Abstract
To fully understand the biological and environmental impacts of nanomaterials requires studies which address both sub-lethal endpoints and multigenerational effects. Here we use a nematode to examine these issues as they relate to exposure to two different types of quantum dots, core (CdSe) and core-shell (CdSe/ZnS), and to compare the effect to those observed after cadmium salt exposures. The strong fluorescence of the core-shell QDs allowed for the direct visualization of the materials in the digestive track within a few hours of exposure. Multiple endpoints, including both developmental and locomotive, were examined at QD exposures of low (10 mg/L Cd), medium (50 mg/L Cd), and high concentrations (100 mg/L Cd). While the core-shell QDs showed no effect on fitness (lifespan, fertility, growth, and three parameters of motility behavior), the core QDs caused acute effects similar to those found for cadmium salts suggesting that biological effects may be attributed to cadmium leaching from the more soluble QDs. Over multiple generations, we commonly found that for lower life-cycle exposures to core QDs the parents response was generally a poor predictor of the effects on progeny. At the highest concentrations, however, biological effects found for the first generation were commonly similar in magnitude to those found in future generations.
Keywords: Caenorhabditis elegans, Quantum Dots, Multigenerational, Toxicity, Cadmium
Introduction
The study of the biological effects of engineered nanoparticles is limited by the challenges associated with rapidly collecting comprehensive toxicological data. While in vitro studies are fast and well suited for evaluating NP libraries, they are limited in capturing more subtle organism impacts particularly over multiple generations.1, 2 More informative in vivo experiments can more accurately capture biological impact, but most animal models require too much time for facile evaluation of tens or hundreds of relevant nanoparticles. The low throughput of animal studies is a severe problem for multi-generational studies where data on multiple biological endpoints (e.g. fertility, development, locomotion) is desired. Such information is precisely the most valuable for ecotoxicological studies where degenerative or adaptive behaviors in progeny may be the most critical impacts.
To address these issues, we present here studies of the nematode Caenorhabditis elegans. Quantitative methodologies are applied to assess multiple endpoints relevant to their fitness after long-term exposures to NPs at three different environmentally-relevant and sub-lethal concentrations. Specifically, automated image analysis is applied to measure the body size and locomotive behavior of C. elegans, which moves in a sinusoidal pattern. Changes in the wavelength, amplitude, and velocity of their movements can indicate adverse neurological effects.3
These studies can be continued over multiple generations due to the short lifespan and large brood size per worm. To study toxicity at all life stages, two generations of exposure are often required because the early embryonic stage of an animal starts when inside the parent.4 Multigenerational studies can reveal cumulative damage, acclimation or adaptive responses.5–7 Cumulative damage in progeny is evident when the parent has no change in fitness but the progeny fitness gets progressively worse. Acclimation occurs when progeny, having been exposed to sublethal concentrations during embryogenesis, has no change in its fitness.5, 7–11
C. elegans has been the subject of a number of toxicology studies focused on the acute effects of NPs such as silver, silica, ceria, titania, and zinc oxide.12–15 However, none of this prior work has addressed the multi-generational impacts of engineered nanoparticle exposures. Here we explore the quantitative impact of both core and core-shell quantum dots over the span of four generations of C. elegans.
For the purposes of illustrating this multigenerational model, we used a standard NP type, quantum dots (QDs), both because they can be imaged within organisms and because of their use in emerging nanomaterial commercial products (QD Vision, Watertown, MA, USA). QD toxicity has been studied in vivo; the materials can be toxic when their largely organic surface coatings are compromised.16–18 Most have attributed this toxicity to the release of soluble cadmium from the CdSe core.19, 20 QDs with an outer ZnS shell, referred to as core-shell QDs, are generally more chemically stable and preferred in products due to their longer lifetime and brighter fluorescence.21 The few in vivo toxicity studies of these core-shell systems have found low or no toxicity in rats.22, 23 In Daphnia and fish, researchers also observed low toxicity unless surface coatings were degraded.19, 24 None of these in vivo studies have yet addressed multigenerational effects. We report for the first time a multigenerational study of the sub-lethal biological effects of both core and core-shell QDs in a nematode. Core QDs had impacts similar but less pronounced than cadmium salts, while the core-shell QDs had little impact on a multitude of endpoints over multiple generations.
