Abstract
The transcriptional regulators of pluripotency, POU5F1 (OCT4), NANOG and SOX2, are highly expressed in embryonal carcinoma (EC). In contrast to OCT4 and NANOG, SOX2 has not been demonstrated in the early human germ cell lineage or carcinoma in situ (CIS), the precursor for testicular germ cell tumours (TGCTs). Here, we have analysed SOX2 expression in CIS and overt TGCTs, as well as normal second and third trimester fetal, prepubertal and adult testes by in situ hybridisation and immunohistochemistry using three different antibodies. In contrast to earlier studies, we detected SOX2 mRNA in most CIS cells. We also detected speckled nuclear SOX2 immunoreactivity in CIS cells with one primary antibody, which was not apparent with other primary antibodies. The results demonstrate SOX2 gene expression in CIS for the first time and raise the possibility of post-transcriptional regulation, most likely sumoylation as a mechanism for limiting SOX2 action in these cells.
Keywords: carcinoma in situ (CIS); intratubular germ cell neoplasia (ITGCN); testicular germ cell tumour (TGCT); Sertoli cell, pluripotency
Introduction
Embryonic stem cells (ESCs), which are derived from the inner cell mass (ICM) of the blastocyst, are defined by self-renewal and the ability to differentiate to representatives of all three germ layers of the embryo; the latter property is termed pluripotency. Studies in mouse and human ESCs indicate that the transcription factors OCT4 (POU5F1), SOX2 and NANOG are crucial for maintenance of pluripotency (Boyer et al. 2005; Loh et al. 2006).
Testicular germ cell tumours (TGCTs), characteristically seen in young adult men, can be divided in two subtypes: the seminomas and non-seminomas. The carcinoma in situ (CIS) cell, also known as intratubular germ cell neoplasia (ITGCN) or testicular intraepithelial neoplasia (TIN), is the preinvasive stage of both subtypes of TGCTs (Skakkebaek 1972). Gene expression profiling data have shown a marked resemblance between CIS and ESCs (Almstrup et al. 2004; Skotheim et al. 2005) as well as between CIS and fetal gonocytes (Biermann et al., 2007; Sonne et al., 2009). Accordingly, CIS cells that follow the non-seminoma fate and undergo neoplastic transformation into embryonal carcinoma (EC) cells, demonstrate a capability for self-renewal and wide-ranging differentiation (Ulbright 1993; Chaganti et al. 2000), reminiscent of ESCs in vitro. EC cells can differentiate to various lineages of somatic cells and give rise to teratomas (TERs). Nonseminomas may also contain elements of choriocarcinoma (CC) cells and yolk sac tumour (YST) that are considered as malignant as EC cells but are defined by restriction to the extra-embryonic lineage (Ulbright et al. 1999). In contrast, seminomas remain capable of metastasis but lack such propensities for differentiation and maintain a closer resemblance to the untransformed germ cell lineage.
Interestingly, OCT4 and NANOG have been described in CIS and the overt TGCTs that are derived from CIS (except for fully differentiated TERs), as well as in normal fetal gonocytes (Looijenga et al. 2003; Avilion et al. 2003; Gidekel et al. 2003; Rajpert-De Meyts et al. 2004; Honecker et al. 2004; Jones et al. 2004; Hoei-Hansen et al. 2005; Hart et al. 2005; Western et al. 2005; Perrett et al. 2008; Kerr et al. 2008). Nuclear SOX2 has been described in nonseminomatous TGCTs (mainly EC) but not in CIS (Korkola et al. 2006; de Jong et al. 2008) and seminomas except for one case in 100 (Santagata et al. 2007). SOX2 has also been reported as absent in germ cells during the first (Perrett et al. 2008), second and third trimester of fetal development, and in adult ovary and testis, but positive in Sertoli cells surrounding CIS (de Jong et al. 2008). In contrast, Sox2 is present in mouse PGCs as both transcripts and protein (Western et al. 2005).
Given the hypothetical origin of CIS cells from the early germ cell lineage, we have extended our previous studies to investigate the expression of SOX2 during pre-pubertal testis development and in CIS at the level of mRNA and protein. As documented here, SOX2 protein was absent from the germ cell lineage during development. In CIS, the SOX2 gene was expressed and data were acquired consistent with post-translational modification that would limit the action of the encoded transcription factor.
