Summary
Transforming growth-factor β (TGFβ) has been implicated in T helper 17 (Th17) cell biology and in triggering expression of interleukin-17A (IL-17A), which is a key Th17 cell cytokine. Deregulated TGFβ receptor (TGFβR) signaling has been implicated in Th17-cell-mediated autoimmune pathogenesis. Nevertheless, the full molecular mechanisms involved in the activation of the TGFβR pathway in driving IL-17A expression remain unknown. Here, we identified protein kinase C α (PKCα) as a signaling intermediate specific to the Th17 cell subset in the activation of TGFβRI. We have shown that PKCα physically interacts and functionally cooperates with TGFβRI to promote robust SMAD2-3 activation. Furthermore, PKCα-deficient (Prkca−/−) cells demonstrated a defect in SMAD-dependent IL-2 suppression, as well as decreased STAT3 DNA binding within the Il17a promoter. Consistently, Prkca−/− cells failed to mount appropriate IL-17A, but not IL-17F, responses in vitro and were resistant to induction of Th17-cell-dependent experimental autoimmune encephalomyelitis in vivo.
Graphical Abstract
Highlights
PKCα-deficient mice are resistant to EAE induction ► PKCα function is specific to the Th17 cell subset ► PKCα is a positive regulator of IL-17A transcription ► PKCα directly regulates TGFβRI-mediated phosphorylation of SMAD2-3
Introduction
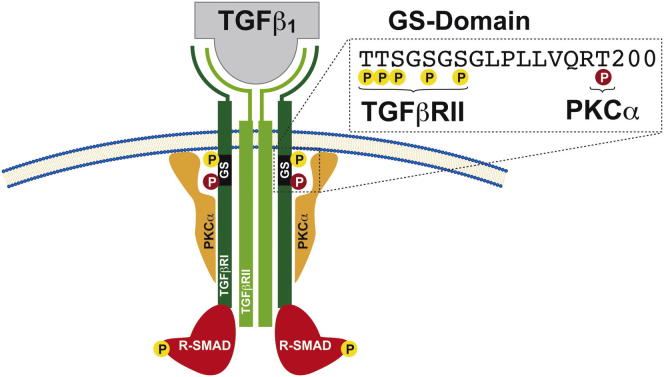
The discovery of interleukin-17 (IL-17)-producing T helper 17 (Th17) cells has markedly changed our view of Th cell differentiation and T-cell-mediated pathogenesis of autoimmune diseases. A major research effort has been focused on the identification of signaling mediators that regulate Th17 cell differentiation, yet how Th17 cell function is initiated and maintained is an area of active research. Deregulated transforming growth-factor β (TGFβ) receptor (TGFβR) signaling has been implicated in Th17 cell autoimmune pathogenesis (Bettelli et al., 2006; Mangan et al., 2006; Veldhoen et al., 2006a). Among other factors, TGFβ critically promotes Th17-cell-mediated IL-17A responses (Lee et al., 2009; Volpe et al., 2008; Wilson et al., 2010). A crucial role of TGFβ in experimental autoimmune encephalomyelitis (EAE) was highlighted by the finding that mice expressing dominant-negative TGFβRII confer resistance to EAE induction through a reduction in pathogenic Th17 cells (Veldhoen et al., 2006b). Accordingly, elevated IL-17A levels have been detected in brain lesions and cerebrospinal fluid of individuals suffering from multiple sclerosis (MS), a Th17-cell-dependent inflammatory CNS-demyelinating disease (Lock et al., 2002; Matusevicius et al., 1999), and of mice affected by EAE (Cua et al., 2003; Langrish et al., 2005). TGFβ initiates its cellular function by binding to TGFβRII, which then activates TGFβRI activity through phosphorylation of several residues in the core of the GS domain. TGFβRI then propagates the signal by inducing SMAD2-3 phosphorylation, which subsequently leads to nuclear influx, SMAD2-3 DNA binding, and the transcriptional activation of several target genes (Shi and Massagué, 2003). However, the complete molecular mechanisms involved in TGFβR signaling, as well as potential Th-cell-selective mechanisms, remain elusive. The protein kinase C (PKC) family comprises nine mammalian isotypes of serine-threonine protein kinases that play distinct roles in signal-transduction pathways. Besides the PKCθ isotype (Isakov and Altman, 2002), key functions for the Ca2+-phospholipid-dependent PKCα isotype during T cell activation have been reported (Gruber et al., 2009; Pfeifhofer et al., 2006). Further evidence for the relevance of PKCα has been provided by the identification of polymorphisms associated with greater risk of MS (Barton et al., 2004; Saarela et al., 2006). Despite this potential importance of PKCα in Th17-cell-mediated autoimmunity, the molecular aspects of PKCα function in Th17 cells and its physiological effector substrates have remained biochemically undefined. In the present study, we reveal a critical positive regulatory role of PKCα as a Th17-cell-selective intermediate of TGFβRI in directly regulating the kinase activity of TGFβRI, which itself activates SMAD2-3, maintains effective IL-17A responses, and thereby drives the pathogenesis of Th17-cell-mediated autoimmune diseases. This PKCα-TGFβRI kinase cooperation extends the paradigm of TGFβRI regulation in Th17 cell biology.
Results
PKCα Positively Regulates IL-17A-Specific Th17 Cell Effector Function
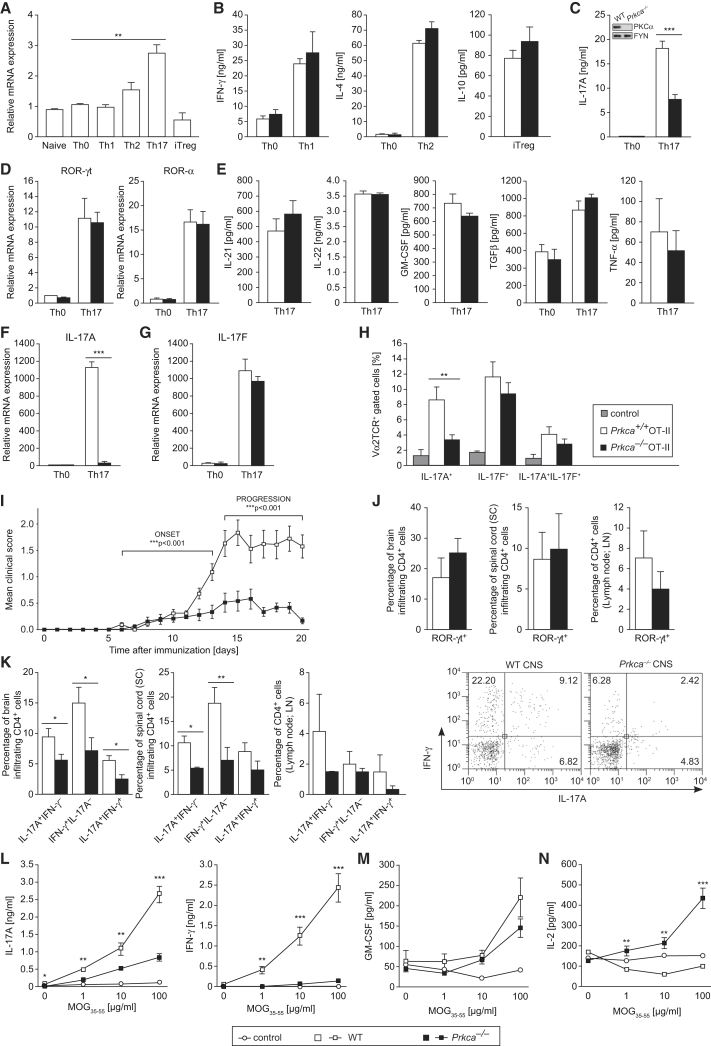
The examination of PKCα mRNA expression in different CD4+ Th cell subsets revealed that PKCα is substantially upregulated in in-vitro-differentiated Th17 cell cultures (Figure 1A). However, expression analysis of classical PKCβ, novel PKCθ, and PKCε, or atypical PKCι and PKCζ family members, revealed no Th17-cell-specific upregulation (Figure S1A, available online). Expression-pattern analysis of key cytokines or transcription factors of wild-type (WT) Th cell types confirmed specific and efficient differentiation into the distinct effector Th cell lineages (Figure S1B). The loss of PKCα preserved the effector responses of Th1-cell-mediated interferon-γ (IFN-γ), Th2-cell-mediated IL-4, and inducible regulatory T (iTreg)-cell-mediated IL-10 (Figure 1B). Strikingly, the secretion of IL-17A was notably reduced in PKCα-deficient (Prkca−/−) Th17 cells (Figure 1C). Of note, expression of the two main Th17-cell-linage-specific transcription factors, ROR-γt and ROR-α (Ivanov et al., 2006; Yang et al., 2008b) (Figure 1D), as well as the transcription factors RUNX1, AHR, and IRF4 (Figure S1C), which are related to Th17 cell development, was comparable between Prkca−/− and WT Th17 cells. The levels of IL-23R and IL-12Rβ2 mRNA (Figure S1D), the surface receptor expression of CCR6 (Figure S1E), and the secretion responses of IL-21, IL-22, granulocyte-macrophage colony-stimulating factor (GM-CSF), TGFβ, and tumor necrosis factor α (TNF-α) (Figure 1E), which are all connected to Th17 cell effector functions (Gutcher et al., 2011; Korn et al., 2009), were not altered between PKCα-proficient and PKCα-deficient Th17 cells. A critical mechanism of effector Th17 cell establishment represents the IL-6-triggered activation of STAT3 (Yang et al., 2007). However, immunoblot experiments showed no differences in (p)STAT3 levels between Prkca−/− and WT CD4+ T cells, stimulated with either IL-6 or TGFβ alone or in combination, suggesting that PKCα does not play a role in the modulation of membrane-proximal signaling events downstream of the IL-6 receptor. In addition, the mRNA of IL-6Rα was equally expressed between both genotypes (Figures S1F–S1G and data not shown). IL-17A and IL-17F, which are encoded within the same locus, are the most homologous IL-17 family members in that they have 50% identity in amino acid sequence (Hymowitz et al., 2001). However, in strict contrast to the barely detectable IL-17A mRNA expression (Figure 1F), IL-17F mRNA expression (Figure 1G) remained comparable between WT and Prkca−/− Th17 cells. To experimentally reconfirm this selective regulation of IL-17A, but not IL-17F, we cocultured naive CD4+ OT-II T cells together with OVA323-339-primed dendritic cells (DCs) under Th17 cell conditions. As a result, when compared to WT OT-II Th17 cells, Prkca−/− OT-II Th17 cells differentiated into a strongly reduced population of IL-17A+IL-17F− cells but an equal population of IL-17A−IL-17F+ cells (Figure 1H and Figure S1H). As a control, defective IL-17A production in Prkca−/− Th17 cells did not correlate with an increased conversion to Th1 or iTreg cells under Th17-cell-polarizing conditions in that they displayed no increase in T-BET or FOXP3, the signature transcription factors of Th1 and iTreg cells, respectively (Figure S2A). The results were attributable neither to survival defects nor to a hindered proliferation of Prkca−/− Th17 cells (Figures S2B and S2C and data not shown). Taken together, these results indicate that the absence of PKCα leads to a profound selective inhibition of Th17 cell effector function at the transcriptional level of IL-17A.
Figure 1.
PKCα Is a Positive Regulator of Th17 Cell Effector Functions In Vitro and In Vivo
Naive CD4+ T cells were cultured under indicated Th-cell-polarizing conditions for 3 days. Error bars represent ± SEM. Data in (A)–(G) were derived from at least three independent experiments. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (See also Figure S1).
(A) PKCα mRNA is highly expressed in Th17 cells.
(B–D) Production of IFN-γ (B, left), IL-4 (B, middle), IL-10 (B, right), and IL-17A (C) was determined. Immunoblotting (IB) (C, inset) indicates the efficient deletion of PKCα in CD4+ T cells. The mRNA expression of ROR-γt (D, left) and ROR-α (D, right) was analyzed by quantitative RT-PCR (qRT-PCR).
(E) Amounts of IL-21, IL-22, GM-CSF, TGFβ, and TNF-α were analyzed.
(F and G) The mRNA expression of IL-17A (F) and IL-17F (G) was analyzed by qRT-PCR.
(H) Prkca+/+ or Prkca−/− naive CD4+ OT-II T cells were stimulated with OVA323-339-pulsed DCs under Th17-cell-polarizing conditions. Intracellular IL-17A and IL-17F (Vα2TCR+ gated) were analyzed by flow cytometry. Data were derived from two independent experiments of three mice per group.
(I) Disease time course combining three independent experiments of EAE in WT (n = 22) or Prkca−/− (n = 19) mice.
(J and K) Mononuclear cells were isolated from the brain, spinal cord, and draining lymph nodes and were stained for intracellular ROR-γt (J) or IL-17A (K) and IFN-γ (CD4+ gated). Representative fluorescence-activated cell sorting (FACS) plots (CD4+-gated) of IL-17A- and IFN-γ-expressing CNS-infiltrating cells in WT and Prkca−/− mice are displayed (right panel). Data were derived from three mice per group per experiment from two independent experiments.
(L–N) Amounts of IL-17A (L, left), IFN-γ (L, right), GM-CSF (M), and IL-2 (N) in the supernatant of EAE-mice splenocytes restimulated with MOG35-55. Data were obtained from five separate mice per group.
PKCα Deficiency Protects against EAE Induced by Myelin Oligodendrocyte Glycoprotein35-55
These observations prompted us to analyze the potential role of PKCα in Th17-cell-based inflammatory immune pathogenesis in vivo. Thus, we determined the susceptibility of Prkca−/− mice to EAE. We immunized WT and Prkca−/− mice with myelin oligodendrocyte glycoprotein35-55 (MOG33-55) and monitored them for clinical signs of EAE. As expected, all WT mice developed EAE; in contrast, Prkca−/− mice displayed a slightly delayed onset, indicating that priming events might be altered. Moreover, the absence of PKCα almost completely inhibited EAE disease development (Figure 1I and Table 1). At the peak of clinical disease signs (day 14), infiltrating CD4+ cells from the brain, spinal cord, and draining lymph nodes were analyzed by flow cytometry. The absolute numbers of CD4+ mononuclear cells (Figure S2D) and the percentage of CD4+ROR-γt+ cells (Figure 1J) remained within a normal range between both genotypes. Although WT and Prkca−/− Th17 cells generated in vitro produce only marginal amounts of IFN-γ (Figures S2E and S2F), Th17 cells generated in vivo often coproduce IFN-γ during EAE (Abromson-Leeman et al., 2009; Hirota et al., 2011; Ivanov et al., 2006). However, IL-17A+ and IFN-γ+ CD4+ CNS-infiltrating cells, as well as IL-17A+IFN-γ+ CD4+ CNS-infiltrating cells, were significantly reduced in Prkca−/− mice compared to WT mice (Figure 1K and right panels). Consistently, ex vivo recall-response analysis at the priming phase of the disease (day 10 after MOG35-55 immunization) revealed strongly decreased values of both IL-17A and IFN-γ (Figure 1L). GM-CSF is also known to play a role during EAE (McQualter et al., 2001), yet its levels remained unaffected in EAE-recall assays (Figure 1M). Strikingly, severely increased levels of IL-2 in MOG35-55-antigen-restimulated Prkca−/− splenocytes were detected (Figure 1N). Collectively, these data demonstrate that PKCα is essential for the priming and effector phases of EAE.
Table 1.
Clinical Parameters of MOG35-55-Induced EAE
| Genotype | Incidence (Score ≥ 0.5) | Onset Day (Mean ± SEM) | Maximum Score (Mean ± SEM) |
|---|---|---|---|
| WT | 100.00% (22/22) | 10.89 ± 0.98 | 2.03 ± 0.22 |
| Prkca−/− | 78.95% (15/19) | 12.00 ± 1.39 | 0.80 ± 0.17∗∗∗ |
The results are shown as the mean ± SEM and indicate the total number of individual mice in three independent experiments. The following abbreviation is used: WT, wild-type. ∗∗∗p < 0.001.
