Abstract
Cell motion in a magnetic field reveals the presence of intracellular paramagnetic elements, such as iron or manganese. Under controlled field and liquid media composition, such motion previously allowed us to compare the paramagnetic contribution to cell magnetic susceptibility in erythrocytes differing in the spin state of heme associated with hemoglobin. The method is now tested on cells with less obvious paramagnetic properties: cell cultures derived from human cancers in order to determine if the magnetophoretic mobility (MM) measurement is sufficiently sensitive to the dysregulation of the intracellular iron metabolism as suggested by reports on loss of iron homeostasis in cancer. The cell lines included hepatocellular carcinoma (Hep 3B 2.1-7 and Hep G2), promyelocytic (HL-60) and chronic myelogenous (K-562) leukemias, histiocytic lymphoma (U-937), tongue (CAL 27) and pharyngeal (Detroit 562) carcinomas, and epitheloid carcinoma (HeLa), whose MM was measured in complete media with standard and elevated soluble iron (ferric nitrate and ferric ammonium citrate), against oxy- and met-hemoglobin erythrocytes used as controls. Different cell lines responded differently to the magnetic field and the soluble iron concentrations in culture media establishing the possibility of single cell elemental analysis by magnetophoresis and magnetic cell separation based upon differences in intracellular iron concentration.
Introduction
A magnetic field-based cell separation and detection method based on the intrinsic magnetic susceptibility of cells is an attractive alternative to current techniques relying on immunomagnetic labeling1–4 because it frees the process from the laborious sample preparation steps and the cost of reagents. A label-free magnetic cell separation has been proposed in the past.5–7 Notable examples include the separation of malaria infected RBCs, which are less diamagnetic than the suspending aqueous media because of the paramagnetic contribution of “malaria pigment”, hemozoin.8, 9
Here we describe studies on extending the approach to label-free separations based on intrinsic magnetic cell susceptibility of mammalian cells of non-erythroid origin, that is, selected human cancer cell lines. Cancer cells are of particular interest because of potential diagnostic applications.4, 10 The proposition that the altered electronic structure of key metabolic compounds is related to cell disease state, including malignant transformation, has been discussed before.11 Independently, it was reported that iron overload could lead to neoplastic transformation 12. Iron is an indispensable requirement for the activity of many vital biochemical processes, such as oxygen transport, electron transfer, and DNA synthesis.13 Rapidly dividing cancer cells have a higher requirement for iron than normal cells, resulting in an increased expression of proteins important for iron transfer into the cell, such as transferrin receptor 1 (TfR1) and iron storage protein, ferritin.12, 13 It was also found that iron overload disrupts the redox balance of the cell and generates excessive reactive oxygen species (ROS) which modulate signaling networks related to malignant transformation.14 Another paramagnetic metal element, manganese, may also play an important role in certain cancers. Increased manganese-superoxide dismutase (MnSOD) expression has been observed in the experimental metastatic cancer animal model.15 We have interpreted those different lines of investigations as pointing to the possibility of paramagnetic additions to the magnetic susceptibility of cancer cells to be sufficiently high as to become detectable by analyzing their motion in a strong magnetic field.
In this work, we have relied on high sensitivity to cell motion of a magnetophoretic mobility analyzer, developed in our laboratories.16, 17 Magnetophoretic mobility is a measure of field-induced cell velocity when exposed to a strong magnetic field gradient in a viscous physiologic electrolyte suspension.18 analogous to the cell electrophoretic mobility when exposed to the electric field, or cell dielectrophoretic mobility when exposed to the oscillating electric field. 19, 20 We have applied magnetophoretic analysis to established human cancer cell lines are routinely used as experimental models for human cancers.19
Experimental Section
Cancer cell lines
Table 1 lists all the cancer cell lines used in this study. The cell preparations were obtained from ATCC (The American Type Culture Collection, Manassas, VA). These cells were cultured in 75 cm2 T-flasks (BD Bioscience, Bedford, MA) in the complete media (as listed in Table 1 and Table S1) and the complete media with iron compound addition (as described below). The cell cultures were maintained at 37°C and 5% CO2 and passaged every two or three days using sterile technique to sustain viability (unless noted otherwise). Up to 20 cell passages were used. Adherent cells were detached by trypsin-EDTA solution (0.05% trypsin, 0.53 mM EDTA, Central Cell Services, Cleveland Clinic). The two iron compounds, Fe(NO3)3 and ferric ammonium citrate (FAC), were purchased from Sigma-Aldrich, St Louis, MO and used as additives to the cell culture media in select experiments. We have selected those two iron compounds because they were used for studies on iron transfer in cells by others and therefore provided convenient reference reagents in our own work. In particular, FAC has been shown by to participate in Fe transfer to physiologically relevant intracellular compartments, including ferritin, and has a pathophysiological relevance as an Fe donor 20–23 whereas iron nitrate is included in complete cell culture media (compare Table S1). Final iron concentrations in media were adjusted as specified for each experiment. Cell size and concentration were evaluated by a Z2™ Coulter Counter® (Beckman Coulter Inc., Fullerton, CA, USA) equipped with a 70 μm aperture. The cell viability was determined by trypan blue (Invitrogen, Grand Island, NY) exclusion test.
Table 1.
