Abstract
Schimke immuno-osseous dysplasia is a rare autosomal recessive multisystem disorder characterized by steroid-resistant nephrotic syndrome, immunodeficiency, and spondyloepiphyseal dysplasia. Mutations in SWI/SNF2 related, matrix associated, actin dependent regulator of chromatin, subfamily a-like 1 (SMARCAL1) gene are responsible for the disease.
The present report describes, for the first time, a Schimke immuno-osseous dysplasia child with SMARCAL1 missense mutation (R561H) and manifestations of intussusception secondary to Epstein-Barr virus-negative non-Hodgkin lymphoma, who expired due to septicemia following chemotherapy. The report emphasizes the necessity of more limited immunosuppressive protocols in Schimke immuno-osseous dysplasia patients with lymphoproliferative disorders.
Key Words: Schimke immunoosseous dysplasia, lymphoproliferative, intussusception
Introduction
Schimke immuno-osseous dysplasia (SIOD) is a fatal syndrome inherited as an autosomal recessive trait, and manifests with facial dysmorphism, growth failure, nephropathy, recurrent infections, hypothyroidism, episodic lymphopenia, and neurologic symptoms.1 Biallelic loss of function mutations of SWI/SNF2- related, matrix associated, actin dependent regulator of chromatin, subfamily a-like 1 (SMARCAL1) gene are the only known cause of SIOD.2 SWI/SNF2 related, matrix associated, actin dependent regulator of chromatin, subfamily a-like 1 protein is homologous to the SWI2/SNF2 family of ATP-dependent chromatin remodeling proteins and has annealing helicase activity.3 In this report, we present an eight-year-old SIOD patient with a missense mutation on a conserved motif of SNF2 domain of SMARCAL1. The patient manifested abdominal mass due to intussusception secondary to Epstein-Barr Virus (EBV)-negative Non-Hodgkin B-cell lymphoma (NHL), and expired due to septicemia following chemotherapy. We did report the first case of SIOD with end stage renal disease due to steroid resistant nephrotic syndrome from Iran,4 Herein, we report on a child with SIOD and intussusception that has not been reported previously.
Case Description
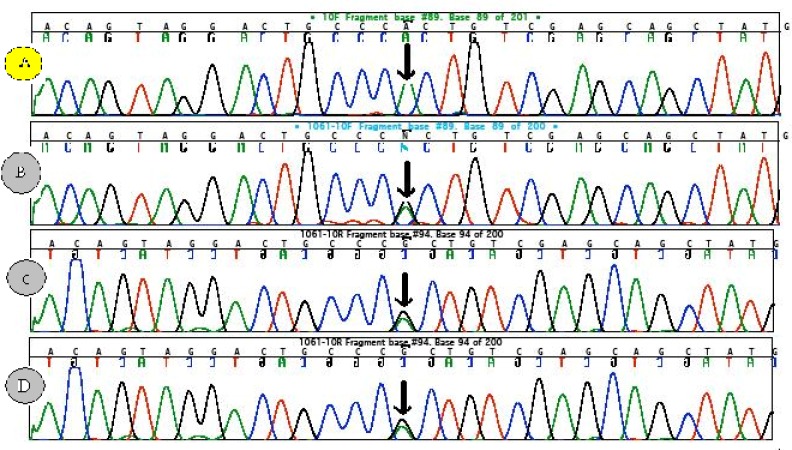
The patient was the third child of consanguineous parents and the product of a normal vaginal delivery at term. His postnatal course did not show anything abnormal, except for a poor growth rate. At the age of four years, he presented withabdominal protrusion. On physical examination he had a peculiar face, short neck, disproportionate short stature and low growth indices as well as extremity edema and hypertension. Laboratory examinations demonstrated nephrotic range proteinuria (2626 mg/day), hyperlipidemia (TG=293 mg/dl, cholesterol=307 mg/dl), hypoalbuminemia (Alb=2.2 mg/dl), T-cell deficiency (CD4/CD8=0.36, normal range: 1.3-3.9) and hypothyroidism. Bone survey revealed generalized osteopenia, platyspondyl of cervical spines, beaking of thoracolumbar vertebrae, epiphyseal dysgenesis of femur and shallow acetabulum. These signs and symptoms are characteristic of SIOD. Therefore, molecular analysis of SMARCAL1 gene in the patient and his family members was performed. The analysis revealed homozygousity for the missense mutation c.1682G>A (R561H) in the patient (panel A figure 1). The parents and one sibling were heterozygous for this mutation (panel B, C and D figure 1).
