Abstract
Glycation is a protein modification, which results in a change in a protein structure. Glycation is believed to be the etiology of various age-related diseases such as diabetes mellitus and Alzheimer’s disease (AD). Activation of microglia and resident macrophages in the brain by glycated proteins with subsequent oxidative stress and cytokine release may be an important factor in the progression of AD. It is also suggested that interaction between an advanced glycation end product (AGE) and its receptor (RAGE) results in glial activation as well as cytokine release and reactive oxygen species release. The use of antioxidants, receptor mediated compounds and reactive oxygen species scavenging enzyme produce an opportunity to intervene with AGE-RAGE signaling pathway, and thereby to slow down the progression of aging-related diseases.
Key Words: Advanced glycation end producs, receptor for advanced glycation end products, oxidative stress, inflammation, signaling pathway
Introduction
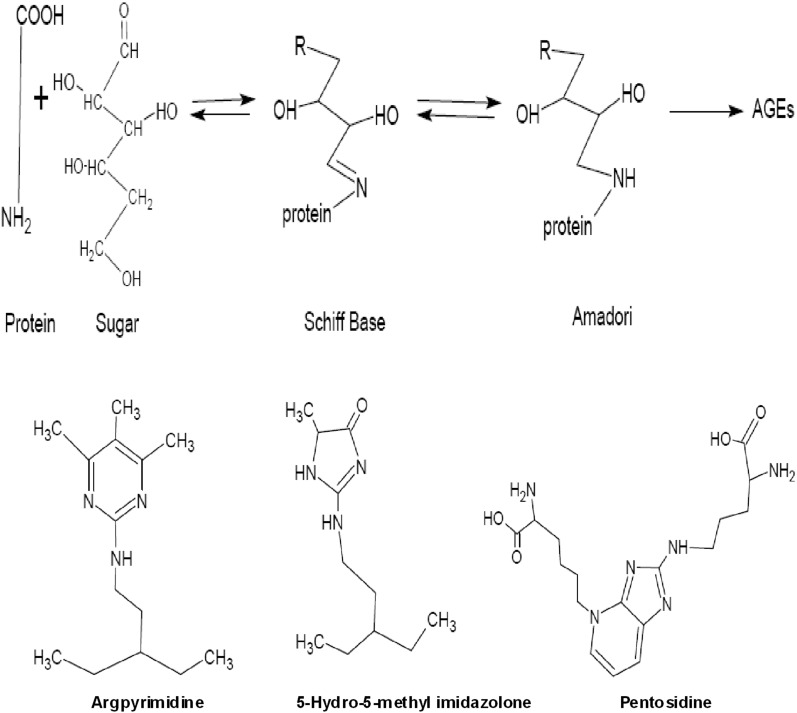
The amino groups of proteins, particularly the side chain of lysine, arginine, and histidine react non-enzymatically with reducing sugars. This post-translational modification called “glycation” or “maillard reaction”, leads via reversible Schiff-base adducts to protein bound amadori products.1-3 By subsequent oxidation and dehydration, a broad range of brown heterogeneous products, mostly fluorescent with nitrogen- and oxygen- containing heterocyclic compounds, called advanced glycation end products (AGEs) are formed. The formation of AEGs is irreversible, and causes a resistant protein deposition to protease.4,5 The Maillard reaction was first described by L.C. Maillard a chemist, who reported the formation of brown products upon heating a solution of amino acid (AA) and sugar.6 Schematic representation of the Maillard reaction (A) and structures of AGEs (B, C and D),7 are shown in figure 1.
Pathological Consequences of AGEs
In vivo Glycation modifies the structural properties of proteins such as albumin and haemoglobin leading to inflammation and oxidative stress. The pathological role of AGEs in diseases such as diabetes mellitus (DM) is not fully understood. In addition to change of the protein structure, the receptor mediated mechanism of AGEs is of special interest.8
Figure 1.
Schematic representation of the Maillard reaction (A) and structure of advanced glycation end products AGEs (B, C and D).
The pathological features of AGEs, which are not receptor mediated, can be observed in the progression of cataracts. Evidence suggests that the glycation of lens protein is one of the causes of cataract,9 and is observed in long-lived proteins such as collagen and eye crystalline.10 However, the pivotal role of AGEs and the interaction with the receptor is not fully understood.
Advanced Glycation End Products and Ageing
Ageing can be characterized as progressive impairment of organs, tissue and cells. It is a progressive process, and not categorized as a disease, unless it interferes with the normal function of the organs. Diabetes mellitus and Alzheimer’s disease (AD) are the most prevalent ageing diseases, and are examples of the tissue impairment by ageing.11 One of the characteristics of ageing is the acceleration of production of glycated proteins and accumulation of them in different tissues. Glycated proteins form aggregations, which are insoluble and resistant to degradation in comparison to non-glycated proteins.2,12
Advanced Glycation End Products and Alzheimer’s Disease
Alzheimer’s disease is the most common type of dementia in elderly people.13 Approximately four million people in the United States have AD, and this number is expected to increase by 2050. The prevalence of AD amongst people aged 85 years or older is estimated to increase seven-fold from 1980 to 2050, however, this rise is slower in people from the age of 65-74 years during the period of 1980 to 2050.14
Alzheimer’s disease is characterized by initial mild memory impairment, and progresses to the loss of mental and physical activities. The cognitive decline is associated with widespread loss of synapses, neuronal cell death and the formation of amyloid plaques and neurofibrillary tangles, markers of AD. Advanced Glycation End Products modification and resulting cross-linking of protein deposits were observed to occur in both plaques and tangles.15
Advanced Glycation End Products Receptor
For the first time in 1992, macrophages were described to uptake AGEs via a specific receptor called Advanced Glycation End Products Receptor (RAGE).16 The receptor has been identified in monocytes, macrophages, microglia, astrocytes, neurons as well as smooth muscle and endothelial cells.17
Different AGE modified proteins such as AGEs and β-sheet fibrils like amyloid proteins and other ligand families such as high-mobility-group B and S100/calgranulin were identified as ligands of RAGE.16 Ability to bind to different families of ligands is a unique characteristic of RAGE.18 It is referred to as pattern recognition receptor . The interaction between RAGE and AGEs is a complicated process, which has been shown to be a cause of problems in different ageing related diseases. It is also known as scavenger receptor in microglia cells.17 Increased expression levels of RAGE were found in the optic nerve of AD patients in proximity to astrocytes.19 While there are many studies regarding AGEs, there is not much information about the receptors. The current data show that glycated modified protein binding to RAGE triggers some components of different signalling pathways. However, the complete network of signalling pathways is still unclear.20
The RAGE and Mitogen-Activated Protein Kinases