Results
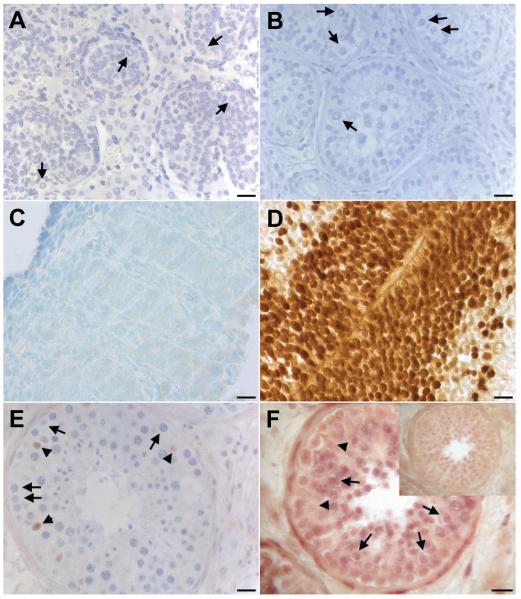
Following previous studies of specimens from the first trimester of human development (Perrett et al. 2008), we investigated the expression of SOX2 during later stages of testis development (as summarised in Table 1). No germ cell staining was detected in several samples of fetal (Fig. 1A) or pre-pubertal testis (Fig. 1B), using either the AB5603 anti-SOX2 antibody from Millipore (except for some weak cytoplasmic background staining) or AF2018 from R&D systems. The latter antibody also failed to detect SOX2 in germ cells during the first trimester while neural tube from the same fetus showed strong staining (Fig. 1 C-D). This is in accordance with our previous report with AB5603 (Perrett et al. 2008). However, SOX2 was detected in a small number of Sertoli cells in adult testis (Fig. 1E), averaging 1-2 positive cells per tubule, consistent with a previous report by others (de Jong et al., 2008). This overlapped with the more widespread detection of SOX2 mRNA that included primary spermatocytes by in situ hybridisation (ISH) (Fig. 1F). Some nuclear staining by ISH was observed, which may be due to nonspecific reaction or the presence of small regulatory RNAs, including possibly sense-antisense transcripts (Katayama et al. 2005), as we hypothesised previously (Novotny et al. 2007a).
TABLE 1.
SOX2 EXPRESSION BY IMMUNOHISTOCHEMISTRY (IHC) AND IN SITU HYBRIDISATION (ISH) DURING NORMAL HUMAN TESTICULAR DEVELOPMENT AND TGCTS
| IHC with AB5603 |
IHC with AF2018 |
Transcripts by ISH |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue and cell type | Age | N | Staining score |
Intensity and location | N | Staining score |
Intensity and location | N | Staining score |
Intensity and location |
| Fetal and prepubertal testis | ||||||||||
| Gonocytes/prespermatogonia | 15-41 wg | 4 | − | Negative (weak cytoplasmic background staining) |
3 | − | None | ND | ||
| Infantile/prepubertal spermatogonia | 2 m - 10 y | 7 | − | Negative (weak cytoplasmic background staining) |
6 | − | None | ND | ||
| Adult testis (including normal tubules in vicinity of CIS) | ||||||||||
| Spermatogonia | Adult | 9 | − to + | Medium nuclear (in a subset of cells) |
9 | − | None | 3 | +− | Weak cytoplasmic |
| Sertoli, undifferentiated in CIS tubules | Adult | 9 | ++ | Medium nuclear | 9 | ++ | Strong nuclear | 2 | ++ | Moderate cytoplasmic |
| Spermatocytes | Adult | 9 | +/− | Weak nuclear | 9 | − | None | 3 | ++ | Weak cytoplasmic and nuclear (some signal in sense controls) |
| Spermatids | Adult | 9 | − | None | 9 | − | None | 3 | + | Weak cytoplasmic |
| Testicular neoplasms | ||||||||||
| CIS | Adult | 11 | ++ | Weak nuclear | 11 | − | None | 2 | ++ | Heterogeneous cytoplasmic |
| Seminoma | Adult | 6 | − | None | 6 | − | None | 3 | ++ | Moderate cytoplasmic |
| Embryonal carcinoma | Adult | 5 | +++ | Strong nuclear; | 5 | +++ | Strong nuclear | 3 | +++ | Strong cytoplasmic |
| Teratoma | Adult | 4 | ++ | Strong nuclear (in a subset of cells positive for OCT4) |
4 | ++ | Strong nuclear (in a subset of cells positive for OCT4) |
ND | ||
| Testicular B cell lymphoma | 58 y | 1 | − | None | 1 | − | None | ND | ||
The proportion of positively stained cells was assessed using a semi-quantitative score (as described in Methods). All fetal, infantile and prepubertal sections were from formalin fixed archival tissues not suitable for ISH. Abbreviations: CIS, carcinoma in situ ; m, months; N, number; ND, not done; wg, weeks of gestation; y, years.
Fig. 1. Expression of SOX2 in normal testis during development.
(A) fetal testis at 17 weeks of gestation; (B) pre-pubertal testis at 7 years (arrows in A and B point to SOX2-negative germ cells); (C) fetal testis at 8 weeks of gestation and (D) neural tube from the same fetus; (E) adult testis (arrows point to SOX2-negative germ cells, arrowheads to positive Sertoli cells). (F) SOX2 transcripts in normal testis (arrows point to primary spermatocytes, arrowheads to Sertoli cells), top right corner shows sense control. Size bars represent 20 μm.