Figure 1.
The sequences of SMARCAL1 related to Schimke immuno-osseous dysplasia (SIOD). Sequence (A), from the patient of this report exhibiting characteristics of Schimke immuno-osseous dysplasia with a homozygote AA sequence (c.1682) leading to the substitution of Arginine (CGC) at position 561 in the protein by histidine (CAC). Panels (B, C and D) show heterozygote (G/A) pattern of the same section from the patient’s parents and siblings.
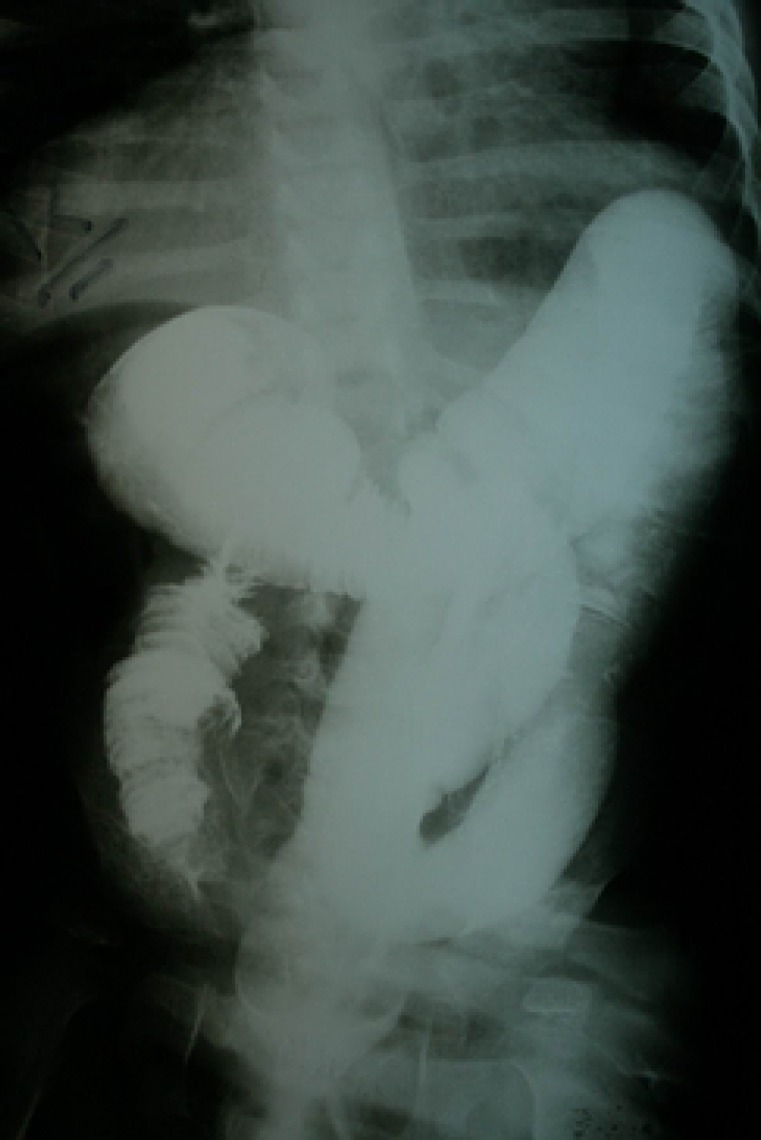
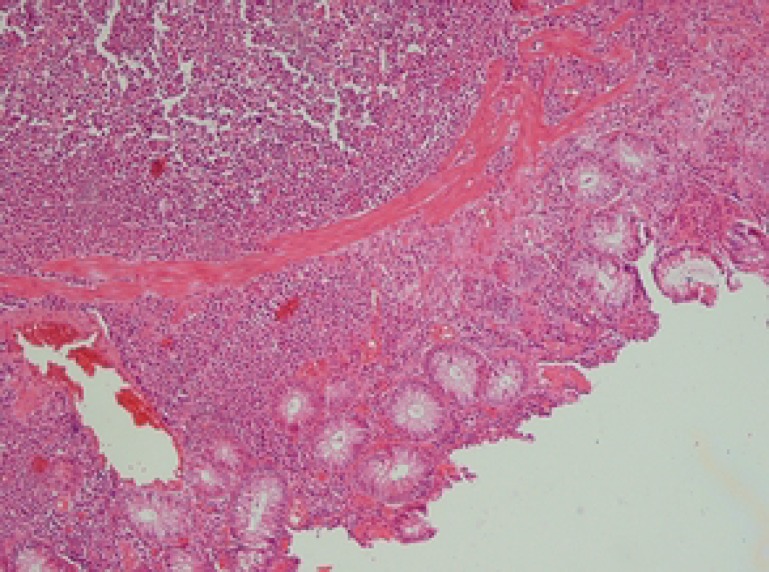
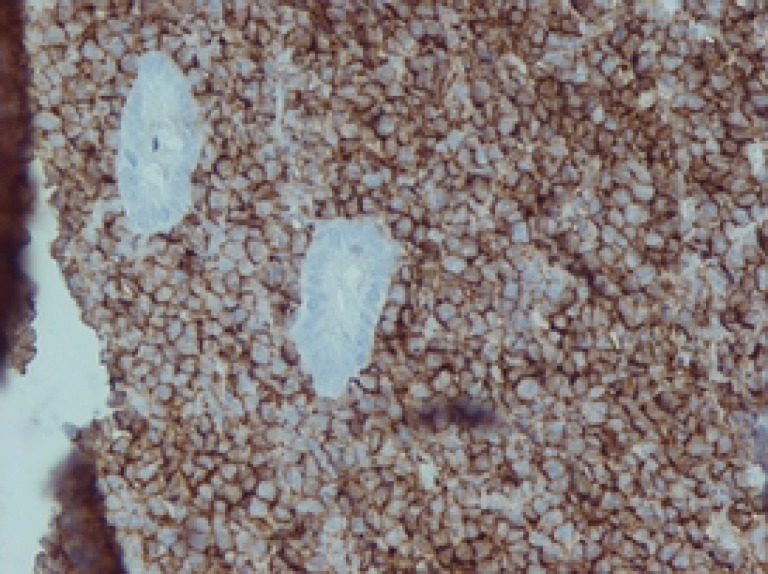
At the age of eight years, he developed colicky abdominal pain and vomiting. Palpation of the abdomen revealed a hard mass in right upper abdomen. A barium enema showed ileocolic intussusception (figure 2). Laparatomy revealed a 2-cm intramural mass in the cecum. Pathologic analysis of the resectioned mass showed diffuse infiltration of medium sized lymphocytic cells with conspicuous nucleoli and high mitotic figures (figure 3). Immunohistochemistry of the lymphoma cells was diffusely reactive for leukocyte common antigen (LCA) and CD20 (figure 4). Latent membrane protein-1 (LMP-1) antigen of EBV was negative. All other markers such as CD3, CD2, CD3, CD5, CD7, CD15, and CD30 were also negative. These findings were indicative of NHL- B cell type (stage III). The patient was treated with chemotherapeutic agents including vincristine, cyclophosphamide, adriamycine and intrathecal methotrexate using half of their usual doses, because of the underlying immunodeficiency. Following chemotherapy, he developed febrile neutropenia (WBC=2000, PMN=10%, Lymph=78%, Eos=5%, Mono=4%, Baso=3%), and despite supportive care and prophylactic antibiotics, expired due to enterobacter sepsis.