The RAGE is a 35 kDa AGE-binding protein belong to the immunoglobulin (Ig) superfamily.18 The mitogen activated protein kinases such as extracellular signal-regulated kinase and c-Jun N-terminal kinase were implicated to be components of the RAGE signalling pathways.21 These MAPKs are induced by cytokines and stressors. Further down in the signal transduction cascade, transcription factors like nuclear factor-kappaB are also activated. Therefore, ligand-RAGE interaction activates NF-κB through these MAPKs signalling pathway. The cascade of signal transduction depends on the binding of AGEs to RAGE, as blocking RAGE with either an excess of sRAGE or anti-RAGE antibody prevents cellular activation.22
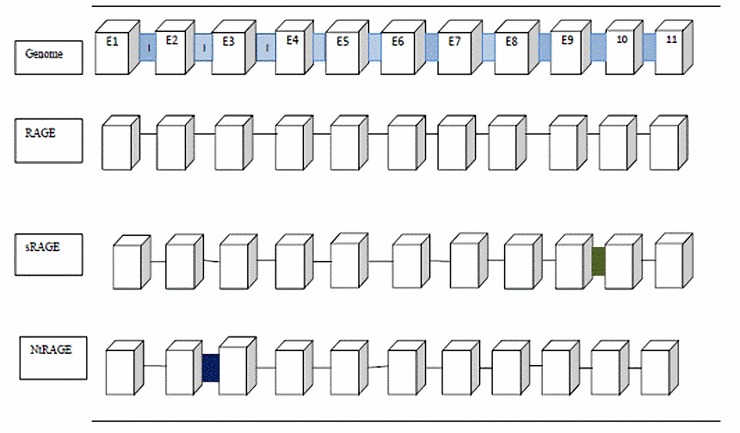
The RAGE is composed of 404 AA.23 It contains an extracellular part with 320 AA, a single transmembrane domain with 21 AA and a short cytoplasmic domain with 40 AA.24 The extracellular part is composed of a variable domain and two constant (C) domains.25 It has 94 AA in Ig-like V-type domain, 98 AA in Ig-like C2-type 1 domain and 91 AA in Ig-like C2-type 2 domain. Alternative splicing plays a major role in the production of different RAGE isoforms.23 During alternative RNA splicing, exons or introns could be retained or removed in different combinations. This process produces different proteins with unique characteristics.26 Over 20 different splice variants of human RAGE have been identified to date.27 Generally there are four main RAGE isoforms: full length -RAGE, N-terminal truncated -RAGE,endogenous secretary (Es)-RAGE, and soluble (s)-RAGE (figure 2). These isoforms are the product of alternative splicing. Different splice variants of RAGE in human brain 28 are shown in figure 2. Soluble-RAGE and Es-RAGE have similar patterns; however, s-RAGE may have some differences compared with Es-RAGE in some AA. Soluble-RAGE is also a product of recombinant technology as well as the cleavage of cell surface RAGE by extracellular metalloproteinases.29 Soluble-RAGE and Es-RAGE do not have the cytoplasmic and transmembrane domains of FL-RAGE.30,31 This intron includes a stop codon in the sequence. Because of this stop codon, s-RAGE lacks exon 10 and 11. This sequence encodes the transmembrane domain of FL-RAGE; therefore, s-RAGE lacks the transmembrane and cytoplasmic domain. Soluble-RAGE contains V, C1 and C2 domains.32 The Nt-RAGE mRNA contains intron 1. Intron 1 contains a stop codon. This stop codon results in the loss of exon 1 and 2. Hence, it lacks the V-type domain of FL-RAGE. It cannot act as a FL-RAGE and different ligands cannot bind to Nt-RAGE.28 The ligands of RAGE such as amphoterin, S100B and Aβ oligomer bind to V domain, however, some ligands such as Aβ also bind to C domain.33 Moreover, there are two N-glycosylation sites in and close to the AGE binding domain. Osawa et al,34 showed that a G82S mutation in the second N-glycosylation motif increased the AGEs affinity in COS-7 cells. As the blood levels of sugar is high in diabetic patients, translational modification of RAGE by de N-glycosylation and N-glycosylation sites could be a potential reason for RAGE activation and deactivation.34
Figure 2.
Different splice variants of Advanced Glycation End Products Receptor (RAGE) in human brain: FL (full length Rage)-RAGE, Nt (N-terminal truncated)-RAGE, Es (Endogenous Secretary)-RAGE and s (soluble)-RAGE. The arrows show the deleted parts of sRAGE and NtRAGE compared to RAGE.
In addition, s-RAGE can suppress the RAGE signalling, and the administration of s-RAGE was shown to suppresses tumor growth and autoimmune response in animal models for cancer and multiple sclerosis suppresses.35 The s-RAGE plasma level is lower in patients with cognitive impairment, non-alcoholic fatty liver disease or type 2 diabetic in comparison with the control.30,31
Soluble-RAGE: a Marker and a Treatment
Soluble-RAGE is the extracellular ligand binding domain of RAGE. It flows in the human plasma and has the potential to function as a decoy for RAGE signalling pathway by binding to circularising plasma AGEs. It has been suggested that s-RAGE can be a biomarker for RAGE-mediated disease development, especially vascular diseases.36
There are controversial studies regarding the s-RAGE plasma level and its relationship to the development of diseases. Some studies showed low s-RAGE plasma level and high N-carboxymethyllysine levels, and abundant natural AGEs in diabetic patients with severe complications.37 On the other hand, the s-RAGE plasma levels are lower in patients with arthritis and hypertension in comparison with healthy patients.38 Yamagishi et al,39 reported a positive relationship between plasma level of AGEs and s-RAGE in non-diabetic population. Interestingly, one study reported the elevated plasma levels of s-RAGE in septic patients. The study was the only to report high s-RAGE plasma levels in acute and non-chronic diseases.40 It was proposed that the s-RAGE plasma level was negatively correlated with body mass index , as overweight people with higher BMI had a lower s-RAGE plasma level.41 Remarkably, higher s-RAGE positively correlates with an increase in inflammatory markers such as tumor necrosis factor -α and monocyte chemo attractant protein-1 in patients with type-2 diabetes.42
The decoy characteristic of s-RAGE is not completely known to function as a negative feedback, as a biomarker, or as a protective factor, which acts by increasing the AGEs level in plasma in different diseases. Recombinant s-RAGE administration was tested in the animal model of DM with atherosclerotic lesions, and was shown to suppress the development of these lesions in the apo E null mice diabetic model.43 In another study, the administration of s-RAGE to diabetic C57/BJ6 and RAGE-transgenic mice with diabetic symptoms inhibited blood-retinal barrier breakdown and leukostasis, which are the signs of retinopathy in diabetic patients.44 These studies suggested an anti-ageing characteristic for s-RAGE in ageing-related diseases.