Summary information about cancer cell lines used in this study, their cell size and the intracellular metal content
| Cell lines | ATCC No. | Mediad | Diameter ± S.D., μm | Fe | Mn | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| fg/cell | ng/mL | fg/cell | ng/mL | ||||
| CAL 27 | CRL-2095 | DMEMa | 13.7 ± 1.80 | 20.7 | 15,000 | - | - |
| Detroit 562 | CCL-138 | AMEMb | 14.5 ± 1.68 | 25.3 | 16,000 | - | - |
| HeLa | CCL-2 | RPMI1640a | 15.8 ± 1.90 | 45.7 | 22,000 | 1.2 | 600 |
| Hep3B 2.1-7 | HB-8064 | EMEMc | 15.5 ± 2.31 | 96.2 | 49,000 | 5.7 | 2,900 |
| Hep G2 | HB-8065 | EMEMc | 14.8 ± 2.55 | 57.9 | 34,000 | 6.9 | 4,000 |
| HL-60 | CCL-240 | IMDMc | 12.1 ± 1.53 | 17.1 | 18,000 | 0.8 | 900 |
| K-562 | CCL-243 | IMDMc | 16.4 ± 2.07 | 47.5 | 21,000 | 0.9 | 400 |
| U-937 | CRL-1593.2 | RPMI1640c | 13.2 ± 1.37 | 0.6 | 500 | - | - |
IMDM: Iscove’s Modified Dulbecoo’s Medium; DMEM: Dulbecco’s Modified Eagle’s Medium; AMEM: Alpha Modification of Eagle’s Medium: EMEM: Eagle’s Minimum Essential Medium; FBS: Fetal Bovine serum; ATCC: American Type Culture Collection, Manassas, VA;
from Central Cell Services, Cleveland Clinic;
from Mediatech, Inc., Manassas, VA;
from ATCC.
All media were supplemented with 10% FBS except of HL60 culture media, supplemented with 20% FBS (from Central Cell Services, Cleveland Clinic).
Red blood cell (RBC) standard
The whole blood (WB) was received from Cleveland Clinic Blood Banking and Transfusion Medicine (discarded tissue from therapeutic phlebotomy, with the approval of institutional ethics committee). A stock suspension was prepared by diluting 0.1 mL whole blood with 10 mL PBS. An aliquot of 2.0 × 106 RBCs from the stock suspension equilibrated with air was used as an oxyHb RBC control (zero magnetophoretic mobility reference standard).24–26 Another aliquot of 2.0 × 106 RBCs was oxidized by exposure to 5 mM sodium nitrite (Sigma-Aldrich Co., Milwaukee, WI) for about 1.5 hr to convert hemoglobin to methemoglobin completely, which was subsequently used as a metHb control (positive magnetophoretic mobility reference standard).24–26
Cell magnetophoretic mobility analysis by CTV
The detection of cell motion in the magnetic field was measured using Cell Tracking Velocimeter (CTV).16, 27, 28 Briefly, the permanent magnet assembly produces a nearly constant (“isodynamic”) magnetic field energy density gradient, Sm (146 ± 1 T.A/mm2), in the region of interest (1.05 mm wide × 0.79 mm high). The horizontal cell velocity component induced by the magnetic field stays constant for the time of the measurement (as does the vertical velocity component induced by the gravitational sedimentation). Before CTV analysis, cell culture media were replaced with PBS containing 0.1% Pluronic F-68. For each sample, 3 mL cell suspension was prepared with a concentration of (2 – 3) × 105 cells/mL. Up to 1,500 cells can be measured in 20 minutes. The final outputs are cell MM (denoted by m) and cell sedimentation coefficient (s) distributions and statistics (including mean, standard deviation and 95% confidence interval). The sensitivity of CTV magnetophoretic mobility analysis to intracellular high spin iron species was validated on oxyHb RBCs and metHb RBCs used as reference standards for which cell size (hydrodynamic diameter), spin density (in Bohr magnetons per unit volume), and the resulting cell volume magnetic susceptibility, χ, are known. 26 The additional details are provided in Supporting Information.
Label-free magnetic cell separation
Cells used for separation were collected from culture flasks into conical falcon tubes and washed once (by centrifugation at 250 g, 4°C for 10 minutes, followed by decanting of supernatant). The pellet was re-suspended in 0.5 mL degassed PBS to a cell number concentration of (2 – 8.5) × 107 cells/mL. The suspension was subsequently passed through a MACS® MS column (Miltenyi Biotec, Bergisch Gladbach, Germany) which was placed in the MiniMACS™ separator. The column was washed with 1 mL PBS twice. The cells that passed through the column on the magnet and the washing effluent were collected together in one tube and labeled as “non-magnetic” fraction. Subsequently, the MS column was removed from the magnet and the cells retained in the column were flushed out with 1 mL PBS by applying the plunger supplied with the column. These cells were referred as “magnetic” fraction. After evaluation of the cell concentration and viability in all three fractions: the negative and positive cells as well as the feed (the cell sample before separation), the fractions were split into three aliquots each for CTV, ICP-MS, and cell cycle analysis.
Intracellular iron and manganese concentrations measurements by ICP-MS
The concentration of paramagnetic elements in the cell and the media responsible for the cell magnetophoretic mobility was measured by atomic mass spectrometry. Although the measurement does not provide information about the high-spin concentration for a given element, it nevertheless provides useful information about the cell spin density upper limit and therefore an upper limit of the expected cell mobility. Such measurements provided useful reference values A Thermo Finnigan™ Element 2 Inductively Coupled Plasma Sector Field Mass Spectrometer (ICP-MS, Thermo Electron Corporation, Bremen, Germany) in medium resolution mode (R = 4,000) was used to quantitatively evaluate the paramagnetic metal element in cells. It was performed at the Trace Element Research Laboratory, The Ohio State University. Before analysis, cells were digested by adding same volume of concentrated nitric acid (HNO3, Trace Metal Grade, Fisher Scientific, Pittsburgh, PA). Digested samples were then diluted 10-fold by distilled water. The concentration of two elements, Fe and Mn were measured in each sample.
