Abstract
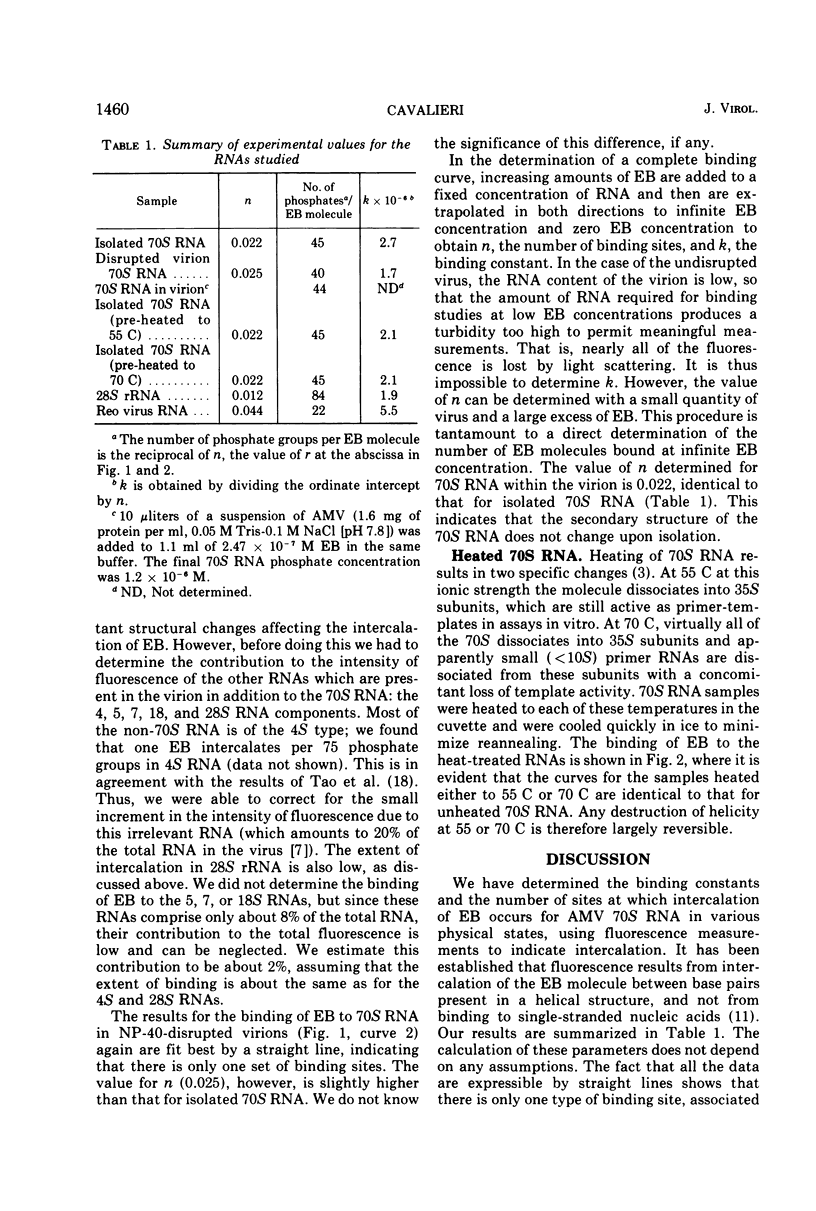
The extent of double strandedness of avian myeloblastosis virus 70S RNA has been determined from fluorescence measurements of the intercalation of ethidium bromide. We have shown that 50% of the nucleotides of 70S RNA in solution are in a stable helical configuration. This value does not include small helical regions that are too unstable to permit intercalation of the dye. The avian myeloblastosis virus RNA as it exists within the virion has the same degree of helicity as the free 70S RNA. Heating the free 70S RNA to 55 or 70 C, followed by cooling, does not measurably change the degree of helicity; the subunits therefore have as much helicity as the parent molecule.
