Abstract
Pallister–Killian syndrome (PKS) is a rare, sporadic genetic disorder caused by tetrasomy 12p mosaicism associated with a supernumerary isochromosome. Craniofacial dysmorphism, learning impairment and seizures are considered characteristic. However, little is known of the seizure and epilepsy patterns seen in PKS. To better define the occurrence and nature of epileptic and non-epileptic paroxysmal events in PKS, we describe our experience with 5 patients and compare their features with data from a larger cohort of PKS patients ascertained via a web-based parental questionnaire. Three of the 5 patients have had definite epileptic seizures, and one other has had paroxysmal events as yet not clarified. Four of the 5 have also had either non-epileptic paroxysmal events or episodes of uncertain nature. In those with epilepsy, all have had some period of relatively refractory seizures, all have required more than one antiepileptic drug, but none experienced status epilepticus. Only one of the patients with epilepsy (the oldest) has gone into remission. In two of the four with non-epileptic events, video-electroencephalographic monitoring has been valuable in clarifying the nature of the events. EEG characteristics include a slow dominant frequency as well as generalized and focal epileptiform features. Brain MRI findings can be normal but are variable. These specific findings correspond well to information reported by parents in a larger cohort of 51 individuals with PKS. Better understanding of the nature of epileptic and non-epileptic events in PKS will result from a more detailed analysis of objective data obtained from this larger cohort, and from deeper understanding of the molecular impact of 12p tetrasomy in selected cell lines.
Keywords: Pallister–Killian syndrome, 12p tetrasomy, Isochromosome 12p, Epilepsy, Non-epileptic paroxysmal events, Seizure semiology, Mosaicism
1. Introduction
Pallister–Killian syndrome (PKS) is a rare, sporadic genetic disorder first described in adults by Pallister [1] and in children by Killian and Teschler-Nicola [2]. Affected individuals present in infancy with dysmorphic features, hypotonia and developmental delay [3,4]. The condition is associated with 12p tetrasomy resulting from the variable expression of a supernumerary isochromosome (i12p) in differing cell lines[3–5]. Diagnosis of this mosaic condition may be elusive due to the absence or reduced expression of the isochromosome in peripheral blood lymphocytes [5,6]. Therefore, identification of the condition depends on a high index of suspicion, and on genotypic studies of cultured skin fibroblasts, buccal mucosal cells or high-sensitivity FISH in peripheral blood lymphocytes.
From a clinical standpoint, the condition is often described as a triad of craniofacial dysmorphism, cognitive impairment and epilepsy [3–5]. However, in contrast to what is known regarding the epilepsy characteristics of several other congenital genetic disorders such as Angelman [7–10], Wolf-Hirschhorn [11,12], Rett [13] and Aicardi [14] syndromes, relatively less is known of the seizure and epilepsy characteristics of people with PKS. The reported incidence of recurrent seizures in individuals with PKS appears to range from 40% to 70% [3,4], but few reports provide much detail regarding the specifics of seizure semiology, treatment options, and response to antiepileptic medications, or prognosis; those that do so describe no more than 2 children per series. Four relatively recent reports [15–18] describe a total of 6 children in fair detail, providing considerable information concerning age of onset, clinical semiology and response to treatment. Long-term follow-up is not described. Based on these reports, it appears that seizure types may be quite variable, ranging from clustered tonic spasms similar to “infantile spasms”, to generalized convulsions and partial seizures. Treatment most often appears to be challenging or incompletely successful, and no particularly unique or common electroencephalographic patterns (as have been suggested with Angelman syndrome [8], for example) have emerged.
Recently, we have added to this existing information by describing the seizure and epilepsy characteristics of a large cohort of PKS patients acquired via a web-based questionnaire in collaboration with the PKS Kids family-based support organization [19]. The self-reported clinical information has been recently summarized, and useful information regarding seizure type, medication management and prognosis so described. This work [19] confirms the rather variable nature of seizures and epilepsy in patients with PKS. In addition, our findings highlight the frequency with which the diagnosis of epilepsy in such patients has proven elusive, and how many may also experience nonepileptic paroxysmal events that make evaluation and management even more challenging.
The accuracy of such self-reported data is, of course, subject to certain limitations including recall bias and variable interpretation of medical terminology. In order to overcome some of these difficulties and to add further to the understanding of epilepsy and paroxysmal disorders in PKS, the following paper summarizes our personal experience with the five patients (also included in our larger PKS cohort) for whom we have provided direct clinical care. The results confirm: 1) the very broad spectrum of seizures, paroxysmal events and epilepsy; 2) the common occurrence of non-epileptic paroxysmal events; 3) the challenge of making a diagnosis of epilepsy in individuals with this condition; and, 4) the potential for remission in patients with PKS.
2. Materials and methods
Collection and analysis of our extended database of PKS subjects has been detailed elsewhere[19]. In brief, individuals with genotypically confirmed PKS were included after parents or guardians provided informed consent. A detailed parent-based, structured questionnaire was completed by a parent or guardian for all subjects included in the database (now containing 51 individuals). Further clarification of questionnaire entries and collection of outside medical records is ongoing.
Of these 51 individuals, the authors have personally provided neurological and medical care for 5 of these patients (Table 1). Families of these patients were contacted again and additional clinical details for this case series were elicited as needed. Permission to conduct this study has been granted by the IRB at the University of Utah. Specific permission to present video-EEG information in a public access publication was solicited and granted by the legal guardians of the children.