Auxiliary analyses: ferritin by TEM, TfR1 (CD71) expression and cell cycle by flow cytometry
For transmission electron microscopy (TEM) preparations, cell cultures were spun down and resuspend into fixative (1 mL 0.1 M sodium cacodylate with 2% glutaraldehyde) overnight for fixation. The fixative was replaced with phosphate/sucrose rinse buffer and the cell pellet transferred into 2.5% molten agar deposited on parafilm till solidification. The TEM images were acquired in the LRI Imaging Core. Transferrin receptor 1 (TfR1, also designated as a cluster of differentiation 71, CD71) is involved in the cellular uptake of iron and is expressed on cells with high proliferative activity such as cancer cells.14, 29 An immunofluorescent labeling protocol was used to target TfR1 expressing cells, as summarized in Supporting Information. Cell cycle analysis was performed by measuring cell DNA content using fluorescent stain (propidium iodide) and flow cytometry, as summarized in Supporting Information.
Statistical analysis
Kruskal-Wallis test was used to compare the equality of sample medians among groups using SigmaStat 3.5 software package (Systat Software, Inc., Chicago, IL). Student’s t-test and Tukey-Kramer test were applied to compare the mean differences between samples, using JMP software (SAS Institute, Cary, NC). The confidence level of P < 0.05 was considered as statistically significant.
Results
MM of cells incubated in the complete media
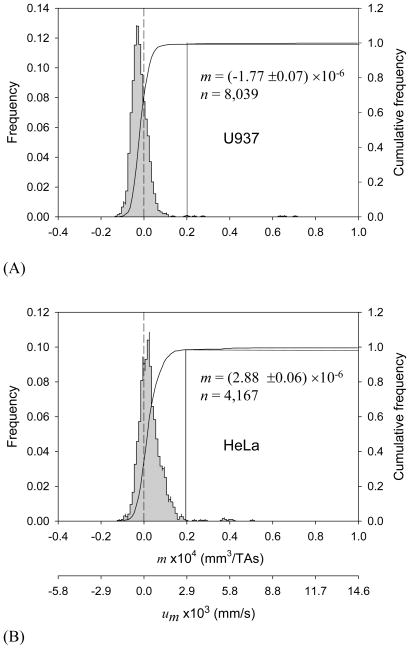
The applied magnetic field had a measurable effect on cell motion for select cancer cell culture suspensions. Representative examples of MM distributions for U-937 and HeLa cells are shown in Figures 1A and 1B, respectively. Both cancer cell lines have broader MM distributions than those of the RBC standards (shown in Supporting Information, Figure S3) which is not unexpected considering a more heterogeneous cell population in cell culture as compared to the mature donor RBCs. Interestingly, the MM distribution of HeLa cells shows a positive mode, not unlike that of metHb RBCs with known paramagnetic contribution from the metHb (Figure S3B). The U-937 cell MM distribution mode is negative, comparable to oxyHb RBC (Figure S3A; note that a negative MM indicates that the cell is more diamagnetic than are the suspending media.)
Figure 1.
Magnetophoretic mobility (MM, m) histograms of (A) U-937 cells (24-hour incubation in complete media, viability 90.9%), and (B) HeLa cells (19-hour incubation, viability 96.1%). The second axis at the bottom shows the corresponding magnetic field-induced velocity (um) values. Drop lines indicate cut-off mobility used to discriminate between “high” and “low” MM fractions (see text).
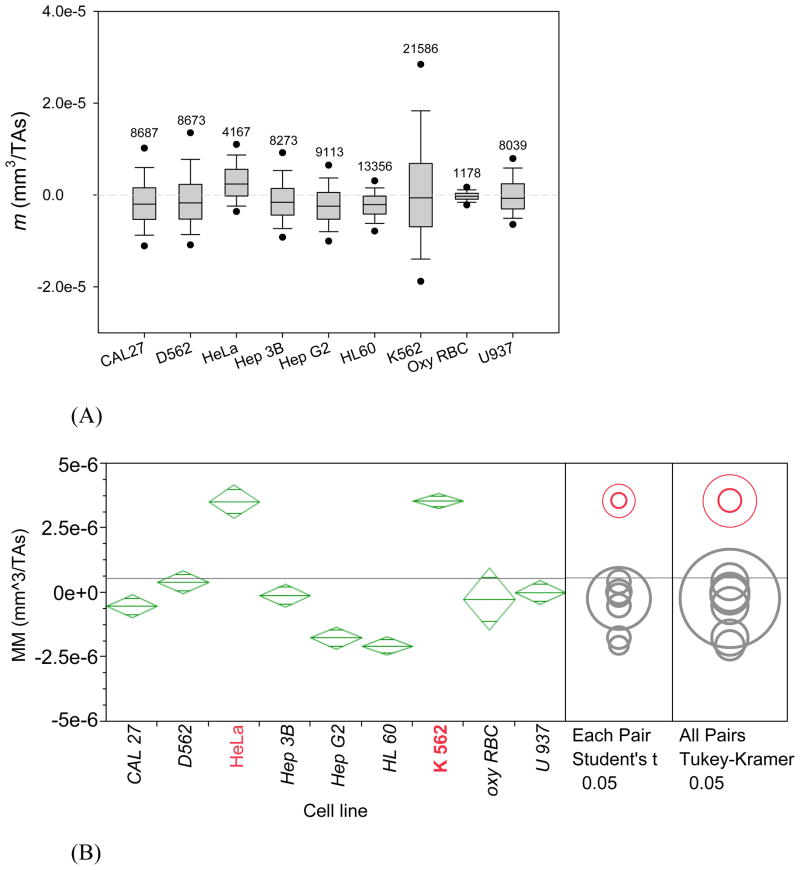
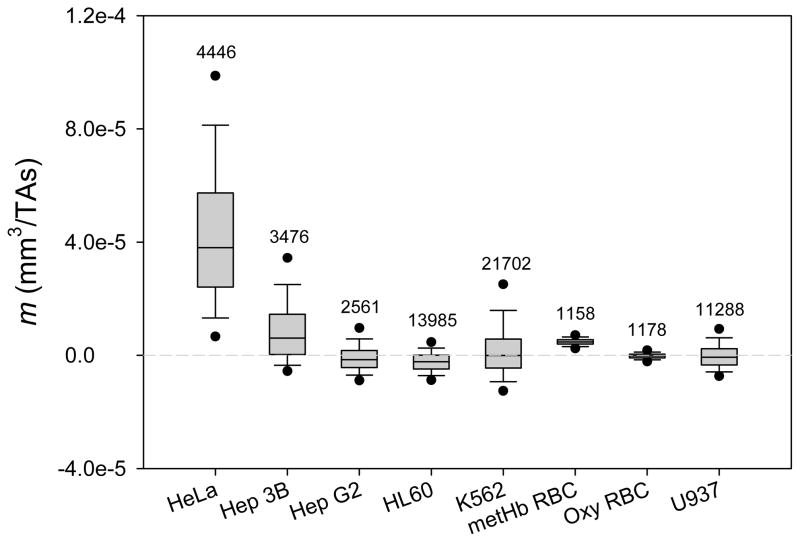
The MM distributions of all the eight cancer cell lines are summarized in Figure 2 (also showing oxyHb RBC reference standard). For large samples (the sample size of over 1,000) the normality test becomes impractical because it may detect statistically significant but experimentally unimportant deviations from normality. Therefore, both non-parametric and parametric methods were used for comparison between cell MM distributions. Non-parametric method, Kruskal-Wallis test was used to compare the MM medians between cell lines. By that test, the MM median of HeLa cells was significantly greater than all other cells, and