fractionation of this extract was done by silica gel column chromatography. Antidermatophytic activities were assayed using agar dilution method. The acute and sub-acute toxicities of oral administrations of the extract were studied in rodents.
Results: The crude extract of C. edulis displayed antidermatophytic activity against the tested microorganisms with highest activity against Microsporum audouinii and Trichophyton mentagrophytes. The fractionation enhanced the antidermatophytic activity in fraction F3 (MIC=0.62-1.25 mg/ml) compared to the crude extract (MIC=1.25-5 mg/ml). Further fractionation and purification of the fractions F2 and F3 gave respectively 3-O-β-D-glucopyranoside of sitosterol (MIC=0.20-0.40 mg/ml) and a mixture of β-sitosterol, stigmasterol and n-hexadecanoid acid (MIC=0.80 mg/ml). The median lethal doses (LD50) of the crude extract were 16.8 and 19.6 g/kg body weight (BW) in male and female mice, respectively. At 200 mg/kg BW, there was a decrease in body weight gain, food and water consumptions. Gross anatomical analysis revealed white vesicles on the liver of the rats treated with the extract at 200 mg/kg BW. This dose also induced significant (P<0.05) changes on hematological and biochemical parameters in rats after 28 days of treatment.
Conclusion: These data suggest that the CH2Cl2-MeOH (1:1 v/v) extract of C. edulis stem bark possesses antidermatophytic properties. They also show that at high doses (≥ 200 mg/kg BW), the extract has significant hepatotoxic and nephrotoxic activities.
Key Words: Coula edulis, fractionation, toxicity, antidermatophytic
Introduction
Dermatophytosis is a group of skin fungal infections caused by dermatophytes (or ring worms), which invade and attack keratinized tissues. Typical symptoms of these infections include inflammation or redness of the infected part, brittleness and fissures of the nails, and loss of hair from the affected parts. A large number of antifungal agents such as griseofulvin, azole derivatives, allylamines and morpholines are used in the treatment of dermatophyte infections. However, they have been shown to exhibit adverse side effects such as gastrointestinal disturbances, cutaneous reactions, hepatotoxicity and leucopoenia.1-2 In addition to such adverse effects, the acquired resistance to certain antifungals,2 and the high cost of synthetic drugs limit the treatment of dermatophytosis. Because of their biodegradable nature, the demand for natural drugs has been increasing, and therefore, the development of antifungal agents from local raw material is still a necessity. This is particularly true in the cases developing countries, which have high levels of their populations.
Coula edulis Bail (Olacaceae), locally known as “African walnut”, is a commonly occurring medicinal plant in Africa. It is an evergreen tree growing to a height of 25-. It can be found in the top canopy of forests as well as the lower story, and has no special soil requirements. Ethnobotanical studies indicate that the stem and fruits of C. edulis are commonly used in West Africa for the treatment of stomach ache and skin diseases. It is also used as tonifiant.3 The bark of the plant is used to produce rinses or enemas for loin pains or kidney problems. Moreover, antibacterial and anti-yeasts activities of C. edulis extracts have been shown in previous studies.4,5 To the best of our knowledge, there is not a published report concerning the antidermatophytic activity of this plant.
This study, therefore, was undertaken to first evaluate the antidermatophytic activity of the CH2Cl2-MeOH (1:1 v/v) extract, fractions and compound isolated from the stem bark of C. edulis, and then to assess the toxicological risk of its extract upon consumption.
Materials and methods
General Experimental Procedures for Structure Elucidations
Melting points (uncorr) were determined on a Kofler apparatus. Infra-red (IR) spectra were recorded using a Shimadzu FTIR-8400S spectrophotometer. Ultra-violet (UV) spectra were measured with a UV-210 PC, UV-Vis scanning spectrophotometer (Analytikjena). Proton Nuclear magnetic resonance (1H-NMR) spectra were recorded in CDCl3 using a Bruker Avance 500 MHz NMR spectrometer and Trimethylsilyl (TMS) as an internal standard. Column chromatography was run on Merck silica gel 60. Thin layer chromatography (TLC) were carried out either on silica gel GF254 pre-coated plates (analytical TLC) or on silica gel 60 PF254 containing gypsum (preparative TLC), with detection accomplished by spraying with 50% H2SO4 followed by heating at 100°C, or by visualizing with a UV lamp at 254 and 366 nm.
Gas chromatography-mass spectrometry (GC-MS) data were obtained with an Agilent 6890N Network GC system/5975 Inert×L Mass selective Detector at 70 eV and 20°C. The GC column was a CP-Sil 8 CB LB, fused silica capillary column ( x , film thickness 0.25 µm). The initial temperature was 50°C for 1 min, and was heated at 10°C/min to 300°C. The fatty acid samples of 0.5 µl were injected. The split ratio was 50:1. The carrier gas was helium at a flow rate of 1.2 ml/min.
Plant Material
The stem bark of C. edulis was collected from Buea (South-West Region of Cameroon) in January 2008. The plant material was identified at the Cameroon National Herbarium in Yaoundé where a voucher specimen (19357/HNC) was conserved. The plant material was air-dried at room temperature. The dried plant material was ground into a fine powder.