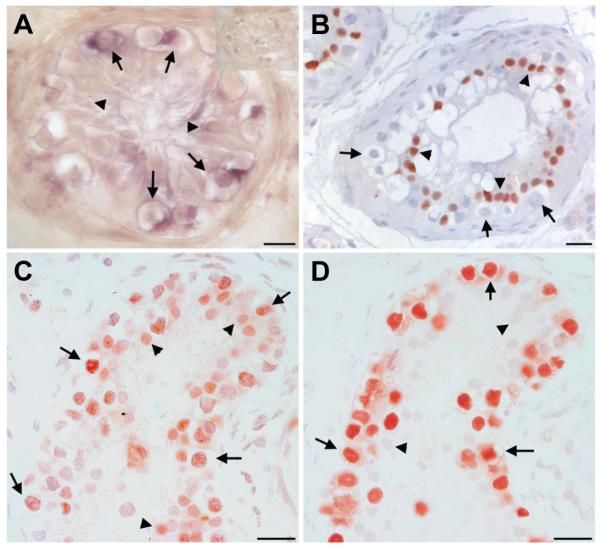
Based on previous findings (Perrett et al. 2008; de Jong et al. 2008), we extended our investigations to the testes from individuals with CIS (results of immunostaining and ISH are summarised in Table 1). In normal seminiferous tubules nuclear SOX2 was detected in a small subset of spermatogonial cells (only with AB5603, data not shown). SOX2 transcripts were detected in CIS cells (arrows in Fig. 2A) with corresponding heterogeneous speckled nuclear immunoreactivity using the AB5603 anti-SOX2 (Fig. 2C) but not the AF2018 anti-SOX2 antibody (Fig. 2B). This unexpected pattern suggested antibody-specific detection of modified forms of the transcription factor present in CIS cells compared to the more characteristic nuclear staining seen in human ESC and EC cells (Perrett et al. 2008). As such modifications would alter SOX2 molecular weight, we conducted immunoblotting of SOX2 using both AF2018 and AB5603 antibodies (Fig. 3). A single band of approximately 45 kD was observed for human ESCs using both anti-SOX2 antibodies. In two CIS testis samples, no bands of SOX2 immunoreactivity were apparent using the AF2013. In contrast, corresponding most likely to the speckled immunostaining observed in CIS cells, a single larger band (approximately 50-55 kD) was apparent using the AB5603 antibody (Fig. 3). Using both antibodies, nuclear SOX2 expression was detected by IHC in poorly differentiated Sertoli cells within CIS tubules (Fig. 2 B,C). The lack of a corresponding band in the Western blot is likely due to the small number of these cells in tissue samples.
Fig. 2. Localisation of SOX2 transcripts and SOX2 protein in testicular carcinoma in situ (CIS).
(A) In situ hybridisation showing the localisation of SOX2 transcripts in CIS testis (arrows), and weak staining in Sertoli cells (arrowheads). Top right corner shows sense control. (B) immunostaining with the AF2018 goat SOX2 antibody from R&D Systems, showing lack of the protein in CIS cells (arrows) but its presence in Sertoli cell nuclei (arrowheads), (C) immunostaining with the AB5603 SOX2 antibody from Millipore showing Sertoli cell nuclear staining (arrowheads) and variable speckled nuclear staining of CIS cells (arrows), and (D) OCT4 used as a marker of CIS cells (arrows) in the serial section for C. Size bars represent 20 μm.
Fig. 3. Immunoblotting for SOX2 in samples of human testicular CIS.
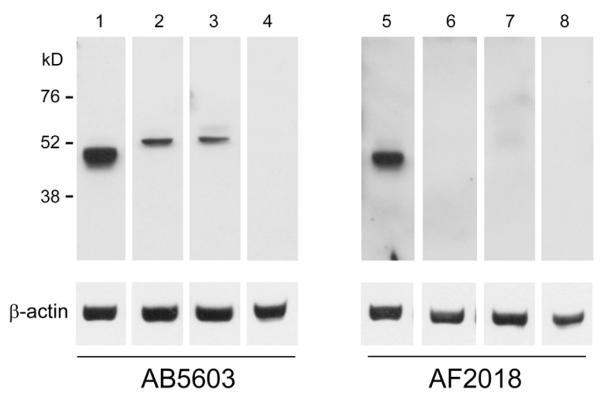
Identical immunoblots for SOX2 using AB5603 (lanes 1-4) and AF2018 (lanes 5-8) antibodies with the detection of beta-actin as loading control. Lane 1 and 5, human ESCs; Lane 2 and 6, CIS testis sample 1; Lane 3 and 7, CIS testis sample 2; Lane 4 and 8, human fetal heart (negative control). Sizes to the left indicate the band positions from the marker lane.