It has been shown that CD4+CD25+FOXP3+ Treg cells contribute to EAE disease amelioration by suppressing autoreactive effector Th cell expansion (O’Connor and Anderton, 2008). By observing reduced FOXP3+ cell infiltrates in Prkca−/− mice (Figure S2G), we excluded the involvement of FOXP3+ Treg cells in the EAE protection of Prkca−/− mice during acute disease. Consistently, and again in contrast to analysis of Th17 cells, phenotypical analysis of Prkca−/− iTreg cells revealed a dispensable role of PKCα in iTreg cell effector responses, as reflected by FOXP3 mRNA expression (Figure S2H), the suppressive capabilities in dampening Th cell effector responses, and the unaltered TGFβR-SMAD2-3 activation responses (data not shown). Furthermore, we could demonstrate that under all investigated doses of TGFβ during iTreg cell differentiation, the fraction of CD4+CD25+FOXP3+ cells was comparable between the genotypes (Figure S2I). Of note, no developmental defect was detected in the generation of CD4+CD25+FOXP3+ natural regulatory T (nTreg) cells in the absence of PKCα (Figure S2J). Collectively, these results provide evidence that PKCα is not required to effectively induce iTreg cell functions and reveal an unexpected, subset-selective role for PKCα in Th17 cells.
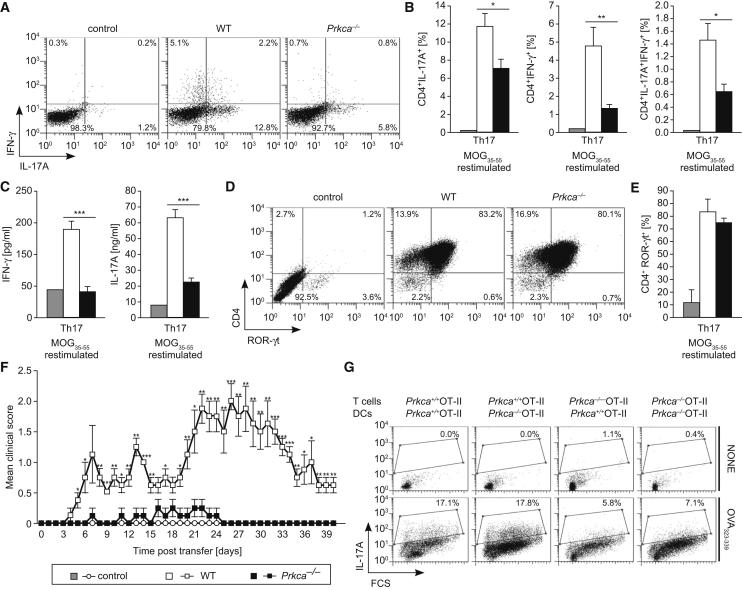
To evaluate a potential CD4+ T-cell-intrinsic function of PKCα in vivo, we transferred ex vivo MOG35-55-antigen-restimulated, CD4+-enriched, Th17-differentiated cells from either MOG35-55-immunized WT or Prkca−/− mice into syngeneic WT recipient mice. Prior to the adoptive cell transfer, the extent of MOG35-55-specific Th17 cell differentiation was validated. A severely impaired antigen-specific immune response, featured by diminished frequencies of IL-17A+IFN-γ−, IL-17A−IFN-γ+, and IL-17A+IFN-γ+ CD4+ T cells, was demonstrated in the Prkca−/− cells (Figures 2A and 2B). In addition, analysis of culture supernatants demonstrated considerably reduced IL-17A and IFN-γ levels in Prkca−/− cells (Figure 2C). However, the total population of CD4+ROR-γt+ cells prior to adoptive cell transfer was comparable between genotypes (Figures 2D and 2E), excluding an artifact due to lower CD4+ Th17 cell frequencies between genotypes in the inoculum. The development of EAE disease severity in mice that received Prkca−/− CD4+ Th17 cells was markedly reduced, and the onset of clinical EAE signs was significantly delayed in these mice compared to those animals receiving WT cells (Figure 2F and Table 2). These observations indicate that MOG35-55-specific CD4+ Th17 cells from Prkca−/− mice are unable to efficiently induce EAE in recipient mice. DCs are critical accessory cells during EAE and produce inflammatory cytokines, which are required for Th17 cell development in vivo (Korn et al., 2009; Veldhoen et al., 2006a). In order to exclude the possibility that impaired Th17 cell development in Prkca−/− mice is caused by a defect in DCs, we evaluated the functionality of Prkca−/− DCs. The critical DC markers CD86 and CD40 were appropriately expressed on the surface of Prkca−/− DCs (Figure S3A). Additionally, lipopolysaccharide (LPS)-stimulated Prkca−/− DCs did not show impaired IL-6, IFN-γ, or TNF-α responses (Figure S3B). Moreover, a coculture of naive WT or Prkca−/− CD4+ OT-II T cells together with OVA323-339-pulsed WT or Prkca−/− DCs indicated that Prkca−/− DCs were capable of supporting Th17 cell development normally. However, the percentage of the CD4+IL-17A+ T cell population of Prkca−/− mice was strongly reduced regardless of the origin of the cocultured DCs (Figure 2G). Although we cannot exclude the existence of a Th17-cell-extrinsic role of PKCα, these experiments validate PKCα as a critical player in Th17-cell-driven neuroinflammatory autoimmune disease.
Figure 2.
PKCα-Deficient Mice Are Resistant to EAE in a Passive Adoptive-Transfer Model
For adoptive EAE, draining lymph-node cells and splenocytes from MOG35-55-immunized WT, Prkca−/−, or PBS-treated WT (control) mice were restimulated with MOG35-55 in the presence of Th17-cell-polarizing cytokines for 3 days. Data in (A)–(E) were derived from five mice per group. (See also Figure S2). Error bars in (B), (C), (E), and (F) represent ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(A and B) Representative FACS dot plots of IL-17A+ and IFN-γ+ cells (CD4+ gated) are displayed in (A), and the quantification is shown in (B).
(C) IFN-γ (left) and IL-17A (right) levels were measured.
(D and E) Representative FACS dot plots of ROR-γt+ cells (CD4+ gated) are displayed in (D), and the quantification is shown in (E).
(F) Disease time course of adoptive EAE in WT recipients, reconstituted with Th17-cell-polarized WT (n = 4), Prkca−/− (n = 4), or control CD4+-enriched (n = 2) cells. Of note, control mice injected with cells from PBS-injected WT mice and stimulated in vitro for 3 days with MOG33-35 under Th17-cell-polarizing conditions did not show any disease signs.
(G) Naive CD4+ OT-II T cells were stimulated with OVA323-339-pulsed DCs under Th17-cell-polarizing conditions. Cells were stained for Vα2TCR and intracellular IL-17A and analyzed by flow cytometry. Representative FACS plots of two independent experiments of three mice per group are shown.
Table 2.
Transfer of MOG35-55-Specific Th17 Cells into WT 129sv Recipients
| Genotype | Incidence (Score ≥ 0.5) | Onset Day (Mean ± SEM) | Maximum Score (Mean ± SEM) |
|---|---|---|---|
| Control | 0% (0/2) | NA | NA |
| WT | 100% (4/4) | 5.00 ± 0.41 | 2.25 ± 0.25 |
| Prkca−/− | 100% (4/4) | 14.00 ± 3.24∗ | 0.50 ± 0.00∗∗∗ |
The results are shown as the mean ± SEM and indicate the total number of individual mice. The following abbreviations are used: WT, wild-type; and NA, not applicable. ∗p < 0.05; ∗∗∗p < 0.001.