HL 60 and Hep G2 had the smallest MM medians (Figure 2A). When the comparison was repeated using parametric tests (pair-wise Student’s t-test and all pairs Tukey-Kramer test, Figure 2B) the HeLa cells again showed distinctly higher mean MM than the other cells. Here K562 also stood out by virtue of a skewed distribution towards positive MM values and a large sample size (N ≈ 1,000) that offset the effect of a relatively broad MM distribution (Figure 2A). Again, Hep G2 and HL 60 placed at the bottom of the mean MM list. Taken together, the data indicated that a fraction of HeLa and K-562 cells may possess sufficiently high intrinsic magnetic susceptibility to qualify it for enrichment by a high-gradient magnetic separation (described in a later section).
Figure 2.
MM distributions (m) of all the eight cancer cell lines incubated in the complete media (plus deoxygenated RBC as a control). (A) Non-parametric box & whiskers plots: (25th, 75th), (10th, 90th), and (5th, 95th) percentile ranges are indicated by boxes vertical extension, whiskers and dots, respectively; the median value by a line inside the box; the number of cells tracked is indicated on top. (B) Same data represented by mean (diamond center line) & 95% confidence intervals (CIs, diamond cuts by vortices). Horizontal grey line shows mean for all data. HeLa and K-562 cells MM values were identified as significantly higher from those of other types of cells at p<0.05 by statistical tests indicated on the right.
The magnetic field-induced cell velocity in viscous media is a direct measure of the magnetic susceptibility of the cell (Supporting Information, Eqs. 1 and 3). It is used to calculate the cell MM value (Supporting Information, Eq. 1) which thus becomes a direct measure of the paramagnetic compounds inside the cell, predominantly the intracellular iron, as discussed below. There is no appreciable paramagnetic interference from the media as more than 95% volume of the cell media is the diamagnetic water along with other media components. An exception is iron complexes, such as ferric nitrate whose contribution is too low to be significant. The cell culture liquid media information is provided in Supporting Information, Tables S1 and S2.
In order to exclude artifacts unrelated to a normal function of a viable cell (such as diffusion of soluble iron into the damaged or a dead cell due to loss of active transmembrane transport) we have tested for a potential effect of cell viability on its magnetophoretic mobility. After inoculation, cell samples were kept in culture without changing the media to progressively lower their viability by starvation. Aliquots were taken every 12 to 24 hours for cell counting, viability determination, and MM measurement by CTV. Before CTV analysis, cells were washed once with PBS containing 0.1% Pluronic F-68 (additional information about fluid media composition is provided in SI). The time course for a selected cell line (HeLa) is shown in Figure S1. The results suggest that the loss of the HeLa cell viability does not affect its magnetophoretic mobility (also true for HeLa cell cultures with iron supplement, Figure S2, and other cell lines, not shown). Nevertheless, in order to avoid potential interference due to loss of cell mechanical integrity on its motion through the viscous fluid only cell samples with the percent viability of not less than 50% were used for subsequent tests.
