Abstract
Parathyroid hormone-related protein producing pancreatic neuroendocrine tumors have been infrequently reported. Herein, we report a case of an Iranian woman who had such a tumor during pregnancy, and gave birth to a female neonate with esophago-tracheal fistula and imperforated anus. Hypercalcemia was diagnosed at postpartum because of elevated serum calcium levels in the neonate and neurologic deterioration of the mother. Extensive literature review revealed 42 cases with pancreatic neuroendocrine tumors and hypercalcaemia. The clinical and laboratory findings of such patients are reviewed in this manuscript.
Key Words: Hypercalcemia, pancreatic tumor, pregnancy
Introduction
Neuroendocrine tumors (NET) are a group of nonhomogeneous malignancies from different organs that share in common the ability to secrete a wide range of peptides. The tumors originate from a group of cells widely dispersed in different organs. These cells were formerly named amine precursor uptake and decarboxylation (APUD) cells, and tumors originating from these cells were called carcinoids or apudomas. At present this cellular system is called diffuse neuroendocrine system (DNES) cells, and the tumors originating from them are collectively called neuroendocrine tumors.1-3 The lungs and the gastrointestinal tract are the major sites for emergence of these neoplasms. The tumors can be either functional or nonfunctional. Nicholls, in 1902, reported the first tumor of this type, an islet cell carcinoma of pancreas. Since then pancreatic NET, either nonfunctional or capable of secreting insulin, glucagon, somatostatin, gastrin and vasoactive intestinal peptide (VIP) have been reported. Secretion of hormones not native to pancreatic endocrine system, such as calcitonin, adrenocorticotropic hormone (ACTH) and parathyroid hormone-related peptide (PTHrP) have also been reported during the last 30 years.4,5
Herein, we present the case of a young Iranian woman who had a pancreatic NET during pregnancy, and was diagnosed at postpartum because of severe hypercalcemia and mental confusion. To the best of our knowledge, there is only one case presentation in the literature,6 whose pregnancy was associated with pancreatic NET. We have also reviewed the clinical and laboratory data of the reported cases of this unusual disease in literature.
Case Description
A 35-year-old gravida one para one, pregnant woman was admitted to hospital because of nausea and vomiting. She was in her 37th week of pregnancy, and had had nausea and vomiting since her second trimester. She also felt that fetal movements had decreased since two to three days before admission. She had had no serious problems until three months earlier when gestational diabetes was diagnosed and successfully treated with insulin. Her total weight gain during pregnancy was around seven kilograms. The patient was admitted to hospital and underwent an immediate cesarean section because of nonreactive non stress test (NST). The baby was a girl with an Apgar score of 10, imperforated anus and tracheoesophageal fistula. Routine laboratory evaluation of the neonate showed a serum calcium level of 17 mg/dl. Neonatal hypercalcemia prompted us to check the mother’s calcium, which was found to be 16.3 and 17 mg/dl on two consecutive measurements. Serum phosphorus levels were 2.1 and 2.6 and 2.8 mg/dl on three separate occasions. Chest X ray and routine laboratory evaluations were within normal limits. Serum PTH was 14 and 15 pg/ml on two different occasions. She had no history of excessive consumption of dairy products, calcium carbonate, nonabsorbable antacids or vitamin D preparations. Serum 25OH vitamin D3 level was 20 ng/ml. Serum levels of calcitonin, α-fetoprotein and carcinoembryonic antigene (CEA) were within normal ranges. Serum concentration of parathyroid hormone-related peptide was not available to us.
Treatment started with normal saline, furosemide and calcitonin. Despite aggressive hydration and continuous intake of furosemide and calcitonin, the patient’s condition gradually deteriorated during the next 48 hours with aggravation of hypercalcemia and deterioration of mental status. Therefore, 90 mg Pamidronate, which resulted in gradual decrement of serum calcium level, was prescribed.