The RAGE: a Transporter of Amyloid Beta in Alzheimer Disease
Deposition of amyloid beta (Aβ) as a senile plaque in the brain and the soluble oligomer form of Aβ have been identified as one of the major causes of AD.45 Salminen et al,46 mentioned that “full-length RAGE was expressed in astrocytes, microglia and neurons. Also endothelial cells can show a high level of RAGE expression in brain”. It is known that the blood brain barrier is important for Aβ brain balance, and that it regulates the transport of Aβ through two receptors: the low density lipoprotein receptor related protein 1 and RAGE. The RAGE protein mediates the influx of amyloid protein from plasma to the brain, whereas, LRP protein mediates the efflux of amyloid protein through the BBB.46 Deane et al,47 suggests that brain CSF is separated from blood by tight junction between endothelial cells. Therefore, Aβ peptide movement through BBB needs a receptor such as RAGE to transfer Aβ from plasma to CSF through endocytosis.47 Generally, efflux which is mediated by LRP1 is greater than influx by RAGE. In AD, changes in RAGE expression might create an imbalance between the rates of influx and efflux of Aβ peptide through the BBB.48
The RAGE Activation and Biological Consequences: Inflammation, Oxidative Stress, Cell Survival and Proliferation
The RAGE is found on the surface of different kinds of cells such as lymphocytes, leukocytes, macrophages/microglia/monocytes, astrocytes, neurons, smooth muscle cells and endothelial cells.49
The RAGE was shown to influence cell survival, cell proliferation, oxidative stress and inflammatory responses. Likewise, AGEs effects on proliferation and cell death were reported in some studies.50,51 These effects were suppressed by the blockade of RAGE in T lymphocytes. Such a blockade shows that AGEs have effect on cell proliferation and cell survival through RAGE. Moreover, several studies demonstrated a role for AGEs in the over-production of intracellular reactive oxygen species , impairments in proteasomal activities, inflammatory responses, and cell insensitivity to insulin in DM. On the other hand, AGEs can induce nitric oxide (NO) production in retinal neurons and N-11 cell line.52,53 Besides, RAGE activation resulted in the activation of nicotinamide adenine dinucleotide phosphate oxidase.54 The product of this enzyme activation is superoxide ion , another ROS. On the other hand, interaction of ligands with RAGE induces the production of cytokines followed by upregulation of multiple signalling pathways. Ligand-induced RAGE activation is shown to drive NF-κB expression, followed by upregulation of inflammatory markers and adhesion molecules, and consequently inflammatory cell recruitment to the site of inflammation.49 In addition, migration of monocytes was reported in AD patients and Aβ-transgenic mice. This migration may play an important role in the RAGE-mediated inflammatory responses in AD patients in the brain.48
C-reactive protein (CRP) is a key marker of inflammation in cardiovascular diseases, and is a mediator for developing atherosclerosis. C-reactive protein increases the expression of cell adhesion molecules , chemokines, RAGE and the production of ROS. Mahajan et al,55,56 suggested that CRP upregulates RAGE expression. They also demonstrated that this upregulation could be reduced by MAPKs inhibitors; therefore, they suggested that p38, ERK and JNK signalling pathways were involved in CRP-induced RAGE expression.55
Donato et al,57 highlighted, in a review article, that S100B, an endogenous RAGE ligand, had protective and neurotrophic effects during brain development in low concentrations. Moreover, in higher concentrations it had a toxic effect, which was through the production of ROS in neuron in a RAGE-dependent manner. He also suggested that S100B-RAGE interaction could stimulate pro-inflammatory responses and ROS production in monocytes/microglia/macrophages.58
Cyclooxygenase is an enzyme responsible for the formation of prostanoids, and its role in inflammation has been pointed out by increasing evidence.59 The interaction of S100B and RAGE upregulates COX-2 expression in BV-2 microglia.57 The upregulation of the COX-2 expression was observed in AGE-BSA induced–human osteoarthritis (OA) chondrocytes.60 This upregulation was also reported in cultured THP-1 monocytes and human peripheral blood monocytes stimulated by AGEs and S100B.61 This data proves the overlap part between COX-2 and RAGE signalling pathway. It was shown that RAGE-/- mice in comparison with wild type (Wt),62 have a lower level of TNF-α and IL-6 plasma concentrations in response to HMGB1 stimulation.63 On the other hand, a high level of circulating TNF-α was reported as a crucial mediator in RAGE/AGE and NF-КB signalling pathway.64
Prostaglandin F2α comprises a class of biologically active products of the arachidonic acid pathway, and is considered a marker of oxidative stress.65 Relevance of urinary 8-iso-PGF2α with s-RAGE in diabetic patients has been considered.66 It was reported that PGF2α was high and had inverse correlation with s-RAGE plasma level.30,65 Furthermore, the overproduction of intracellular ROS was observed in THP1 monocytes after treatment with glycoxidized albumin.67 Moreover, human umbilical vein endothelial cells (HUVECs) induced by TNF-α have demonstrated a high level of RAGE expression. This expression was reduced by N-acetyl-L-cysteine , a ROS scavenger.68 E-selectin is a cell adhesion molecule, which is expressed in activated endothelial cells in inflammatory conditions and by AGEs.69,70 It has been reported that E-selectin was unregulated in human saphenous vein endothelial cells in response to heterogeneous AGEs.69 It was observed that E-selectin expression induced by Glc-human serum albumin (GHSA) was reduced by NADPH oxidase inhibitors and scavengers of ROS, NAC in human saphenous vein endothelial cells. However, it was reported that such E-selectin expression could not be abolished by anti-RAGE antibody.54 This information suggests that RAGE/AGE interaction stimulates pro-inflammatory responses through NF-КB signalling pathway in an oxidative stress- dependent manner.
The RAGE and Signaling Pathways
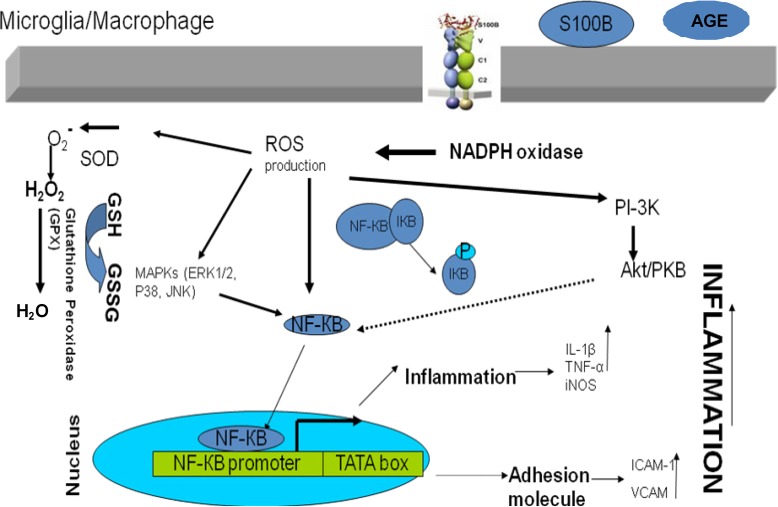
Nuclear factor kappa B (NF-κB) and MAPKs such as ERK1/2, JNK, Akt and p38 were implicated to be involved in the RAGE signalling pathway (figure 3).71-74
Figure 3.
The proposed schematic AGE-RAGE signalling pathways. The thickness of arrows shows the available supportive data.