Table 1.
Summary of clinical characteristics of five PKS patients.
| Patient ID | Age/sex | (Yr) Sz onset | Seizure types | Medications | DQ | Non-epi events | Sleep dis. | Rmssn |
|---|---|---|---|---|---|---|---|---|
| 1 | 32/F | 0.5 | M, CPS | CLN, PHB, CBZ | <20 | Yes | Yes | Yes |
| 2 | 17/F | 12.5 | GT, GTC, CPS, ?Drop attacks, other | TPM, OXC, LEV, LTG, ZNS | 50 | Yes | Yes | No |
| 3 | 13/M | 4 | M, GT, GTC | CLN, TPM, OXC, LEV | <20 | Yes | Yes | No |
| 4 | 11/F | NA | NA | None | 60 | No | No | NA |
| 5 | 4/M | NA | Uncertain | None | 40 | Yes? | No | NA |
Abbreviations: F, female; M, male; Yr, year; Sz, seizure; NA, Not applicable; M, myoclonic; CPS, complex-partial seizure; GT, generalized tonic; GTC, generalized tonic-clonic seizure; CLN, clonazepam; PHB, phenobarbital; CBZ, carbamazepine; TPM, topiramate; OXC, oxcarbazepine; LEV, levetiracetam; LTG, lamotrigine; ZNS, zonisamide; DQ, Developmental quotient (In most children formal IQ testing was not available. The DQ was calculated as estimated developmental age/chronological age); Non-epi events, non-epileptic paroxysmal events or events of uncertain nature; Rmssn, remission.
3. Case Summaries (from oldest to youngest in chronological age)
3.1. Case 1
This 32 years old woman was born of an uncomplicated pregnancy, labor and delivery. She was diagnosed with PKS in early childhood. She developed myoclonic jerks at 6 months of age. Treatment with clonazepam was successful in controlling seizures. At 17 years of age she was again evaluated for possible epileptic seizures and sleep disturbances. These episodes consisted of abrupt arousal “as if from a nightmare” and occurred up to 8 times per night. During the episodes, her arms and legs would stiffen and she would be poorly responsive and “glassy-eyed”.
Electroencephalography demonstrated a slow dominant posterior frequency. A single mild episode captured during the recording could not be clearly confirmed as epileptic in nature. Nevertheless, itwas believed that these episodes represented nocturnal complexpartial or frontal lobe seizures and treatment with carbamazepine and phenobarbital eventually provided complete seizure control. This treatment also appeared to improve her sleep disturbance. These anticonvulsants were subsequently discontinued without seizure recurrence. Recent evaluation by an adult neurology colleague for sleep disturbance identified episodes, occurring in clusters, of exaggerated startle responses during which eyes open wide sometimes associated with scissoring of the legs. EEG at age 31 years continued to show slow and poorly regulated dominant posterior frequencies but no definite epileptiform discharges. Unusual arousal responses were suspected.
She has intellectual and cortical visual impairment, mild hearing loss and is non-verbal. Her mother believes she may understand simple language and enjoy music. She is non-ambulatory. Examination demonstrates typical coarse facial features seen in PKS and related craniofacial dysmorphism. She exhibits dysconjugate gaze with roving eye movements but seems to regard her mother. She does not have a feeding tube. She has limb and axial hypotonia.
3.2. Case 2
This 17 years old young woman was born of a term, uncomplicated pregnancy. Birth weight was 3.1 kg. She was diagnosed with PKS at 14 months of age.
At 12½ years of age she developed daily unprovoked, generalized tonic seizures lasting “a few seconds” which were associated with loss of postural tone as well as post-ictal somnolence and confusion. Electroencephalography demonstrated a fairly well-regulated 8 Hz dominant posterior rhythm and rare irregular generalized spike and wave discharges (Fig. 1).
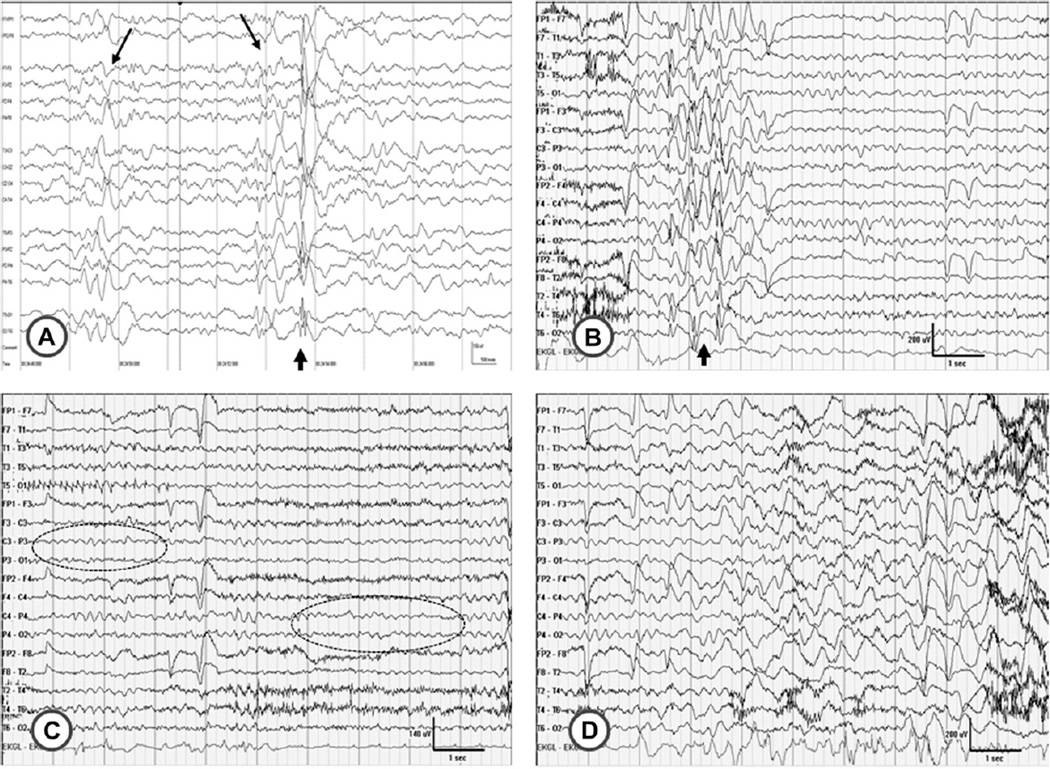
Fig. 1.