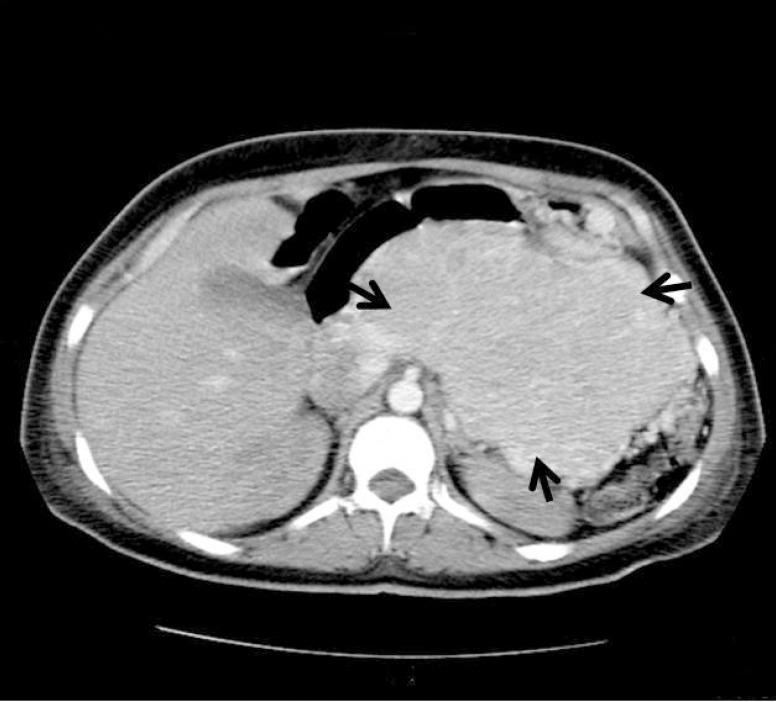
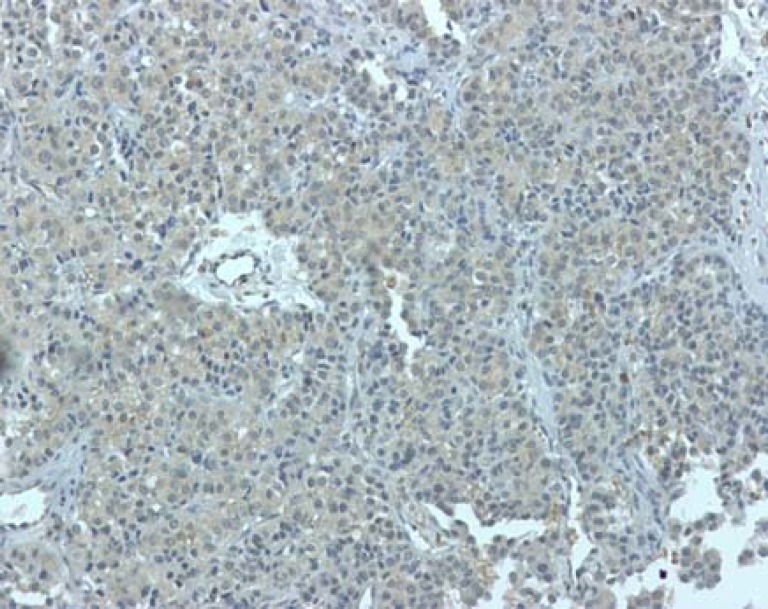
Ultrasonographic evaluation of the abdomen revealed a 150 mm lobulated mass in the upper part of abdomen which was confirmed by CT scan (figure 1). The patient underwent surgery, during which a large, lobulated, hard, hypervascular and irregular mass occupying the body and tail of the pancreas was observed. The mass could not be totally excised because of hypervascularity and severe bleeding potentials. Histopathologic evaluation revealed that the mass was a neuroendocrine tumor. Immunohistochemistry (IHC) staining, done in Iran, was positive for synaptophysin, alpha 1 antitrypsin and vimentin. Re-evaluation of IHC, done at the Department of Pathology, St. Michael Hospital, Toronto, Ontario, Canada, disclosed cytoplasmic immunopositivity for PTHrP (figure 2), somatostatin, calcitonin, serotonin and chromogranin. Ki-67 nuclear labeling index was estimated at 1-3%.