Figure 2.
Barium enema showing ileocolic intussusception in the patient with Schimke immuno-osseous dysplasia.
Figure 3.
Sections from intestine show diffuse infiltration of intermediate –sized cells in the mucosa.
Figure 4.
The lymphoma cells are diffusely positive for CD20.
Discussion
Non-Hodgkin lymphoma is a heterogeneous group of malignancies originating from B and T lymphocytes, and has different etiologic, morphologic, immuno-phenotypic, genetic, and clinical features.5 There are several risk factors for NHL including congenital and acquired immunodeficiency states, infection with chronic antigen stimulation, autoimmune disorders, and environmental factors.6
According to the comprehensive database from the Immunodeficiency Cancer Registry, the most common tumors in primary immunodeficiencies are lymphomas.7 Immunodeficiency is the strongest described risk factor for NHL.8 The incidence of NHL is increased 10-100 or more in people with acquired or congenital immunodeficiency.8,9 Such an association is not surprising, because the immune system plays a critical role in the recognition and destruction of malignant cells, and successful elimination of these cells requires an intact immune surveillance system. Therefore, excessive generation of malignant cells coupled with immunodeficiency may result in the increased risk of cancer.10
Some immune defects are associated with abnormalities in other organs. These syndromic immunodeficiencies present more with other symptoms rather than immune abnormalities.11 The present patient with SIOD is an example of a syndromic immunodeficiency that presented with edema and poor growth; however, immunodeficiency was not the major clinical problem.
The mechanism by which SMARCAL1 protein deficiency causes SIOD is still unknown. The arginine residue at position 561 is located in a conserved SNF2 motif (IIa) that contributes to the enzyme active site, and is in close proximity to Walker B magnesium binding site. Therefore, we suspect that the nonconservative substitution of histidine for arginine affects function of the active site and possibly DNA binding.12
Patients with SIOD have T cell deficiency, which generally affects CD4+ cells in the most severe manner.13 Although our patient had episodic lymphopenia, low CD3, CD4, CD4/CD8 ratio, and low IgG level, he did not have prominent symptoms of immunodeficiency such as recurrent infections prior to presenting with large B cell lymphoma at the age of eight.
The only reported case of SIOD with lymphoproliferative disorder in the literature is a 5-year-old Saudi Arabian boy who presented fever of unknown origin and EBV-related non-Hodgkin lymphoma.14 In contrast, lymphoma in our patient was EBV negative. The cause of this difference is not clear; however, it might be due to a milder immunodeficiency state in our patient. It has been suggested that milder, but measurable immunodeficiency, is mostly unrelated to EBV infection.15 The other possibilities include unknown lymphotrophic virus and dysregulation of B cell proliferation with resultant malignant proliferation. Also, given the recent findings of a role for SMARCAL1 in DNA repair and replication,16 SIOD patients may have an increased cancer risk, although their short lifespan limits the manifestation of such a risk.
The poor prognosis of NHL in immune deficiency states is accompanied by increased risk of complications such as sepsis following chemotherapy. Although our patient did not have a history of increased infection, he developed severe septicemia and unfortunately died following chemotherapy. This is consistent with increased rate of opportunistic infections in the presence of immunosuppressive agents after renal transplantation in SIOD patients.17
Conclusion
The signs and symptoms of the present case, who expired of enterobacter sepsis following chemotherapy, showed that he had SIOD with intussusception secondary to EBV-negative non-Hodgkin lymphoma. The patient's history might be taken as evidence to recommend supportive care and more limited immunosuppressive protocols in SIOD patients.