MM of cells incubated in the complete media with 0.5 mg/mL Fe(NO3)3 addition
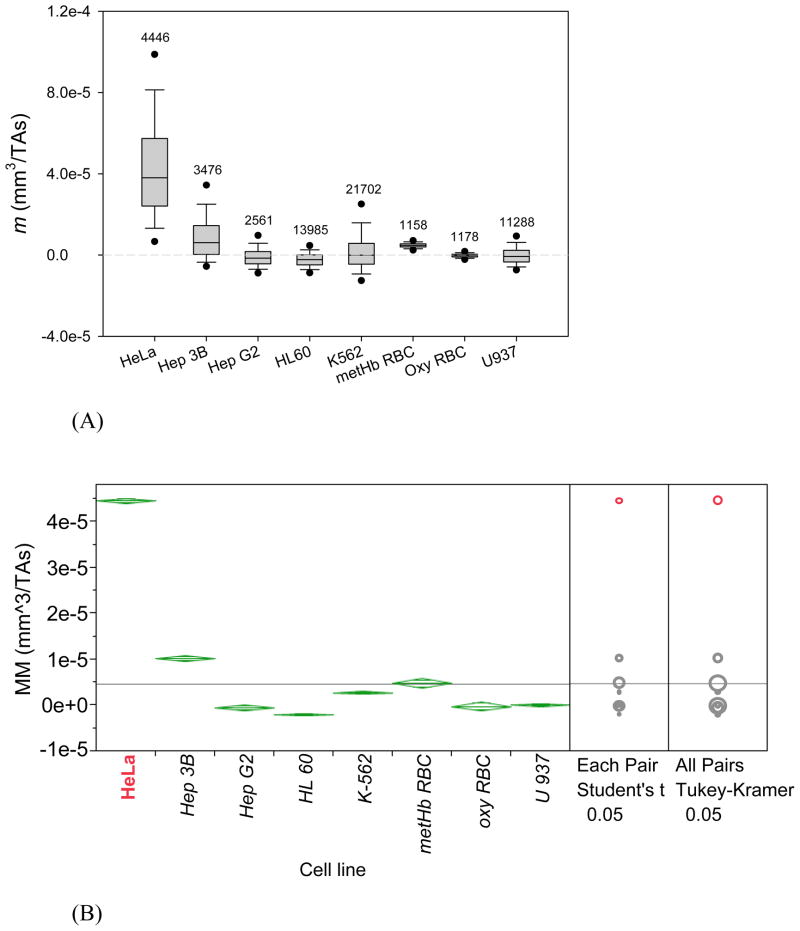
In order to test the effect of the elevated soluble iron in the media on cell magnetophoresis we have selected iron compounds used in culture media as described in the literature.13, 14, 29 Two different soluble iron compounds typically used for studies on iron transfer in cells are ferric nitrate, Fe(NO3)3, and ferric ammonium citrate (FAC, described in the next section). For the effect of Fe(NO3)3 addition to the media, at a final concentration of 0.5 mg/mL was used in the complete media (described in the preceding section). CAL-27 and D562 cells did not grow well in these modified media. Their viability dropped to less than 50% following 20 hours after inoculation and therefore they were excluded from further analysis. The measurements of cell MM as described in the preceding section were repeated for HeLa, Hep3B, HepG2, HL60, K562 and U937 following incubation in media enriched in ferric nitrite and the results are summarized in Figure 3. There was a pronounced effect of soluble iron addition to culture media on HeLa cell magnetophoresis: an increase over 10-fold from 3.5 × 10−6 to 5 × 10−5 mm3/TAs (Figure 3A v. Figure 2A). Both Kruskal-Wallis test and t-test show that the MM of HeLa cells is significantly higher than all other cells (Figure 3B). There was a lesser effect on one other cell line, Hep 3B, which showed a moderate increase in MM above that of the positive control standard, metHb RBC, with other cells remaining unaffected (their MM values staying in the range bracketed by negative and positive controls, oxyHb RBC and metHb RBC, respectively, compare Figures 2B and 3B). The addition of 0.5 mg/mL Fe(NO3)3 to cell culture media did not change cells’ growth rate, viability and cell size (with the exception of CAL-27 and D562, as noted above, data not shown).
Figure 3.
MM distributions (m) of six cancer cell lines incubated in media supplemented with Fe(NO3)3 (plus oxygenated and metHb RBC controls). (A) and (B) are non-parametric and parametric plots of the same data, analogous to presentation in Figure 2. Note change in vertical scale as compared to Figure 2. HeLa cell MM is by far the most responsive to Fe(NO3)3 addition by statistical tests indicated in (B) on the right.
MM of cells incubated in the complete media with 0.5 mM FAC addition
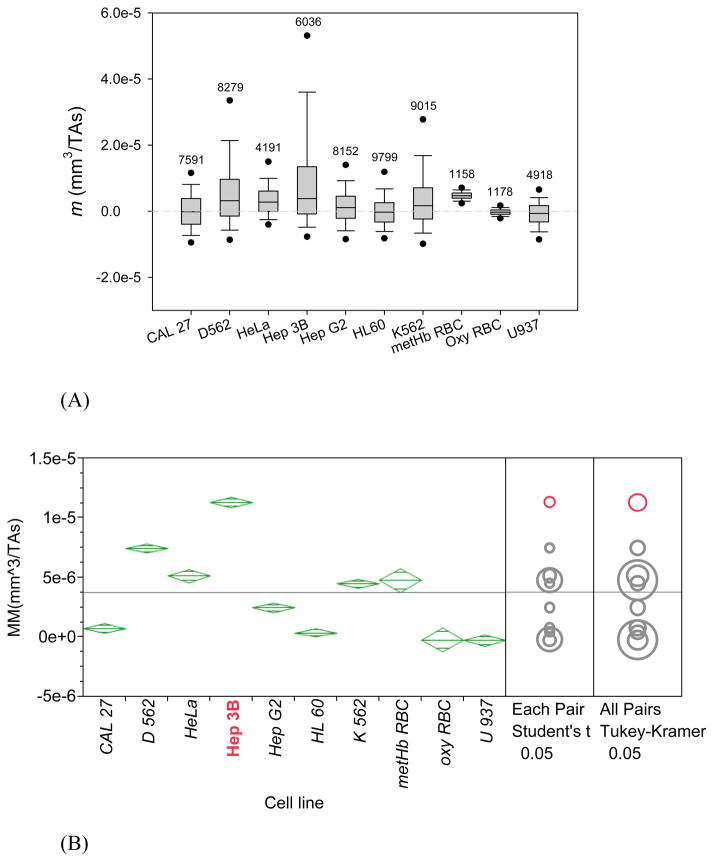
Another soluble iron compound used for studies on intracellular iron is ferric ammonium citrate (FAC).30, 31 FAC was added to media with a final concentration of 0.5 mM (0.132 mg/mL) in the complete cell culture media. The same protocol was followed as that used for experiments with and without ferric nitrate addition, described above. Unlike the addition of ferric nitrate, the addition of FAC did not have a significant effect on cell viability as compared to the original, complete media and all cell lines met the criteria of MM analysis; the results are summarized in Figure 4. The effect of FAC addition was different from that of the ferric nitrite addition, shown in the preceding section. Here MM of most of the cell lines increased after addition of FAC to the cell culture, albeit to a lesser extent than after addition of the ferric nitrite. Interestingly, the effect on HeLa cells was essentially nil (compare Figures 4B and 2B). The most affected cell line by FAC addition to culture media was Hep 3B, which showed a comparable increase in mean MM as that following addition of the ferric nitrite to the media (compare Figures 4B and 3B).