Figure 1.
Abdominal computed tomography scan showing the pancreatic tumor
Figure 2.
Immunohistochemical staining showing positivity for parathyroid hormone related protein.
After one week, because of paresthesia and serum calcium concentration of 7.1 mg/dl, calcium carbonate and calcitriol were prescribed followed by chemotherapy with Etoposide and VP16. After six months the patient underwent surgery for a second time in another hospital. This surgery was also unsuccessful at complete removal of the pancreatic mass. The neonate was also operated on by a team of pediatric surgeons; however, unfortunately she expired the day after the surgery.
Discussion
Present case is unique because of the large invasive tumor spanning whole length of pregnancy, severe post partum hypercalcemia, and birth of a baby with above-mentioned malformations secondary to a pancreatic NET.
Neuroendocrine tumors are rare neoplasms. The annual incidence is 2-3/100,000 and 30-50% of the tumors are functional.4,7 Pancreatic NET presenting with hypercalcemia secondary to PTHrP production constitute a small minority of these tumors. Because of the similarity of the clinical picture with multiple endocrine neoplasia type 1 (MEN1), the pathogenesis of hypercalcemia in patients with pancreatic NET was a real challenge.8 Indeed, hypercalcemia of first series of patients was presumed to be due to concomitant primary hyperparathyroidism, and based on this untoward assumption, at least three patients underwent unnecessary parathyroidectomy.9 Since Albright’s novel statement in 1941 about the humoral nature of tumor hypercalcemia,10 many efforts have been made to prove the secretion of either ectopic PTH or a substance that has functional similarity to PTH. The enthusiasm and the ensuing hard work led to the discovery of PTHrP in 1988,11 which was a turning point in the correct interpretation of tumor hypercalcemia.12
In an extensive review of the literature we could find 42 patients with pancreatic NET and hypercalcemia. Clinical and laboratory data of the reviewed cases as well as the present case are shown in table 1. The patients are 20 men and 22 women with a mean age of 45 years (age range 8-77 years). The largest size of the tumor was 3.9-18 cm with a mean of 10.2 cm. All patients were hypercalcemic with serum calcium concentrations ranging from 10.6-26.4 mg/dl with a mean of 15.5 mg/dl. Serum concentrations of PTH were low or undetectable in 31 cases, and within normal range in 11 cases. Of 25 patients whose serum PTHrP had been measured, 24 had elevated levels ranging from 2.3-40 pmol/L with a mean of 10.8 pmol/L, which was about 10 times the upper limit of normal range. Data for IHC, available for 17 patients, showed positivity for PTHrP in all except for two cases.13 chromogranin (CgA), synaptophysin (Syn), neuron specific enolase (NSE), somatostatin (So) and calcitonin (Cal) were positive in varying combinations in all cases except two.14 Moreover, KI 67 in those who were analyzed was less than 10%, which was in agreement with the low growth rate and long survival of those patients.
Table1.