It was shown that S100A8 and S100A9-induced human prostate cancer cells could activate NF-ҚB transcription in vivo.75 Sun et al,73 showed that phosphorylated p38 was not associated with Aβ plaques and neurofibrillary tangles in subregions of hippocampus of patients with AD. This suggests that p38/MAPKs signalling pathway is not crucial for the neurotixicity of Aβ, which is a RAGE ligand.73 Moreover, p38 MAPK phosphorylation increased in cultured neurons treated with a nontoxic concentration of Aβ42, soluble amyloid beta peptide. This effect was reduced by anti-RAGE antibody.72 This contradicted the findings with regards to RAGE and pp38. Further studies are in need to examine the role of pp38 in the RAGE signalling pathway.
Cyclooxygenase-2 enzyme has been implicated in the pathogenesis of several inflammatory diseases. Increase in the expression level of COX-2, which was induced by S100B, another RAGE ligand, was reported in human peripheral blood monocytes from healthy donors.61 Moreover, the involvement of NF-КB in the expression of COX-2 induced by IL-1β in mesenchymal cells of human amnion was shown.76 These studies implied that the role of COX-2 in the inflammatory response was RAGE-ligand dependent through NF-КB signaling pathway. These studies are controversial, and more investigations are in need to examine the role of COX-2 in the RAGE signalling pathway.
The proliferation of the mouse microglial cell line Ra2 was stimulated through increasing the expression of macrophage colony-stimulating factor in Aβ treated cell line. This stimulation was blocked by an Akt inhibitor.77,78 Furthermore, an electromobility shift assay showed that the M-CSF promoter region with a putative NF-κB binding site was associated with Aβ-induced M-CSF expression in the Ra2 cells treated with Aβ.74 This gives an indication that Aβ, a RAGE ligand, might act through the RAGE and Akt/NF-КB signaling pathway. The proposed schematic AGE-RAGE signalling pathways,21,58,79 are shown in figure 3.
Potential Therapeutic Approaches According to the AGEs Hypothesis
A number of therapeutic approaches were proposed for ageing-related diseases with relevance to glycated proteins. These approaches include the use of AGE inhibitors, AGE breakers, AGE signalling blocking, anti-RAGE antibody and RAGE antagonists, and antioxidants such as ascorbic acid and α-tocopherol (vitamin E).80,81 Anti-RAGE antibody treatment in uremic apoE−/− mice showed a decrease in the development of atherosclerotic lesions.82 Moreover, vitamin C treatment of patients with renal failure showed a decrease in AGEs plasma level.83 Oxidative stress and ROS production, based on the AGEs hypothesis, may potentially benefit from antioxidant treatment, especially in AD.
Inflammation Induced By Glycated Proteins, Interferon Gamma and Lipopolysaccharide
It has been reported that glycated proteins trigger the inflammation. The pathophysiology of inflammation includes the recruitment of white blood cells and production of cytokines which are the messenger among the cells.84 In addition, lipopolysaccharide (LPS), which is the outer membrane of gram negative bacteria, elicits the production of a number of cytokines.85 Cytokines can bind to specific receptors and trigger the complex cytokine network signalling. An excessively elevated level of cytokines was observed in AD patients. Among those, INF-γ, which was originally called macrophage-activating factor, is of potential interest.86 Macrophages are sensitive to INF-γ. In addition, the non lethal dose of LPS was shown to be toxic in combination with INF-γ in mice. The involvement of cytokines with LPS in Shwartzman-like lethal inflammatory response was observed in rabbits and mice.87,88 There is evidence that this protein modification results in the elevated level of cytokines.89
Inhibition of Glycated Protein Induced-Inflammation by Different Antioxidants
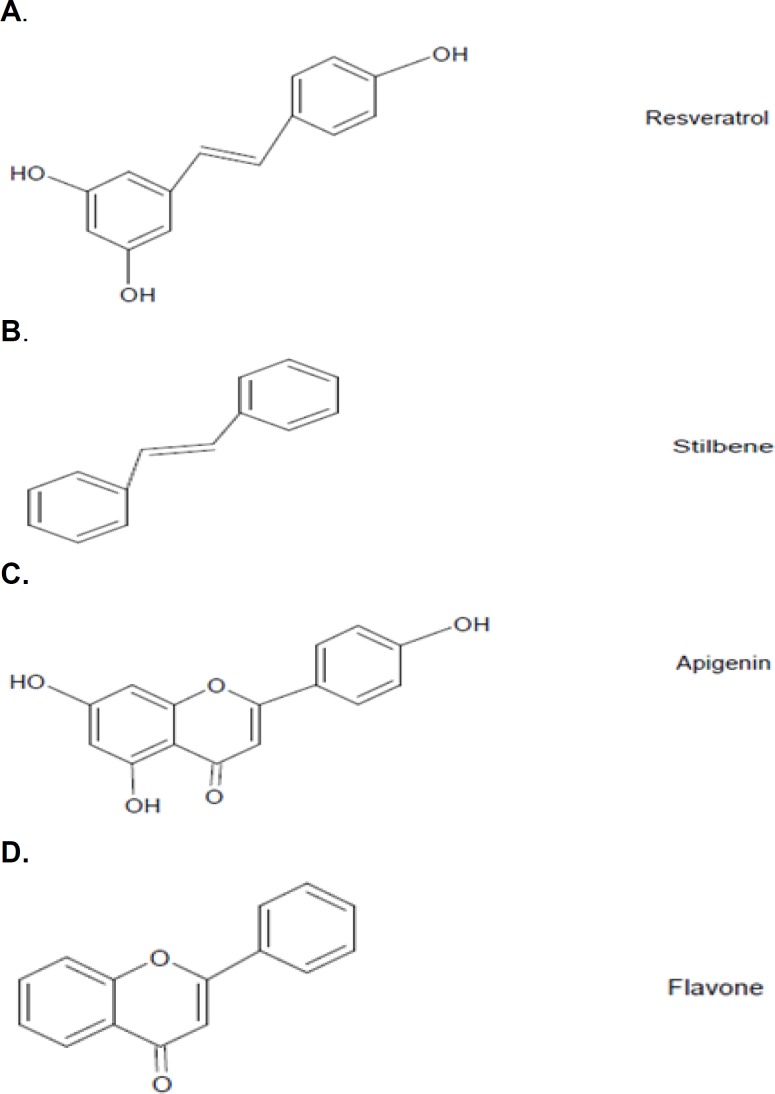
Inflammation is known as a potential cause of neurodegenerative diseases such as AD and Parkinson’s. Several studies have shown that neuronal cell death is regulated by inflammation. Microglia, the resident macrophages in the brain, can produce inflammation markers such as NO and TNF-α. The inflammation, which results in neurodegenerative diseases, has a deleterious effect on neurons.90 Reduction of inflammation might help to decrease the level of induced damages. Polyphenols are a group of compounds with defined structures and are abundant in fruit and vegetables. They are well known as antioxidants and antiinflammatory compounds.91 So far, the use of polyphenols as antiinflammatories has been studied extensively.92 The flavone structure is known as the antiinflammatory structure in a wide range of plant extracts. Resveratrol; however, belongs to a different structural family of plant extracts, and has stilbene structure (figure 4).93 The chemical structures of apigenin and resveratrol, their core structures, and related chemical groups are shown in figure 4.94
Inhibition of AGE-Induced Inflammation by Compounds Acting on the Receptors
Protein glycation is a protein modification, and is observed in ageing. Glycated proteins can be up taken via the RAGE receptor.49 There are various ways to intervene at the receptor level in AGE-RAGE signalling pathway such as the blockade of the receptor by antagonist and antibody.95
The RAGE was implicated to be involved in AD and diabetic vascular diseases.96 Blockade of RAGE-ligand interaction by soluble RAGE, RAGE antibody and RAGE antagonist were considered in the reduction of plaque formation.97 The RAGE-ligand interaction triggers the AGE-RAGE signalling pathway. It leads to further inflammation and oxidative stress.30 Any intervention along this signalling pathway could reduce the inflammation and consequently, induce damages.
