Abstract
This review addresses the cellular and molecular mechanisms of cadherin-based tissue morphogenesis. Tissue physiology is profoundly influenced by the distinctive organizations of cells in organs and tissues. In metazoa, adhesion receptors of the classical cadherin family play important roles in establishing and maintaining such tissue organization. Indeed, it is apparent that cadherins participate in a range of morphogenetic events that range from support of tissue integrity to dynamic cellular rearrangements. A comprehensive understanding of cadherin-based morphogenesis must then define the molecular and cellular mechanisms that support these distinct cadherin biologies. Here we focus on four key mechanistic elements: the molecular basis for adhesion through cadherin ectodomains; the regulation of cadherin expression at the cell surface; cooperation between cadherins and the actin cytoskeleton; and regulation by cell signaling. We discuss current progress and outline issues for further research in these fields.
I. Introduction to classical cadherins
The physiology of metazoan organisms is profoundly influenced by the distinctive histoarchitectures of their tissues and organs. For example, the efficacy of transporting epithelia or endothelia requires their constituent cells to assemble into biological barriers that separate distinct body compartments (73, 189, 312). Similarly, neuronal connectivity involves the precise guidance of axons to their target cells and assembly of cell-cell connections at synapses (83, 323). Such tissue patterning is established during development, maintained in the face of cellular turnover in post-embryonic life, and characteristically perturbed in a range of diseases, notably inflammation and cancer. Important advances in genetics, developmental and cell biology have begun to elucidate the mechanisms responsible for tissue morphogenesis. These often entail complex interactions between cells that reflect interplay between cell signaling, physical contact, the cytoskeleton and membrane trafficking. The challenge is to identify key determinants of tissue organization and understand the mechanisms responsible for their morphogenetic impact.
This review focuses on classical cadherin adhesion receptors, mediators of cell-cell interactions that play important roles in the establishment and maintenance of tissue architecture. We will discuss the several distinct contributions that classical cadherins make to morphogenesis, and then review the cellular and molecular mechanisms of cadherin biology that are likely to contribute to these morphogenetic effects. Ultimately, any comprehensive analysis of cadherin-based morphogenesis must map mechanisms onto specific morphogenetic outcomes. We are not there yet, but hope to highlight promising lines of research in this article.
A. Classical cadherins and the cadherin superfamily
The cadherins were first identified by the labs of Takeichi, Kemler and Jacob as membrane proteins that supported calcium-dependent cell-cell adhesion (147, 363, 385). Molecular cloning allowed the identification of a large superfamily of cell surface glycoproteins, based on sequence homology with a unique domain first found in the extracellular regions of E- and N-cadherin (reviewed in (264, 368, 411)(Fig 1). These cadherin repeats (also called cadherin domains or cadherin motifs) bear negatively charged DXD, DRE, and DXNDNAPXF sequence motifs thought to be involved in Ca2+ binding (411). Sequence homology combined with genomic and phylogenetic analysis make it possible to define 6 major subgroups within the superfamily - classical (or Type 1) cadherins, atypical (Type II) cadherins, desmosomal cadherins, flamingo cadherins and protocadherins – as well as a number of solitary members (264).
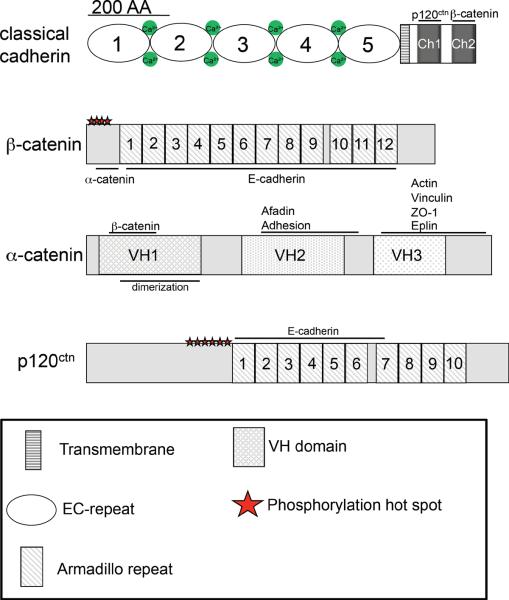
Figure 1. Domain structure of the cadherin-catenin complex components.