Electrographic features of Case 2. A, EEG at 12 years of age. An example of a brief, irregular burst of generalized spike-wave activity (short arrow) is shown; these were rare during the recording. Normal sleep features were seen (long arrows). B, C, D, EEG at 14 years of age. B, another example of a brief, irregular generalized spike-wave discharge is seen (arrow). C, EEG pattern at beginning of the paroxysmal event shown in Video 1. The child is awake and the slow dominant posterior rhythm is evident (ellipses). D, EEG pattern at peak of the event, toward end of video when she is seen to be rocking and swaying forward. Note the diffuse high amplitude delta waves. This is felt to be due to breath-holding which parents reported during many of these events. No epileptiform discharges are seen during this event.
Treatment with topiramate followed by levetiracetam was ineffective. With time, parents noted that episodes of breath holding lasting 20–40 s appeared to precede the onset of her seizures. Two additional types of paroxysmal events emerged consisting of drop-attacks and staring spells. Lamotrigine was instituted empirically, but was also of limited benefit. The paroxysmal events evolved. By age 14 she would “tense up, look up and to the side, get a smile on her face, and then laugh”. She would often twist to the right and one arm would extend and elevate more than the other. Consciousness was not clearly impaired.
Video-EEG monitoring (at 14.5 years of age) demonstrated a fairly well-regulated 5–6 Hz posterior rhythm. Infrequent interictal irregular generalized spike-wave discharges were noted (Fig. 1). Numerous but varied stereotypic events of concern were recorded (Videos 1 and 2). These consisted of stereotyped or repetitive behaviors in which she would tense and sometimes rock or sway her body from side-to-side (Video 1). At other times, she would become abruptly still other than perhaps rotating her head or twisting her face. Though none of these phenomena were associated with definite epileptiform activity, they were often associated with the abrupt onset of high amplitude, frontally dominant, diffuse, rather regular, 2–4 Hz delta activity (see Video 1 and Fig. 1). During sleep, apparent arousals were often associated with periodic, quasi-generalized sharp and slow wave discharges (Video 2,Fig. 2). Definitive diagnosis was, therefore elusive, though it was concluded that most of these events likely did not represent epileptic seizures. Accordingly, lamotrigine was discontinued.
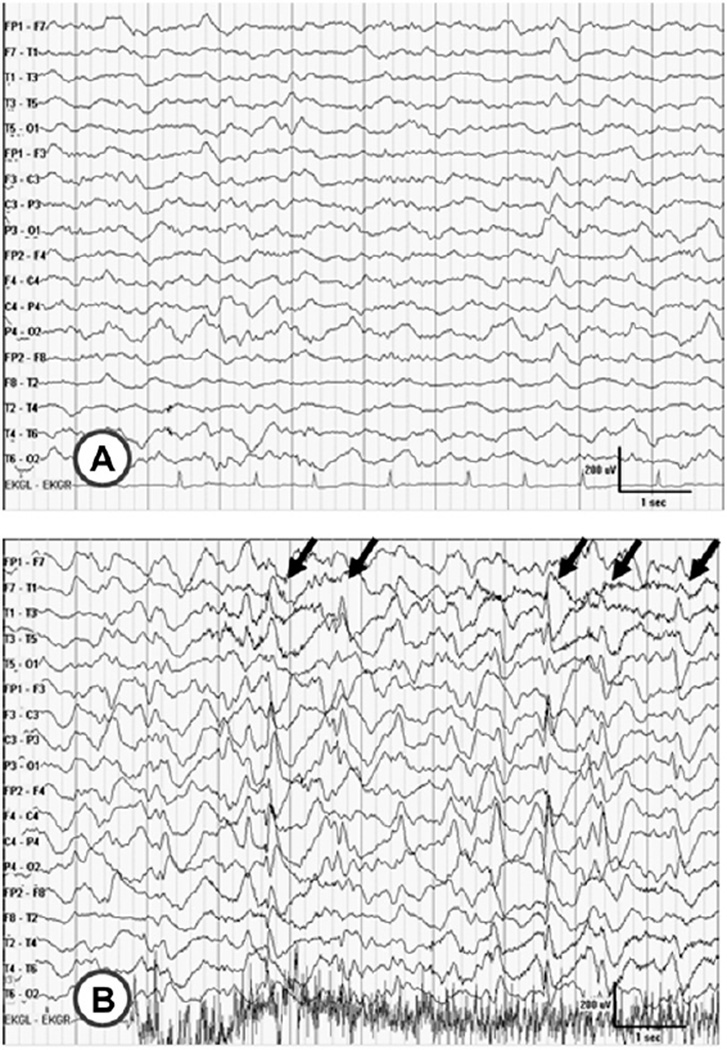
Fig. 2.