Figure 4.
The chemical structures of apigenin and resveratrol (A and C), the core structures and related chemical groups (B and D).
Several RAGE blockers such as TTP488 and PF-04494700 have been tested. In addition, naturally occurring biopolymers such as hyaluronic acid is shown to have antagonizing activity.98 Hyaluronic acid injection has also been introduced as a treatment for osteoarthritis.81 In addition to chemicals and polymers as antagonists for RAGE, antibodies against the receptor can be an alternative way of antagonizing the receptor.
Inhibition of AGE-Induced Inflammation by Catalase, NADPH Oxidase Inhibitors
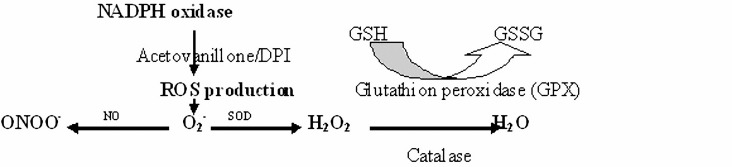
The physiological role of NO signalling as an extracellular signalling molecule is now widely appreciated.99 Hydrogen peroxide (H2O2) is another messenger with similar biochemical characteristics similar to NO, and is predominantly involved in pro-inflammatory signalling.100,101 Hydrogen peroxide can act as a messenger and transmit pro-inflammatory signals between adjacent cells.102 It can act both as a mediator molecule and a toxic substance. In higher concentrations it is toxic, and in lower concentrations it is a messenger.103 The special role of H2O2 in inflammation by some types of cells such as T-cells has been extensively investigated.104 Hydrogen peroxide belongs to ROS, and it has been hypothesised that H2O2 acts as a messenger in gene regulation and signal transduction pathways.105 It has also been reported that the concentration of H2O2 in leukocytes increases from 1 to 100 mM during phagocytosis.106 Various activities within the cells are related to H2O2 production. Schematic presentation of the chemical reactions involved in antioxidant defense mechanisms,107 are shown in figure 5.
Figure 5.
Schematic presentation of the chemical reactions involved in antioxidant defense mechanisms.
Conclusion
While there are several studies about glycated proteins, the number of reports regarding the signalling network triggered by glycated proteins is limited. The publications reviewed herein might indicate that AGE-RAGE signalling pathway was a possible signalling pathway. In addition to inflammatory response, oxidative stress is another mechanism through which the destructive effects of glycated protein in AD and other age-related diseases are prominent. It is still unclear what the second messenger of the RAGE, which results in the receptor activation, is. It might also be possible to conclude that the understanding of the pathway can help in finding an inhibitory compound useful in AD.
Conflict of Interest: None declared
References
- 1.Kikuchi S, Shinpo K, Takeuchi M, et al. Glycation--a sweet tempter for neuronal death. Brain Res Brain Res Rev. 2003;41:306–23. doi: 10.1016/s0165-0173(02)00273-4. [DOI] [PubMed] [Google Scholar]
- 2.Sattarahmady N, Moosavi-Movahedi AA, Habibi-Rezaei M, et al. Detergency effects of nanofibrillar amyloid formation on glycation of human serum albumin. Carbohydr Res. 2008;343:2229–34. doi: 10.1016/j.carres.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Sattarahmady N, Moosavi-Movahedi AA, Ahmad F, et al. Formation of the molten globule-like state during prolonged glycation of human serum albumin. Biochim Biophys Acta. 2007;1770:933–42. doi: 10.1016/j.bbagen.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Münch G, Lüth HJ, Wong A, et al. Crosslinking of [alpha]-synuclein by advanced glycation endproducts - an early pathophy-siological step in Lewy body formation? J Chem Neuroanat. 2000;20:253–7. doi: 10.1016/s0891-0618(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 5.Lapolla A, Traldi P, Fedele D. Importance of measuring products of non-enzymatic glycation of proteins. Clin Biochem. 2005;38:103–15. doi: 10.1016/j.clinbiochem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Danehy JP. Maillard reactions: nonenzymatic browning in food systems with special reference to the development of flavor. Adv Food Res. 1986;30:77–138. doi: 10.1016/s0065-2628(08)60348-1. [DOI] [PubMed] [Google Scholar]
- 7.Reddy VP, Beyaz A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov Today. 2006;11:646. doi: 10.1016/j.drudis.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Cooper ME. Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens. 2004;17:31S–38S. doi: 10.1016/j.amjhyper.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Hennessey PJ, Ford EG, Black CT, Andrassy RJ. Wound collagenase activity correlates directly with collagen glycosylation in diabetic rats. J Pediatr Surg. 1990;25:75–8. doi: 10.1016/s0022-3468(05)80167-8. [DOI] [PubMed] [Google Scholar]
- 10.Scalbert P, Birlouez-Aragon I. Relationship Between Lens Protein Glycation and Membrane Structure in Human Cataract. Exp Eye Res. 1993;56:335–40. doi: 10.1006/exer.1993.1043. [DOI] [PubMed] [Google Scholar]
- 11.Puglielli L. Aging of the brain, neurotrophin signaling, and Alzheimer's disease: Is IGF1-R the common culprit? Neurobiol Aging. 2008;29:795–811. doi: 10.1016/j.neurobiolaging.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rondeau P, Navarra G, Cacciabaudo F, et al. Thermal aggregation of glycated bovine serum albumin. Biochim Biophys Acta. 2010;1804:789–98. doi: 10.1016/j.bbapap.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Malloy P, Tremont G, Grace J, Frakey L. The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer's disease. Alzheimers Dement. 2007;3:200–3. doi: 10.1016/j.jalz.2007.04.374. [DOI] [PubMed] [Google Scholar]
- 14.Evans DA. Estimated Prevalence of Alzheimer's Disease in the United States. Milbank Q. 1990;68:267–89. [PubMed] [Google Scholar]
- 15.Cummings BJ, Pike CJ, Shankle R, Cotman CW. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer's disease. Neurobiology of Aging. 1996;17:921–33. doi: 10.1016/s0197-4580(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 16.Gu L, Hagiwara S, Fan Q, et al. Role of receptor for advanced glycation end-products and signalling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytes. Nephrol Dial Transplant. 2006;21:299–313. doi: 10.1093/ndt/gfi210. [DOI] [PubMed] [Google Scholar]
- 17.Hoppmann S, Steinbach J, Pietzsch J. Scavenger receptors are associated with cellular interactions of S100A12 in vitro and in vivo. Int J Biochem Cell Biol. 2010;42:651–61. doi: 10.1016/j.biocel.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann MA, Drury S, Fu C, et al. RAGE Mediates a Novel Proinflammatory Axis: A Central Cell Surface Receptor for S100/ Calgranulin Polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 19.Alarcón R, Fuenzalida C, Santibáñez M. Expression of Scavenger Receptors in Glial Cells: Comparing the Adhesion of Astrocytes and Microglia from Neonatla Rats to Surface-Bound β-Amyloid. J Biol Chem. 2005;280:30406–15. doi: 10.1074/jbc.M414686200. [DOI] [PubMed] [Google Scholar]