PKCα Is an Essential Regulator of the TGFβR-SMAD-Signaling Pathway
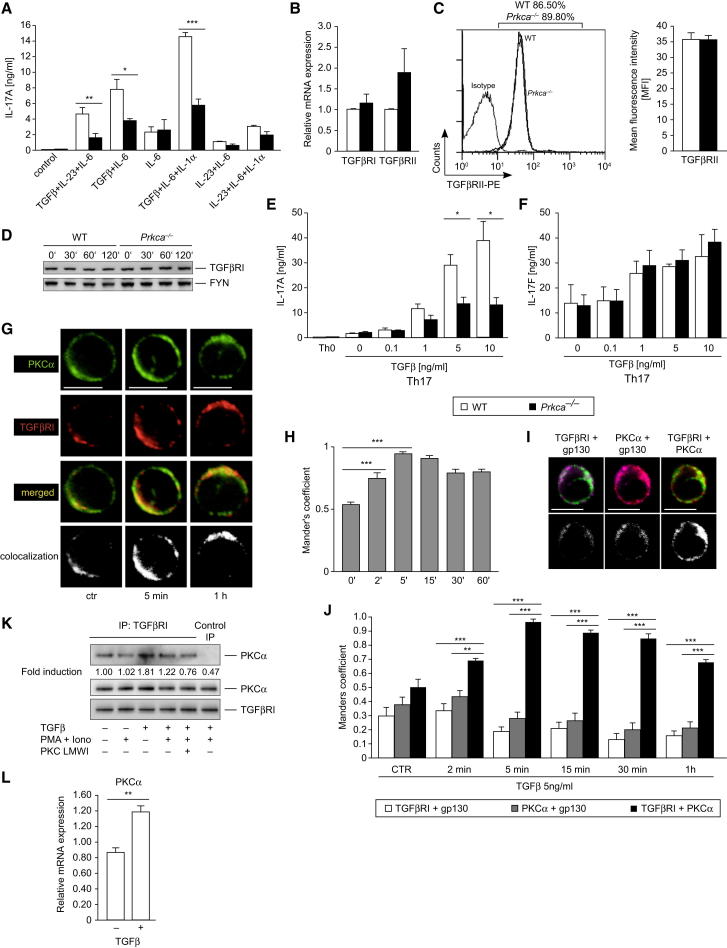
To elucidate the mechanistic basis of the decreased IL-17A expression found in Prkca−/− Th17 cells, we cultured WT and PKCα-deficient naive CD4+ T cells under distinct Th17 cell cytokine milieus. The stimulations that were performed in the absence of TGFβ did not reveal gross differences in IL-17A expression. However, when TGFβ was applied in combination with IL-6, IL-23, or IL-1α, Prkca−/− Th17 cells, compared to WT Th17 cells, exhibited remarkably reduced IL-17A secretion responses (Figure 3A), indicating defective TGFβR signaling. This finding was secondary to neither downregulated expression of TGFβRI and TGFβRII mRNA nor reduced TGFβRI and TGFβRII protein expression (Figures 3B–3D). Remarkably, the observed reduced TGFβR-mediated IL-17A-secretion response in Prkca−/− Th17 cells was especially prominent at high TGFβ concentrations (Figure 3E). However, IL-17F remained unaffected under these conditions (Figure 3F). Strikingly, confocal-microscopy analysis of endogenously stained PKCα and TGFβRI identified a constitutive and TGFβ-inducible colocalization of these two proteins in primary human CD4+ peripheral-blood mononuclear cells (PBMCs) (Figures 3G and 3H). This PKCα-TGFβRI-colocalization complex peaked at 5 minutes and remained elevated for at least 1 hour of TGFβ stimulation. As a control, neither PKCα nor TGFβRI colocalized with the transmembrane IL-6R subunit gp130 (Figures 3I and 3J). Furthermore, a constitutive and TGFβ-inducible physical interaction between the endogenous PKCα and TGFβRI proteins in primary T cells was confirmed by coimmunoprecipitation (Figure 3K). Interestingly, PKCα mRNA expression in CD4+ T cells was inducible by TGFβ (Figure 3L). However, mRNA expression of PKCθ under similar conditions remained unaffected (data not shown). Several groups showed that TGFβ directly upregulates PKCα protein (Chen et al., 2010; Zhou et al., 2010) and mRNA levels (Gao et al., 2003; Ranganathan et al., 2007). To investigate the PKCα domains that are capable of physically interacting with TGFβRI, we cotransfected the HIS6-tagged WT PKCα, the constitutively active PKCα mutant, or the catalytic subdomain of PKCα with a FLAG-tagged WT TGFβRI expression vector in human embryonic kidney (HEK)293T cells. Here, pulldown assays showed that the catalytic fragment of PKCα is sufficient to bind TGFβRI, suggesting a kinase-substrate relationship (Figure S4A).
Figure 3.
Physical Interaction between PKCα and TGFβRI
(A) IL-17A levels were assessed of cells differentiated with the indicated Th17-cell-favoring cytokines.
(B) The mRNA transcript levels of TGFβRI and TGFβRII of naive CD4+ T cells were determined with qRT-PCR. The results are presented relative to WT levels.
(C) Flow-cytometry analysis of TGFβRII surface expression of naive CD4+ T cells. A representative FACS histogram (CD4+ gated) and quantification of the mean fluorescence intensity (MFI) per cell are shown.
(D) The expression level of TGFβRI in CD4+ T cells, stimulated with TGFβ as indicated, was detected by IB. One representative blot from at least four independent experiments that yielded similar results is shown.
(E and F) Levels of IL-17A (E) and IL-17F (F) were assessed of cells differentiated with the indicated TGFβ concentrations after 3 days of Th17 cell differentiation.
(G and H) Representative confocal immunofluorescence (G) and quantification (H) of the colocalization of PKCα and TGFβRI in untreated (ctr) or TGFβ-treated human CD4+ PBMCs.
(I and J) Representative single confocal section overlays of cells stimulated with TGFβ (2 min) (I). Quantitative colocalization analysis is shown in (J).
(G and I) Colors are as follows: green, PKCα; red, TGFβRI; yellow, merged; and purple, gp130. The scale bars represent 5 μm. All pixels colocalized are represented as white spots.
(K) Endogenous binding between PKCα and TGFβRI in CD3+ T cells. TGFβRI antibody or normal IgG antibody (control IP) was used for the immunoprecipitation and analyzed by IB with a PKCα antibody.
(L) Naive WT CD4+ T cells were stimulated with TGFβ and analyzed for expression of PKCα.
Data in (A)–(C), (E), and (F) were derived from two independent experiments of three to four mice per group. Data in (D), (L), and (K) were derived from at least three independent experiments. Error bars in (A)–(C), (E), (F), (H), (J), and (L) represent ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (See also Figure S3).
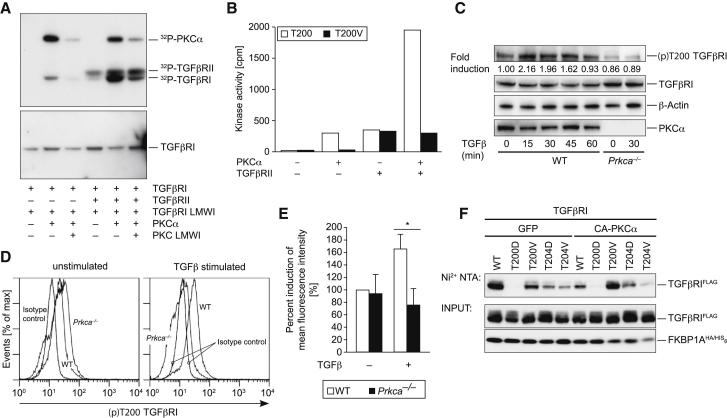
TGFβRI Is a Direct Substrate of PKCα
By using in vitro kinase assays, we showed that PKCα is able to induce TGFβRI phosphorylation. Here, inclusion of TGFβRII, together with PKCα, led to a synergistic phosphorylation of TGFβRI. In addition, TGFβRI phosphorylation was reduced by a PKCα-specific inhibitor (Figure 4A). Predicting PKC-mediated phosphorylation sites led to the identification of T200 on TGFβRI as a bona fide candidate PKC-mediated phosphorylation site. The experimental investigation indicated that the T200WT TGFβRI peptide, but not the T200V mutant motif, was phosphorylated by PKCα (Figure 4B). TGFβRII additively acts with PKCα to induce phosphorylation of T200WT, but not the T200V mutant, TGFβRI motif. Next, we evaluated the presence of this phosphosite on endogenous TGFβRI by using a (p)T200-site-specific antiserum that was raised for this investigation (Figure S4B). Both immunoblot (Figure 4C) and intracellular flow cytometry showed that phosphorylation of T200 on TGFβRI was induced by TGFβ in WT, but not Prkca−/−, CD4+ T cells (Figures 4D and 4E). Although we cannot exclude the existence of additional PKCα-mediated phosphorylation sites, the above results from the use of the (p)T200 TGFβRI antibody suggest that T200 on TGFβRI is a PKCα-mediated phosphorylation site in intact T cells. Notably, the T200 site is in close proximity to the established binding site of the immunophilin FKBP1A on TGFβRI. During steady state, FKBP1A bound to TGFβRI counteracts ligand-independent unspecific phosphorylation of TGFβRI by TGFβRII. However, TGFβ-ligand-induced receptor activation causes the release of FKBP1A from TGFβRI (Chen et al., 1997; Shi and Massagué, 2003). Cotransfection with constitutively active PKCα significantly diminished binding of FKBP1A to the WT TGFβRI (Figure 4F; lanes 1 and 6), which was rescued when the phosphorylation-defective T200V TGFβRI mutant was cotransfected (Figure 4F; lanes 6 and 8). Moreover, the phosphomimic T200D TGFβRI mutant completely failed to bind FKBP1A (Figure 4F; lanes 2 and 7). As a control, T204 alterations in TGFβRI did not show this effect. These data suggest that phosphorylation of T200 on TGFβRI by PKCα might lead to an induced displacement of FKBP1A from the TGFβRI. Collectively, these data suggest a physical and functional TGFβRI-PKCα interaction that might regulate efficient TGFβRI kinase-activation responses.