Clinical and laboratory data of 42 patients with pancreatic neuroendocrine tumor and hypercalcemia
| No | Sex |
Age
(Year) |
Size
(cm) |
Calcium (mg/dl) | PTH | PTHrP (pmol/l) | IHC | Description of the tumor | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 57 | 10 | 16 | Nl | NA | NA | Islet cell carcinoma, Liver metastasis | DeWys, 1973 |
| 2 | Male | 8 | NA | 14.4 | L | NA | NA | Islet cell carcinoma, Liver metastasis | Friesen, 1975 |
| 3 | Female | 62 | NA | 19.6 | Nl | NA | NA | Islet cell carcinoma, Liver metastasis | Cryer, 1977 |
| 4 | Female | 68 | 10 | 13.3 | Nl | NA | NA | Islet cell carcinoma, Liver metastasis | Rasbach, 1985 |
| 5 | Male | 41 | 14x10 | 16.8 | Nl | NA | NA | Endocrine carcinoma, Liver metastasis | Arp, 1986 |
| 6 | Female | 47 | 9 | 16.4 | Nl | NA | NA | Islet cell carcinoma | Vair, 1987 |
| 7 | Male | 44 | NA | 15.3 | Nl | NA | NA | Islet cell carcinoma, Liver metastasis | Shetty, 1987 |
| 8 | Female | 52 | 13x9 | 14.8 | Nl | NA | NA | Benign endocrine tumor | Heitz, 1989 |
| 9 | Female | 37 | 5x4 | 14 | U | 17.5 | PTHrP, NSE | Malignant islet cell tumor, liver metastasis | Wyvick, 1990 |
| 10 | Female | 42 | Large | 19.6 | L | 2.4 | PTHrP | Malignant islet cell tumor, liver metastasis | Dodwel, 1990 |
| 11 | Male | 30 | 8 | 14 | Nl | NA | PTHrP,CgA,NSE | Malignant islet cell tumor, liver metastasis | Rizzoli, 1990 |
| 12 | Female | 60 | large | 14.4 | L | NA | PTHrP | Malignant islet cell tumor, liver metastasis | Rizzoli, 1990 |
| 13 | Male | 45 | 12 | 14.4 | L | NA | Neg | Islet cell carcinoma, liver metastasis | Bresler, 1991 |
| 14 | Female | 77 | NA | 13.1 | L | 12.8 | NA | Islet cell carcinoma, liver metastasis | Mitlak, 1991 |
| 15 | Female | 47 | 11x7x7 | 18 | Nl | NA | PTHrP, NSE,SYN | Malignant endocrine tumor | Miraliakbari, 1992 |
| 16 | Male | 30 | NA | 17.2 | U | NA | NA | Malignant Somatostatinoma | Starv, 1992 |
| 17 | Male | 30 | L | 16.1 | L | 20 | PTHrP | Neuroendocrine tumor of pancreas | Williams,1992 |
| 18 | Male | 36 | L | 16 | L | 7.8 | NA | Neuroendocrine tumor of pancreas, liver metastasis | Tarver, 1992 |
| 19 | Female | 39 | 12x9 | 18.8 | L | 12.9 | CgA,PTHrP | Neuroendocrine tumor of pancreas | Ratcliff, 1994 |
| 20 | Male | 41 | 18x16x10 | 26.4 | L | NA | neg | Islet cell carcinoma, liver metastasis | Mao, 1995 |
| 21 | Male | 43 | 15 | 13.6 | L | 6.2 | PTHrP | Islet cell carcinoma, liver metastasis | Mao, 1995 |
| 22 | Male | 64 | 7x5x2 | 16.4 | Nl | NA | PTHrP | Islet cell carcinoma, liver metastasis | Mao, 1995 |
| 23 | Male | 66 | NA | 12 | L | U | NA | Islet cell tumor, liver & spleen metastasis | Wu, 1997 |
| 24 | Female | 42 | NA | 10.7 | U | 4 | NA | Islet cell tumor, liver & spleen metastasis | Wu, 1997 |
| 25 | Female | 45 | NA | 11.6 | L | 9.1 | NA | Islet cell tumor, liver & spleen metastasis | Wu, 1997 |
| 26 | Male | 64 | NA | 13.7 | L | 9.6 | NA | Islet cell tumor, liver metastasis | Wu, 1997 |
| 27 | Male | 61 | NA | 11.5 | L | 14 | NA | Islet cell tumor, liver metastasis | Wu, 1997 |
| 28 | Female | 38 | NA | 15 | L | 16 | NA | Islet cell tumor, liver metastasis | Wu, 1997 |
| 29 | Male | 20 | NA | 10.8 | U | 18 | NA | Islet cell tumor, liver metastasis | Wu, 1997 |
| 30 | Female | 47 | NA | 10.8 | U | 23 | NA | Islet cell tumor, liver metastasis | Wu, 1997 |
| 31 | Female | 51 | NA | 13.6 | U | 40 | NA | Islet cell tumor, liver metastasis | Wu, 1997 |
| 32 | Male | 34 | 7x11 | 19.6 | L | 12.3 | CgA,CyK,Syn | Neuroendocrine tumor of pancreas | Clemens, 2001 |