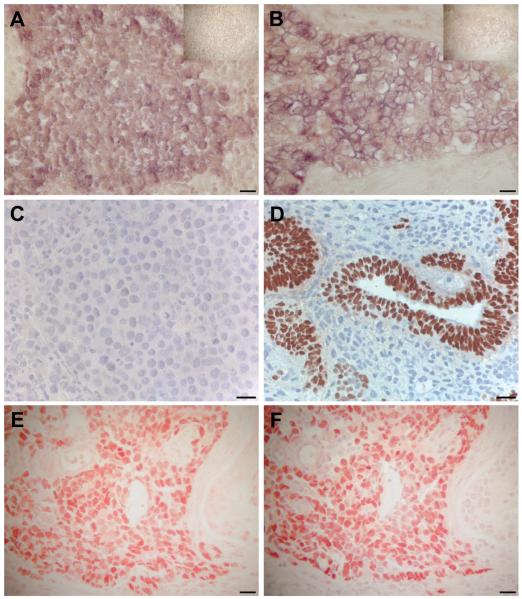
In overt TGCTs, SOX2 transcripts were detected in focal areas of seminomas by ISH (Fig. 4A). Conversely, by IHC, SOX2 protein was not detected in seminomas using either the AB5603 (Perrett et al. 2008) or AF2018 antibodies (Fig. 4C). In EC, SOX2 transcripts (Fig. 4B) and protein (Fig. 4 D-E) were detected; the latter using both anti-SOX2 antibodies. Immunostaining for OCT4 in EC showed exactly the same pattern as for SOX2 (AB5603) (Fig. 4F). In addition, SOX2 was expressed within some differentiated areas of teratomas, particularly papillary structures, which were OCT4-negative.
Fig. 4. Localisation of SOX2 mRNA and protein in testicular germ cell tumours.
In situ hybridisation with an anti-sense SOX2 probe in (A) seminoma, (B) embryonal carcinoma (EC). Top right corners show sense controls. Note that only tumour cells are positive, while the stromal compartment is negative in both tumour types. Immunostaining with two SOX2 antibodies, AF2018 (from R&D) in (C,D), and AB5603 from Millipore in (E) demonstrating: (C) the lack of SOX2 protein in seminoma, (D) the presence of SOX2 protein in EC areas of nonseminomas, while the more differentiated teratomatous components are negative. Identity of EC is confirmed in the lowest panel by staining of serial sections for SOX2 (E) and OCT4 (F). Size bars represent 20 μm.
Discussion
We previously showed that SOX2, unlike OCT4 and NANOG, is absent from PGCs and gonocytes during the first trimester of human development (Perrett et al. 2008). These data are confirmed here using an alternative anti-SOX2 antibody and are in stark contrast to data from mouse PGCs, which express Sox2 (Perrett et al. 2008). We decided to investigate whether or not the same is true in preinvasive germ cell neoplasia and found evidence of SOX2 gene expression in CIS cells accompanied by heterogeneous speckled nuclear SOX2 protein using the Millipore AB5603 antibody. Staining in CIS was absent using the R&D Systems AF2018 antibody consistent with two previous reports that used the same antibody (Korkola et al. 2006; de Jong et al. 2008). Similar negative protein data were published by Biermann et al., 2007 (Biermann et al. 2007), who used a rabbit polyclonal antibody that is no longer available.
It is possible that the additional staining observed in different germ cell derivatives (some spermatogonia and CIS cells) using the Millipore AB5603 antibody is due to cross-reaction with targets others than SOX2; possibly other transcription factors from the SOX family. Of note, cross-reactivity with SOX17 is highly unlikely as this latter protein is readily detected in human fetal gonad during the first trimester when staining using anti-SOX2 antibodies AB5603 (or AF2018) is absent (N Hanley, unpublished findings). We propose an alternative possibility, that the AB5603 antibody detects a modified form of SOX2 not recognised by AF2018. The manufacturer of AB5603 details SOX2 as a 34 kD band on immunoblotting of isolates from mouse ESCs and human NTERA2-d1 EC cells, with additional bands at ~45 and ~50-55 kD. In fact, in human ESCs, we only detected SOX2 at approximately 45 kD using either antibody. Using AB5603, we detected a single band of 50-55 kD in the CIS samples, which is the previously recognised size of sumoylated SOX2 (Tsuruzoe et al. 2006), and compatible with the speckled pattern detected in CIS cells (Weger et al. 2003). Consistent with this hypothesis is the fact that the R&D AF2018 antibody is raised against the region of the SOX2 protein (amino acid 135-317) that contains the sumoylation site at amino acid 247. Thus, sumoylation, predicted to alter transcription factor conformation, would also be predicted to alter recognition by the AF2018 antibody.