- 20.Tsoporis JN, Izhar S, Huttunen HJ, Parker TG. S100b induces myocyte apoptosis via a signaling pathway that includes rage, Erk1/2-p38 Mapk and p53. Journal of Molecular and Cellular Cardiology. 2007;42 [Google Scholar]
- 21.Shen CP, Tsimberg Y, Salvadore C, Meller E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5 doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Liang C, Liu X, et al. AGEs increased migration and inflammatory responses of adventitial fibroblasts via RAGE, MAPK and NF-κB pathways. Atherosclerosis. 2010;208:34–42. doi: 10.1016/j.atherosclerosis.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Srikanth V, Maczurek A, Phan T, et al. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging. 2011;32:763–77. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Kalea AZ, Reiniger N, Yang H, et al. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J. 2009;23:1766–74. doi: 10.1096/fj.08-117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talts JF, Andac Z, Göhring W, et al. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, [alpha]-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–70. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez AJ. Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu Rev Genet. 1998;32:279–305. doi: 10.1146/annurev.genet.32.1.279. [DOI] [PubMed] [Google Scholar]
- 27.Sparvero LJ, Asafu-Adjei D, Kang R, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and their role in Cancer and Inflammation. J Transl Med. 2009;7 doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Q, Keller JN. Splice variants of the receptor for advanced glycosylation end products (RAGE) in human brain. Neurosci Lett. 2005;373:67–72. doi: 10.1016/j.neulet.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 29.Hanford LE, Enghild JJ, Valnickova Z, et al. Purification and Characterization of Mouse Soluble Receptor for Advanced Glycation End Products (sRAGE) J Biol Chem. 2004;279:50019–24. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devangelio E, Santilli F, Formoso G, et al. Soluble RAGE in type 2 diabetes: Association with oxidative stress. Free Radic Biol Med. 2007;43:511–8. doi: 10.1016/j.freeradbiomed.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Ghidoni R, Benussi L, Glionna M, et al. Decreased plasma levels of soluble receptor for advanced glycation end products in mild cognitive impairment. J Neural Trans. 2008;115:1047–50. doi: 10.1007/s00702-008-0069-9. [DOI] [PubMed] [Google Scholar]
- 32.Xie J, Reverdatto S, Frolov A, et al. Structural Basis for Pattern Recognition by the Receptor for Advanced Glycation End Products (RAGE) J Biol Chem. 2008;283:27255–69. doi: 10.1074/jbc.M801622200. [DOI] [PubMed] [Google Scholar]
- 33.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: An update. Biochimica et Biophysica Acta (BBA). Biochim Biophys Acta. 2009;1793:993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Osawa M, Yamamoto Y, Munesue S, et al. De-N-glycosylation or G82S mutation of RAGE sensitizes its interaction with advanced glycation endproducts. Biochimica et Biophysica Acta (BBA) Biochim Biophys Acta. 2007;1770:1468–74. doi: 10.1016/j.bbagen.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Ostendorp T, Weibel M, Leclerc E, et al. Expression and purification of the soluble isoform of human receptor for advanced glycation end products (sRAGE) from Pichia pastoris. Biochem Biophys Res Commun. 2006;347:4–11. doi: 10.1016/j.bbrc.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 36.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Grossin N, Wautier MP, Meas T, et al. Severity of diabetic microvascular compli-cations is associated with a low soluble RAGE level. Diabetes Metab. 2008;34:392–5. doi: 10.1016/j.diabet.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Chen YS, Yan W, Geczy C, et al. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R39. doi: 10.1186/ar2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamagishi S, Adachi H, Nakamura K, et al. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism. 2006;55:1227–31. doi: 10.1016/j.metabol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Bopp C, Hofer S, Weitz J, et al. sRAGE is Elevated in Septic Patients and Associated With Patients Outcome. J Surg Res. 2008;147:79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Norata GD, Garlaschelli K, Grigore L, et al. Circulating soluble receptor for advanced glycation end products is inversely associated with body mass index and waist/hip ratio in the general population. Nutr Metab Cardiovasc Dis. 2009;19:129–34. doi: 10.1016/j.numecd.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K, Yamagishi S, Adachi H. Circulating advanced glycation end products (AGEs) and soluble form of receptor for AGEs (sRAGE) are independent determin-ants of serum monocyte chemoattractant protein-1 (MCP-1) levels in patients with type 2 diabetes. Diabetes Metab Res Rev. 2008;24:109–14. doi: 10.1002/dmrr.766. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt AM. Suppression of accelerated diabetic atherosclerosis by soluble receptor for age (sRAGE) Biomedecine & Pharma-cotherapy. 1998;52:321. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 44.Kaji Y, Usui T, Ishida S, et al. Inhibition of Diabetic Leukostasis and Blood-Retinal Barrier Breakdown with a Soluble Form of a Receptor for Advanced Glycation End Products. Invest Ophthalmol Vis Sci. 2007;48:858–65. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- 45.White JA, Manelli AM, Holmberg KH, et al. Differential effects of oligomeric and fibrillar amyloid-[beta]1-42 on astrocyte-mediated inflammation. Neurobiol Dis. 2005;18:459–65. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Salminen A, Ojala J, Kauppinen A, et al. Inflammation in Alzheimer's disease: Amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–94. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Deane R, Wu Z, Zlokovic BV. RAGE (Yin) Versus LRP (Yang) Balance Regulates Alzheimer Amyloid β-Peptide Clearance Through Transport Across the Blood-Brain Barrier. Stroke. 2004;35:2628–31. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 48.Wegiel J, Imaki H, Wang K-C, et al. Cells of monocyte/microglial lineage are involved in both microvessel amyloidosis and fibrillar plaque formation in APPsw tg mice. Brain Res. 2004;1022:19–29. doi: 10.1016/j.brainres.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 49.Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004;6:1219–25. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Moser B, Wendt TM, Ankersmit JH, et al. Blockade of receptor for age (RAGE) suppresses T lymphocyte proliferation in mixed lymphocyte culture. The Journal of Heart and Lung Transplantation. 2003;22 [Google Scholar]