Experimental
QD Preparation and Characterization
The CdSe core and the CdSe/ZnS core-shell quantum dots (QDs) used in this study were prepared following procedures described in our previous work.21 To ensure their stability in biologically relevant conditions, the amphiphilic copolymer poly(maleic anhydride-alt-1-octadecene, Sigma) (PMAO) (Mn = 30,000–50,000) - PEG (Mn = 1000, Sigma) (molar ratio PMAO:PEG=1:10) was formed through an anhydride coupling reaction to coat and to water-solubilize the QDs from organic solutions.25
Before beginning these toxicity studies, we carefully evaluated the physicochemical properties of these systems. Transmission electron microscopy (Joel 2010 TEM) revealed monodisperse core QDs with 3.4 nm core diameter and core-shell QDs with 4.1 nm core diameter (Figure S1). Dynamic light scattering (Malvern Zetasizer Nano-ZS) showed that both core and core-shell QDs had a hydrodynamic diameter of approximately 17 nm (Figure S1); the polymer coating contributes substantially to the size of the particles in water.21, 25 The cadmium concentration was determined by inductively coupled plasma-optical emission spectroscopy (Perkin Elmer, ELAN9000 ICP-OES). Concentrations were reported as milligrams of the cadmium ion in the solution. Three sub-lethal concentrations of [Cd] = 10, 50, and 100 mg/L were tested for each QD sample.
C. elegans Maintenance
The wild type N2 strain was obtained from the Caenorhabditis Genetics Center (MN, USA). C. elegans were cultured at 20 °C on nematode growth medium agar, using Escherichia coli strain OP50 as the food source following standard cultivation protocols.26, 27 To obtain a synchronized population, gravid nematodes were collected and treated with hypochlorite.26
Assessment of E. coli Viability
To study the effects on food source, QD or chemical exposure plates were prepared as described above except that no C. elegans were placed on the plates. The plates were incubated at 20 °C for 24 h. After the inoculation time, 1 mL of sterile H2O was used to rinse and collect the bacteria from the surface of each well to prepare serial dilutions. 20 μL of each dilution was spread onto a 6 cm LB plate and incubated overnight at 37 °C. The number of colonies was counted to evaluate the concentration of live bacteria in the original solution.
Quantum Dot and Chemical Control Exposure
Experiments were carried out on 24-well plates with 1 mL of NGM-agar added into each well. The wells were seeded with 10 μL of fresh overnight-culture of E. coli OP50 and kept for 3 days at room temperature to form a bacterial lawn. To the surface of each well, 30 μL of the QD in borate buffer solution (50 mM, pH=10) or chemical controls was added to cover the bacterial lawn. After about 30 min at 20 °C, one C. elegans was placed into each well. The plates were cultivated in a 20 °C incubator. C. elegans were transferred to freshly dosed plates every 24 h. Since the QDs were suspended in borate buffer, this solution was used as untreated and negative controls for multiple generations to examine variations for each endpoint measured (Table S1).
The acute toxicity of QDs and CdSO4 on parent worms was done before performing multigenerational testing. From these data, sub-lethal concentrations for the main study were set at three sub-lethal concentrations equivalent to that found in the QD samples ([Cd] = 10, 50, 100 mg/L). All reagents were of analytical grade and supplied by Aldrich.
Multigenerational Toxicity
In the multi-generation experiment, first-generation C. elegans were continuously exposed to QDs or chemicals at the fourth larval (L4) stage. L4 is the larval stage when germs cells start to divide and develop. In subsequent generations, C. elegans L4s were randomly selected from the first-day progeny of the previous generation and continuously exposed to QDs or chemicals throughout their life span.
Bioimaging and Microscopy
To visualize red fluorescence from internalized NPs, the exposure concentration for core and core-shell QDs used was [Cd] = 300 mg/L. After the inoculation time, C. elegans were immobilized with 10% sodium azide (NaN3, Sigma) and mounted on slides following standard procedures.28 The nematodes were imaged using a compound microscope (Axio Imager M2m, Carl Zeiss) with Nomarski objectives. Core-shell QD fluorescence was detected using a Texas Red filter (Carl Zeiss). Fluorescence and Nomarski images were acquired using a CCD camera (AxioCam, Carl Zeiss). All images were acquired and processed using the AxioVision (Rel.4.8, Carl Zeiss) software. Core QDs could not be directly imaged inside the C. elegans due to their low quantum yields (data not shown).
Quantification of Internalized Cd
To determine the uptake rate, the exposure concentration for core and core-shell QDs used was [Cd] = 300 mg/L. The metal content of exposed adults was measured using an inductively coupled plasma-optical emission spectroscopy instrument (Perkin Elmer, ELAN9000 ICP-OES) and reported as milligrams of the cadmium ion in the solution.
One-hundred nematodes were picked and transferred to clean NGM-plates to remove the bacteria. The nematodes were rinsed off with water and transferred into pre-weighed glass tubes to digest in a block heater at 90 °C for 4 h after addition of 1 mL of 70% HNO3. After digestion the samples were filtered and transferred to polypropylene tubes and diluted with ultrapure water to achieve a final acid concentration of 1% by volume.