The presence of SOX2 transcripts but variable protein detection in CIS cells is reminiscent of a similar experience during our analysis of the CDH1 gene, which was highly expressed in CIS cells at the RNA level but undetectable as protein with two out of three commercial antibodies, despite clear immunohistochemical staining in some overt TGCTs (Sonne et al. 2006). We concluded that the expression of CDH1 was post-transcriptionally down-regulated in CIS cells but not in the overt TGCTs. Such a pattern of gene expression appears to be more common in CIS cells than previously thought. We believe that post-translational or post-transcriptional regulation of certain genes in CIS cells may be a common physiological mechanism operating during germ cell differentiation; for instance, via sumoylation or via regulatory function of small RNAs, as we also previously demonstrated for the E2F1 gene (Novotny et al. 2007b).
SOX2 was detected by both antibodies in a subset of Sertoli cells, especially in association with CIS cells, in agreement with previous observations (de Jong et al. 2008). Function of the transcription factor in this location remains to be established. The presence of SOX2 in Sertoli cells adjacent to CIS cells may reflect their immaturity, as we often see morphologically immature Sertoli cells in patients with CIS and testicular dysgenesis. However, Sertoli cells during fetal or pre-pubertal development did not express SOX2.
In conclusion, we have extended findings in this study that the pluripotency-linked SOX2 transcription factor is not expressed in normal germ cells during development. SOX2 mRNA is detected in a number of CIS cells accompanied by SOX2 immunoreactivity that is consistent with sumoylation. Progression from CIS into overt TGCTs sees loss of speckled nuclear SOX2 immunoreactivity in seminomas and robust detection of nuclear transcription factor in pluripotent EC. Unravelling of the mechanisms that regulate these changes remains a challenge but one that is important to elucidate in order to gain full understanding of how germ cell tumourigenesis occurs.
Materials and Methods
Tissue samples
The Regional Committee for Medical Research Ethics in Denmark approved the use of all human tissue for this project. The tissue samples from adults with testicular neoplasms were obtained directly after orchidectomy and macroscopic pathological evaluation. Testicular samples were fixed overnight at 4°C in formalin or paraformaldehyde (PFA), and subsequently embedded in paraffin. A series of 16 overt testicular tumours were analysed by immunohistochemistry (IHC), including six classical seminomas (mean age 32 years / range 28-37), nine nonseminomatous tumour components (mean age 29 years / range 24-35) and one non-germinal testicular neoplasm, a B-cell lymphoma from an adult man as an additional control. Eleven samples of testicular CIS were analysed (mean age 30 years / range 26-37). We included four samples of normal fetal testicular tissue from 15-41 weeks of gestation (wg), (obtained from induced or spontaneous abortion or autopsy of stillbirths), and seven samples of infantile and prepubertal tissues (age 2 months - 10 years). Finally, we examined normal adult testis using nine specimens of tissue removed because of TGCT, but in which there were normal preserved tubules with complete spermatogenesis (mean age 31 years / range 26-37).
Immunohistochemical staining and immuno-blotting
Immunohistochemical staining (IHC) was performed using commercially available polyclonal antibodies: rabbit anti-SOX2 (AB5603, Millipore, Temecula, CA, US) and goat anti-human SOX2 (AF2018, R&D Systems, Minneapolis, MN, US). In addition, a monoclonal anti-SOX2 antibody (MAB2018, R&D Systems) was tested in a subset of samples and discontinued because of a lack of appropriate staining in positive control tissue. To identify CIS cells and EC cells in non-seminomas we used the following antibodies: rabbit anti-PLAP (placental-like alkaline phosphatase; Neomarkers, Fremont, CA, US) or goat anti-OCT4 (Santa Cruz Biotechnology, Santa Cruz, CA, US). The anti-SOX2 antibody from Millipore was thoroughly tested in a previous study, including analysis of several tissue types by western blotting (Perrett et al. 2008). The IHC stainings were performed using a standard indirect peroxidase method, according to the same protocol as previously described (Rajpert-De Meyts et al. 2004; Perrett et al. 2008). For SOX2 antibodies, dewaxed and rehydrated sections were heated in a microwave oven in sodium citrate buffer (10 mM, pH 6) for AB5603 or TEG buffer (10 mM TRIS, 0.5 mM EGTA, pH 9) for AF2018 to unmask the antigen. Subsequently, the sections were incubated with 0.5% H2O2 to inhibit endogenous peroxidase, followed by diluted non-immune goat serum (Zymed, San Francisco, CA, US) or horse serum (Vector Laboratories, Burlingame, CA, US) to block unspecific binding sites. The sections were incubated overnight at 4°C with the primary antibodies against SOX2 diluted 1:1000 (AB5603) and 1:250 (AF2018). As a negative control, a serial section from each block was incubated with the dilution buffer alone. Subsequently, a secondary biotinylated goat anti-rabbit (for AB5603) or rabbit anti-goat antibody (for AF2018) was applied (Zymed), followed by the horseradish peroxidase-streptavidin complex (Zymed). The sections were washed with TBS buffer between each step. The bound antibody was visualised using aminoethyl carbazole substrate (Zymed). Sections were counterstained with Mayer’s haematoxylin.