Full text
PDF
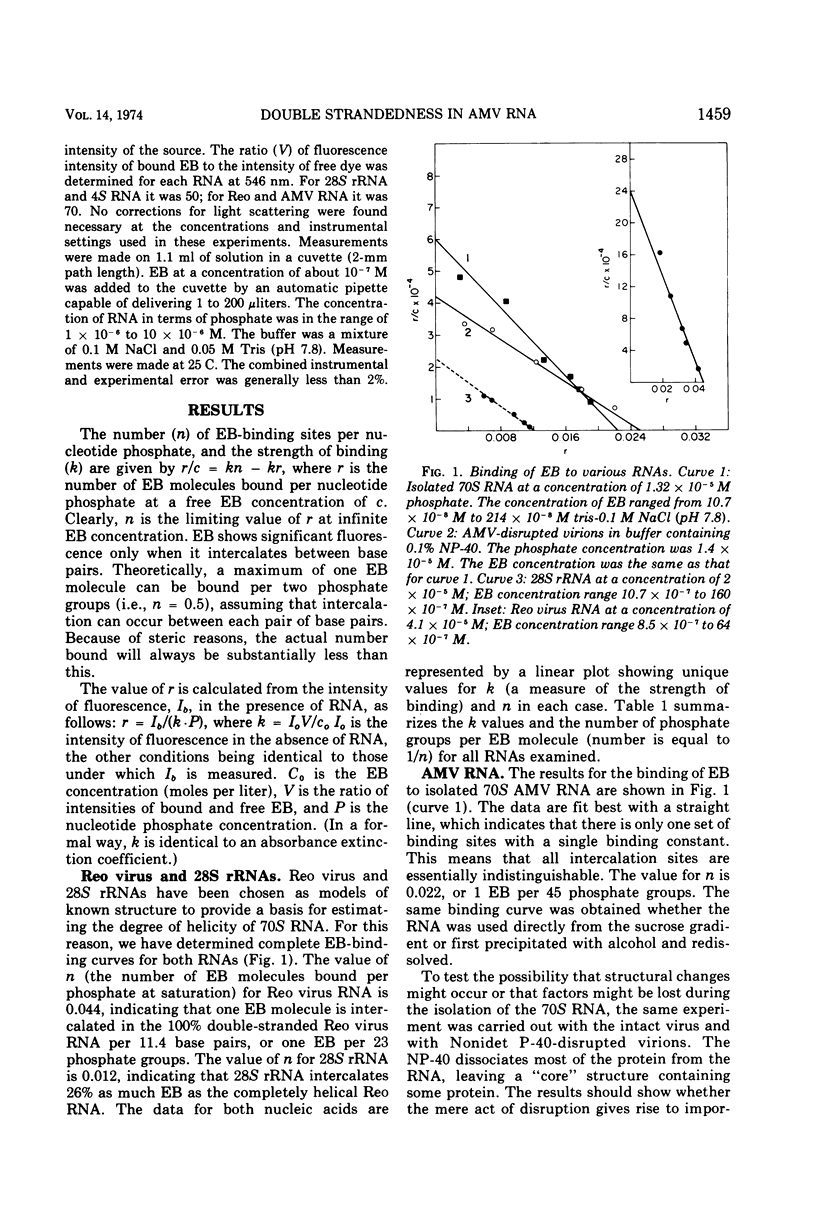

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. The secondary structure of ribosomal ribonucleic acid in solution. Biochem J. 1966 Mar;98(3):841–857. doi: 10.1042/bj0980841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. H., Laskowski M., Sr Mung bean nuclease I. II. Resistance of double stranded deoxyribonucleic acid and susceptibility of regions rich in adenosine and thymidine to enzymatic hydrolysis. J Biol Chem. 1970 Feb 25;245(4):891–898. [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulski A. J., Laskowski M., Sr Mung bean nuclease I. 3. Purification procedure and (3') omega monophosphatase activity. J Biol Chem. 1970 Oct 10;245(19):5026–5031. [PubMed] [Google Scholar]
- Montagnier L., Goldé A., Vigier P. A possible subunit structure of Rous sarcoma virus RNA. J Gen Virol. 1969 Apr;4(3):449–452. doi: 10.1099/0022-1317-4-3-449. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Baluda M. A. The nucleic acid from avian myeloblastosis virus compared with the RNA from the Bryan strain of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1686–1692. doi: 10.1073/pnas.54.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER M., FULLER W., WILKINS M. H., BROWN G. L. Determination of the helical configuration of ribonucleic acid molecules by X-ray diffraction study of crystalline amino-acid-transfer ribonucleic acid. Nature. 1962 Jun 16;194:1014–1020. doi: 10.1038/1941014a0. [DOI] [PubMed] [Google Scholar]
- Tao T., Nelson J. H., Cantor C. R. Conformational studies on transfer ribonucleic acid. Fluorescence lifetime and nanosecond depolarization measurements on bound ethidium bromidee. Biochemistry. 1970 Sep 1;9(18):3514–3524. doi: 10.1021/bi00820a004. [DOI] [PubMed] [Google Scholar]
- Trávnícek M., Ríman J. Subunits of oncornavirus high-molecular-weight RNA. I. Stepwise conversion of 60 S AMV (avian myeloblastosis virus) RNA to subunits. Biochem Biophys Res Commun. 1973 Jul 2;53(1):217–223. doi: 10.1016/0006-291x(73)91422-8. [DOI] [PubMed] [Google Scholar]
- Trávnícek M., Ríman J. Subunits of oncornavirus high-molecular-weight RNA. II. Detection of double-stranded-regions in 60S AMV (avian myeloblastosis virus) RNA. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1347–1355. doi: 10.1016/0006-291x(73)91135-2. [DOI] [PubMed] [Google Scholar]