Measurement of Life Span and Fertility
Life span was measured as the number of days that a nematode was alive: from egg to time of death. Fertility was measured as the total progeny number (brood size) of each nematode.
Measurement of Body Length and Locomotive Behavior
If cadmium leaches from QDs it may have neurotoxic effects.29 For this reason we analyzed the body length and locomotive behavior of adult nematodes using an automated Worm Tracker to measure sinusoidal movement and velocity.30
The hardware of the system consisted of a dissecting microscope (Unitron) equipped with a motorized stage (Prior) and a Firewire camera (Unibrain Fire-i). The same computer controlled the motorized stage and the camera. The entire setup was placed at a constant temperature of 20 °C. To start the measurement, a first-day adult nematode was transferred to the middle of a 10 cm NGM plate that was spread with fresh OP50. The software suite tracked the C. elegans and recorded a video of the nematode movement for 4 minutes. This video was then analyzed for body length and several motility parameters: amplitude, wavelength and velocity.
Analysis of Variance and Statistics
All samples were analyzed using OrginPro 8.5.1 (OriginLab Corporation, USA). Data was compared against the buffer control by one-way ANOVA post-hoc Tukey test at 99% confidence interval.
Results & Discussion
Toxicity studies in C. elegans are directly relevant to the assessment of the environmental impact of NPs because nematodes are one of the most abundant animals in the soil. Also, NPs are often engineered for good dispersion in water as this is important for many of their applications; such modifications also have the consequence of increasing NP accessibility to terrestrial ecosystems, land disposals or wastewater treatment plants containing NPs.31–33 In such an exposure scenario, nematodes are likely to be among the first organisms to encounter nanomaterial waste.
Uptake of Quantum Dots and Reference Toxicant
For these studies, we designed polymer-coated QDs to be monodisperse and non-aggregating under aqueous conditions. But before any QD toxicity could be interpreted, it was necessary to evaluate the impact of these NPs on the bacteria which are the food source for this organism.
E. coli growth showed no change after exposure to core QD, core-shell QD, or the cadmium salt controls after 24 h (Table 1). QD materials can be anti-microbial which can lead to starvation and developmental defects in nematodes.17, 34 However, these particular QDs and respective control solutions pose no threat to the food source allowing a straightforward interpretation of the biological impact of the QDs on C. elegans. Because the QD materials were added to the surface of an agar plate supporting the worms, we anticipated that the main route of exposure would be through the digestive tract.
Table 1.
Bacterial viability. Number of live cells after 24 h exposure to high [Cd] = 300 mgL−1 and low [Cd] = 10 mgL−1 concentrations of chemical control solutions and to nanoparticles at [Cd] = 10 mgL−1. The results are expressed as the log of CFU/mL (colony-forming units/mL).
| Sample | CFU | Log CFU | p-value |
|---|---|---|---|
| Buffer (Control) | 1.5E+09 | 9.2 | - |
| 300 mgL−1 CdSO4 | 1.2E+09 | 9.1 | 0.07 |
| 10 mgL−1 CdSO4 | 1.5E+09 | 9.2 | 0.76 |
| Core QD | 1.5E+09 | 9.2 | 0.80 |
| Core/shell QD | 1.8E+09 | 9.3 | 0.13 |
Data shown are mean + SE from four independent experiments; p-values are derived from student t-test.
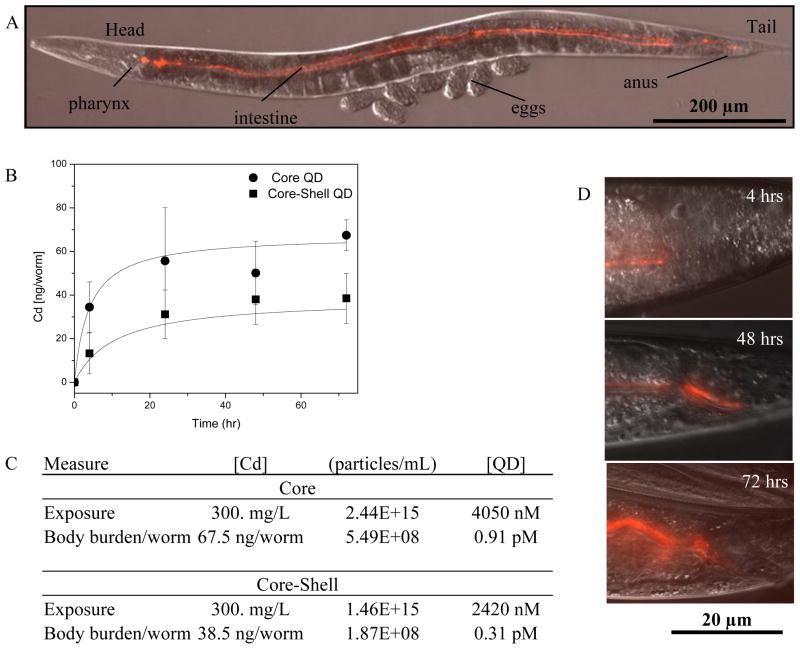
We confirmed this exposure route by using fluorescent microscopy. The transparent body of C. elegans and the red fluorescence from core-shell QDs enabled us to directly visualize them in a live, intact animal. Over the timescale of these experiments we only found red fluorescence in the digestive tissue of the nematodes (Figure 1). Moreover, the emission remained bright and consistently red over the duration of the study which indicates the core-shell QD do not appreciably change while ingested (Figure 1D).20 This is in agreement with other studies of QDs in aquatic organisms such as crustaceans and Daphnia magna.24, 35
Figure 1.