The sections were examined under a light microscope (Zeiss Oberkochen, D) and scored systematically by two investigators (RMP and SBS). The staining was assessed using an arbitrary semi-quantitative score of the proportion of cells stained: +++, nearly all cells stained; ++, approximately half of the cells stained; +, a low percentage of cells stained; +/-, only single cells stained; -, no positive cells detected. The staining intensity was furthermore evaluated as strong, medium, weak, very weak or absent.
Immunoblotting was carried out as described previously (Perrett et al. 2008), using representative samples of frozen testis tissue with CIS, as well as human ESC (for a positive control) and a sample of human heart tissue (for a negative control). The blots were incubated with two primary antibodies for SOX2 (AB5603 and AF2018) and mouse anti β-actin for loading control.
mRNA in situ hybridisation
The DNA templates for in situ hybridisation (ISH) probes were prepared by two rounds of PCR using specific primers designed for the 5′UTR of the SOX2 transcript: first primer combination; forward 5′ GGAAAGTAGTTTGCTGCCTCT 3′ and reverse 5′ CGAGGAAAATCAGGCGAAGAA 3′; second nested primer combination with T3- and T7-promotor sequences added, forward 5′ AATTAACCCTCACTAAAGGGCTGCCTCTTTAAGA 3′ and reverse 5′ TAATACGACTCACTATAGGGCGAAGAATAATTTG 3′ (T3 and T7 sequences underlined). First round PCR conditions were as follows: 5 min 95°C; 40 cycles of 30 sec at 95°C, 1 min at 62°C and 1 min at 72°C; and finally 5 min 72°C. Second round PCR conditions were: 3 min 95°C; 5 cycles of 30 sec at 95°C, 1 min at 42 °C and 1 min at 72°C; followed by 35 cycles of 30 sec at 95 °C, 1 min at 62°C and 1 min at 72°C; and finally 5 min at 72°C. The PCR product was run on a 1% agarose gel, the PCR band extracted and purified by the Nucleospin extract II kit (Macherey Nagel) and sequenced from both ends using Cy5 labelled primers complementary to the T3- and T7-tags. Aliquots of ~200 ng were used for in vitro transcription labelling with biotin-16-UTP, using the MEGAscript-T3 or MEGAscript-T7 kits (Ambion, Houston, TX, US) for sense and anti-sense probes, respectively. To estimate quantity and labelling efficiencies, aliquots of the labelled RNA product were analysed by agarose gel-electrophoresis. ISH was performed on three different samples of seminoma and nonseminoma (EC), two samples of CIS and three of normal testicular tissue, as described previously (Nielsen et al. 2003; Hoei-Hansen et al. 2004).
Acknowledgements
The authors wish to thank Pernille Timmerby, Brian Vendelbo Hansen and Heidi Kistrup for excellent technical assistance. The study was supported by grants from the Danish Cancer Society (to ERDM), Svend Andersen’s Foundation (ERDM); International Research Mobility Award from the Worldwide Universities Network (www.wun.ac.uk, RMP / NAH); and a project grant from the Welcome Trust (NAH). NAH acknowledges support from the NIHR Manchester Biomedical Research Centre.
Abbreviations used in this paper
- CC
choriocarcinoma
- CIS
carcinoma in situ
- EC
embryonal carcinoma
- ESC
embryonic stem cell
- IHC
immunohistochemistry
- ISH
in situ hybridisation
- PGC
primordial germ cell
- TER
teratoma
- TGCT
testicular germ cell tumour
- YST
yolk sac tumour
References
- ALMSTRUP K, HOEI-HANSEN CE, WIRKNER U, BLAKE J, SCHWAGER C, ANSORGE W, NIELSEN JE, SKAKKEBAEK NE, RAJPERT-DE MEYTS E, LEFFERS H. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 2004;64:4736–4743. doi: 10.1158/0008-5472.CAN-04-0679. [DOI] [PubMed] [Google Scholar]
- AVILION AA, NICOLIS SK, PEVNY LH, PEREZ L, VIVIAN N, LOVELL-BADGE R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIERMANN K, HEUKAMP LC, STEGER K, ZHOU H, FRANKE FE, GUETGEMANN I, SONNACK V, BREHM R, BERG J, BASTIAN PJ, MULLER SC, WANG-ECKERT L, SCHORLE H, BUTTNER R. Gene expression profiling identifies new biological markers of neoplastic germ cells. Anticancer Res. 2007;27:3091–3100. [PubMed] [Google Scholar]