Conflict of Interest: None declared
References
- 1.Boerkoel CF, Takashima H, John J, et al. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno osseous dysplasia. Nat Genet. 2002;30:215–20. doi: 10.1038/ng821. [DOI] [PubMed] [Google Scholar]
- 2.Elizondo LI, Cho KS, Zhang W, et al. Schimke immuno-osseous dysplasia: SMARCAL1 loss-of-function and phenotypic correlation. J Med Genet. 2009;46:49–59. doi: 10.1136/jmg.2008.060095. [DOI] [PubMed] [Google Scholar]
- 3.Yusufzai T, Kadonaga JT. HARP is an ATP-driven annealing helicase. Science. 2008;322:748–50. doi: 10.1126/science.1161233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basiratnia M, Fallahzadeh MH. Schimke immuno-osseous dysplasia. Saudi Med J. 2007;28:457–60. [PubMed] [Google Scholar]
- 5.Alexander DD, Mink PJ, Adami HO, et al. The non Hodgkin lymphoma: a review of epidemiologic literature. Int J Cancer. 2007;120:1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 6.Grulich AE, Vajdic CM. The epidemiology of non-Hodgkin lymphoma. Pathology. 2005;37:409–19. doi: 10.1080/00313020500370192. [DOI] [PubMed] [Google Scholar]
- 7.Filipovich AH, Mathur A, Kamat D, et al. Lymphoproliferative disorders and other tumor complicating immunodeficiencies. Immunodeficiency. 1994;5:91–112. [PubMed] [Google Scholar]
- 8.Grulich AE, Wan X, Law MG, et al. B cell-stimulation and prolonged immune deficiency are risk factors for non Hodgkin's lymphoma in people with AIDS. AIDS. 2000;14:133–40. doi: 10.1097/00002030-200001280-00008. [DOI] [PubMed] [Google Scholar]
- 9.Filipovich AH, Mathur A, Kamat D, Shapiro RS. Primary immunodeficiencies: genetic risk factors for lymphoma. Cancer Res. 1992;52:5465s–7s. [PubMed] [Google Scholar]
- 10.Nugent DJ, Kunicki TJ. Immune deficiency and malignancy. In: Stiehm-Ochs-Winkel Stein., editor. Immunologic disorders in infant and children. 5th ed. Philadelphia: Elsevier; 2004. pp. 1253–81. [Google Scholar]
- 11.Ming JE, Stiehm ER. Syndromic immunodeficiencies: genetic syndromes associated with immune abnormalities. Crit Rev Clin Lab Sci. 2003;40:587–642. doi: 10.1080/714037692. [DOI] [PubMed] [Google Scholar]
- 12.Dürr H, Körner C, Müller M, et al. X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA. Cell. 2005;121:363–73. doi: 10.1016/j.cell.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Boerkoel CF, O'Neill S, André JL, et al. Manifestations and treatment of Shimke immono- osseous dysplasia: 14 new cases and a review of the literature. Eur J Pediatr. 2000;159:1–7. doi: 10.1007/s004310050001. [DOI] [PubMed] [Google Scholar]
- 14.Taha D, Boerkoel CF, Balfe JW, et al. Fatal lymphoproliferative disorder in a child with schimke immuno osseous dysplasia. Am J Med Genet. 2004;131:194–9. doi: 10.1002/ajmg.a.30356. [DOI] [PubMed] [Google Scholar]
- 15.Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:405–8. doi: 10.1158/1055-9965.EPI-06-1070. [DOI] [PubMed] [Google Scholar]
- 16.Driscoll R, Cimprich KA. HARPing on about the DNA damage response during replication. Genes Dev. 2009;23:2359–65. doi: 10.1101/gad.1860609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lücke T, Kanzelmeyer N, Baradaran-Heravi A, et al. Improved outcome with immunosuppressive monotherapy after renal transplantation in Schimke-immuno-osseous dysplasia. Pediatr Transplant. 2009;13:482–9. doi: 10.1111/j.1399-3046.2008.01013.x. [DOI] [PubMed] [Google Scholar]