(A) Internalized QDs. DIC image with color overlay of the entire nematode showing the anatomy: head, body, and tail. (B) The uptake profile of QDs based on body burden of internalized cadmium and (C) the exposure concentrations for each QD by ICP-OES. Data shown are mean + SE (n = 5). (D) Red fluorescence channel showing the tail region near the anus of the worm after 4, 48 and 72 h of exposure. The exposure concentration was [Cd] = 300 mg/L. As time goes on, more fluorescence is observed consistent with the measurements of the body burden for cadmium (B). Anterior is left and dorsal is up in all of the figures.
To complement the imaging observations which are semi-quantitative and applicable only to core-shell QDs, we also quantitatively assessed the total cadmium body-burden by ICP-OES. After 72 hours of continual exposure, we found an average of 67.5 ng Cd/worm [118.6 ng/mg worm] for core QDs and 38.5 ng Cd/worm [67.7 ng/mg worm] for core-shell QDs (Figure 1B, C). The higher body burden for the core QD reflects that this material is more soluble and likely to leach cadmium into the organism in a molecular form.20 Also, we note that these body burdens are similar to studies of PEG conjugate PMAO QDs in organisms such as D. magna, where Lewinski, et al. found that the average uptake was 41 ng Cd/daphnia.24 More comprehensive biokinetics studies that address the time dependence of the uptake, the role of depuration, and the compartmentalization of cadmium in the gut as opposed to whole body, are underway.
Range-Finding Toxicity Response
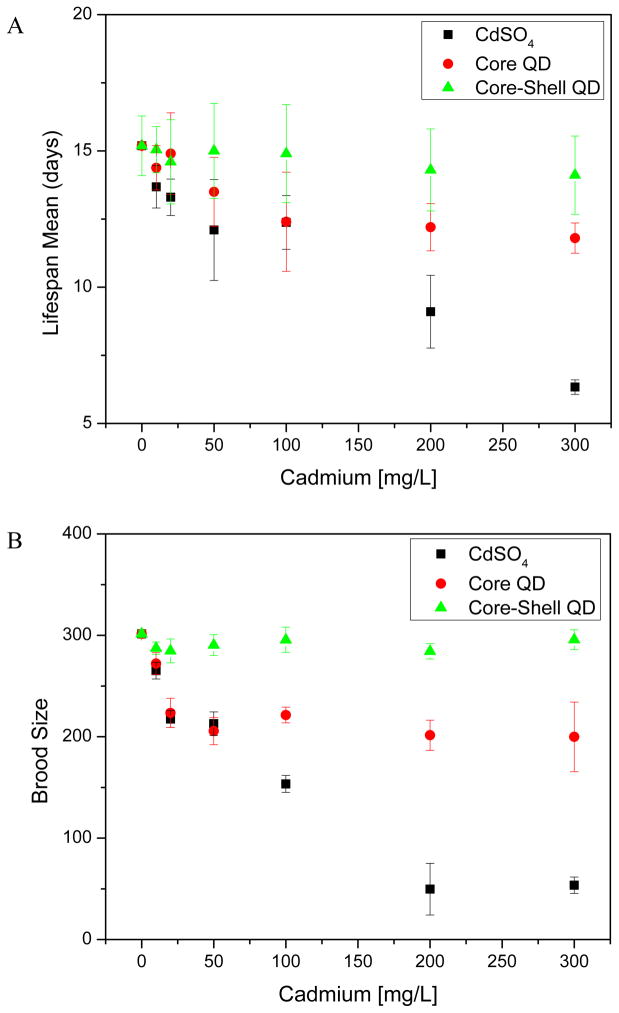
The sub-lethal effects of the core QD on these organisms are similar to the effects of CdSO4, but generally occur at net higher doses of QD. Figure 2 shows that after 72 h of exposure to core QDs (300 mg/L Cd), adult nematodes have a significantly decreased (p < 0.01) lifespan. Similarly, CdSO4, the acute reference toxicant, caused a similar effect on lifespan albeit at even lower concentrations (Figure 2A, black). When exposed to core QDs (20 mg/L), brood size decreased (p < 0.01) by 25.8% (Figure 2B, red), with a dose-response relationship similar to CdSO4. In stark contrast, core-shell QD exposures at concentrations up to 100 ppm had no impact on lifespan or brood size.