- BOYER LA, LEE TI, COLE MF, JOHNSTONE SE, LEVINE SS, ZUCKER JP, GUENTHER MG, KUMAR RM, MURRAY HL, JENNER RG, GIFFORD DK, MELTON DA, JAENISCH R, YOUNG RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAGANTI RS, HOULDSWORTH J. Genetics and biology of adult human male germ cell tumors. Cancer Res. 2000;60:1475–1482. [PubMed] [Google Scholar]
- DE JONG J, STOOP H, GILLIS AJ, VAN GURP RJ, VAN DE GEIJN GJ, BOER M, HERSMUS R, SAUNDERS PT, ANDERSON RA, OOSTERHUIS JW, LOOIJENGA LH. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J. Pathol. 2008;215:21–30. doi: 10.1002/path.2332. [DOI] [PubMed] [Google Scholar]
- GIDEKEL S, PIZOV G, BERGMAN Y, PIKARSKY E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- HART AH, HARTLEY L, PARKER K, IBRAHIM M, LOOIJENGA LH, PAUCHNIK M, CHOW CW, ROBB L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005;104:2092–2098. doi: 10.1002/cncr.21435. [DOI] [PubMed] [Google Scholar]
- HOEI-HANSEN CE, ALMSTRUP K, NIELSEN JE, SONNE SB, GRAEM N, SKAKKEBAEK NE, LEFFERS H, RAJPERT-DE MEYTS E. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology. 2005;47:48–56. doi: 10.1111/j.1365-2559.2005.02182.x. [DOI] [PubMed] [Google Scholar]
- HOEI-HANSEN CE, NIELSEN JE, ALMSTRUP K, HANSEN MA, SKAKKEBAEK NE, RAJPERT-DE MEYTS E, LEFFERS H. Identification of genes differentially expressed in testes containing carcinoma in situ. Mol.Hum Reprod. 2004;10:423–431. doi: 10.1093/molehr/gah059. [DOI] [PubMed] [Google Scholar]
- HONECKER F, STOOP H, DE KRIJGER RR, CHRIS LAU YF, BOKEMEYER C, LOOIJENGA LH. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J.Pathol. 2004;203:849–857. doi: 10.1002/path.1587. [DOI] [PubMed] [Google Scholar]
- JONES TD, ULBRIGHT TM, EBLE JN, CHENG L. OCT4: A sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin.Cancer Res. 2004;10:8544–8547. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- KATAYAMA S, TOMARU Y, KASUKAWA T, WAKI K, NAKANISHI M, NAKAMURA M, NISHIDA H, YAP CC, SUZUKI M, KAWAI J, SUZUKI H, CARNINCI P, HAYASHIZAKI Y, WELLS C, FRITH M, RAVASI T, PANG KC, HALLINAN J, MATTICK J, HUME DA, LIPOVICH L, BATALOV S, ENGSTROM PG, MIZUNO Y, FAGHIHI MA, SANDELIN A, CHALK AM, MOTTAGUI-TABAR S, LIANG Z, LENHARD B, WAHLESTEDT C. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- KERR CL, HILL CM, BLUMENTHAL PD, GEARHART JD. Expression of pluripotent stem cell markers in the human fetal testis. Stem Cells. 2008;26:412–421. doi: 10.1634/stemcells.2007-0605. [DOI] [PubMed] [Google Scholar]
- KORKOLA JE, HOULDSWORTH J, CHADALAVADA RS, OLSHEN AB, DOBRZYNSKI D, REUTER VE, BOSL GJ, CHAGANTI RS. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 2006;66:820–827. doi: 10.1158/0008-5472.CAN-05-2445. [DOI] [PubMed] [Google Scholar]
- LOH YH, WU Q, CHEW JL, VEGA VB, ZHANG W, CHEN X, BOURQUE G, GEORGE J, LEONG B, LIU J, WONG KY, SUNG KW, LEE CW, ZHAO XD, CHIU KP, LIPOVICH L, KUZNETSOV VA, ROBSON P, STANTON LW, WEI CL, RUAN Y, LIM B, NG HH. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat.Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- LOOIJENGA LH, STOOP H, DE LEEUW HP, GOUVEIA BRAZAO CA, GILLIS AJ, VAN ROOZENDAAL KE, VAN ZOELEN EJ, WEBER RF, WOLFFENBUTTEL KP, VAN DEKKEN H, HONECKER F, BOKEMEYER C, PERLMAN EJ, SCHNEIDER DT, KONONEN J, SAUTER G, OOSTERHUIS JW. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- NIELSEN JE, HANSEN MA, JORGENSEN M, TANAKA M, ALMSTRUP K, SKAKKEBAEK NE, LEFFERS H. Germ cell differentiation-dependent and stage-specific expression of LANCL1 in rodent testis. Eur J Histochem. 2003;47:215–222. doi: 10.4081/830. [DOI] [PubMed] [Google Scholar]