Figure 4.
PKCα Phosphorylates T200 on TGFβRI
(A) Kinase assays of the glutathione S-transferase (GST) fusion protein of the cytoplasmic subdomain of TGFβRI (amino acids 148–503) were incubated with recombinant PKCα and TGFβRII as indicated. Phosphorylation was detected by autoradiography. The GST-antibody immunoblot (lower panel) confirmed equal loading.
(B) The phosphorylation rates of T200WT and T200V mutant motifs were measured by the incorporation of 32Pi from γ32P-ATP incubated with PKCα and/or TGFβRII kinases. The following abbreviation is used: cpm, counts per minute.
(C) Naive CD4+ T cells were stimulated with TGFβ as indicated. TGFβ-inducible phosphorylation of T200 on TGFβRI was observed in WT cells, but not Prkca−/− cells, as shown by a representative IB.
(D and E) The intracellular induction by (p)T200 on TGFβRI with the (p)T200-specific antibody and the quantification of the percent induction of MFI are shown. In (E), the error bars represent ± SEM. ∗p < 0.05.
(F) Cotransfection of Jurkat T cells with constitutively active A25E PKCα significantly diminished binding of FKBP1A to the WT TGFβRI, but not the neutral-exchange T200V mutant of TGFβRI. Representative data were derived from at least two independent experiments.
(See also Figure S4).
PKCα Does Not Play a Role in Noncanonical TGFβ-Mediated Signaling Pathways
TGFβ is known to also signal via noncanonical pathways (Moustakas and Heldin, 2005). However, to this point, we have been unable to detect a substantial TGFβ-induced activation of the noncanonical TGFβ-signaling pathways p38 mitogen-activated protein (MAP) kinase, stress-activated protein kinase (SAPK)/Jun amino-terminal kinase (JNK), extracellular-signal-regulated kinases 1 and 2 (ERK1/2), or protein kinase B (PKB or AKT) in primary T cells (Figures S5A–S5H), consistent with observations of other groups (Cejas et al., 2010; Chang et al., 2011). Nevertheless, we observed no difference in the activation levels between WT and Prkca−/− cells. Furthermore, SMAD-independent TGFβ-mediated downregulation of eomesodermin (Ichiyama et al., 2011) remains unaffected in the absence of PKCα (Figure S5I).
PKCα Is a Critical Signaling Intermediate of the SMAD-Dependent TGFβRI-Signaling Pathway
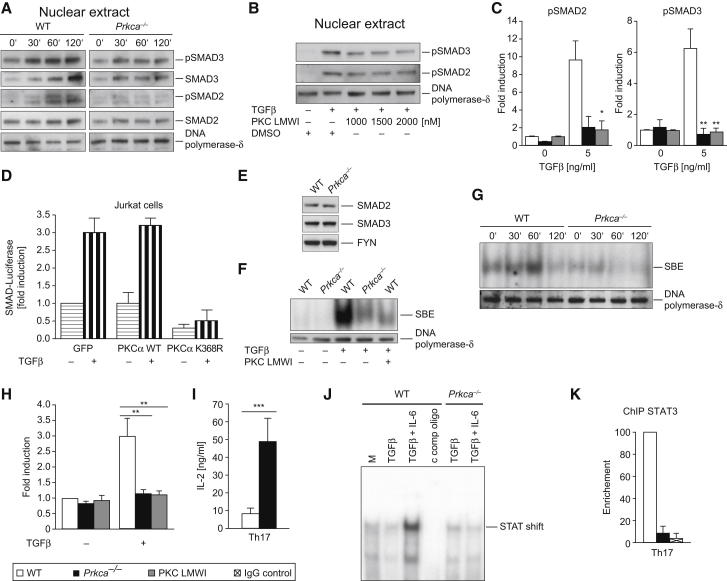
The investigation of the canonical, SMAD-dependent TGFβRI-signaling pathway led to the striking observation that the phosphorylation of both SMAD2 and SMAD3 is impaired in Prkca−/− CD4+ T cells (Figures 5A and 5C). In addition, the treatment of WT CD4+ T cells with a PKCα-specific inhibitor led to a complete phenocopy of Prkca−/− cells, indicating that the PKCα catalytic activity is required to activate the canonical TGFβRI-SMAD2-3 pathway (Figures 5B and 5C). Similarly, transfection of Jurkat T cells with a kinase-dead PKCα mutant construct repressed the induction of a SMAD-dependent promoter luciferase reporter (Figure 5D). At the same time, both WT and Prkca−/− naive CD4+ T cells expressed similar SMAD2, SMAD3, and SMAD4 levels (Figure 5E and data not shown). By using band-shift assays, we identified a strong defect in SMAD2-3 DNA-binding activity in both genetically deleted and pharmacologically PKCα-inhibited cells (Figures 5F–5H). Taken together, our data suggest that PKCα acts as a positive regulator of SMAD2-3-mediated TGFβ signaling in T cells and that its deletion results in reduced sensitivity to the biologic effects of TGFβ. Interestingly, the SBE has been located upstream of the Il2 promoter, which is important for SMAD-mediated transcriptional suppression of IL-2 (Tzachanis et al., 2001). Moreover, it was demonstrated that TGFβ suppresses IL-2 production of T cells (Brabletz et al., 1993) in a SMAD3-dependent manner (McKarns et al., 2004). In order to elucidate the connection between the impaired TGFβ-mediated SMAD-signaling cascade and the severely reduced IL-17A Th17 cell effector responses in the absence of PKCα, we investigated IL-2 amounts. Interestingly, we observed substantially elevated IL-2 amounts in Th17 cell cultures (Figure 5I), as well as in MOG35-55 EAE-recall samples (Figure 1N) in the absence of PKCα. A recent study (Yang et al., 2011) supports a model in which the balance of IL-6-induced STAT3- and IL-2-triggered STAT5-DNA-binding capabilities directly dictates the outcome of IL-17A production. Indeed, by using band-shift assays, we observed a severely impaired STAT binding to the minimal Il17a promoter region in Prkca−/− CD4+ T cells, stimulated under Th17-cell-polarizing conditions (TGFβ + IL-6) (Figure 5J). Furthermore, ChIP analysis revealed a notably reduced STAT3 DNA binding to the same Il17a promoter region in WT Th17 cells treated with a PKCα-specific inhibitor (data not shown), as well as in Prkca−/− Th17 cells (Figure 5K).
Figure 5.
The Activation of SMAD2 and SMAD3 by TGFβRI Critically Depends on PKCα
(A and B) IB shows that TGFβ stimulation leads to SMAD2 and SMAD3 phosphorylation in WT cells yet is hindered in both Prkca−/− cells (A) and cells preincubated with PKC LMWI (low-molecular-weight inhibitor) (B). CD4+ T cells were treated with TGFβ as indicated, and nuclear extracts (NEs) were generated.
(C) Graphs represent the fold induction of normalized nuclear levels of selected TGFβ concentrations (0 and 5 ng/ml) of (p)SMAD2 (left) and (p)SMAD3 (right).