Figure 4.
MM distributions (m) of six cancer cell lines incubated in media supplemented with FAC (plus oxygenated and metHb RBC controls). (A) and (B) are non-parametric and parametric representations of the same data, analogous to Figures 2 and 3. Note change in vertical scale as compared to Figures 2 and 3. Here Hep 3B cells MM is the most responsive to Fe(NO3)3 addition but not HeLa cells (compare with Figure 3) by statistical tests indicated in (B) on the right.
Label-free magnetic separation of K-562 and HeLa cells
The skewed distribution of the MM data of K-562 (Figure 2) and HeLa (Figures 1B and 2) cells suggests the presence of high mobility fraction mixed with the population of mostly low MM cells. In order to further characterize the mobility distribution of the cancer cell lines we have considered them as consisting of a mixture of low and high mobility cell subpopulations, the high mobility cells defined as more magnetophoretically mobile than at least 95% of the unfractionated cell population. The cut-off MM separating “non-magnetic” from “magnetic” cell fractions was set as equal to 2×10−5 mm3/TAs resulting in 2% high-mobility fraction for HeLa cells (Figure 1B) and negligibly small high-mobility fraction for U937 cells (Figure 1A)
The presence of high MM fraction in HeLa and K-562 cell samples suggests the possibility of its enrichment by magnetic separation. A commercially available high-gradient magnetic separator (HGMS) column was used with HeLa and K-562 cells, according to the manufacturer’s specifications but without any magnetic cell labeling (MACS® MS column and magnet, Miltenyi Biotec, Bergisch Gladbach, Germany). Only cells cultured in the complete media without soluble iron addition were used. The results were in agreement with the expected segregation of cell properties between the “magnetic” and “non-magnetic” fractions collected from the HGMS column based on the MM distribution in the original sample (Tables S3, S4 and Figure S4). Thus, the “magnetic” HeLa cell fraction consisted of a small fraction (1.1%) of the original sample with a significantly higher mean MM (5.51 × 10−5 mm3/TAs) and the intracellular Fe content (430 fg/cell) than the original sample (3.3×10−6 mm3/TAs and 46 fg/cell, respectively). Notably, these values are also significantly higher than those for the metHb RBC used as a positive control (the mean MM of 4.7 × 10−6 mm3/TAs and Fe content of 100 fg/cell, Figure S3B and Table S3, correspondingly). Also as expected, the “non-magnetic” fraction’s mean MM and intracellular Fe concentration were lower than their respective values in the “non-magnetic” fraction and the original sample (Table S3 and Figure S4). There was no significant change in cell viability before and after separation. In an attempt to better characterize the “magnetic” and “non-magnetic” fractions, they were re-cultivated in the same complete media as the original cell sample. After about 72 hrs incubation, cells were harvested and analyzed by CTV and ICP-MS again. Rather unexpectedly, the “magnetic” cell isolates appeared to lose their magnetic attributes during incubation, which was reflected in the reduced value of MM and a lower average Fe content over time (shown in columns marked “B” in Table S3, with additional information provided in SI). A possible explanation is that the decrease in Fe concentration per cell was the result of Fe redistribution across daughter cells in culture.
Similar results were obtained following magnetic separation of the un-labeled K-562 cell line, which also confirmed the notion of a small paramagnetic cell fraction present in the original sample as suggested by skewed MM distribution in the original K-562 cell sample, Figure 2 (additional data provided in SI, Table S4). The ICP-MS analysis of the original and sorted samples indicated the role of the intracellular iron in the magnetic separation but not the manganese (shown in SI).
Intracellular ferritin by TEM and TfR1 (CD71) expression and cell cycle analysis by flow cytometry
Transmission electron microscopy was used to check for evidence of iron storage protein, ferritin, inside HeLa cells incubated in culture media with and without Fe(NO3)3 addition. No evidence of ferritin molecules was found by scanning HeLa cell TEM images from cells incubated in complete media only, Figure S7B. By comparison, the samples obtained after 72 hr incubation with 0.5 mg/mL Fe(NO3)3 clearly showed ferritin particle inclusions within the lysosome matrix (Figure 7A). This result suggests that iron added to the cell culture media was apparently taken up by endocytosis and passed to the cell lysosomal system. The elevated intracellular ferritin concentration is consistent with the elevated MM values for this cell line (Figure 3) and our previous work on magnetic separation of leukocytes decorated with cationized ferritin.32
The effect of exogenous soluble iron in cell culture media on the mechanism of the intracellular iron transport was further investigated by measuring levels of surface transferrin receptors (TfR1) in both K-562 and HeLa cells after FAC or Fe(NO3)3 addition. The experimental details are provided in SI. Rather unexpectedly, the treatment of cells with 0.5 mM FAC or 0.5 mg/mL Fe(NO3)3 initially reduced the number of TfR1s by 25 – 40% within 1–5 hrs incubation time for both K-562 and HeLa cells before returning to the control TfR1 signal level after about 24 hrs incubation (Figure S6). At that time point, the surface TfR1s expression reached the level of control cells for HeLa cells or increased above the control level for K-562 cells. Thus the data indicate that the addition of soluble iron to the cell media at high concentration may initially suppress the intracellular iron transport and may require additional time of approximately 24 hrs before the desired effects of iron incorporation and concomitant increase of the cell magnetic susceptibility take place.30 Interestingly, the data show that K-562 cells express higher level of TfR1s than HeLa cells as a result of soluble iron addition to culture media, contrary to the expectation based on their lower mean MM as compared to that of HeLa cells (Figures 3 and 4). The possible explanation is that the uptake of iron probably does not correlate with the number of TfR1 molecules on the cell surface but rather with the rate of TfR1 internalization. This observation agrees with the report by others31 that TfR1s in K-562 cells are internalized slowly, whereas those in HeLa cells are internalized quickly.