Extraction, Fractionation and Isolation
Previously dried and powdered stem bark of C. edulis () was extracted with dichloromethane-methanol (1:1) () for 48 hours. The filtrate was concentrated under reduced pressure at 40°C using rotary vacuum evaporator to give a brown paste crude extract (). One hundred and four grams () of this extract was then subjected to fractionation as previously described.4 Briefly, the crude extract was subjected to a column chromatography with silica gel 40 (particle size 0.2-) as stationary phase. The column was successively eluted with hexane, hexane-ethyl acetate and ethyl acetate-methanol gradients. One hundred and seventy one (171) fractions of 250 ml each were collected and combined on the basis of their thin layer chromatography (TLC) profiles to afford eight main fractions: F1 (1-59), F2 (60-122), F3 (123-124), F4 (125-126), F5 (127-138), F6 (139-149), F7 (150-156) and F8 (157-171). These fractions were tested for their antidermatophytic activities. Fraction F2 () was purified on a silica gel 60 (0.063-, ) column (×) to give glucosterol (28 mg). Fraction F3 () was rechromatographed on a silica gel 60 (0.063-, ) column giving a mixture of sterols and fatty acids (32 mg).
Identification of the Compounds
The structure of the isolated compound was established on the basis of spectroscopic analysis (IR, UV, 1H NMR) and by comparison of the data with those reported in literature.6 The mixture of sterols and fatty acids was identified by Gas Chromatography-Mass Spectrometry (GC-MS) after saponification and methylation of fatty acids.7 The separated compounds were identified by comparisons of their mass spectra to those of compounds registered in NIST 89 and Wiley 237 spectral libraries of GC-MS instrument.
Micro-organisms
The microorganisms used in this study included four strains of dermatophytes, namely: Trichophyton mentagrophytes E1425, Trichophyton terrestre E1501, Microsporum gypseum E1420 and Epidermophyton floccosum E1423 obtained from “Ecole nationale vétérinaire d’Aford” (France), and one clinical isolate of Microsporum audouinii characterized in our laboratory. These fungi were grown at room temperature (25±2°C) and maintained on sabouraud dextrose agar (SDA, Biomerieux).
In Vitro Antidermatophytic Test
The antidermatophytic activities of the crude CH2Cl2-MeOH (1:1 v/v) extract, fractions and pure compound from C. edulis were evaluated using the agar dilution method as reported by Kuiate and co-workers.8 Stock solutions of the test samples (100 mg/ml) were prepared using a 10% solution of dimethylsulfoxide (DMSO, Mehr). From these stock solutions, dilutions (in melted Sabouraud Dextrose Agar, SDA, Biomerieux) were made to give serial two-fold dilutions with concentrations ranging from 0.312 to 5 mg/ml. The Petri dishes ( diameters) were filled with samples containing SDA to a final volume of 10 ml. The Petri dishes were then inoculated at their centre with a disk ( diameters) cut from the periphery of 10 days-old cultures. Negative control dishes contained a 10% final concentration of DMSO. Griseofulvin was used as a positive control. The test and the negative control Petri dishes were incubated at room temperature for 10 days. The radial growth of each fungus was recorded every day at the same time and the percentages of inhibition were calculated using the following formula:
Where dc was the diameter of colony of negative control culture and dt was the diameter of colony of test culture.
Each assay was repeated trice. The minimum inhibitory concentration (MIC) was defined as the lowest concentration that prevented a visible growth of fungal strain.
The fungicidal/fungistatic natures of the extracts were determined as described by Thompson.9 The inhibited fungal discs from the extract treated dishes were sub-cultured in a fresh medium, and revival of their growth indicated fungistatic effect, while absence of any growth indicated fungicidal effect.
Experimental Animals
In this study, 72 Swiss albino mice (36 males and 36 females; 7–8 weeks old; 20–) and 50 Wistar albino rats (25 males and 25 females; 6–8 weeks old; 120–) were used. They were bred in the animal house of the Department of Biochemistry, University of Dschang, Cameroon. The animals were housed two or three per cage in elevated wire mesh cages, and were provided with standard animal food, grower’s mash (Grand Cereals LTD, Jos-Nigeria) and water ad libitum.
Acute Toxicity Study
This study was carried out on Swiss albino mice (males and females) as described by Emerson and co-workers.10 A total of 72 mice, 6-8 weeks old were used. The mice of each sex were divided into 6 subgroups of 6 animals each. Stock solution of crude extract (0.8 g/ml) was prepared using 1% aqueous solution of DMSO. Mice in subgroup 1 (control) were given (oral gavage) 1% aqueous solution of DMSO (1 ml per BW) while those of subgroups 2, 3, 4, 5 and 6 were administrated 2, 8, 16, 24 and 28 g/kg BW of crude extract, respectively. All the animals were fasted for 18 hours prior to the extract administration. After a single dose administration, the animals were observed for the first 3 hours for behavioral changes.11 Deaths were counted for the first 48 hours. The survived mice were closely observed for two weeks, and were monitored daily for behavioral changes, signs of toxicity and the latency of death. The median lethal dose (LD50) was determined by calculation.12
Sub-Acute Toxicity Study
Fifty Wistar albino rats (25 males and 25 females) were used. Animals of each sex were divided into 5 subgroups of 5 animals each. They were kept under the same conditions as described above. Rats in subgroup 1 (control) were given daily administration of 1% DMSO (1.5 ml per BW) by oral gavage, while those of subgroups 2, 3, 4 and 5 were given 25, 50, 100 and 200 mg/kg BW of the crude extract, respectively for four weeks. All animals were provided with food and water ad libitum. They were then observed for physiological and behavioral changes.
Body Weight Trend
The body weight of each rat was determined during the acclimatization period, once before the commencement of vehicle or extract administration, once every day during the administration period, and once on the day of the sacrifice. The relative body weight (RBW) of each animal was calculated as follows:
Food and Water Consumptions
The amounts of food and water consumed (AC) were measured daily from the quantity of food and water supplied and the amount remaining after 24 hours as followed:
AC (in g or ml)=Total amount of food/water given – Amount of food/water remaining.