(D) Transfection of the catalytically inactive kinase-dead K368R PKCα mutant (but not the WT) expression vector in Jurkat T cells repressed the expression of a SMAD2-3-dependent promoter luciferase reporter.
(E) IB analysis confirmed equal total-protein expression levels of SMAD2 and SMAD3 between WT and Prkca−/− CD4+ T cell lysates.
(F and G) Reduced SMAD2-3 DNA binding to the SMAD-binding element (SBE) in both Prkca−/− cells and PKC-LMWI-preincubated cells, as determined by electromobility shift assays (EMSA). CD4+ T cells were treated with TGFβ as indicated, and NEs were generated.
(H) The graph represents the fold induction of normalized SMAD2-3-DNA-binding efficiency.
(I) IL-2 levels were analyzed in Th17-cell-differentiated WT and Prkca−/− cells. Data are from four independent experiments.
(J) EMSA analysis of the binding capability of an Il17a minimal promoter oligonucleotide, containing a consensus STAT binding site and incubated with WT or Prkca−/− NEs of cells that were stimulated as indicated (2 hr). Resting cells were used as unspecific controls.
(K) Chromatin immunoprecipitation (ChIP) analysis (for 16 hr) of the binding capability of STAT3 to the minimal Il17a promoter in Th17-cell (TGFβ + IL-6)-primed, WT, or Prkca−/− CD4+ T cells. Data are from two independent experiments.
Representative blots are from two (J) or at least three (A, B, and E–G) independent experiments that yielded similar results. In (C) and (H), the results are presented relative to unstimulated WT levels and are normalized to the expression levels of DNA polymerase-δ, and data were pooled from at least three independent experiments. Error bars in (C), (D), (H), (I), and (K) represent the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (See also Figure S5).
Discussion
TGFβR signaling plays an essential role in the generation of iTreg and Th17 cells, yet little is known about whether TGFβR-triggered pathways might make use of linage-specific activators of TGFβR signaling. Here, we report that CD4+ T cells lacking PKCα function exhibit a specific Th17 cell defect in vitro and during neuroinflammatory disease in vivo. Our data of the hyposusceptibility of Prkca−/− mice to EAE induction and the substantial reduction of IL-17A+ and IFN-γ+ CNS cell infiltrates demonstrate that PKCα is critical for the control of IL-17A transcription. Supporting our findings of IL-17A+IFN-γ+ cells in Prkca−/− mice during EAE, there exists sophisticated evidence that Th17 cells have considerable plasticity toward a Th1 cell phenotype in EAE by producing both IL-17A and IFN-γ (Ivanov et al., 2006; Zhou et al., 2009). With the use of mouse systems tracking reporter Th cell fate, it has been shown that IL-17A-producing cells are reprogrammed to produce both IL-17A and IFN-γ or even IFN-γ alone during EAE (Hirota et al., 2011; Kurschus et al., 2010). Thus, we speculate that this robust IFN-γ-production defect observed in Prkca−/− Th17 cells during EAE disease progression is secondary to the IL-17A defect.
Mice deficient in IL-17A exhibit delayed onset, reduced scores of maximum severity, ameliorated histological changes, and early recovery of EAE (Ishigame et al., 2009; Komiyama et al., 2006; Nakae et al., 2003; Yang et al., 2008a). Moreover, it has been shown that in vivo neutralization of IL-17A significantly reduces the severity of EAE (Hofstetter et al., 2005; Komiyama et al., 2006; Uyttenhove and Van Snick, 2006). However, there exist some contradictory data on the relative importance of IL-17A during EAE (El-Behi et al., 2011; Haak et al., 2009), but the consensus view is that IL-17A clearly plays a pathogenic role in EAE and MS. Nevertheless, we cannot exclude additional PKCα-mediated effects (next to IL-17A) for the apparent EAE benefit in Prkca−/− mice.
We observed that the decreased IL-17A levels do not correlate with a decreased ROR-γt expression in Prkca−/− Th17 cells. Consistently, changes in IL-17A expression can be independent of ROR-γt expression, as observed in other studies (Acosta-Rodriguez et al., 2007; Kaminski et al., 2011; Tzartos et al., 2008).
Interestingly, we observed a reduced population of FOXP3+ cells in Prkca−/− CNS tissues during acute EAE. This counterintuitive finding might merely reflect a reduced recruitment of FOXP3+ iTreg cells as a result of the curtailed inflammatory stimulus in Prkca−/− mice. Interestingly, several reports (Chen et al., 2011; Veldhoen et al., 2006b) underpinned a potential pathologic function of iTreg cells by showing that FOXP3+ iTreg cells empowered rather than inhibited Th17 cell differentiation during chronic inflammatory conditions in vivo. Thus, we could speculate that the reduced presence of FOXP3+ cells in CNS tissues of Prkca−/− mice during EAE even represents a beneficial, though until this point undefined, mechanism. Our results support the notion that PKCα is not required to effectively induce iTreg cell functions and reveal an unexpectedly selective role for PKCα in TGFβR signaling in Th17 cells.
Mechanistically, we have shown that PKCα deficiency renders T cells less sensitive to TGFβ-induced SMAD2-3 activation. Of note, the cellular role of SMAD2-3 signaling in Th17 and iTreg cells is not without controversy in the literature given that two studies showed that SMAD2 is especially important for the optimal induction of Th17 cells (Malhotra et al., 2010; Takimoto et al., 2010), whereas another report (Martinez et al., 2009) indicated that SMAD3-activation defects increase Th17 cell effector functions. However, in agreement with our investigations, a recent study demonstrated that the enhanced generation of Th17 cells is associated with increased TGFβ-induced SMAD2-3 activation (Cejas et al., 2010).
Th17 cell commitment represents a dynamic balance between the DNA-binding of STAT3 and STAT5 to sites along the single Il17a–Il17f locus. Furthermore, STAT5 DNA binding is associated with displacement of STAT3 within the Il17a promoter region (Yang et al., 2011). Here, we provide strong experimental data that the absence of PKCα abrogates STAT3 DNA accessibility to the minimal Il17a promoter region in Th17 cells. Thus, it is tempting to speculate that in Prkca−/− Th17 cells, IL-2 hyperproduction, which is secondary to the defects in SMAD2-3 activation, might lead to increased efficiency of STAT5 DNA binding, thereby displace STAT3 from this locus, and ultimately result in the observed inhibition of IL-17A transcription. Importantly, another study (Cejas et al., 2010) has also suggested that a main function of TGFβ in early Th17 cell differentiation might be the inhibition of IL-2-mediated suppression of Th17 cell generation.
Given the selective IL-17A expression defect, which does not affect IL-17F expression, in Prkca−/− Th17 cells, it is important to mention that the Il17a promoter is established to be primarily responsive to STAT3, whereas the Il17f promoter is largely responsive to ROR-γt (Thomas et al., 2012). Accordingly, it has been demonstrated that at high IL-6 concentrations, IL-17F, in strict contrast to IL-17A, is insensitive to IL-2-mediated inhibition (Yang et al., 2011). Given the present data, we can hypothesize that this selective PKCα-dependent Th17 cell defect during TGFβR signaling is due to constrained STAT3 binding to the Il17a promoter.
In this study, direct regulation of the canonical TGFβRI-SMAD pathway by PKCα appeared to be mediated at the level of T200 phosphorylation on TGFβRI. Intriguingly, the T200V alteration has been identified to severely inhibit TGFβRI kinase activity and consequently TGFβ-dependent activation responses (Wieser et al., 1995). However, the protein kinase that is responsible for the physiological phosphorylation of this candidate phosphosite has not been defined to date. Given the obtained results, one possible function of PKCα might be to modulate FKBP1A binding on TGFβRI, and the T200 phosphoswitch, located within the critical interaction surfaces of these two binding partners, might alter local electrostatic potential to perturb the intermolecular interaction with FKBP1A, required for effective negative TGFβRI kinase regulation.
Collectively, this study extends the paradigm underlying TGFβRI activation in the efficient generation of Th17 cell immune responses. Consistent with human genetic data that link polymorphisms of PRKCA (encoding PKCα) to the Th17-cell-based pathogenesis of MS (Barton et al., 2004; Saarela et al., 2006), our findings reveal an essential immunomodulatory function of PKCα. Intriguingly, because PKC inhibitors are in clinical trials (Baier and Wagner, 2009), our findings could provide a rational mechanistic basis for the treatment of certain Th17-cell-mediated immune pathologies.