| 33 | Female | 25 | 9 | 23.6 | U | 3.9 | PTHrP | Neuroendocrine tumor of pancreas | Abraham, 2002 |
| 34 | Female | 56 | 4.4x3.9 | 11.8 | U | NA | NSE, Syn, Cal | Neuroendocrine tumor of pancreas | Mullerpatan, 2004 |
| 35 | Male | 59 | large | 18.9 | L | 7.3 | PTHrP,Cal, CgA | Neuroendocrine tumor of pancreas | Eynden, 2006 |
| 36 | Male | 40 | 6x3.5 | 18.9 | L | 8.5 | PTHrP, So | Neuroendocrine tumor of pancreas | Mussieg, 2007 |
| 37 | Female | 25 | NA | 22 | L | 5.1 | NA | Neuroendocrine tumor of pancreas | Srirajaskanthan et al, 2007 |
| 38 | Female | 44 | NA | 11.7 | L | 3.6 | NA | Neuroendocrine tumor, liver metastasis | Srirajaskanthan et al, 2007 |
| 39 | Male | 26 | NA | 13.5 | L | 2.4 | NA | Neuroendocrine tumor, liver metastasis | Srirajaskanthan et al, 2007 |
| 40 | Female | 64 | NA | 11.9 | L | 2.6 | NA | Panctreatic tumor, local invasion | Srirajaskanthan et al, 2007 |
| 41 | Female | 34 | NA | 13.5 | L | 5.6 | NA | Panctreatic tumor, liver metastasis | Srirajaskanthan et al, 2007 |
| 42 | Female | 35 | 15 | 17 | L | NA | PTHrP, Syn, CgA, Cal, So | Neuroendocrine tumor of pancreas | Present study, 2010 |
Cal: Calcitonin, CgA: Chromogranine A, Cyk: Cytokeratin, L: Low, NA: Not available, Nl: Normal, NSE: Neuron Specific Enolase, So: Somatostatin, Syn: Synaptophisin, U: Undetectable, PTH: Parathyroid Hormone, PTHrP: Parathyroid Hormone Related Peptide, IHC: immunohistochemistry. Serum calcium reference range=8.5-10.3 mg/dl; PTHrP, Reference range: <1.3 pmol/L
Surgical removal of the tumor was the main therapeutic option. However, complete removal of the tumor could be done in only seven cases because of multiple liver metastasis, local invasion and hypervascularity of the tumors. Indeed multiple liver metastases were seen in 80 % of the patients. In these cases, distal pancreatectomy and/or debulking were done. Due to such limitations for surgery, nonsurgical treatment modalities are of utmost significance.
Somatostatin analogues have been used in patients with NET for the last two decades. Their alleviating effects on hypercalcemia as well as their potential anti tumor effects have also been reported. In last couple of years, Sandostatin LAR or similar analogues have been used in almost all cases in which complete surgical removal of the tumor has been impossible. It should be noted that anti proliferative effects of the drug is weak, and in some cases the tumor has progressed with time.15,16 Interferon α is another biotherapeutic agent approved for patients with NET. A previous study,16 reported that it was effective in reducing serum calcium and maintaining normocalcemia for a period of six months. Apparently it did have no effects on tumor growth in a limited number of patients in whom the drug had been advised.16
Chemotherapy has also been advised in all cases in which surgery has not been successful. Cisplatin, streptozotocin and, 5 fluorouracil were the most frequently-used agents. Partial improvement in patients' condition and stabilization of serum calcium has been reported by some researchers.16,17
Liver transplantation was done in a limited number of patients, especially in whom metastasis to the liver was established. The transplantation seems promising in such patients and the five year survival after transplantation has been reported.16 Hepatic arterial embolization and external radiation have been used in limited numbers of patients with minimal clinical benefits.