EEG features associated with arousal in Case 2. EEG at 14 years of age corresponding to Video 2. A, normal NREM sleep prior to event. B, quasi-periodic generalized sharp and slow wave discharges occurring with arousal (arrows). It is unclear if this is a clinical seizure or not.
Supplementary video related to this article can be found at doi:10.1016/j.ejmg.2012.01.006.
Episodes subsided somewhat in frequency and severity and over time often seemed to be interruptible, as though the patient’s behavior could be redirected. However, at 16.5 years of age, she experienced several generalized tonic-clonic seizures of several minutes duration followed by typical post-ictal somnolence. She experienced a drug eruption with Zonisamide and is now responding well to levetiracetam.
On examination she is an alert, happy and interactive young woman with craniofacial features consistent with PKS. She has a short neck and short digits. She has central obesity anteriorly with an unusually stocky body habitus. She is communicative, but has cognitive difficulties. She is able to follow multiple step instructions and can communicate in short phrases and sentences. She engages in stereotypic behaviors. She has mild generalized hypotonia, normal strength, and is clumsy with awkward motor skills but without frank tremor or ataxia. She can easily arise from a squatting or sitting position. MRI of the brain (at age 12 years) was normal.
3.3. Case 3
This 13 years old boy was born of a pregnancy complicated by polyhydramnios and antenatal diagnosis of PKS was made by amniocentesis. Multiple congenital anomalies were noted including omphalocele, coarctation of the aorta, bicommissural aortic valve with mild aortic stenosis, abnormal mitral valve, hypoplasia of the auditory canals, and imperforate anus. Multiple corrective surgeries were required.
Stereotypic abnormal movements were first observed during sleep at around 15 months of age. Two initial routine EEGs were normal. At four years of age, long-term video-EEG monitoring demonstrated a slow dominant posterior rhythm and frequent, brief, irregular, high amplitude, frontally dominant generalized spike-wave discharges, many of which were associated with subtle generalized myoclonic jerks. Clonazepam and then topiramate were both transiently effective. At nearly six years of age, frequent, intractable, nocturnal generalized tonic seizures lasting 2–3 min appeared. These occurred during sleep numerous times per week and were associated with apnea and cyanosis.
Over time, treatment with oxcarbazepine plus topiramate and/ or levetiracetam resulted in periods of complete seizure control lasting up to 5 months. However, he continues to have frequent generalized tonic-clonic seizures, typically when ill (but not necessarily febrile). The longest seizure-free interval since 8 years of age has been about 2 months. Electroencephalography (at 10 years of age) showed slowing, hemispheric asymmetries, and variable interictal epileptiform features (Fig. 3).
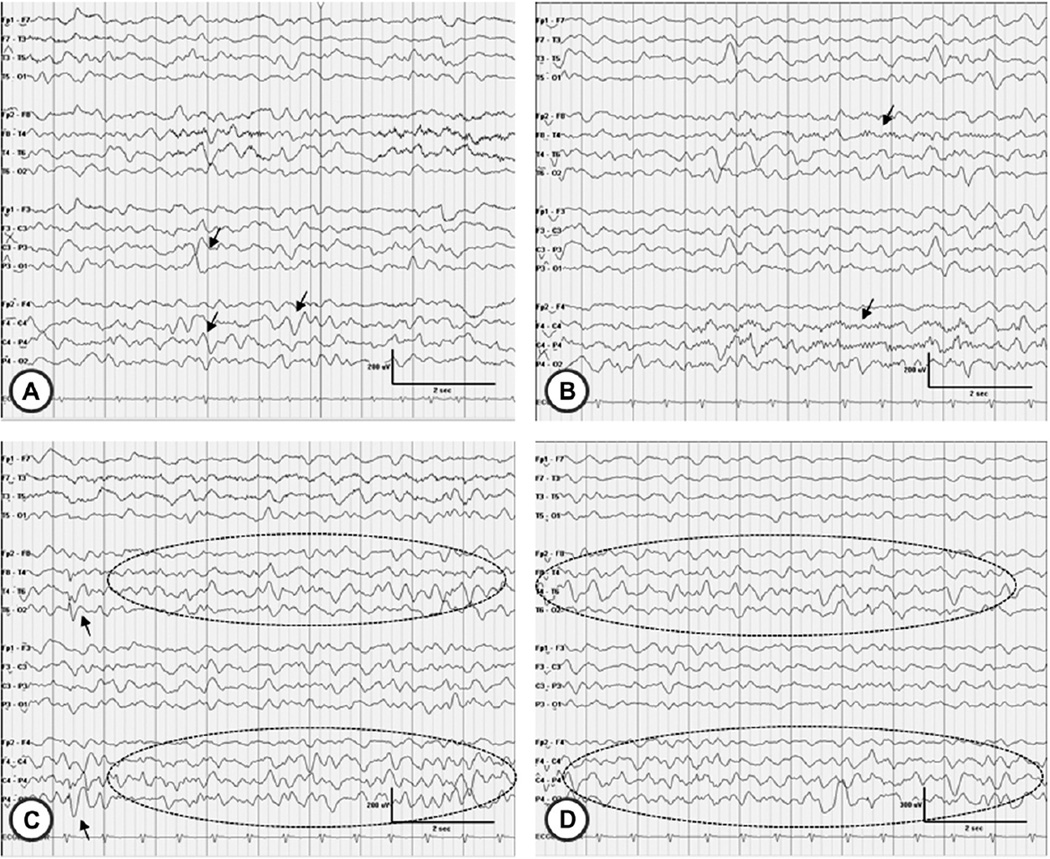
Fig. 3.