Figure 2.
Range-finding toxicity response curves. (A) C. elegans life span mean of generation 1 after QD and chemical acute exposures. (B) Exposure related effect on brood size of adult nematodes of generation 1. Error bars indicate standard error of means from 12 independent experiments for each data point.
One mode of toxicity suggested for quantum dots is the release of soluble cadmium from the CdSe core into solution; even small amounts of leached cadmium could have significant effects as this trace metal is acutely toxic.19 We find in this data a similar pattern of biological response in multiple endpoints to both the positive cadmium salt control and the core QDs. The primary difference is that the core QD caused these impacts at higher doses than the cadmium salt. These observations suggest that not all cadmium in the core QD is biologically available in contrast to the cadmium derived from soluble salts.
The lack of toxicity of core-shell QDs as compared to core QDs can be attributed to the zinc-containing shell of the core-shell QD. As zinc is incorporated into cadmium sulfide, the overall solubility of both metals decreases.20, 36 These data are consistent with other in vivo studies showing that QDs have low or no toxicity if their surfaces are appropriately designed to stabilize the nanoparticle and prevent dissolution.17, 25
From these dose-response data, it was possible to select sub-lethal exposures for the multi-generational study; these correspond to cadmium concentrations of 10, 50, and 100 mg Cd/L for the positive control, core QD and core-shell QD.
Multigenerational Toxicity Response
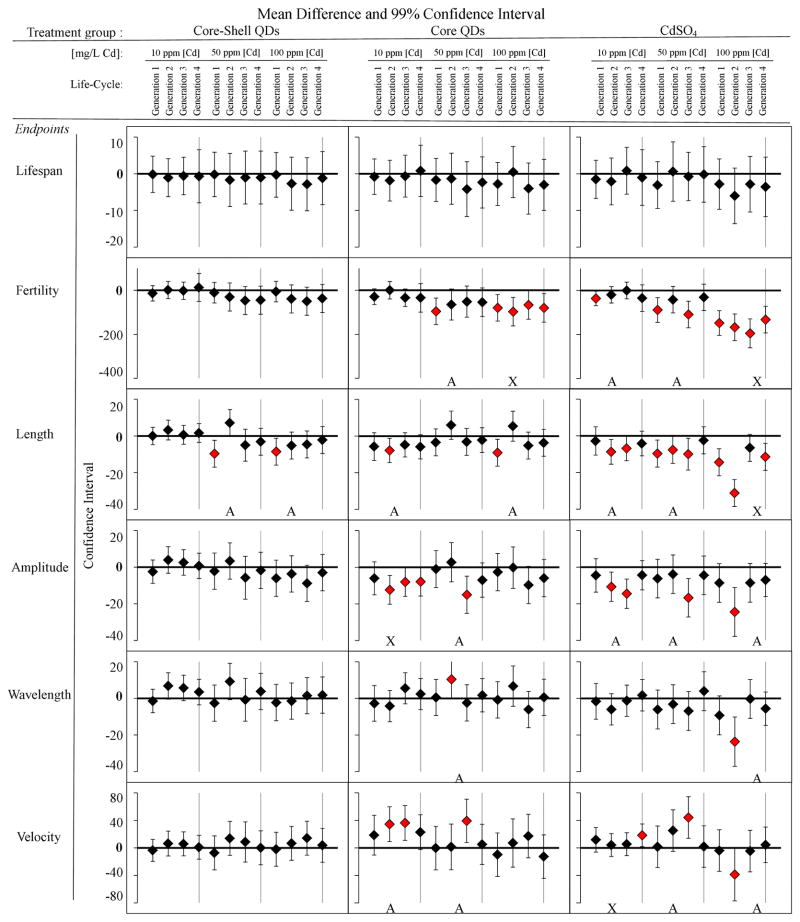
Figure 3 shows multigenerational toxicity data for several biological endpoints after QD exposures. These data illustrate that at higher toxicant exposures, the biological response of the first generation is commonly mirrored in subsequent generations. For example, multigenerational exposure to the positive control, CdSO4, showed significant adverse toxicity in fertility (brood size) and length at high concentration [Cd] =100 mg/L (Figure 3). This significant change in population and growth may be due to the bioaccumulation of the trace metal toxicant. Similar to the impact of cadmium salts, exposure to low (10 mg/L), medium (50 mg/L) and high (100 mg/L) concentrations of core QDs had the most notable impact on fertility. For fertility, a normal progeny count for unaffected adult worms is 300 offspring, but with a chronic exposure to core QDs at high concentrations [Cd] = 100 mg/L, brood size decreased significantly (p < 0.01) by 20% or more in population for all generations (Figure 3).