Blood Analysis
Twenty-four hours after the last administration, rats were anesthetized with chloroform vapor, and blood samples were collected through cardiac punctures using heparinized and non heparinized centrifuge tubes. The heparinized blood was used for the total red blood cell (RBC) and white blood cell (WBC) counts,13 and heamatocrit.14 The non heparinized blood samples were allowed to clot before centrifugation (4000 rpm at +4°C for 10 min) to obtain serum samples, which were assessed for alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose, creatinine, total cholesterol and protein levels by standard methods using relevant kits (Biosystem Reagents and Instruments).
Organ Analysis
Immediately after the blood collection, the liver, lung, heart, spleen and kidneys were carefully dissected out, blotted, observed macroscopically and weighed immediately using a sartorius electronic balance. The relative organ weight (ROW) of each animal was then calculated as follows:
Organs tissues were thawed and homogenized 20 times (w/v) by homogeniser in ice-cold Tris-HCl KCl buffer (pH 7.4). The homogenate samples were centrifuged at 6000 rpm for 30 min, and the supernatants were then used for enzyme and total protein assays using the method cited above.
Statistical Analysis
Statistical analysis was carried out using Statistical Package for Social Science (SPSS, version 12.0). The experimental results were expressed as the mean±Standard Deviation (SD). Group comparisons were performed using One Way ANOVA followed by Waller-Duncan Post Hoc test. A p value ≤0.05 was considered statistically significant.
Ethics
The experiments were carried out observing the welfare of animals as recommended by World Health Organization (WHO).15 Moreover, all procedures involving animals were carried out in strict compliance with the rules and regulations of local Ethics Committee.
Results
Chemical Analysis
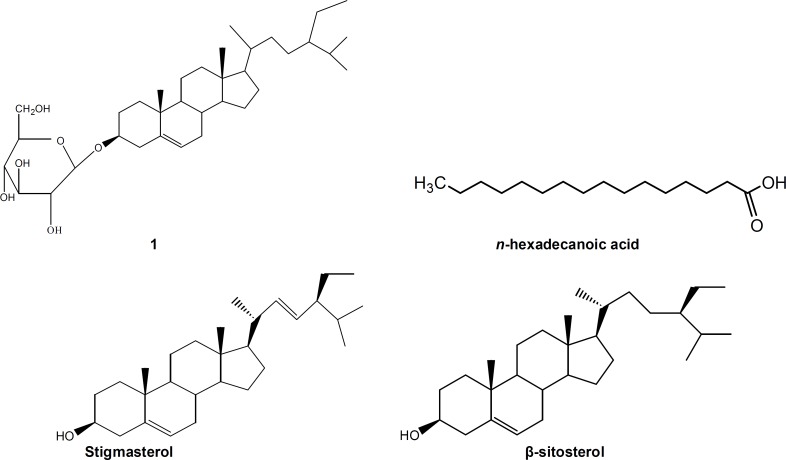
One known compound: 3-O-β-D-glucopyranoside of sitosterol (1), and a mixture of β-sitosterol, stigmasterol and n-hexadecanoid acid (2) were isolated from CH2Cl2 : MeOH (1:1) extract of C. edulis stem bark (figure 1).
Figure 1.
Chemical structures of 3-O-β-D-glucopyranoside of sitosterol (1) and a mixture of β-sitosterol, stigmasterol and n-hexadecanoid acid (2)
Antidermatophytic Activity
The results of the antidermatophytic activities of the crude extract, fractions and compounds from C. edulis are presented in tables 1 and 2. It appeared that the extract and fractions F2 and F3 were able to prevent the total growth of all studied microorganisms at the concentrations examined (table 1). The other samples showed less antifungal activities. The most sensitive fungi were Microsporum audouinii and Epidermophyton floccoseum (table 2). Fraction F3 showed the best antidermatophytic activity (MIC=0.62–1.25 mg/ml), as compared to that of the crude extract (MIC=1.25-5 mg/ml) or other fractions (MIC=1.25->5 mg/ml). However, none of the samples tested was as active as the reference antibiotic, griseofulvin (MIC=0.001-0.020 mg/ml) (table 2).
Table 1.
The percentage inhibitions (%) of fungal growth by the crude extract of stem bark of Coula edulis and the fractions from the extract at the concentration of 5 mg/ml against the tested dermatophytes.
| Test samples | M. audouinii | M. gypseum | T. terrestre | T. mentagrophytes | E. floccoseum |
|---|---|---|---|---|---|
| Crude extract | 100±0.00a | 100±0.00a | 100±0.00a | 100±0.00a | 100±0.00a |
| Fraction F1 | 95.50±1.46b | 95.76±0.75b | 96.72±0.67b | 25.11±5.60f | 100±0.00a |
| Fraction F2 | 100±0.00a | 100±0.00a | 100±0.00a | 100±0.00a | 100±0.00a |
| Fraction F3 | 100±0.00a | 100±0.00a | 100±0.00a | 100±0.00a | 100±0.00a |
| Fraction F4 | 100±0.00a | 65.69±2.53d | 92.01±1.18c | 91.39±1.83b | 100±0.00a |
| Fraction F5 | 100±0.00a | 71.33±2.60c | 82.55±2.99d | 65.07±3.93c | 100±0.00a |
| Fraction F6 | 100±0.00a | 65.54±2.80d | 82.70±2.63d | 54.58±3.42d | 100±0.00a |
| Fraction F7 | 100±0.00a | 46.56±1.55e | 69.54±2.54e | 41.07±3.10e | 97.46±0.61b |
| Fraction F8 | 100±0.00a | 67.20±2.73d | 70.97±3.78e | 57.68±4.37d | 95.37±0.87c |
M; Microsporum, T: Trichophyton, E: Epidermophyton. Values are expressed as mean±SD for three trials. For the same column, values signified by different letters (a-f) are significantly (P < 0.05) different from each other.