- 51.Peterszegi G, Molinari J, Ravelojaona V, et al. Effect of advanced glycation end-products on cell proliferation and cell death. Pathol Biol (Paris) 2006;54:396–404. doi: 10.1016/j.patbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi T, Oku H, Komori A, et al. Advanced glycation end products induce death of retinal neurons via activation of nitric oxide synthase. Exp Eye Res. 2005;81:647–54. doi: 10.1016/j.exer.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Berbaum K, Shanmugam K, Stuchbury G, et al. Induction of novel cytokines and chemokines by advanced glycation endpr-oducts determined with a cytometric bead array. Cytokine. 2008;41:198–203. doi: 10.1016/j.cyto.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Higai K, Shimamura A, Matsumoto K. Amadori-modified glycated albumin predo-minantly induces E-selectin expression on human umbilical vein endothelial cells through NADPH oxidase activation. Clin Chim Acta. 2006;367:137–43. doi: 10.1016/j.cca.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Mahajan N, Bahl A, Dhawan V. C-reactive protein (CRP) up-regulates expression of receptor for advanced glycation end products (RAGE) and its inflammatory ligand EN-RAGE in THP-1 cells: Inhibitory effects of atorvastatin. Int J Cardiol. 2010;142:273–8. doi: 10.1016/j.ijcard.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Mahajan N, Malik N, Bahl A, Dhawan V. Receptor for advanced glycation end products (RAGE) and its inflammatory ligand EN-RAGE in non-diabetic subjects with pre-mature coronary artery disease. Atherosclerosis. 2009;207:597–602. doi: 10.1016/j.atherosclerosis.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging. 2010;31:665–77. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 58.Donato R, Sorci G, Riuzzi F, et al. S100B's double life: Intracellular regulator and extracellular signal. Biochimica et Biophysica Acta (BBA) Biochim Biophys Acta. 2009;1793:1008–22. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Scheuren N, Jacobs M, Ertl G, et al. Cyclooxygenase-2 in Myocardium Stimulation by Angiotensin-II in Cultured Cardiac Fibroblasts and Role at Acute Myocardial Infarction. J Mol Cell Cardiol. 2002;34:29–37. doi: 10.1006/jmcc.2001.1484. [DOI] [PubMed] [Google Scholar]
- 60.Nah SS, Choi IY, Lee CK, et al. Effects of advanced glycation end products on the expression of COX-2, PGE2 and NO in human osteoarthritic chondrocytes. Rheumatology (Oxford) 2008;47:425–31. doi: 10.1093/rheumatology/kem376. [DOI] [PubMed] [Google Scholar]
- 61.Shanmugam N, Kim YS, Lanting L, Natarajan R. Regulation of Cyclooxygenase-2 Expression in Monocytes by Ligation of the Receptor for Advanced Glycation End Products. J Biol Chem. 2003;278:34834–44. doi: 10.1074/jbc.M302828200. [DOI] [PubMed] [Google Scholar]
- 62.Donnelly LE, Newton R, Kennedy GE, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–83. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 63.Kokkola R, Andersson A, Mullins G. RAGE is the Major Receptor for the Proinflammatory Activity of HMGB1 in Rodent Macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Park Y, Wu J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–30. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dimopoulos N, Piperi C, Psarra V, et al. Increased plasma levels of 8-iso-PGF2 [alpha] and IL-6 in an elderly population with depression. Psychiatry Res. 2008;161:59–66. doi: 10.1016/j.psychres.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 66.Saenger AK, Laha TJ, Edenfield MJ, Sadrzadeh SM. Quantification of urinary 8-iso-PGF2α using liquid chromatography-tandem mass spectrometry and association with elevated troponin levels. Clin Biochem. 2007;40:1297. doi: 10.1016/j.clinbiochem.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 67.Rondeau P, Singh NR, Caillens H, et al. Oxidative stresses induced by glycoxidized human or bovine serum albumin on human monocytes. Free Radic Biol Med. 2008;45:799–812. doi: 10.1016/j.freeradbiomed.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Mukherjee TK, Mukhopadhyay S, Hoidal JR. The role of reactive oxygen species in TNFα-dependent expression of the receptor for advanced glycation end products in human umbilical vein endothelial cells. Biochimica et Biophysica Acta (BBA) Biochim Biophys Acta. 2005;1744:213–23. doi: 10.1016/j.bbamcr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Basta G, Lazzerini G, Massaro M, et al. Advanced Glycation End Products Activate Endothelium Through Signal-Transduction Receptor RAGE: A Mechanism for Amplification of Inflammatory Responses. Circulation. 2002;105:816–22. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 70.Belch JJF, Shaw JW, Kirk G, et al. The White Blood Cell Adhesion Molecule E-Selectin Predicts Restenosis in Patients With Intermittent Claudication Undergoing Percutaneous Transluminal Angioplasty. Circulation. 1997;95:2027–31. doi: 10.1161/01.cir.95.8.2027. [DOI] [PubMed] [Google Scholar]
- 71.Ishihara K, Tsutsumi K, Kawane S, et al. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 2003;550:107–13. doi: 10.1016/s0014-5793(03)00846-9. [DOI] [PubMed] [Google Scholar]
- 72.Origlia N, Righi M, Capsoni S, et al. Receptor for Advanced Glycation End Product-Dependent Activation of p38 Mitogen-Activated Protein Kinase Contributes to Amyloid-α-Mediated Cortical Synaptic Dysfunction. J Neurosci. 2008;28:3521–30. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun A, Liu M, Nguyen XV, Bing G. P38 MAP kinase is activated at early stages in Alzheimer's disease brain. Exp Neurol. 2003;183:394–405. doi: 10.1016/s0014-4886(03)00180-8. [DOI] [PubMed] [Google Scholar]
- 74.Ito S, Sawada M, Haneda M, et al. Amyloid-[beta] peptides induce cell proliferation and macrophage colony-stimulating factor exp-ression via the PI3-kinase/Akt pathway in cultured Ra2 microglial cells. FEBS Lett. 2005;579:1995–2000. doi: 10.1016/j.febslet.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 75.Hermani A, Medunjanin S, et al. S100A8 and S100A9 activate MAP kinase and NF-κB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312:184–97. doi: 10.1016/j.yexcr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Yan X, Sun M, et al. N uclear factor kappa B activation and regulation of cyclooxygenase type-2 expression in human amnion mesenchymal cells by interleukin-1beta. Biol Reprod. 2002;66:1667–71. doi: 10.1095/biolreprod66.6.1667. [DOI] [PubMed] [Google Scholar]