Electroencephalographic features of case 3 (at 10 years of age). A, the slow dominant posterior rhythm of 3–6 Hz is apparent; intermixed sharply contoured delta waves (arrows) are evident. B, asymmetric sleep spindles are seen, of higher amplitude over the right hemisphere (arrows). C, a right hemisphere spike (arrow) is seen along with quasi-periodic, sharply contoured delta waves (ellipse) more prominent over the right than the left hemisphere. D, another example of right greater than left hemisphere delta activity (ellipse) is shown.
Other medical problems have included severe gastroesophageal reflux, obstructive and central apnea, upper airway congestion with stertorous respirations and recurrent upper respiratory infections. A percutaneous gastrostomy tube was placed at 6 years of age.
On examination, he is profoundly neurologically impaired. He has coarse facial features with the typical craniofacial dysmorphism of PKS. He has very small external auditory canals. He is non-verbal, does not appear to understand language, has marked cortical visual impairment and cannot roll over, sit up, stand or walk independently. He has marked generalized hypotonia. MRI of the brain (at age 4 months) demonstrated asymmetry of the lateral ventricles with enlargement of the left lateral ventricle, atrophy of the left hemisphere, and hypoplasia of the anterior falx.
3.4. Case 4
This 11 years old girl was born at term following a pregnancy complicated by polyhydramnios. Birth weight was 3.63 kg. She has had mild developmental delays, attends a regular school with resource help, and is friendly and sociable. She has not had seizures. She sleeps 9–10 h per night and takes naps during the day but otherwise does not have evidence of disturbed sleep. She has had headaches and possibly intermittent hypertension though the latter has not been recently confirmed.
On examination, she is an alert, happy and engaging girl who enjoys communicating with others. She has mild, but typical, craniofacial features of PKS, dysconjugate gaze with nystagmus, mild abdominal fullness and central obesity. She speaks in full sentences. She has mild generalized hypotonia and, though awkward in her motor skills, does not have frank tremor or ataxia of limb or gait. MRI of the brain (at 8 months of age) demonstrated mild delay in central myelination along with mild prominence of the lateral ventricles and of the subarachnoid space overlying the cerebral convexities.
3.5. Case 5
This 4 years old boy was born at 36 weeks gestation following an uncomplicated pregnancy. He had neonatal hypoglycemia. His birth weight was 3.04 kg. Dysmorphic features were noted at birth and he was treated for hyperbilirubinemia. PKS was diagnosed at 10 months of age when he was evaluated for developmental delay.
At two and a half years of age (i.e., at his last visit with us), parents described some episodes during which he would stop and stare fixedly for a second or two, after which he would sometimes make a grunting sound. However, he also made this same sound intermittently at other times. One such episode was witnessed during the visit and it was not clear what this represented. Neurological consultation was declined by parents.
4. Discussion
Although the common occurrence of seizures in PKS is well supported by existing literature [3–5], relatively little is known regarding the semiologic characteristics, the spectrum of severity or the prognosis of seizures and epilepsy in individuals with PKS. Taken together, previously described case series and case reports strongly suggest a fairly broad variability in the epilepsy phenotype among these patients [3–5,20,21]. Observations in the five children described here, when combined with our recent web-based questionnaire study [19] of 51 individuals with PKS (Table 2), support this impression. Furthermore, they allow us to draw some general conclusions regarding the seizure characteristics in PKS: 1) Seizures are common in PKS (~60%); 2) No single seizure or epilepsy pattern is most common in PKS patients, though myoclonic and generalized convulsive seizures occur more often than other seizure types (~50%); 3) The age of onset is variable, with neonatal seizures being very rare; 4) Many patients experience one or more period(s) of seizure intractability; and 5) Only a minority of patients who develop seizures appear to undergo remission. It is also worth noting that none of the patients in this clinical series experienced status epilepticus, despite the fact that at least 2 of the 5 had fairly problematic seizures as judged by high seizure frequency and use of multiple anticonvulsants.
Table 2.