Schematic overview of the domain structure of a classical cadherin and its three associated catenins, β-catenin, α-catenin and p120-ctn.
The capacity for classical cadherins to support cell-cell adhesion is most clearly demonstrated by experiments where exogenous expression of specific cadherins increases the adhesiveness of cadherin-deficient cells that otherwise adhere poorly to one another (e.g. Drosophila Schneider cells, mouse fibroblastic L cells, Chinese hamster ovary cells) (14, 225, 231, 248, 418)1. The predicted increase in cell-cell adhesion has been evaluated by a number of means, but the most intuitively obvious assays test the ability of freshly isolated cells to aggregate in agitated suspensions (77, 248, 249). This approach has the advantage of examining the ability of cells to adhere to one another independent of cell-matrix adhesive interactions. Furthermore, aggregation under conditions of shaking or stirring further tests the ability of cells to resist detachment forces imposed by fluid shear stress, providing an additional measure of relative adhesiveness. This capacity of cadherins to resist disruptive forces was first demonstrated for E-cadherin and N-cadherin (240, 248) and has since been confirmed for many other classical cadherins.
Although the cadherin domain was first identified in proteins that are established adhesion molecules, its presence does not necessarily predict an adhesion function for all members of this superfamily. For example, in Xenopus embryos paraxial protocadherin (PAPC) contributes to patterning the gastrulating mesoderm, but does not appear to support homophilic cell adhesion (50). Instead, PAPC influences morphogenetic movements by down-regulating the adhesive activity of the classical cadherin, C-cadherin, through an as-yet-unknown mechanism. Similarly, the flamingo cadherins, which are serpentine (7TM-spanning) molecules, are genetically implicated in planar cell polarity, but may exert their effects through cell signaling rather than adhesion (336, 408).
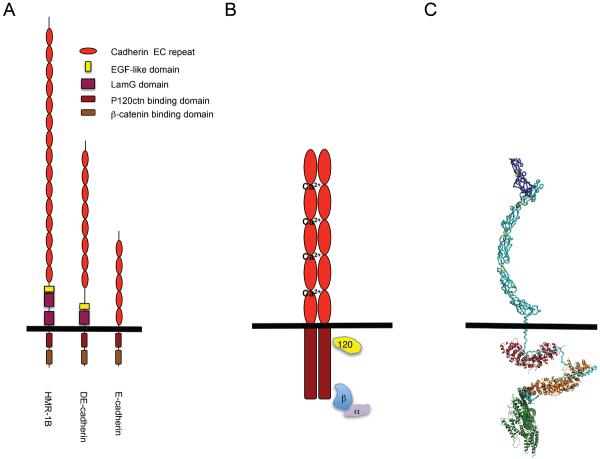
Accordingly, we will concentrate our attention on the classical cadherins, which have been confirmed as adhesion molecules and have established effects on tissue patterning and organization. However, the functional and mechanistic distinction between classical/Type I and atypical/type II cadherins is not clear-cut. Although these can be segregated by sequence divergence and phylogenetic clustering, they share important common features. In vertebrates both subgroups of proteins possess a common domain organization that includes five cadherin repeats in their extracellular domains (the fifth repeat, closest to the plasma membrane, being more divergent in sequence than the other repeats) (Fig 1). Functionally, they also share many similarities. For example, VE-cadherin which segregates more closely with Type II cadherins, engages cell signaling and trafficking pathways similar to the classical cadherin, E-cadherin (54, 191, 409). Furthermore, the extracellular domains of invertebrate classical cadherins are highly variable (47, 123, 271, 272) (Fig 2A). Thus the ability to interact directly with p120-ctn and β-catenin is the best defining feature of classical cadherins (123, 264), and this will be implied when we use the term “cadherin” in this review.
Figure 2. The cadherin-catenin complex.
(A) Schematic representation of classical cadherins in C. elegans, Drosophila and vertebrates. (B) Schematic representation of the vertebrate cadherin-catenin complex. (C) Structural model of the cadherin catenin complex. This model is based on the crystal structures of the C-cadherin extracellular domain, the cadherin cytoplasmic domain bound to the armadillo repeats of β-catenin, the cadherin juxtamembrane domain bound to the p120-4AΔins and a-catenin fragments. Model reproduced with permission from (152).