Experimental Procedures
Mice
The generation of the Prkca−/− mice was described previously (Pfeifhofer et al., 2006). All animal studies complied with the current laws and were approved by the authors’ respective institutional review boards.
Th Cell Differentiation
Naive CD4+ T cells were isolated with a CD4+CD62Lhi T cell isolation kit II (Miltenyi Biotec), and the Th-cell-subset differentiation (3 days) was performed in RPMI or IMDM (for Th17 cell differentiation) as previously described (Hermann-Kleiter et al., 2008). In brief, the Th-cell-neutral (Th0) conditions contained neither exogenous cytokines nor blocking antibodies. The other conditions were as follows: Th1 = mIL-12 (10 ng/ml) and αIL-4 (5 μg/ml); Th2 = IL-4 (10 ng/ml), αIL-12 (5 μg/ml), and IFN-γ (5 μg/ml); Th17 = TGFβ (5 ng/ml), IL-6 (40 ng/ml), IL-1α (20 ng/ml), αIL-4 (2 μg/ml), and αIFN-γ (2 μg/ml); and iTreg = TGFβ (10 ng/ml), IL-2 (10 ng/ml), αIFN-γ (5 μg/ml), αIL-12 (5 μg/ml), and αIL-4 (5 μg/ml). Naive CD4+ OT-II T cells were stimulated with 2.5 × 105 LPS (100 ng/ml)-activated splenic CD11c+ DCs (DC:T cell = 1:4) and pulsed with 1 μM OVA-peptide323-339 (Genscript) in the presence of Th17-cell-polarizing cytokines for 3 days.
Analysis of Cytokine Production
The cytokine amount in culture supernatants was determined with BioPlex multianalyte technology (Biorad) on day 3 of Th cell differentiation according to the manufacturer’s instructions. For IL-4 and TGFβ analysis in Th2 and Th17 cell cultures, respectively, cells were washed after 3 days of differentiation and restimulated with 2 μg/ml plate-bound CD3 antibody for 24 hr in serum-free X-vivo 20 medium. The supernatant was used for cytokine analysis.
EAE Assay and Preparation of CNS Mononuclear Cells
The EAE assay and the preparation of CNS mononuclear cells have been previously described (Hermann-Kleiter et al., 2008).
Passive Adoptive EAE
The general procedure was adapted, with minor modifications, to reports in Axtell et al., 2010, Jäger et al., 2009, and Komiyama et al., 2006. Splenocyte suspensions were generated from MOG35-55-immunized (day 10) WT, Prkca−/−, or WT PBS-treated control mice. Splenocytes were restimulated with 25 μg/ml MOG35-55 under Th17-cell-polarizing conditions for 3 days. CD4+ T cells were purified with a MACS (Miltenyi Biotec) kit, and 1.5 × 107 cells were transferred into healthy WT recipient mice (intraperitoneally [i.p.]). In addition, pertussis toxin (Sigma; 200 ng/mouse) was administered i.p. on the day of the adoptive transfer and 48 hr later. Signs of EAE were assigned scores on a scale of 0–4 (Hermann-Kleiter et al., 2008).
Coimmunoprecipitation Analysis
The coimmunoprecipitation analysis was described previously (Gruber et al., 2009). In brief, a total of 5 × 107 murine CD3+ T cells were lysed, precleared, and incubated with 2 μg of PKCα antibody (Millipore) overnight. Unspecific IgG Ab was used as a negative control. Thereafter, samples were incubated with protein G sepharose (Amersham-Pharmacia), washed in lysis buffer, and resolved by SDS-PAGE.
Gel Shift Assay
NEs were harvested from 1 × 107 to 2 × 107 CD4+ T cells according to standard protocols. In brief, CD4+ T cells were isolated with the CD4+ T cell Isolation Kit (Miltenyi Biotec) and rested for 1.5 hr in X-vivo 20 (37°C, 5% CO2); this was followed by various stimulation conditions (as indicated). Cells were resuspended in 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol (DTT), and protease inhibitors. Cells were incubated on ice for 15 min. NP-40 was then added to a final concentration of 0.6%, the cells were vigorously mixed, and the mixture was centrifuged for 5 min at 2,300 rpm × g. The nuclear pellets were resuspended in 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and protease inhibitors, and the tubes ware rocked for 30 min at 4°C. After centrifugation at 13,400 rpm × g, the supernatants were collected and stored at −80°C for further analysis. Nuclear protein extracts (2–7 μg) were incubated in binding buffer with the end-labeled, double-stranded oligonucleotide SBE probe (SBE consensus oligonucleotide [sc-2603]: 5′-AGTATGTCTAGACTGA-3′) as described previously (Kaminski et al., 2011). The following WT and mutated oligonucleotides were used, and the core binding motifs for STAT are underlined for the minimal Il17a promoter: 5′-TCTGTTCAGCTCCCAAGAAGTCATGCTTCTTTGCATAGTGAACTTCTGCC-3′ (Stat) and 5′-TCTGTACAGCTCCCAAGCAGTCATGCTACTTTGCATAGTGTACTGCTGCC-3′ (Stat mu). The band shifts were resolved on a 5% polyacrylamide gel.
RNA Transcript Analysis by qRT-PCR
The gene-expression analysis has been previously described (Kaminski et al., 2011). For qRT-PCR analysis, cells were restimulated with plate-bound CD3 antibody (2 μg/ml) for 4 hr. Data were normalized to GAPDH mRNA and are presented relative to Th0 levels.
Statistical Analysis
The p values were calculated with an unpaired Student’s t test. Significant differences are indicated as ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Acknowledgments
We are grateful to N. Haas and N. Krumböck for providing animal care and technical assistance. This work was supported by grants from the Austrian Science Fund (MCBO-DK, SFB-021, T264-B13, P22207, P23537, and P25044), funds from the Austrian Ministry of Science and Research, and European Community Program SYBILLA grant agreement HEALTH-F4-2008-201106.
Published: January 3, 2013
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2012.09.021.