- 77.Ito S, Sawada M, Haneda M, et al. Amyloid-β peptides induce several chemokine mRNA expressions in the primary microglia and Ra2 cell line via the PI3K/Akt and/or ERK pathway. Neurosci Res. 2006;56:294–9. doi: 10.1016/j.neures.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 78.Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 79.Fukunaga K, Miyamoto E. Role of MAP kinase in neurons. Mol Neurobiol. 1998;16:79–95. doi: 10.1007/BF02740604. [DOI] [PubMed] [Google Scholar]
- 80.Peyroux J, Sternberg M. Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathologie Biologie. 2006;54:405–19. doi: 10.1016/j.patbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Sabbagh MN, Agro A, Bell J, et al. PF-04494700, an Oral Inhibitor of Receptor for Advanced Glycation End Products (RAGE), in Alzheimer Disease. Alzheimer Dis Assoc Disord. 2010:28. doi: 10.1097/WAD.0b013e318204b550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bro S, Flyvbjerg A, Binder CJ, Pallotta G. A neutralizing antibody against receptor for advanced glycation end products (RAGE) reduces atherosclerosis in uremic mice. Atherosclerosis. 2008;201:274–80. doi: 10.1016/j.atherosclerosis.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 83.Ferretti G, Bacchetti T, Masciangelo S, et al. Lipid peroxidation in hemodialysis patients: Effect of vitamin C supplementation. Clin Biochem. 2008;41:381–6. doi: 10.1016/j.clinbiochem.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 84.Johnston JB, Rahman MM, McFadden G. Strategies that modulate inflammasomes: insights from host-pathogen interactions. Semin Immunopathol. 2007;29:261–74. doi: 10.1007/s00281-007-0080-5. [DOI] [PubMed] [Google Scholar]
- 85.Dueñas AI, Aceves M, Orduña A, et al. Francisella tularensis LPS induces the production of cytokines in human monocytes and signals via Toll-like receptor 4 with much lower potency than E. coli LPS. Int Immunol. 2006;18:785–95. doi: 10.1093/intimm/dxl015. [DOI] [PubMed] [Google Scholar]
- 86.Reyes-Gibby CC, Wu X, Spitz M, et al. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008;9:777–85. doi: 10.1016/S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heremans H, Dillen C, et al. Interferon gamma, a mediator of lethal lipopolysaccharide-induced Shwartzman-like shock reactions in mice. J Exp Med. 1990;171:1853–69. doi: 10.1084/jem.171.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heremans H, Dijkmans R, Sobis H, et al. Regulation by interferons of the local inf-lammatory response to bacterial lipopol ysaccharide. J Immunol. 1987;138:4175–9. [PubMed] [Google Scholar]
- 89.Hein G, Franke S. Are advanced glycation end-product-modified proteins of pathog-enetic importance in fibromyalgia? Rheumatology. 2002;41:1163–7. doi: 10.1093/rheumatology/41.10.1163. [DOI] [PubMed] [Google Scholar]
- 90.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience. 2009;158:1021–9. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 91.Lee JS, Oh TY, Kim YK, et al. Protective effects of green tea polyphenol extracts against ethanol-induced gastric mucosal damages in rats: Stress-responsive trans-cription factors and MAP kinases as potential targets. Mutat Res. 2005;579:214–24. doi: 10.1016/j.mrfmmm.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 92.Nichols J, Katiyar S. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeong GS, Lee SH, Jeong SN, et al. Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. Int Immunopharmacol. 2009;9:1374–80. doi: 10.1016/j.intimp.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 94.Li BH, Ma XF, Wang Y, Tian WX. Structure-Activity Relationship of Polyphenols That Inhibit Fatty Acid Synthase. J Biochem. 2005;138:679–85. doi: 10.1093/jb/mvi171. [DOI] [PubMed] [Google Scholar]
- 95.Gilliam-Davis S, Payne VS, Kasper SO, et al. Long-term AT1 receptor blockade improves metabolic function and provides renoprotection in Fischer-344 rats. Am J Physiol Heart Circ Physiol. 2007;293:H1327–33. doi: 10.1152/ajpheart.00457.2007. [DOI] [PubMed] [Google Scholar]
- 96.Natarajan R, Nadler JL. Lipid Inflammatory Mediators in Diabetic Vascular Disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–8. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 97.Maillard-Lefebvre H, Boulanger E, Daroux M, et al. Soluble receptor for advanced glycation end products: a new biomarker in diagnosis and prognosis of chronic inf-lammatory diseases. Rheumatology (Oxford) 2009;48:1190–6. doi: 10.1093/rheumatology/kep199. [DOI] [PubMed] [Google Scholar]
- 98.Neumann A, Schinzel R, Palm D, et al. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-[kappa]B activation and cytokine expression. FEBS Letters. 1999;453:283–7. doi: 10.1016/s0014-5793(99)00731-0. [DOI] [PubMed] [Google Scholar]
- 99.Neill SJ, Desikan R, Clarke A, et al. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot. 2002;53:1237–47. [PubMed] [Google Scholar]
- 100.Orozco-Cardenas ML, Ryan CA. Nitric Oxide Negatively Modulates Wound Signaling in Tomato Plants. Plant Physiol. 2002;130:487–93. doi: 10.1104/pp.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA. Hydrogen Peroxide Acts as a Second Messenger for the Induction of Defense Genes in Tomato Plants in Response to Wounding, Systemin, and Methyl Jasmonate. Plant Cell. 2001;13:179–91. [PMC free article] [PubMed] [Google Scholar]
- 102.Sobey CG, Miller AA. Signalling pathways activated by hydrogen peroxide in vascular smooth muscle. J Hypertens. 2005;23:1961–2. doi: 10.1097/01.hjh.0000184409.96672.ab. [DOI] [PubMed] [Google Scholar]
- 103.Park IJ, Hwang JT, Kim YM, et al. Differential Modulation of AMPK Signaling Pathways by Low or High Levels of Exogenous Reactive Oxygen Species in Colon Cancer Cells. Ann N Y Acad Sci. 2006;1091:102–9. doi: 10.1196/annals.1378.059. [DOI] [PubMed] [Google Scholar]
- 104.Song Y, Driessens N, Costa M, et al. Roles of Hydrogen Peroxide in Thyroid Physiology and Disease. J Clin Endocrinol Metab. 2007;92:3764–73. doi: 10.1210/jc.2007-0660. [DOI] [PubMed] [Google Scholar]
- 105.Xing Y, Jia W, Zhang J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008;54:440–51. doi: 10.1111/j.1365-313X.2008.03433.x. [DOI] [PubMed] [Google Scholar]
- 106.Ikeda Y, Taira K. Biologically Important Reactions Catalyzed by RNA Molecules. Chem Rec. 2002;2:307–18. doi: 10.1002/tcr.10031. [DOI] [PubMed] [Google Scholar]
- 107.Guglielmotto M, Giliberto L, Tamagno E, Tabaton M. Oxidative stress mediates the pathogenic effect of different Alzheimer& rsquo;s disease risk factors. Front Aging Neurosci. 2010;2 doi: 10.3389/neuro.24.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]