Comparison of current clinical PKS cases with questionnaire-based series.
| All patients |
Patients with epilepsy |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (yrs) (mean/median) |
% with Sz | Sz types (order of frequency) |
% other paroxysm events |
% > 1 AED | % > 1 Sz type | % neo Sz | % Sz >15 min | % remission | |
| Current Clinical Cases | 15.4/13 | 60 | GTC, GT, M > others | 80 | 100 | 100 | 0 | 0 | 33 |
| Questionnaire-Based Series | 8.3/5.8 | 53 c 63 s | M ~ GTC, GC > others | 35a | 56 | 48 | 2 | 22b | 30 |
Abbreviations: yrs, years; Sz, seizure; paroxysm, paroxysmal; AED, antiepileptic drugs; neo, neonatal; min, minute; GTC, generalized tonic-clonic seizure; GT, generalized tonic; M, myoclonic; GC, generalized clonic; c, confirmed; s, suspected.
Probably an underestimate due to nature of questionnaire.
Probably an overestimate due to failure to distinguish between clusters of seizures and continuous seizure activity.
Our experience indicates that non-epileptic paroxysmal events are common in individuals with PKS. In four of the five patients in this clinical series and in at least 35% of our questionnaire-based patients (Table 2), paroxysmal events have at times been difficult to characterize. For example, in a period of just a few years our Case 2 experienced a variety of similar phenomena that continue to challenge diagnosis. While the first few seizure-like spells and more recent paroxysmal events appear to have been typical convulsive seizures, many other events appear to be repetitive occurrences that could fall into the category of “self-stimulation” or “motor” or “behavioral” stereotypies [22] as observed in other developmentally impaired children with autistic spectrum disorders [22,23].We postulate that some of the EEG changes (e.g. high amplitude slow waves) that accompanied (and often preceded) these events (see Video 1) could have represented effects of breath holding or hyperventilation. However, the possibility of complex-partial seizures of deep frontal lobe origin could not be completely ruled out either. The ability of her caretakers to interrupt or to influence the duration and pattern of these events supports the hypothesis that these represent some form of stereotypy [22], while the unusual ictal EEG findings make it difficult to exclude the possibility of frontal lobe seizures. This conundrum is further complicated by the incontrovertible interictal epileptiform abnormalities that certainly would be consistent with an epileptic disorder (Fig. 1).
The challenge of distinguishing epileptic from non-epileptic paroxysmal events is, of course, not limited to PKS. In fact, the problem is well recognized in autism [23,24] and appears to be particularly common in individuals with neurologic disorders in which autistic features and automatisms are prominent, such as Rett syndrome [25,26] and Tuberous sclerosis. In autism spectrum disorders, the high rate of interictal epileptiform abnormalities [24] raises the index of suspicion for clinical epileptic seizures. Thus, stereotypic events accompanied by withdrawal, staring, and other automatisms such as unusual posturing, flapping and rocking may be misinterpreted as epileptic in nature [23]. Video-EEG may be useful in differentiating between these episodes [25]. In PKS, the distinction between epileptic and non-epileptic phenomena may prove similarly problematic.
The underlying molecular and neurophysiological disturbances leading to epileptic seizures in PKS are at present not known. Chromosome 12 contains more than 1400 coding genes and 487 loci that have been associated with human disease [27]. Gene mutation or altered expression may lead to epilepsy via a number of mechanisms including disturbances in ligand- or voltage-gated ion channels [28], synaptic function [29,30], or brain formation [31]. While the pathophysiology of seizure generation in such conditions may result from gross organizational defects of neuronal circuits, mutations within some of these same genes may lead to epilepsy even in the absence of visible brain malformation [31]. Potential candidate genes regulating ion channels, synaptic physiology or CNS development exist on chromosome 12p. For example, SLC6A12 (BGT-1) and SLC6A13 (GAT2) encode transporter molecules involved in GABA reuptake, and ERC (RAB6-interacting protein 2 isoform beta) encodes a protein involved in cytomatrix organization at the active zone of nerve terminals which regulates neurotransmitter release [32]. Therefore, study of the specific effects of 12p tetrasomy on gene expression may lead to better understanding of the pathophysiology of seizures in PKS as well as in other conditions sharing common molecular disturbances.
To date, no consistent imaging abnormality has been demonstrated in PKSto suggest a common structural disturbance accounting for altered synaptic physiology. Broad phenotypic variability does appear to be typical of human conditions associated with genetic mosaicism [33,34]. The variation in seizure type and severity in individuals with PKS could then reflect the differential expression of the chromosomal defect in the progenitors of particular neural or glial cell lines.
Though limited to thorough clinical observation in five patients, the results of this case series, combined with parental report in our cohort of 51 (i.e. 46 additional) individuals, do provide some guidance for practitioners caring for children with PKS. These findings suggest that physicians should maintain a high index of suspicion for seizures in children with PKS. However, careful clinical evaluation and a healthy degree of attention to detail are needed as these children are likely to have both epileptic and non-epileptic events. Reliance on the interictal EEG alone to confirm a diagnosis of epilepsy is probably unwise. Video-EEG monitoring will often be necessary to better characterize paroxysmal events and to optimally guide treatment. Finally, while many of these children have refractory seizures, most will not require recurrent hospitalization for intractable epilepsy and few will experience status epilepticus. These facts, coupled with the chance of remission, do offer some hope for parents and families as they care for a loved one with PKS.
Supplementary Material
Acknowledgments
The authors thank the families of the PKS Kids organization for their support and participation. We thank Annie Johnson and Alyssa Goerdt for assistance in preparing the figures.
Appendix. Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejmg.2012.01.006.