Table 2.
Minimum inhibitory concentration/minimum fungicidal concentration (MIC/MFC) (in mg/ml) of the crude extract of Coula edulis stem bark and fractions and compounds isolated from the extract against the tested dermatophytes.
| Test samples | M. audouinii | M. gypseum | T. terrestre | T. mentagrophytes | E. floccoseum |
|---|---|---|---|---|---|
| Crude extract | 1.25 / 1.25 | 2.50 / 2.50 | 5 / 5 | 1.25 / 1.25 | 2.50 / 2.50 |
| Fraction F1 | >5 / >5 | >5 / >5 | >5 / >5 | >5 / >5 | 2.50 / >5 |
| Fraction F2 | 1.25 / 1.25 | 2.50 / >5 | 5 / >5 | 5 / >5 | 1.25 / 2.50 |
| Fraction F3 | 0.62 / 1.25 | 2.50 /2.50 | 1.25 / 1.25 | 1.25 / 1.25 | 0.62 / 1.25 |
| Fraction F4 | 1.25 / 2.50 | >5 / >5 | >5 / >5 | >5 / >5 | 2.50 / 2.50 |
| Fraction F5 | 2.50 / 5 | >5 / >5 | >5 / >5 | >5 / >5 | 2.50 / 5 |
| Fraction F6 | 2.50 / 5 | >5 / >5 | >5 / >5 | >5 / >5 | 2.50 / 5 |
| Fraction F7 | 2.50 / 5 | >5 / >5 | >5 / >5 | >5 / >5 | >5 / >5 |
| Fraction F8 | 2.50 / 5 | >5 / >5 | >5 / >5 | >5 / >5 | >5 / >5 |
| Compound 1 | 0.40 / 0.40 | nt | nt | Nt | 0.20 / 0.20 |
| Compound 2 | 0.80 / >0.80 | nt | nt | Nt | 0.80 / >0.80 |
| Griseofulvin | 0.001 / >0.10 | 0.01 / > 0.10 | 0.020 / > 0.10 | 0.001 / > 0.10 | 0.001 / >0.10 |
M; Microsporum, T: Trichophyton, E: Epidermophyton.1: 3-O-β-D-glucopyranoside of sitosterol; 2: β-sitosterol, stigmasterol and n-hexadecanoid acid. Nt; not tested
Acute Toxicity
The results of the acute toxicity study indicated that female mice were more tolerant than male ones to oral administration of the crude extract. For doses up to 16 g/kg BW, the animal’s reaction to pinch and noise were reduced. All animals developed diarrhea within 3 hours after the administration of doses ≥16 g/kg BW. None of the treated mice survived within 48 hours after the administration of 24 and 28 g/kg BW in males and females, respectively. The calculated LD50 values were 16.80 and 19.60 g/kg BW for male and female mice, respectively.
Sub-acute Toxicity
General Symptoms, Body and Organ Weight Changes
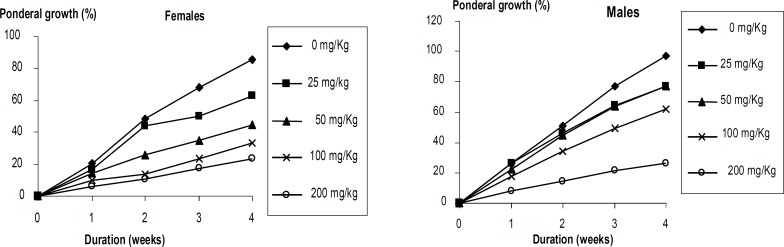
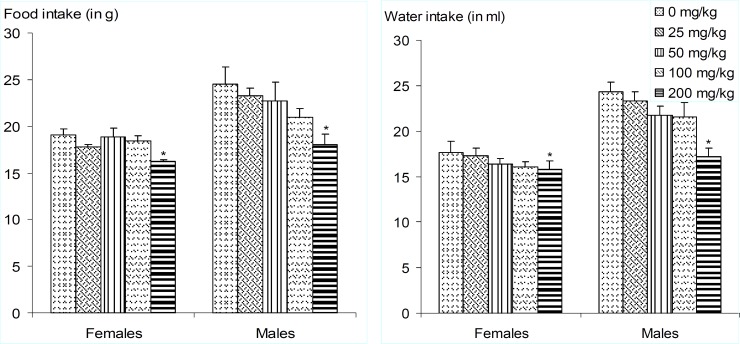
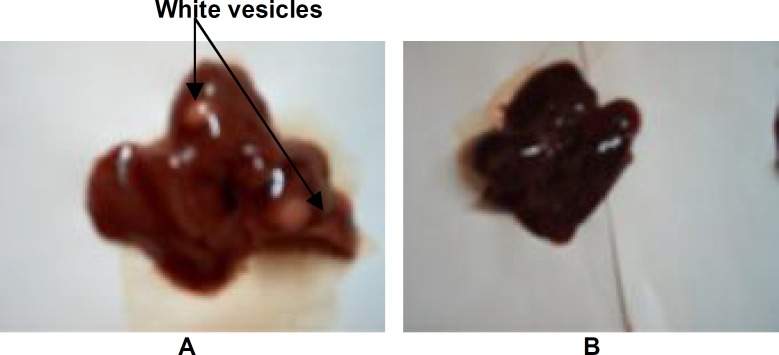
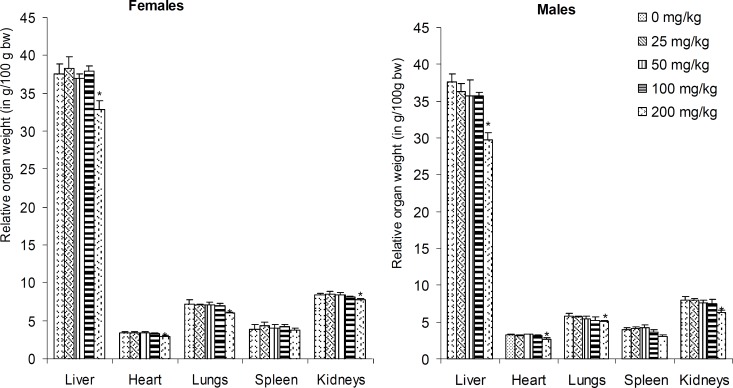
No death did occur following daily administration of vehicle or the extract for 28 days in control or extract treated group, respectively. The animals did not show any significant changes in their general behaviur. However, there were significant dose-dependent decreases in animals' body weight gain as well as food and water consumptions. (figure 2-3). These reductions were most pronounced (P<0.05) in the animals that were administered the extract at the highest dose (200 mg/kg BW). Moreover, the macroscopic observation of livers of animals revealed the presence of white vesicles on the surface of this organ at the dose of 200 mg/kg BW (figure 4). Also, significant decreases (P<0.05) were recorded in the relative liver, heart, lung and kidney weights at the same dose irrespective of the sex (figure 5). None of the screened organ showed significant (P≥0.05) variation in the parameters evaluated above at doses ≤100 mg/kg BW (figure 5).