Supplemental Information
References
- Abromson-Leeman S., Bronson R.T., Dorf M.E. Encephalitogenic T cells that stably express both T-bet and ROR γ t consistently produce IFNgamma but have a spectrum of IL-17 profiles. J. Neuroimmunol. 2009;215:10–24. doi: 10.1016/j.jneuroim.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Axtell R.C., de Jong B.A., Boniface K., van der Voort L.F., Bhat R., De Sarno P., Naves R., Han M., Zhong F., Castellanos J.G. T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier G., Wagner J. PKC inhibitors: Potential in T cell-dependent immune diseases. Curr. Opin. Cell Biol. 2009;21:262–267. doi: 10.1016/j.ceb.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Barton A., Woolmore J.A., Ward D., Eyre S., Hinks A., Ollier W.E., Strange R.C., Fryer A.A., John S., Hawkins C.P., Worthington J. Association of protein kinase C α (PRKCA) gene with multiple sclerosis in a UK population. Brain. 2004;127:1717–1722. doi: 10.1093/brain/awh193. [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Brabletz T., Pfeuffer I., Schorr E., Siebelt F., Wirth T., Serfling E. Transforming growth factor β and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol. Cell. Biol. 1993;13:1155–1162. doi: 10.1128/mcb.13.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejas P.J., Walsh M.C., Pearce E.L., Han D., Harms G.M., Artis D., Turka L.A., Choi Y. TRAF6 inhibits Th17 differentiation and TGF-β-mediated suppression of IL-2. Blood. 2010;115:4750–4757. doi: 10.1182/blood-2009-09-242768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Liu F., Wang X., Lin A., Zhao H., Su B. The kinases MEKK2 and MEKK3 regulate transforming growth factor-β-mediated helper T cell differentiation. Immunity. 2011;34:201–212. doi: 10.1016/j.immuni.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.G., Liu F., Massague J. Mechanism of TGFbeta receptor inhibition by FKBP12. EMBO J. 1997;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yu G., Yu D., Zhu M. PKCalpha-induced drug resistance in pancreatic cancer cells is associated with transforming growth factor-β1. J. Exp. Clin. Cancer Res. 2010;29:104. doi: 10.1186/1756-9966-29-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Haines C.J., Gutcher I., Hochweller K., Blumenschein W.M., McClanahan T., Hämmerling G., Li M.O., Cua D.J., McGeachy M.J. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G.X., Dittel B.N., Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P.J., Li Y., Sun A.J., Liu J.J., Ji K.D., Zhang Y.Z., Sun W.L., Marche P., Zhu D.L. Differentiation of vascular myofibroblasts induced by transforming growth factor-β1 requires the involvement of protein kinase Calpha. J. Mol. Cell. Cardiol. 2003;35:1105–1112. doi: 10.1016/s0022-2828(03)00207-4. [DOI] [PubMed] [Google Scholar]
- Gruber T., Hermann-Kleiter N., Pfeifhofer-Obermair C., Lutz-Nicoladoni C., Thuille N., Letschka T., Barsig J., Baudler M., Li J., Metzler B. PKC θ cooperates with PKC α in alloimmune responses of T cells in vivo. Mol. Immunol. 2009;46:2071–2079. doi: 10.1016/j.molimm.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Gutcher I., Donkor M.K., Ma Q., Rudensky A.Y., Flavell R.A., Li M.O. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34:396–408. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak S., Croxford A.L., Kreymborg K., Heppner F.L., Pouly S., Becher B., Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann-Kleiter N., Gruber T., Lutz-Nicoladoni C., Thuille N., Fresser F., Labi V., Schiefermeier N., Warnecke M., Huber L., Villunger A. The nuclear orphan receptor NR2F6 suppresses lymphocyte activation and T helper 17-dependent autoimmunity. Immunity. 2008;29:205–216. doi: 10.1016/j.immuni.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Duarte J.H., Veldhoen M., Hornsby E., Li Y., Cua D.J., Ahlfors H., Wilhelm C., Tolaini M., Menzel U. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter H.H., Ibrahim S.M., Koczan D., Kruse N., Weishaupt A., Toyka K.V., Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hymowitz S.G., Filvaroff E.H., Yin J.P., Lee J., Cai L., Risser P., Maruoka M., Mao W., Foster J., Kelley R.F. IL-17s adopt a cystine knot fold: Structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K., Sekiya T., Inoue N., Tamiya T., Kashiwagi I., Kimura A., Morita R., Muto G., Shichita T., Takahashi R., Yoshimura A. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of eomesodermin. Immunity. 2011;34:741–754. doi: 10.1016/j.immuni.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Isakov N., Altman A. Protein kinase C(θ) in T cell activation. Annu. Rev. Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jäger A., Dardalhon V., Sobel R.A., Bettelli E., Kuchroo V.K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski S., Hermann-Kleiter N., Meisel M., Thuille N., Cronin S., Hara H., Fresser F., Penninger J.M., Baier G. Coronin 1A is an essential regulator of the TGFβ receptor/SMAD3 signaling pathway in Th17 CD4(+) T cells. J. Autoimmun. 2011;37:198–208. doi: 10.1016/j.jaut.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Komiyama Y., Nakae S., Matsuki T., Nambu A., Ishigame H., Kakuta S., Sudo K., Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kurschus F.C., Croxford A.L., Heinen A.P., Wörtge S., Ielo D., Waisman A. Genetic proof for the transient nature of the Th17 phenotype. Eur. J. Immunol. 2010;40:3336–3346. doi: 10.1002/eji.201040755. [DOI] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock C., Hermans G., Pedotti R., Brendolan A., Schadt E., Garren H., Langer-Gould A., Strober S., Cannella B., Allard J. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Malhotra N., Robertson E., Kang J. SMAD2 is essential for TGF β-mediated Th17 cell generation. J. Biol. Chem. 2010;285:29044–29048. doi: 10.1074/jbc.C110.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan P.R., Harrington L.E., O’Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Martinez G.J., Zhang Z., Chung Y., Reynolds J.M., Lin X., Jetten A.M., Feng X.H., Dong C. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J. Biol. Chem. 2009;284:35283–35286. doi: 10.1074/jbc.C109.078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusevicius D., Kivisäkk P., He B., Kostulas N., Ozenci V., Fredrikson S., Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- McKarns S.C., Schwartz R.H., Kaminski N.E. Smad3 is essential for TGF-β 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J. Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- McQualter J.L., Darwiche R., Ewing C., Onuki M., Kay T.W., Hamilton J.A., Reid H.H., Bernard C.C. Granulocyte macrophage colony-stimulating factor: A new putative therapeutic target in multiple sclerosis. J. Exp. Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A., Heldin C.H. Non-Smad TGF-β signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Nakae S., Nambu A., Sudo K., Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- O’Connor R.A., Anderton S.M. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J. Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Pfeifhofer C., Gruber T., Letschka T., Thuille N., Lutz-Nicoladoni C., Hermann-Kleiter N., Braun U., Leitges M., Baier G. Defective IgG2a/2b class switching in PKC alpha-/- mice. J. Immunol. 2006;176:6004–6011. doi: 10.4049/jimmunol.176.10.6004. [DOI] [PubMed] [Google Scholar]
- Ranganathan P., Agrawal A., Bhushan R., Chavalmane A.K., Kalathur R.K., Takahashi T., Kondaiah P. Expression profiling of genes regulated by TGF-β: Differential regulation in normal and tumour cells. BMC Genomics. 2007;8:98. doi: 10.1186/1471-2164-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela J., Kallio S.P., Chen D., Montpetit A., Jokiaho A., Choi E., Asselta R., Bronnikov D., Lincoln M.R., Sadovnick A.D. PRKCA and multiple sclerosis: Association in two independent populations. PLoS Genet. 2006;2:e42. doi: 10.1371/journal.pgen.0020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Takimoto T., Wakabayashi Y., Sekiya T., Inoue N., Morita R., Ichiyama K., Takahashi R., Asakawa M., Muto G., Mori T. Smad2 and Smad3 are redundantly essential for the TGF-β-mediated regulation of regulatory T plasticity and Th1 development. J. Immunol. 2010;185:842–855. doi: 10.4049/jimmunol.0904100. [DOI] [PubMed] [Google Scholar]
- Thomas R.M., Sai H., Wells A.D. Conserved intergenic elements and DNA methylation cooperate to regulate transcription at the il17 locus. J. Biol. Chem. 2012;287:25049–25059. doi: 10.1074/jbc.M112.351916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzachanis D., Freeman G.J., Hirano N., van Puijenbroek A.A., Delfs M.W., Berezovskaya A., Nadler L.M., Boussiotis V.A. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat. Immunol. 2001;2:1174–1182. doi: 10.1038/ni730. [DOI] [PubMed] [Google Scholar]
- Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C., Van Snick J. Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur. J. Immunol. 2006;36:2868–2874. doi: 10.1002/eji.200636662. [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Flavell R.A., Stockinger B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- Volpe E., Servant N., Zollinger R., Bogiatzi S.I., Hupé P., Barillot E., Soumelis V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Wieser R., Wrana J.L., Massagué J. GS domain mutations that constitutively activate T β R-I, the downstream signaling component in the TGF-β receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S., Madala S.K., Ramalingam T.R., Gochuico B.R., Rosas I.O., Cheever A.W., Wynn T.A. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yang X.O., Chang S.H., Park H., Nurieva R., Shah B., Acero L., Wang Y.H., Schluns K.S., Broaddus R.R., Zhu Z., Dong C. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y., Ma L., Shah B., Panopoulos A.D., Schluns K.S. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR α and ROR γ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.P., Ghoreschi K., Steward-Tharp S.M., Rodriguez-Canales J., Zhu J., Grainger J.R., Hirahara K., Sun H.W., Wei L., Vahedi G. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Chong M.M., Littman D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhou H.Y., Chen W.D., Zhu D.L., Wu L.Y., Zhang J., Han W.Q., Li J.D., Yan C., Gao P.J. The PDE1A-PKCalpha signaling pathway is involved in the upregulation of alpha-smooth muscle actin by TGF-beta1 in adventitial fibroblasts. J. Vasc. Res. 2010;47:9–15. doi: 10.1159/000231716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.