Contributor Information
Francis M. Filloux, Email: francis.filloux@hsc.utah.edu.
John C. Carey, Email: john.carey@hsc.utah.edu.
Ian D. Krantz, Email: Ian2@mail.med.upenn.edu.
Jeffrey J. Ekstrand, Email: jeffrey.ekstrand@hsc.utah.edu.
Meghan S. Candee, Email: meghan.candee@hsc.utah.edu.
References
- 1.Pallister, Meisner, et al. The Pallister mosaic syndrome. Birth Defects Orig. Artic. Ser. 1977 [PubMed] [Google Scholar]
- 2.Killian W. Teschler-Nicola, Case report 72: mental retardation, unusual facial appearance, abnormal hair. Synd. Ident. 1981;7:6–7. [Google Scholar]
- 3.Reynolds JF, Daniel A, Kelly TE, Gollin SM, Stephan MJ, Carey J, Adkins WN, Webb MJ, Char F, Jimenez JF, Opitz JM. Isochromosome 12p mosaicism (Pallister mosaic aneuploidy or Pallister-Killian syndrome): report of 11 cases. Am. J. Med. Genet. 1987;27:257–274. doi: 10.1002/ajmg.1320270204. [DOI] [PubMed] [Google Scholar]
- 4.Bielanska MM, Khalifa MM, Duncan MV. Pallister-Killian syndrome: a Mild case diagnosed by fluorescene in situ hybridization. Review of the literature and expansion of the phenotype. Am. J. Med. Genet. 1996;65:104–108. doi: 10.1002/(SICI)1096-8628(19961016)65:2<104::AID-AJMG4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Speleman F, Leroy JG, Van Roy N, De Paepe A, Suijkerbuijk R, Brunner H, Looijenga L, Verschraegen-Spae MR, Orye E. Pallister-Killian syndrome: characterization of the isochromosome 12p by fluorescent in situ hybridization. Am. J. Med. Genet. 1991;41:381–387. doi: 10.1002/ajmg.1320410321. [DOI] [PubMed] [Google Scholar]
- 6.Smigiel R, Pilch J, Makowsa I, Buska H, Slezak R, Sasiadek MM. The Pallister-Killian syndrome in a child with rare karyotypeda diagnostic problem. Eur. J. Pediatr. 2008;167:1063–1065. doi: 10.1007/s00431-007-0608-7. [DOI] [PubMed] [Google Scholar]
- 7.Conant KD, Thibert RL, Thiele EA. Epilepsy and the sleep-wake patterns found in Angelman syndrome. Epilepsia. 2009;50:2497–2500. doi: 10.1111/j.1528-1167.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 8.Korff CM, Kelley KR, Nordli DR., Jr Notched delta, phenotype and Angelman syndrome. J. Clin. Neurophysiol. 2005;22:238–243. doi: 10.1097/01.wnp.0000167930.90824.0f. [DOI] [PubMed] [Google Scholar]
- 9.Laan LA, Brouwer OF, Begeer CH, Zwinderman AH, van Dijk JG. The diagnostic value of the EEG in Angelman and Rett syndrome at a young age. Electroencephalogr. Clin. Neurophysiol. 1998;106:404–408. doi: 10.1016/s0013-4694(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 10.Minassian BA, DeLorey TM, Olsen RW, Philippart M, Bronstein Y, Zhang Q, Guerrini R, Van Ness P, Livet MO, Delgado-Escueta AV. Angelman syndrome: correlations between epilepsy phenotypes and genotypes. Ann. Neurol. 1998;43:485–493. doi: 10.1002/ana.410430412. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia A, Filippia T, South ST, Carey JC. Spectrum of epilepsy and electroencephalogram patterns in Wolf-Hirschhorn syndrome: experience with 87 patients. Dev. Med. Child. Neurol. 2009;51:373–380. doi: 10.1111/j.1469-8749.2008.03233.x. [DOI] [PubMed] [Google Scholar]
- 12.Worthington JC, Rigby AS, Quarrell OW. Seizure frequency in adults with Wolf-Hirschhorn syndrome. Am. J. Med. Genet. Part A. 2008;146:2528–2531. doi: 10.1002/ajmg.a.32483. [DOI] [PubMed] [Google Scholar]
- 13.Buoni S, Zannolli R, Felice CD, Saponari S, Strambi M, Dotti MT, Castrucci E, Corbini L, Orsi A, Hayek J. Drug-resistant epilepsy and epileptic phenotype-EEG association in MECP2 mutated Rett syndrome. Clin. Neurophysiol. 2008;119:2455–2458. doi: 10.1016/j.clinph.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsuka Y, Oka E, Terasaki T, Ohtahara S. Aicardi syndrome: a longitudinal clinical and electroencephalographic study. Epilepsia. 1993;34:627–634. doi: 10.1111/j.1528-1157.1993.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 15.Cerminara C, Compagnone E, Bagnolo V, Galasso C, Lo-Castro A, Brinciotti M, Curatolo P. Late-onset epileptic spasms in children with Pallister-Killian syndrome: a report of two new cases and review of the electroclinical aspects. J. Child. Neurol. 2010;25:238–245. doi: 10.1177/0883073809336933. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Carpintero R, McLellan A, Parmeggiani L, Cockwell AE, Ellis RJ, Cross JH, Eckhardt S, Guerrini R. Pallister-Killian syndrome: an unusual cause of epileptic spasms. Dev. Med. Child. Neurol. 2005;47:776–779. doi: 10.1017/S0012162205001623. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H, Fukuda M, Murakami H, Kamiyama N, Miyamoto Y. A case of Pallister-Killian syndrome associated with West syndrome. Pediatr. Neurol. 2007;37:226–228. doi: 10.1016/j.pediatrneurol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Gerstner T, Bell N, Koenig SA. Valproate-associated reversible encephalopathy in a 3-year-old girl with Pallister-Killian syndrome. Ther. Clin. Risk Manag. 2008;4:645–647. doi: 10.2147/tcrm.s2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candee M, Carey JC, Krantz I, Filloux F. Seizure characteristics in Pallister-Killian syndrome, manuscript submitted for publication. doi: 10.1002/ajmg.a.35567. [DOI] [PubMed] [Google Scholar]