Figure 2.
Variation of relative body weight of rats as a function of the duration and dose of Coula edulis CH2Cl2/MeOH (1:1) stem bark extract.
Figure 3.
Daily amounts of food and water consumed as affected by doses of Coula edulis CH2Cl2/MeOH (1:1) stem bark extract during sub-acute toxicity study.
Values are expressed as mean±SD of five animals. For the same parameter and sex, values bearing asterisk (*) are significantly different (p<0.05) to the control and other groups.
Figure 4.
Livers from rats treated with the CH2Cl2/MeOH (1:1) extract of Coula edulis stem bark (A) or vehicle (Dimethylsulfoxide, DMSO) (B). They can be differentiated by the presence of white vesicles on liver from rats receiving the extract.
Figure 5.
Relative organ weights (as a percent of body weight) of rats after sub-acute treatment (28 days) with different doses of Coula edulis CH2Cl2/MeOH (1:1) stem bark extract.
Values are expressed as mean±SD of five animals. * Indicate significant (P <0.05) difference from the control group for the each organ and gender.
Hematological and Biochemical Observations
There was no gender-dependent differences in RBC and WBC, but a significant decrease in hematocrit (P<0.05) was observed in males at the dose of 200 mg/ kg BW as compared to that of the control (table 3). This dose also induced a significant (P<0.05) increase in serum creatinine in both sexes. Also, a significant (P<0.05) decrease in serum total cholesterol levels, and a significant (P<0.05) increase in serum and liver levels of proteins as well as serum levels of ALT and AST were observed in both male and female rats compared to the control group (tables 4-5).
Table 3.
Peripheral blood changes observed in rats after 82 days treatment with different doses of C. edulis CH2Cl2/MeOH (1:1) stem bark extract.
| Sex of animal | Dose (mg/kg BW) | Hematocrit (%) | WBC counts (x10 3 /mm 3 ) | RBC counts (x10 6 /mm 3 ) |
|---|---|---|---|---|
| Females | 0 | 49.41±1.93a | 5.32±0.68a | 4.25±0.32a |
| 25 | 48.28±2.32a | 5.40±0.93a | 4.10±0.61a | |
| 50 | 47.07±2.63a | 6.16±0.64a | 3.96±0.52a | |
| 100 | 47.61±1.47a | 5.40±0.59a | 3.71±0,29a | |
| 200 | 46.09±1.81a | 5.20±0.82a | 3.66±0.33a | |
| Males | 0 | 48.64±1.26a | 5.84±1.13a | 4.14±0.32a |
| 25 | 50.42±1.84a | 5.32±0.38a | 4.46±0.60a | |
| 50 | 47.04±1.35ab | 5.68±0.61a | 3.86±0.39a | |
| 100 | 46.83±1.77ab | 5.48±0.69a | 3.73±0.47a | |
| 200 | 45.06±1.24b | 4.92±0.33a | 3.64±0.57a |
Values are expressed as mean±SD of five animals. Doses are expressed in mg/kg body weight (BW), WBC: white blood cells; RBC: red blood cells. For the same sex and in the same column, values bearing different superscript letters are significantly (p < 0.05) different from each other.
Table 4.