Figure 3.
Multigenerational chronic exposure effects in multiple endpoints: lifespan, fertility, growth, and locomotion (amplitude, wavelength, velocity) for four generations at low (10 mg/L) medium (50 mg/L), and high (100 mg/L) concentrations of equivalent cadmium for each sample tested: core-shell QDs, core QDs, and CdSO4. An A represents multi-generational data in which the effect in subsequent generations is different than the first generation. An X indicates continual adverse effects over multiple generations. Each horizontal bar represents the mean differences against the untreated control (ANOVA and post-hoc Tukey test with 99% confidence interval). If the interval excludes 0, then the difference is considered significant (red marker) for that pair-wise comparison (n=12).
This agrees with previous reports of a 20% inhibition in fertility (brood size) of C. elegans in highly metal-contaminated soils.37 Even the core QDs caused consistent impact on fertility at the highest exposures (100 mg/L Cd). Also, growth abnormality is a common indicator of developmental and metabolic defects.34 Höss et al. (2009) investigated the growth of C. elegans in sediments polluted with trace metals and showed a similar magnitude of effect on such parameters as length and fertility.37 Qualitatively, of the three samples studied CdSO4 caused the most significant changes to fertility, growth and to locomotive behaviors. In contrast, the multi-generational data for the core-shell QDs showed no evidence of any biological impact both in the first generation or subsequent generations.
While at the higher exposures, the first generation response predicted the response in subsequent generations, at the lower exposures for the core and core-shell QD first generation effects did not reflect the changes in subsequent generations.8 In particular, it was commonly observed that when the first generation had a significant response (p < 0.01) to cadmium salt, core QDs, or core-shell QDs, subsequent generations had either less of an effect or no effect at all (Figure 3). This is most clearly seen in the core-shell QD exposures, particularly the measure of nematode length (Figure 3). The first generation in the 50 and 100 ppm exposures both had significantly reduced (p < 0.01) length, but this effect was not seen in subsequent generations. For core QD at medium concentration [Cd] = 50 mg/L, fertility significantly decreased (p < 0.01) in the first generation, but returned to normal by generation 2. Similarly, nematodes exposed to CdSO4 at varying Cd concentrations had more notable changes in the first generation than in subsequent ones. This was true at low concentrations with respect to fertility.
Such data may reflect that over generations nematodes can acclimate to low QD exposures as much as they acclimate to trace metal contaminants in their environment.7, 38 Acclimation of aquatic organisms to trace metal exposures has been linked to the upregulation of metallothionein-like proteins that bind to trace metals and lower their bioavailability.6, 35, 38 This phenomenon has been seen in other multi-generational toxicity studies of chemical agents such as endocrine disrupters and pharmaceutical waste.6, 9, 11 Further studies to test whether and how the organisms are acclimating to the presence of core QDs are ongoing with a particular focus on the hypothesis that this acclimation is linked to regulation of metallothionein-like proteins.
To conclude, the biological effects of QDs of different surface coating and their precursor salts were extensively evaluated in this first multigenerational study of C. elegans. From this study, we conclude that QDs do not fully dissolve in nematodes as their biological effects are far less severe than cadmium salts; they are in effect much less toxic than free cadmium, a fact we attribute to the low solubility of cadmium selenide and the even lower solubility of cadmium selenide surrounded by zinc sulfide.20, 36 We note, however, that any biological or environmental process that results in the full dissolution of these QDs could have significant and exaggerated consequences for this organism due to the intrinsic toxicity of their constituent cadmium. The multi-generational data suggests that nematodes may have the capacity to adapt or acclimate to the presence of low levels of trace metals. It illustrates the importance of considering effects over multiple generations and provides one effective animal model well suited for evaluating different types of engineered nanomaterials.
Supplementary Material
Acknowledgments
We thank Dr. Marta L. Derkowska, W. Duncan Wadsworth, and Dr. Boanerges Aleman-Meza for their assistance in various aspects of this research. We also thank the Caenorhabditis Genetic Center for strains. E.Q.C. was supported by the Center for Biological and Environmental Nanotechnology (NSF EEC 0647452) and by the EPA grant RD834557501-0 at Rice University. Additional funding was provided by the NIH grant R00HG004724 and the Kinship Foundation through a Searle Scholars grant to W.Z.