The HeLa cell cycle (Figure S8) distribution was shifted slightly towards the actively dividing cells (S and G2 phases, Table S5) in the “magnetic” fraction as compared to the original sample and the “non-magnetic” fraction without reaching statistical significance, however.
Discussion
Iron metabolism in cancer cells is of particular interest because of its importance to diagnostic applications and new anti-cancer drug development.12 Out of the eight cell lines tested, two, HeLa and K-562, showed significant motion in the imposed magnetic field even without addition of soluble iron supplement to media, with their mean magnetophoretic mobility, or MM, approaching that of paramagnetic (methemoglobin-containing) RBCs (Figure 3B). The HeLa cell motion induced by the magnetic field was reported before, albeit in a more limited study33. The magnetic susceptibility of those cells was sufficiently high to make possible their separation in a high-gradient magnetic separator (HGMS) column. Such “label-free” magnetic separation was demonstrated before for high-spin RBCs but not for cells of non-erythroid origin.5, 34 The intracellular iron concentration in the “magnetic” HeLa and K-562 cell fractions, measured by ICP-MS, was high, as expected of cells enriched in the HGMS column, even higher than that in the RBC control (Tables S3 and S4). The iron could have been sequestered in an ubiquitous iron storage protein, ferritin. Ferritin has an average capacity of 2,000 iron atoms with a property of a super-paramagnetic nanoparticle35, 36 that may act as an intrinsic, naturally occurring “magnetic label”. We have shown before that native (cationized) ferritin and a reconstituted “magnetoferritin” conjugated to monoclonal antibodies possess sufficiently high magnetic moment to separate cells in a magnetic field.32, 37 Upon additional culture of the “magnetic” fraction its high intracellular iron concentration and magnetophoretic mobility decreased over time, indicating that it is a transient phenomenon (Tables S3 and S4). There was a slight shift towards higher percentage of dividing HeLa cells in the “magnetic” fraction as compared to the “non-magnetic” fraction and the original sample but the difference was not statistically significant under present experimental conditions (Table S5). Thus, although the skewed MM distributions for HeLa and K-562 cell cultures suggested presence of high mobility, high intracellular iron cell subpopulations, their other distinguishing characteristics could not have been ascertained at this time.
Our combined results provided a picture consistent with the current understanding of the molecular mechanism of iron control in the cell.13, 14, 29 In the case of HeLa cell culture supplemented with 0.5 mg/mL Fe(NO3)3, the observed initial suppression of TfR1 expression was consistent with the large increase in mean MM and the presence of numerous ferritin molecule inclusion in the cytosol, seen in TEM images. It is known that TfR1 expression is primarily regulated at the post-transcriptional level in response to the intracellular iron concentration.8 For high intracellular iron conditions (here resulting from high iron concentration in the culture media) iron regulatory proteins 1 and 2 (IRP1 and IRP2) do not bind to the five iron response elements (IREs) in the 3′ un-translated region (UTR) of the TfR1 mRNA resulting in TfR1 mRNA degradation and loss of TfR1 expression (Figure S9A). Concurrently, high iron concentration impedes binding of IRP1 and IRP2 to IRE in the 5′ UTR of the ferritin mRNA allowing the translation to proceed and resulting in high ferritin expression (Figure S9B). Thus the high iron concentration in the media is expected to have a two-fold effect on the cell: suppression of the transferrin receptor and increase in ferritin synthesis, which is in agreement with our HeLa data. Also, significantly higher MM of HeLa cells as compared to that of K-562 cells after addition of Fe(NO3)3 to cell culture agree with the reported higher rate of TfR1 internalization in HeLa cells.31 The less pronounced and more varied responses from other cell lines indicate that there are differences between individual cell lines that may require a more sophisticated experimental approach than used in this study. Considering that a better understanding between the cell iron regulation and the cell magnetic susceptibility and its magnetophoretic mobility may lead to improved methods of label-free magnetic cell separation, such follow-up studies are well worth pursuing.
Conclusions
Taken together, the results show the existence of non-erythroid cell lines derived from human cell cancers that are sufficiently paramagnetic to be possible to affect their motion by application of a strong magnetostatic field gradient. These findings point to a new method of single cell magnetometry by cell tracking velocimetry in application to biologically important questions related to iron metabolism dysregulation in cancer cells, and suggest new applications of magnetic cell separation that do not require attachment of magnetic ligands but rather rely on soluble iron transfer across the cell membrane.
Supplementary Material
Acknowledgments
This work has been supported by the National Institutes of Health (R01 CA62349). Expert technical help of Lee R. Moore in instrument setup and preliminary data analysis is thankfully acknowledged. Helpful comments from Reviewers are appreciated.