- NOVOTNY GW, NIELSEN JE, SONNE SB, SKAKKEBAEK NE, RAJPERT-DE-MEYTS E, LEFFERS H. Analysis of gene expression in normal and neoplastic human testis: new roles of RNA. Int. J. Androl. 2007a;30:316–326. doi: 10.1111/j.1365-2605.2007.00773.x. [DOI] [PubMed] [Google Scholar]
- NOVOTNY GW, SONNE SB, NIELSEN JE, JONSTRUP SP, HANSEN MA, SKAKKEBAEK NE, RAJPERT-DE-MEYTS E, KJEMS J, LEFFERS H. Translational repression of E2F1 mRNA in carcinoma in situ and normal testis correlates with expression of the miR-17-92 cluster. Cell Death. Differ. 2007b;14:879–882. doi: 10.1038/sj.cdd.4402090. [DOI] [PubMed] [Google Scholar]
- PERRETT RM, TURNPENNY L, ECKERT JJ, O’SHEA M, SONNE SB, CAMERON IT, WILSON DI, RAJPERT-DE-MEYTS E, HANLEY NA. The Early Human Germ Cell Lineage Does Not Express SOX2 During In vivo Development or Upon In vitro Culture. Biol. Reprod. 2008;78:852–858. doi: 10.1095/biolreprod.107.066175. [DOI] [PubMed] [Google Scholar]
- RAJPERT-DE MEYTS E, HANSTEIN R, JORGENSEN N, GRAEM N, VOGT PH, SKAKKEBAEK NE. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004;19:1338–1344. doi: 10.1093/humrep/deh265. [DOI] [PubMed] [Google Scholar]
- SANTAGATA S, LIGON KL, HORNICK JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am. J. Surg. Pathol. 2007;31:836–845. doi: 10.1097/PAS.0b013e31802e708a. [DOI] [PubMed] [Google Scholar]
- SKAKKEBAEK NE. Possible carcinoma-in-situ of the testis. Lancet. 1972;2:516–517. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- SKOTHEIM RI, LIND GE, MONNI O, NESLAND JM, ABELER VM, FOSSA SD, DUALE N, BRUNBORG G, KALLIONIEMI O, ANDREWS PW, LOTHE RA. Differentiation of human embryonal carcinomas in vitro and in vivo reveals expression profiles relevant to normal development. Cancer Res. 2005;65:5588–5598. doi: 10.1158/0008-5472.CAN-05-0153. [DOI] [PubMed] [Google Scholar]
- SONNE SB, HOEI-HANSEN CE, NIELSEN JE, HERLIHY AS, ANDERSSON AM, ALMSTRUP K, DAUGAARD G, SKAKKEBAEK NE, LEFFERS H, RAJPERT-DE MEYTS E. CDH1 (E-cadherin) in testicular germ cell neoplasia: suppressed translation of mRNA in pre-invasive carcinoma in situ but increased protein levels in advanced tumours. APMIS. 2006;114:549–558. doi: 10.1111/j.1600-0463.2006.apm_445.x. [DOI] [PubMed] [Google Scholar]
- SONNE SB, ALMSTRUP K, DALGAARD M, JUNCKER AS, EDSGARD D, RUBAN L, HARRISON NJ, SCHWAGER C, ABDOLLAHI A, HUBER PE, BRUNAK S, GJERDRUM LM, MOORE HD, ANDREWS PW, SKAKKEBAEK NE, RAJPERT-De MEYTS E, LEFFERS H. Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res. 2009;69:5241–5250. doi: 10.1158/0008-5472.CAN-08-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSURUZOE S, ISHIHARA K, UCHIMURA Y, WATANABE S, SEKITA Y, AOTO T, SAITOH H, YUASA Y, NIWA H, KAWASUJI M, BABA H, NAKAO M. Inhibition of DNA binding of Sox2 by the SUMO conjugation. Biochem. Biophys. Res. Commun. 2006;351:920–926. doi: 10.1016/j.bbrc.2006.10.130. [DOI] [PubMed] [Google Scholar]
- ULBRIGHT TM. Germ cell neoplasms of the testis. Am. J. Surg. Pathol. 1993;17:1075–1091. doi: 10.1097/00000478-199311000-00001. [DOI] [PubMed] [Google Scholar]
- ULBRIGHT TM, AMIN MB, YOUNG RH. Tumors of the Testis, Adnexa, Spermatic Cord and Scrotum. In: Rosai J, Sobin LH, editors. Atlas of Tumor Pathology. Armed Forces Institute of Pathology; Washington DC: 1999. [Google Scholar]
- WEGER S, HAMMER E, ENGSTLER M. The DNA topoisomerase I binding protein topors as a novel cellular target for SUMO-1 modification: characterization of domains necessary for subcellular localization and sumolation. Exp. Cell Res. 2003;290:13–27. doi: 10.1016/s0014-4827(03)00292-1. [DOI] [PubMed] [Google Scholar]
- WESTERN P, MALDONADO-SALDIVIA J, VAN DEN BERGEN J, HAJKOVA P, SAITOU M, BARTON S, SURANI MA. Analysis of Esg1 expression in pluripotent cells and the germline reveals similarities with Oct4 and Sox2 and differences between human pluripotent cell lines. Stem Cells. 2005;23:1436–1442. doi: 10.1634/stemcells.2005-0146. [DOI] [PubMed] [Google Scholar]