Footnotes
Supporting Information- Characterization of QDs coated with PMAO-PEG copolymers and coefficients of variation (%CV) of motility endpoints used to measure N2 worms. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Choi JY, Lee SH, Bin Na H, An K, Hyeon T, Seo TS. In vitro cytotoxicity screening of water-dispersible metal oxide nanoparticles in human cell lines. Bioprocess Biosyst Eng. 2010;33(1):21–30. doi: 10.1007/s00449-009-0354-5. [DOI] [PubMed] [Google Scholar]
- 2.Sohaebuddin S, Thevenot P, Baker D, Eaton J, Tang L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part Fibre Toxicol. 2010;7(1):22. doi: 10.1186/1743-8977-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhawan R, Dusenbery DB, Williams PL. Comparison of lethality, reproduction, and behavior as toxicological endpoints in the nematdoe Caenorhabditis elegans. J Toxicol Environ Health, Part A. 1999;58(7):451–462. doi: 10.1080/009841099157179. [DOI] [PubMed] [Google Scholar]
- 4.Wright PL. Test procedures to evaluate effects of chemical exposure on fertility and reproduction. Environ Health Perspect. 1978;24:39–43. doi: 10.1289/ehp.782439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lock K, Janssen CR. Multi-generation toxicity of zinc, cadmium, copper and lead to the potworm Enchytraeus albidus. Environ Pollut. 2002;117(1):89–92. doi: 10.1016/s0269-7491(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 6.Reinecke SA, Prinsloo MW, Reinecke AJ. Resistance of Eisenia fetida (Oligochaeta) to cadmium after long-term exposure. Ecotoxicol Environ Saf. 1999;42(1):75–80. doi: 10.1006/eesa.1998.1731. [DOI] [PubMed] [Google Scholar]
- 7.Spurgeon DJ, Hopkin SP. The development of genetically inherited resistance to zinc in laboratory-selected generations of the earthworm Eisenia fetida. Environ Pollut. 2000;109(2):193–201. doi: 10.1016/s0269-7491(99)00267-5. [DOI] [PubMed] [Google Scholar]
- 8.Brennan SJ, Brougham CA, Roche JJ, Fogarty AM. Multi-generational effects of four selected environmental oestrogens on Daphnia magna. Chemosphere. 2006;64(1):49–55. doi: 10.1016/j.chemosphere.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Clubbs RL, Brooks BW. Daphnia magna responses to a vertebrate estrogen receptor agonist and an antagonist: A multigenerational study. Ecotoxicol Environ Saf. 2007;67(3):385–398. doi: 10.1016/j.ecoenv.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Muyssen BTA, Janssen CR. Multigeneration zinc acclimation and tolerance in Daphnia magna: Implications for water-quality guidelines and ecological risk assessment. Environ Toxicol Chem. 2001;20(9):2053–2060. [PubMed] [Google Scholar]
- 11.Tominaga N, Kohra S, Iguchi T, Arizono K. A Multi-Generation Sublethal Assay of Phenols Using the Nematode Caenorhabditis elegans. J Health Sci. 2003;49(6):459–463. [Google Scholar]
- 12.Pluskota A, Horzowski E, Bossinger O, von Mikecz A. In Caenorhabditis elegans Nanoparticle-Bio-Interactions Become Transparent: Silica-Nanoparticles Induce Reproductive Senescence. PLoS One. 2009;4(8):e6622. doi: 10.1371/journal.pone.0006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roh JY, Park YK, Park K, Choi J. Ecotoxicological investigation of CeO2 and TiO2 nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environ Toxicol Pharmacol. 2010;29(2):167–172. doi: 10.1016/j.etap.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Roh J-y, Sim SJ, Yi J, Park K, Chung KH, Ryu D-y, Choi J. Ecotoxicity of Silver Nanoparticles on the Soil Nematode Caenorhabditis elegans Using Functional Ecotoxicogenomics. Environ Sci Technol. 2009;43(10):3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- 15.Wang HH, Wick RL, Xing BS. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut. 2009;157(4):1171–1177. doi: 10.1016/j.envpol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Kirchner C, Javier A, Susha A, Rogach A, Kreft O, Sukhorukov G, Parak W. Cytotoxicity of nanoparticle-loaded polymer capsules. Talanta. 2005;67(3):486– 491. doi: 10.1016/j.talanta.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Mahendra S, Zhu H, Colvin VL, Alvarez PJ. Quantum Dot Weathering Results in Microbial Toxicity. Environ Sci Technol. 2008;42(24):9424–9430. doi: 10.1021/es8023385. [DOI] [PubMed] [Google Scholar]