- 20.Horneff G, Majewski F, Hildebrand B, Voit T, Lenard HG. Pallister-Killian syndrome in older children and adolescents. Pediatr. Neurol. 1993;9:312–315. doi: 10.1016/0887-8994(93)90071-j. [DOI] [PubMed] [Google Scholar]
- 21.Stalker HJ, Gray BA, Bent-Williams A, Zori RT. High cognitive functioning and behavioral phenotype in Pallister-Killian syndrome. Am. J. Med. Genet. 2006;140:1950–1954. doi: 10.1002/ajmg.a.31403. [DOI] [PubMed] [Google Scholar]
- 22.Freeman RD, Soltanifar A, Baer S. Stereotypic movement disorder: easily missed. Dev. Med. Child. Neurol. 2010;52:733–738. doi: 10.1111/j.1469-8749.2010.03627.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim HL, Donnelly JH, Tournay AE, Book TM, Filipek P. Absence of seizures despite high prevalence of epileptiform EEG abnormalities in children with autism monitored in a tertiary care center. Epilepsia. 2006;47:394–398. doi: 10.1111/j.1528-1167.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- 24.Parmeggiani A, Barcia G, Posar A, Raimondi E, Santucci M, Scaduto MC. Epilepsy and EEG paroxysmal abnormalities in autism spectrum disorders. Brain Dev. 2010;32:783–789. doi: 10.1016/j.braindev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Glaze DG, Schultz RJ, Frost JD. Rett syndrome: characterization of seizures versus non-seizures. Electroencephalogr. Clin. Neurophysiol. 1998;106:79–83. doi: 10.1016/s0013-4694(97)00084-9. [DOI] [PubMed] [Google Scholar]
- 26.Cardoza G, Clarke A, Wilcox J, Gibbon F, Smith PE, Archer H, Hryniewiecka-Jaworska A, Kerr M. Epilepsy in Rett syndrome: association between phenotype and genotype, and implications for practice. Seizure. 2011;20:646–649. doi: 10.1016/j.seizure.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Scherer SE, Muzny DM, Buhay CJ. et al., Baylor College of Medicine Human Genome Sequencing Center Sequence Production Team, The finished DNA sequence of human chromosome 12. Nature. 2006;440:346–351. doi: 10.1038/nature04569. [DOI] [PubMed] [Google Scholar]
- 28.Nicita F, De Liso P, Danti FR, Papetti L, Ursitti F, Castronovo A, Allemand F, Gennaro E, Zara F, Striano P, Spalice A. The genetics of monogenic idiopathic epilepsies and epileptic encephalopathies. Seizure. 2011 Sep 12; doi: 10.1016/j.seizure.2011.08.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, Jorgensen EM. Defects in synaptic vesicle docking in unc-18 mutants. Nat. Neurosci. 2003;6:1023–1030. doi: 10.1038/nn1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molinari F, Kaminska A, Fiermonte G, Boddaert N, Raas-Rothschild A, Plouin P, Palmieri L, Brunelle F, Palmieri F, Dulac O, Munnich A, Colleaux L. Mutations in the mitochondrial glutamate carrier SLC25A22 in neonatal epileptic encephalopathy with suppression bursts. Clin. Genet. 2009;76:188–194. doi: 10.1111/j.1399-0004.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- 31.Sherr EH. The ARX story (epilepsy, mental retardation, autism, and cerebral malformations): one gene leads to many phenotypes. Curr. Opin. Pediatr. 2003;15:567–571. doi: 10.1097/00008480-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Morar B, Zhelyazkova S, Azmanov DN, Radionova M, Angelicheva D, Guergueltcheva V, Kaneva R, Scheffer IE, Tournev I, Kalaydjieva L, Sander JW. A novel GEFS+ locus on 12p13.33 in a large Roma family. Epilepsy Res. 2011 Sep 12; doi: 10.1016/j.eplepsyres.2011.08.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Griffith CB, Vance GH, Weaver DD. Phenotypic variability in trisomy 13 mosaicism: two new patients and literature review. Am. J. Med. Genet. A. 2009;149:1346–1358. doi: 10.1002/ajmg.a.32883. [DOI] [PubMed] [Google Scholar]
- 34.Tucker ME, Garringer HJ, Weaver DD. Phenotypic spectrum of mosaic trisomy 18: two new patients, a literature review, and counseling issues. Am. J. Med. Genet. A. 2007;143:505–517. doi: 10.1002/ajmg.a.31535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.