Footnotes
Supporting Information Available
This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pankhurst QA, Connolly J, Jones SK, Dobson J. Journal of Physics D: Applied Physics. 2003;36:R167–R181. [Google Scholar]
- 2.Pamme N, Wilhelm C. Lab Chip. 2006;6:974–980. doi: 10.1039/b604542a. [DOI] [PubMed] [Google Scholar]
- 3.Grutzkau A, Radbruch A. Cytometry A. 2010;77:643–647. doi: 10.1002/cyto.a.20918. [DOI] [PubMed] [Google Scholar]
- 4.Zborowski M, Chalmers JJ. Anal Chem. 2011;83:8050–8056. doi: 10.1021/ac200550d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roath S, Smith AR, Watson JHP. Journal of Magnetism and Magnetic Materials. 1990;85:285–289. [Google Scholar]
- 6.Furlani EP. Journal of Physics D-Applied Physics. 2007;40:1313–1319. [Google Scholar]
- 7.Han KH, Frazier AB. IEE Proceedings-Nanobiotechnology. 2006;153:67–73. doi: 10.1049/ip-nbt:20050019. [DOI] [PubMed] [Google Scholar]
- 8.Hackett S, Hamzah J, Davis TM, St Pierre TG. Biochim Biophys Acta. 2009;1792:93–99. doi: 10.1016/j.bbadis.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Moore LR, Fujioka H, Williams PS, Chalmers JJ, Grimberg B, Zimmerman PA, Zborowski M. Faseb J. 2006;20:747–749. doi: 10.1096/fj.05-5122fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Lang JC, Balasubramanian P, Jatana KR, Schuller D, Agrawal A, Zborowski M, Chalmers JJ. Biotechnol Bioeng. 2009;102:521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szent-Györgyi A. Electronic biology and cancer: a new theory of cancer. Marcel Dekker; New York: 1976. [Google Scholar]
- 12.Richardson DR, Kalinowski DS, Lau S, Jansson PJ, Lovejoy DB. Biochim Biophys Acta. 2009;1790:702–717. doi: 10.1016/j.bbagen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Aisen P, Enns C, Wessling-Resnick M. The International Journal of Biochemistry & Cell Biology. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 14.MacKenzie EL, Iwasaki K, Tsuji Y. Antioxid Redox Signal. 2008;10:997–1030. doi: 10.1089/ars.2007.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor KM, Hempel N, Nelson KK, Dabiri G, Gamarra A, Belarmino J, Van De Water L, Mian BM, Melendez JA. Cancer Res. 2007;67:10260–10267. doi: 10.1158/0008-5472.CAN-07-1204. [DOI] [PubMed] [Google Scholar]
- 16.Chalmers JJ, Haam S, Zhao Y, McCloskey K, Moore L, Zborowski M, Williams PS. Biotechnol Bioeng. 1999;64:519–526. [PubMed] [Google Scholar]
- 17.Jin X, Zhao Y, Richardson A, Moore L, Williams PS, Zborowski M, Chalmers JJ. Analyst. 2008;133:1767–1775. doi: 10.1039/b802113a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing Y, Mal N, Williams PS, Mayorga M, Penn MS, Chalmers JJ, Zborowski M. Faseb J. 2008;22:4239–4247. doi: 10.1096/fj.07-105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masters JR. Nat Rev Mol Cell Biol. 2000;1:233–236. doi: 10.1038/35043102. [DOI] [PubMed] [Google Scholar]
- 20.Hirsh M, Konijn AM, Iancu TC. J Hepatol. 2002;36:30–38. doi: 10.1016/s0168-8278(01)00221-5. [DOI] [PubMed] [Google Scholar]
- 21.Neumannova V, Richardson DR, Kriegerbeckova K, Kovar J. In Vitro Cell Dev Biol Anim. 1995;31:625–632. doi: 10.1007/BF02634316. [DOI] [PubMed] [Google Scholar]
- 22.Popovic Z, Templeton DM. Mol Cell Biochem. 2004;265:37–45. doi: 10.1023/b:mcbi.0000044313.19574.c6. [DOI] [PubMed] [Google Scholar]
- 23.Richardson DR, Ponka P. Biochim Biophys Acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- 24.Coryell C, Stitt F, Pauling L. J Am Chem Soc. 1937;59:633–642. [Google Scholar]
- 25.Pauling L, Coryell CD. Proc Natl Acad Sci USA. 1936;22:210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN. Biophys J. 2003;84:2638–2645. doi: 10.1016/S0006-3495(03)75069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin X, Yazer MH, Chalmers JJ, Zborowski M. Analyst. 2011;136:2996–3003. doi: 10.1039/c0an01018a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura M, Zborowski M, Lasky LC, Margel S, Chalmers JJ. Exp Fluids. 2001;30:371–380. [Google Scholar]
- 29.Richardson DR. Curr Med Chem. 2005;12:2711–2729. doi: 10.2174/092986705774462996. [DOI] [PubMed] [Google Scholar]
- 30.Koorts AM, Viljoen M. Arch Physiol Biochem. 2007;113:55–64. doi: 10.1080/13813450701422575. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi N, Kojima C, Harada A, Koiwai K, Emi N, Kono K. Bioconjug Chem. 2008;19:1588–1595. doi: 10.1021/bc800126s. [DOI] [PubMed] [Google Scholar]
- 32.Zborowski M, Fuh CB, Green R, Sun L, Chalmers JJ. Anal Chem. 1995;67:3702–3712. doi: 10.1021/ac00116a014. [DOI] [PubMed] [Google Scholar]
- 33.Kashevskii BE, Kashevskii SB, Prokhorov IV, Aleksandrova EN, Istomin Iu P. Biofizika. 2006;51:1026–1032. [PubMed] [Google Scholar]
- 34.Karl S, David M, Moore L, Grimberg BT, Michon P, Mueller I, Zborowski M, Zimmerman PA. Malar J. 2008;7:66. doi: 10.1186/1475-2875-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Awschalom DD, Smyth JF, Grinstein G, DiVicenzo DP, Loss D. Physical Review Letters. 1992;68:3092–3095. doi: 10.1103/PhysRevLett.68.3092. [DOI] [PubMed] [Google Scholar]
- 36.Bulte JW, Douglas T, Mann S, Frankel RB, Moskowitz BM, Brooks RA, Baumgarner CD, Vymazal J, Strub MP, Frank JA. J Magn Reson Imaging. 1994;4:497–505. doi: 10.1002/jmri.1880040343. [DOI] [PubMed] [Google Scholar]
- 37.Zborowski M, Fuh CB, Green R, Baldwin NJ, Reddy S, Douglas T, Mann S, Chalmers JJ. Cytometry. 1996;24:251–259. doi: 10.1002/(SICI)1097-0320(19960701)24:3<251::AID-CYTO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.