Serum levels of different parameters in rats treated for 28 days with different doses of C. edulis CH2Cl2/MeOH (1:1) stem bark extract.
|
Sex of
animal |
Dose
(mg/kg BW) |
ALT
(U/l) |
AST
(U/l) |
Proteins
(g/l) |
Glucose
(g/l) |
Total
Cholesterol (mg/dl) |
Total Creatinine
(mg/dl) |
|---|---|---|---|---|---|---|---|
| Females | 0 | 21.4±1.6a | 14.3±0.5a | 89.6±3.2a | 1.0±0.0a | 86.6±7.8a | 0.9±0.0a |
| 25 | 22.1±1.3a | 13.3±1.7a | 93.4±3.0a | 1.0±0.1a | 99.1±7.8a | 0.9±0.0a | |
| 50 | 21.3±3.2a | 14.1±3.1a | 95.0±6.8a | 1.1±0.1a | 110.7±19.8a | 0.9±0.0a | |
| 100 | 24.4±1.6a | 15.3±0.9a | 96.0±6.9a | 0.9±0.0a | 83.4±11.3a | 1.0±0.1a | |
| 200 | 29.2±3.0b | 20.9±0.6b | 119.0±5.7b | 1.0±0.0a | 61.1±2.0b | 1.2±0.0b | |
| Males | 0 | 15.6±1.4a | 14.6±2.0a | 97.1±2.1a | 1.0±0.1a | 82.7±5.5a | 0.8±0.0a |
| 25 | 16.5±1.6a | 14.9±1.9a | 98.5±3.0a | 1.1±0.1a | 87.4±7.6a | 0.8±0.0a | |
| 50 | 18.6±1.9a | 16.8±1.6a | 100.7±3.5a | 1.1±0.0a | 93.9±5.7a | 0.8±0.0a | |
| 100 | 17.9±2.1a | 17.6±2.8a | 99.5±2.4a | 1.0±0.0a | 81.6±6.9a | 0.9±0.10a | |
| 200 | 26.9±1.8b | 21.1±0.3b | 112.2±1.6b | 1.2±0.1a | 63.7±4.3b | 1.5±0.0b |
Values are expressed as mean±SD of 5 animals. BW: body weight, AST: aspartate aminotransferase; ALT: alanine aminotransferase. For the same sex and in the same column, values bearing different superscript letters are significantly (P < 0.05) different from each other.
Table 5.
The concentrations of different parameters in the liver of the rats treated for 28 days with different doses of stem bark crude extract of C. edulis.
| Sex of animal |
Dose
(mg/kg BW) |
ALT
(UI/g of liver) |
AST
(UI/g of liver) |
Proteins
(g/100 g of liver) |
|---|---|---|---|---|
| Females | 0 | 353±08a | 343±32a | 145.21±3.29a |
| 25 | 356±17a | 346±13a | 144.88±1.48a | |
| 50 | 364±09a | 376±14ab | 146.30±1.86a | |
| 100 | 387±18ab | 378±16ab | 145.96±2.08a | |
| 200 | 419±16b | 394±07b | 133.73±9.70b | |
| Males | 0 | 358±07a | 249±30ab | 149.96±3.95a |
| 25 | 349±19a | 247±09a | 149.02±1.70a | |
| 50 | 367±13a | 260±42ab | 147.12±2.22a | |
| 100 | 373±11a | 283±09b | 148.02±1.63a | |
| 200 | 428±08b | 353±06c | 133.11±2.34b |
Values are expressed as mean±SD of five animals. BW: body weight, AST: aspartate aminotransferase; ALT: alanine aminotransferase. For the same sex and in the same column, values bearing different superscript letters are significantly (P < 0.05) different from each other.
Discussion
Antidermatophytic Activity
The antidermatophytic activities of the CH2Cl2-MeOH (1:1 v/v) extract from stem bark of C. edulis may be attributed to the presence of various classes of compounds of biological interest, namely alkaloids, terpenoids, flavonoids, tannins and anthraquinones as shown by Tamokou and co-workers.4 Differences observed in the antidermatophytic activities of crude extract and its fractions can be linked to the differences in chemical composition of these test samples.4 Fraction F3 was more active than the crude extract, indicating that fractionation increased its antidermatophytic activity. This could be due to the exclusion, by fractionation, of some constituents of the extract, which may tend to dilute the active principle and reduce its activity. On the other hand, fractionation may have increased the concentrations and the activities of antidermatophytic principles in this fraction. A keen look of the MFC results, indicated that none of the noticeable values obtained with many samples were more than 4 fold their corresponding MIC, postulating a fungicidal effect of the studied samples.16,17 Compounds 1 and 2 displayed antidermatophytic activities. Comparable results were obtained by Arwind and co-workers,18 and Nazif.19 These results reveal the potential of C. edulis as a source of antidermatophyte drugs and support its use in folk medicine for the treatment of fungal skin infections.
In developing countries, phytotherapy often represents the main, if not the lone, therapeutic approach to which a majority of the people are referred to for their primary health care.20 The increase in the number of users medicinal plants in the face of the scarcity of scientific evidences on their safety have raised concerns regarding the toxicity and detrimental effects of these remedies,21 and the same applies for C. edulis. This plant, like most others, contains several bioactive principles which are able to induce beneficial and/or detrimental effects. To optimize its safety use as a plant-based medicine, one should, beside the historical documentation on C. edulis, have a toxicity assessment of this medicinal plant. Thus, the evaluation of the acute and sub-acute toxicities of C. edulis in the present study appears to be biologically essential.
Acute Toxicity
With the LD50 of 16.8 and kg in male and female mice respectively, the crude extract of C. edulis may be considered fairly toxic.22,23 These result indicate that female mice are more tolerant to the C. edulis extract than males after oral administration. This is in contrary to the observation of Drici and Clement,24 and Liechti and co-workers,25 who showed that the adverse effects of drugs and toxic substances were more pronounced in women than in men. A reduced reaction to noise was observed suggesting that the extract may have a depressant or sedative effect on the central nervous system.11 The administration of the extract to mice caused a reduced reaction to pinch. This decreased sensitivity may be due to the action of the extract on the nociceptors or to the inhibition of the production of algogenic substances (e.g. prostaglandins or histamines), or to the inhibition of the painful message transmission at the central level.26
Sub-Acute Toxicity
Changes in body weight are used as an indicator of adverse effects of drugs and chemicals.27 In the sub-acute toxicity study, significant decreases in total weight gain were observed in the rats, which received the extract at the dose of 200 mg/kg BW as compared to the control. This suggests that C. edulis had negative effect on the normal growth of rats. The reduction in total weight gain may be due to less food and water intake,28 after the administration of C. edulis extract. This growth retardation may also be due to the antilipidaemic effect of C. edulis extract as shown by the decrease of serum total cholesterol.