- 18.Ryman-Rasmussen J, Riviere J, Monteiro-Riviere N. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Invest Dermatol. 2007;127:143– 153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- 19.Pelley JL, Daar AS, Saner MA. State of Academic Knowledge on Toxicity and Biological Fate of Quantum Dots. Toxicol Sci. 2009;112(2):276–296. doi: 10.1093/toxsci/kfp188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett KA, McBride MB. Dissolution of Zinc-Cadmium Sulfide Solid Solutions in Aerated Aqueous Suspension. Soil Sci Soc Am J. 2007;71(2):322–328. [Google Scholar]
- 21.Zhu HG, Prakash A, Benoit DN, Jones CJ, Colvin VL. Low temperature synthesis of ZnS and CdZnS shells on CdSe quantum dots. Nanotechnology. 2010;21(25):255604. doi: 10.1088/0957-4484/21/25/255604. [DOI] [PubMed] [Google Scholar]
- 22.Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WCW. In vivo Quantum-Dot Toxicity Assessment. Small. 2010;6(1):138–144. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YB, Chen W, Zhang J, Liu J, Chen GP, Pope C. In vitro and in vivo toxicity of CdTe nanoparticles. J Nanosci Nanotechnol. 2007;7(2):497–503. doi: 10.1166/jnn.2007.125. [DOI] [PubMed] [Google Scholar]
- 24.Lewinski NA, Zhu H, Jo HJ, Pham D, Kamath RR, Ouyang CR, Vulpe CD, Colvin VL, Drezek RA. Quantification of Water Solubilized CdSe/ZnS Quantum Dots in Daphnia magna. Environ Sci Technol. 2010;44(5):1841–1846. doi: 10.1021/es902728a. [DOI] [PubMed] [Google Scholar]
- 25.Yu WW, Chang E, Falkner JC, Zhang J, Al-Somali AM, Sayes CM, Johns J, Drezek R, Colvin VL. Forming Biocompatible and Nonaggregated Nanocrystals in Water Using Amphiphilic Polymers. J Am Chem Soc. 2007;129(10):2871–2879. doi: 10.1021/ja067184n. [DOI] [PubMed] [Google Scholar]
- 26.Stiernagle T. Maintenance of C. elegans. WormBook, editor. The C. elegans Research Community, WormBook. 2006 doi: 10.1895/wormbook.1.101.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 27.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71– 94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaham S. Methods in Cell Biology. WormBook, editor. The C. elegans Research Community, WormBook. 2006 doi: 10.1895/wormbook.1.49.1. http://www.wormbook.org. [DOI]
- 29.López E, Figueroa S, Oset-Gasque MJ, González MP. Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br J Pharmacol. 2003;138(5):901–911. doi: 10.1038/sj.bjp.0705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronin C, Mendel J, Mukhtar S, Kim YM, Stirbl R, Bruck J, Sternberg P. An automated system for measuring parameters of nematode sinusoidal movement. BMC Genetics. 2005;6(1):5. doi: 10.1186/1471-2156-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balbus JM, Maynard AD, Colvin VL, Castranova V, Daston GP, Denison RA, Dreher KL, Goering PL, Goldberg AM, Kulinowski KM, Monteiro-Riviere NA, Oberdorster G, Omenn GS, Pinkerton KE, Ramos KS, Rest KM, Sass JB, Silbergeld EK, Wong BA. Meeting report: Hazard assessment for nanoparticles - Report from an interdisciplinary workshop. Environ Health Perspect. 2007;115(11):1654–1659. doi: 10.1289/ehp.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21(10):1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 33.Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17(5):372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 34.Boyd WA, Cole RD, Anderson GL, Williams PL. The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ Toxicol Chem. 2003;22(12):3049–3055. doi: 10.1897/02-565. [DOI] [PubMed] [Google Scholar]
- 35.Bodar CWM, van der Sluis I, van Montfort JCP, Voogt PA, Zandee DI. Cadmium resistance in Daphnia magna. Aquat Toxicol. 1990;16(1):33–39. [Google Scholar]
- 36.de Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG. Cadmium solubility in paddy soils: Effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ. 2011;409(8):1489–1497. doi: 10.1016/j.scitotenv.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Höss S, Jänsch S, Moser T, Junker T, Römbke J. Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicol Environ Saf. 2009;72(7):1811–1818. doi: 10.1016/j.ecoenv.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Swain SC, Keusekotten K, Baumeister R, Stürzenbaum SRC. elegans Metallothioneins: New Insights into the Phenotypic Effects of Cadmium Toxicosis. J Mol Biol. 2004;341(4):951–959. doi: 10.1016/j.jmb.2004.06.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.