Concerning the outcome, it should be noted that in accordance with other studies,4,14,18 functional pancreatic NETs seems to have an indolent behavior and a more favorable prognosis. While 80% of the patients had liver metastasis, eight lived for more than five years and 11 lived for more than three years.
Our patient had some unique features, which merit special considerations. Having an invasive large pancreatic carcinoma during pregnancy is quite unusual because, and as a rule, pregnancy occurs in females with an optimum health status. Indeed, we do not know whether the patient had the tumor before pregnancy, or the cancer developed during pregnancy. Whatever the course of events, it was quite unusual to proceed to full-term pregnancy with such a big tumor. The finding is also in accordance with the slow growth rate of the tumor during pregnancy. There was no a sign or symptom that could be attributed to the abdominal mass or hypercalcemia, and postpartum detection of high serum calcium level in the mother was a result of accidental finding of hypercalcemia in the neonate during routine laboratory evaluations.
Aggravation of our patient’s hypercalcemia in the immediate postpartum period, which resulted in the deterioration of the patient’s mental status, was due to the elimination of the fetoplacental unit that worked as a buffer to alleviate hypercalcemia in prepartal period. Indeed a substantial amount of mother's calcium transfers to fetus during pregnancy. While this passage of calcium ameliorates mother's hypercalcemia; undoubtedly has some deleterious effects on the neonate. In this case we do not know if we could attribute the neonate’s problems such as imperforated anus and tracheoesophagal fistula to severe and longstanding hypercalcemia during pregnancy. We could not find any report about the association of these complications and maternal hypercalcemia, however, the association of complications such as intrauterine growth retardation, low birth weight, preterm delivery, intrauterine fetal demise and postpartum neonatal tetany with maternal hypercalcemia have been reported.2,7,8,16,19,20
Regarding limitations of the study, measurement of serum levels of PTHrP and 1-25(OH)2 Vitamin D3 was not available to us, and due to the unaffordability of long acting somatostatin analogue we could not use the drug for our patient.
Conclusion
Our presented case was unique as neuroendocrine pancreatic tumor-induced hypercalcemia was presented during pregnancy. Of note was the concealment of hypercalcemia during pregnancy and aggravation after parturition. The findings suggest that in the case of hypercalcemia in postpartum state, the possibility of tumor induced hypercalcemia should be kept in mind.
Acknowledgement
We would like to express our deep appreciation to Professor Ashley Grossman for his comments on the case, Mrs. N. Shiva for editing the text and Mrs. T. Fakhimi for preparation of the manuscript.
Conflict of Interest: None declared
References
- 1.Ferolla P, Faggiano A, Mansueto G, et al. The biological characterization of neuroendocrine tumors: the role of neuroendocrine markers. J Endocrinol Invest. 2008;31:277–86. doi: 10.1007/BF03345602. [DOI] [PubMed] [Google Scholar]
- 2.Vazquez DE. Hyperparathyroidism and pregnancy. Am Surg. 76:648–9. [PubMed] [Google Scholar]
- 3.Metastatic pancreatic islet cell carcinoma with peptic ulcer disease and hypercalcemia. Am J Med. 1977;63:142–51. doi: 10.1016/0002-9343(77)90126-7. [DOI] [PubMed] [Google Scholar]