The hematopoietic system is one of the most sensitive targets for toxic compounds, and is an important index of physiological and pathological status in man and animal,29 In this study, a significant decrease in hematocrit values was also observed in male from the dose of 200 mg/kg BW as compared to that of the control group, suggesting that the extract at high doses may have some effect on the red blood cells. This was confirmed by the decrease, though not significant, observed in red blood cells count of rats treated with the same doses. However, the normal values for hematocrit range from 34% to 48% in Wistar albino rats.30 In the present study, hematocrit value (45.0±1.2) of the male rats receiving the extract at the dose of 200 mg/kg BW was within the normal range.
The biochemical parameters (i.e. serum levels of ALT, AST and creatinine) also showed significant increases in the group receiving the highest dose as compared to that of the control group. Indeed, the transaminases (AST and ALT) are well-known enzymes used as good indicators of liver function,27 and as biomarkers predicting possible toxicity.31 Generally, any damage to the parenchymal liver cells results in the elevations of both transaminases in the blood.32 In addition, AST, found in the serum, is of both mitochondrial and cytoplasmic origins and any rise can be taken as a first sign of cell damage that leads to the outflow of the enzyme into the serum.33 Thus, the significant increases observed in ALT and AST activities strongly suggest that the sub-acute oral administration of C. edulis extract did affect the hepatocytes, and consequently the metabolism of the rats. Equally, there was also a significant rise in creatinine in group receiving the highest dose when compared to that of the control group. Indeed, creatinine is known as a good indicator of renal function.27 Any rise in creatinine level is only observed, if there is a marked damage at the nephrons.34 Therefore, the results recorded in this study similarly suggest that C. edulis extract might have altered the renal function. Clearly, this only serves as a preliminary test, and for a better estimation of renal function a creatinine clearance test is required.
At last, significant decreases were recorded in the relative liver, heart, lung and kidney weights at the dose of 200 mg/kg BW indicating that the sub-acute oral administration of C. edulis extract had a detrimental effect on the normal growth of these organs. This corroborates with the white vesicles observed on the liver surface indicating damages at the level of this organ. Endogenous proteins ensure not only the transportation of xenobiotics in blood toward target organs, but also their biotransformation in the liver in order to activate, excrete or detoxify them.35 The increased protein levels in the serum and liver could be due to the response of hepatic cells to the toxic substances.
This study is the first to show that C. edulis, which is claimed to be a cure for stomach ache and infectious diseases, is a medicinal plant with detrimental biological properties. If an extrapolation of the above results is to be made to humans, it might be possible to suggest that precautions during its use is necessary, especially when used at high doses (≥200 mg/kg BW) or over a long period of time.
Conclusion
This study provides valuable data on the antidermatophytic activity as well as acute and sub-acute oral toxicity profiles of C. edulis extract that might be very useful for any future in vivo and clinical studies of this medicinal plant. Fraction F3 is the most active fraction, and Microsporum audouinii and Epidermophyton floccoseum are the most sensitive microorganisms to the plant fractions. The C. edulis CH2Cl2-MeOH (1:1) extract at high doses (≥200 mg/kg BW) had significant hepatotoxic and nephrotoxic activities. Further studies to determine the effects of this plant on animal fetuses, and pregnant animals and their reproductive capacity are necessary to complete the safety profile of this plant
Conflict of Interest: None declared
References
- 1.Gupta AK, del RossoJQ, Lynde CW, et al. Hepatitis associated with terbinafine therapy: three case reports and a review of the literature. Clin Exp Dermatol. 1998;23:64–7. doi: 10.1046/j.1365-2230.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 2.Carrillo-Muñoz AJ, Giusiano G, Ezkurra PA, Quindós G. et al. Antifungal agents: Mode of action in Yeast cells. Rev EspQuimioter. 2006;19:130–9. [PubMed] [Google Scholar]
- 3.Iwu MM. Handbook of African Medicinal Plants. Boca Raton, Florida, London, Tokyo: CRC Press; 1993. pp. 435–3. [Google Scholar]
- 4.Tamokou JD, Kuiate JR, Njateng GSS, et al. Antimicrobial activity of dichloromethane-methanol (1:1 v/v) extract from the stem bark of Coula edulis Bail. (Olacaceae) Res J Microbiol. 2008;3:414–22. [Google Scholar]
- 5.Bukola C, Adebayo-Tayo , Kola KA. Antimicrobial activities of Coula edulis. Res J Med Plant. 2008;2:86–91. [Google Scholar]
- 6.Aziz Shahid, Habib-Ur-Rehman Studies on the chemical constituents of Thymus serphyllum. Turk J Chem. 2008;32:605–14. [Google Scholar]
- 7.Silva MD, Manfio GP, Candros VP. Characterization of selected strains of mucorales using fatty acid profiles. Revista de Microbiologia. 1998;29:1–8. [Google Scholar]
- 8.Kuiate JR, Mouokeu S, Wabo KH, Tane P. Antidermatophytic Triterpenoids from Syzygium jambos (L.) Alston (Myrtaceae) Phytother Res. 2007;21:149–52. doi: 10.1002/ptr.2039. [DOI] [PubMed] [Google Scholar]
- 9.Thompson DP. Fungitoxic activity of essential oil components on food storage fungi. Mycologia. 1989;81:151–3. [Google Scholar]
- 10.Solomon FE, Sharada AC, Devi PU. Toxic effects of crude extract of Plummuneae rosea (Rokta chitcakal) J Ethnopharmacol. 1993;38:79–84. doi: 10.1016/0378-8741(93)90081-f. [DOI] [PubMed] [Google Scholar]
- 11.Gatsing D, Aliyu R, Kuiate JR, et al. Toxicological evaluation of the aqueous extract of Allium sativum bulbs on laboratory mice and rats. Cameroon J Exp Biol. 2005;1:39–45. [Google Scholar]
- 12.Behrens B, Karber G. Mathematics for naturalists and agriculturalists, PWN, Warszawa1983. Warszawa: PWN; 1983. p. 218. [Google Scholar]
- 13.Theml H. Atlas de poche d’hématologie. Flammarion Médecine-Science. Paris: pour la tradition Française; 2000. pp. 2–21. [Google Scholar]
- 14.Benson JP, Cales B. Animal anatomy and physiology Laboratory text book. owa, USA: Dubuque, Wm.C. Brown Communication; 1992. pp. 325–41. [Google Scholar]
- 15.WHO. Research guidelines for evaluating the safety and efficacy of herbal medicines. Manila: World Health Organisation/Western Pacific Regional Office (WHO/WPRO); 1993. pp. 3–33. [Google Scholar]
- 16.Mims CA, Playfair JHL, Roitt IM, et al. Antimicrobials and chemotherapy. In: Mims, C.A, et al., editors. Med Microbiol Rev. Vol. 35. 1993. pp. 1–34. [Google Scholar]
- 17.Tamokou JD, Tala FM, Wabo KH, et al. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. Journal of Ethnopharmacology. 2009;124:571–5. doi: 10.1016/j.jep.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 18.Shah A, Cross RF, Palombo EA. Identification of antimicrobial component of an ethanolic extract of Australian medicinal plant, Eremophila duttonii. Phytother Res. 2004;18:615–8. doi: 10.1002/ptr.1507. [DOI] [PubMed] [Google Scholar]
- 19.Nazif NM. Phytoconstituents of Zizyphus spina-christi L. fruits and their antimicrobial activity. Food Food Chemistry. 2002;76:77–81. [Google Scholar]
- 20.WHO. WHO Guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Geneva: World Health Organization; 2007. [Google Scholar]
- 21.Saad B, Azaizeh H, Abu-Hijleh G, Said O. Safety of traditional Arab herbal medicine. Evid Based Complement Alternat Med. 2006;3:433–9. doi: 10.1093/ecam/nel058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu FC. Toxicologie : Données générales, procédures d’évaluation, organes cibles, évaluation du risque. Paris: Masson; 1992. pp. 3–87. [Google Scholar]
- 23.Schorderet M. Pharmacologie des concepts fondamentaux aux applications thérapeutiques. Éditions Slatkine Genève. Paris-Grenoble: Édition Frison-Roche; p. 1992. [Google Scholar]
- 24.Drici MD, Clément N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT Syndrome. Drug Saf. 2001;24:575–85. doi: 10.2165/00002018-200124080-00002. [DOI] [PubMed] [Google Scholar]
- 25.Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology. 2001;154:161–8. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- 26.Nguelefack TB, Fotio AL, Watcho P, et al. Analgesic properties of the aqueous and ethanol extracts of the leaves of Kalanchoe crenata (Crassulaceae) Phytother Res. 2004;18:385–8. doi: 10.1002/ptr.1444. [DOI] [PubMed] [Google Scholar]
- 27.El HilalyJ, Israili ZH, Lyoussi B. Acute and chronic toxicological studies of Ajuva iva in experimental animals. J Ethnophamacol. 2004;91:43–50. doi: 10.1016/j.jep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Joseph PK, Rao KR, Sundaresh CS. Toxic effects of garlic extract and garlic oil in rats. Indian J Exp Biol. 1989;27:977–9. [PubMed] [Google Scholar]
- 29.Mukinda JT, Syce JA. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J Ethnopharmacol. 2007;112:138–44. doi: 10.1016/j.jep.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Derelanko MJ, Hollinger MA. CRC Handbook of Toxicology. New York, USA: CRC Press. Inc; 1995. p. 976. [Google Scholar]
- 31.Rahman MF, Siddiqui MK, Jamil K. Effects of Vepacide (Azadirachta indica) on aspartate and alanine aminotransferase profiles in a subchronic study with rats. Hum Exp Toxicol. 2001;20:243–9. doi: 10.1191/096032701678227730. [DOI] [PubMed] [Google Scholar]
- 32.Wolf PL, Williams D, Tsudaka T, et al. Methods and techniques in clinical chemistry. USA: John Wiley & Sons; 1972. pp. 132–383. [Google Scholar]
- 33.Mdhluli M. Toxicological and antifertility investigations of oleanolic acid in male vervet monkeys (Chlorocebus aethiops). PhD Thesis. Discipline of Physiological Sciences. University of the Western Cape: Cape Town, South Africa; 2003. [Google Scholar]
- 34.Lameire NH, Van BiesenW, Vanholder R. Acute renal failure. Lancet. 2005;365:417–30. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 35.Koolman J, Röhm KH. Atlas de Poche de Biochimie. Paris: Ed medicine-sciences Flammarion; 1995. 426 pp. [Google Scholar]