- 4.Rothenstein J, Cleary SP, Pond GR, et al. Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am J Clin Oncol. 2008;31:64–70. doi: 10.1097/COC.0b013e31807a2f49. [DOI] [PubMed] [Google Scholar]
- 5.Mullerpatan PM, Joshi SR, Shah RC, et al. Calcitonin-secreting tumor of the pancreas. Dig Surg. 2004;21:321–4. doi: 10.1159/000080901. [DOI] [PubMed] [Google Scholar]
- 6.Abraham P, Ralston SH, Hewison M, et al. Presentation of a PTHrP-secreting pancreatic neuroendocrine tumour, with hypercalcaemic crisis, pre-eclampsia, and renal failure. Postgrad Med J. 2002;78:752–3. doi: 10.1136/pmj.78.926.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vair DB, Boudreau SF, Reid EL. Pancreatic islet-cell neoplasia, with secretion of a parathormone-like substance and hypercalcemia. Can J Surg. 1987;30:108–10. [PubMed] [Google Scholar]
- 8.Underdahl LO, Woolner LB, Black BM. Multiple endocrine adenomas; report of 8 cases in which the parathyroids, pituitary and pancreatic islets were involved. J Clin Endocrinol Metab. 1953;13:20–47. doi: 10.1210/jcem-13-1-20. [DOI] [PubMed] [Google Scholar]
- 9.Rasbach DA, Hammond JM. Pancreatic islet cell carcinoma with hypercalcemia. Primary hyperparathyroidism or humoral hypercalcemia of malignancy. Am J Med. 1985;78:337–42. doi: 10.1016/0002-9343(85)90446-2. [DOI] [PubMed] [Google Scholar]
- 10.Stewart AF, Broadus AE. Malignancy associated hypercalcemia. In: Degroot LJ, Jameson JL, editors. Endocrinology. 5th ed. Philadelphia: Saunders; 2006. pp. 1555–6. [Google Scholar]
- 11.Stewart AF, Mangin M, Wu T, et al. Synthetic human parathyroid hormone-like protein stimulates bone resorption and causes hypercalcemia in rats. J Clin Invest. 1988;81:596–600. doi: 10.1172/JCI113358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strewler GJ. The parathyroid hormone-related protein. Endocrinol Metab Clin North Am. 2000;29:629–45. doi: 10.1016/s0889-8529(05)70154-7. [DOI] [PubMed] [Google Scholar]
- 13.Bresler L, Boissel P, Conroy T, Grosdidier J. Pancreatic islet cell carcinoma with hypercalcemia: complete remission 5 years after surgical excision and chemotherapy. Am J Gastroenterol. 1991;86:635–8. [PubMed] [Google Scholar]
- 14.Mao C, Carter P, Schaefer P, et al. Malignant islet cell tumor associated with hypercalcemia. Surgery. 1995;117:37–40. doi: 10.1016/s0039-6060(05)80227-2. [DOI] [PubMed] [Google Scholar]
- 15.Srirajaskanthan R, McStay M, Toumpanakis C, et al. Parathyroid hormone-related peptide-secreting pancreatic neuroendocrine tumours: case series and literature review. Neuroendocrinology. 2009;89:48–55. doi: 10.1159/000151222. [DOI] [PubMed] [Google Scholar]
- 16.Schnatz PF, Curry SL. Primary hyperparathyroidism in pregnancy: evidence-based management. Obstet Gynecol Surv. 2002;57:365–76. doi: 10.1097/00006254-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Mitlak BH, Hutchison JS, Kaufman SD, Nussbaum SR. Parathyroid hormone-related peptide mediates hypercalcemia in an islet cell tumor of the pancreas. Horm Metab Res. 1991;23:344–6. doi: 10.1055/s-2007-1003693. [DOI] [PubMed] [Google Scholar]
- 18.Kazanjian KK, Reber HA, Hines OJ. Resection of pancreatic neuroendocrine tumors: results of 70 cases. Arch Surg. 2006;141:765–9. doi: 10.1001/archsurg.141.8.765. [DOI] [PubMed] [Google Scholar]
- 19.Neyret A, Fumey G, et al. PTHrP, calcitonin and calcitriol in a case of severe, protracted and refractory hypercalcemia due to a pancreatic neuroendocrine tumor. Bone. 2007;40:1166–71. doi: 10.1016/j.bone.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Vázquez DE. Hyperparathyroidism and pregnancy. Am Surg. 2010:648–9. [PubMed] [Google Scholar]