B. The architecture(s) of the cadherin molecular complex
Classical cadherins function as membrane-spanning macromolecular complexes. The cadherins themselves are single-pass Type 1 transmembrane glycoproteins. Their N-terminal ectodomains mediate adhesive binding to cadherins presented on the surfaces of neighbouring cells, while the C-terminal cytoplasmic domains (commonly referred to as the cadherin cytoplasmic “tails”) interact with a range of cytoplasmic proteins.
The best understood cytoplasmic binding partners are the catenins (Fig 1, 2B): β-catenin, α-catenin and p120-catenin (p120-ctn). β-catenin and α-catenin were first identified as metabolically-labelled polypeptides that co-immunoprecipitated with E-cadherin (226, 249, 250, 275). A third polypeptide, initially named γ-catenin, was subsequently identified as plakoglobin (288). A homologue of β-catenin that can substitute for it under some circumstances, plakoglobin is more consistently found in association with desmosomes (64), rather than with classical cadherins. p120-ctn, in contrast, was first identified in a screen for substrates of the Src protein tyrosine kinase (165, 307), and was only later discovered to immunoprecipitate with classical cadherins (66, 306).
When fully incorporated into complexes with cadherins these three catenins associate with a stoichiometry of one of each catenin per cadherin molecule (160, 275) (Fig 2B). β-catenin binds directly to the distal ~ 76 amino acids of the cadherin cytoplasmic tail where it serves as an anchor for α-catenin, which does not itself bind directly to the cadherin molecule (Fig 2B,C). p120-ctn binds independently to the membrane-proximal region of the cadherin cytoplasmic tail (66, 306). β-catenin appears to associate with cadherins co-translationally (52). It is less clear when α-catenin and p120-ctn associate with cadherins (237, 391). Of note, each of these catenins has cellular functions independent of the cadherin. β-catenin is well-understood to function as a signal transducer in the Wnt signaling pathway (93, 290); α-catenin is implicated in intracellular traffic in association with the dynactin complex (207) and can regulate actin dynamics (26); and p120-ctn can regulate cell locomotion, Rho GTPase signaling and activity of the transcription factor, Kaiso (12, 112, 282). The biological impact in each of these cases is attributable to a cytosolic pool of the catenin. Whether any of these mechanisms are indirectly involved in the morphogenetic effects of cadherins remains unknown. For example, although cadherins can affect Wnt signaling, by sequestering β-catenin at the membrane (159), there is no clear evidence that this contributes to cadherin-driven morphogenesis.
Although the catenins are the best-studied proteins to associate with the cadherin cytoplasmic tail, they are neither exclusive nor necessarily the only functionally important cytoplasmic molecules that interact with cadherins. Indeed, there is accumulating evidence that many cellular regulators can interact, directly or indirectly with the cytoplasmic tails of the classical cadherins. These include many cytoskeletal regulators and signaling molecules (141), some of which will be discussed further below. Many of these molecules are unlikely to interact constitutively with cadherins, but may be dynamic or regulated by cellular context (213), presumably in response to cell signaling. An important point to note, however, is that these interactions have been identified for a relatively limited number of cadherins, most especially E-cadherin, and may not be shared with other classical cadherins (91).
II. The diverse morphogenetic impacts of classical cadherin adhesion receptors
While the ability of cadherins to support cell-cell adhesion was first demonstrated in tissue culture systems, analysis of their function in organisms has identified several different impacts on tissue organization.
A. Cadherins and tissue integrity
The most commonly-understood impact of cadherin adhesion receptors lies in their contribution to the preservation of cell-to-cell cohesion in solid tissues of the body (Fig 3). This effect is most evident in early embryos. Expression of mutant cadherin constructs in the early Xenopus embryo caused a range of defects in tissue integrity, which include discontinuties in the ectodermal layer that covers the embryo and the dissociation of blastomeres from one another (171, 203, 206). Decreased cell-cell adhesion was independently demonstrated by the observation that isolated blastomeres expressing mutant forms of cadherin failed to aggregate in culture (171). Such dominant-negative effects were reported with mutant constructs lacking the cytoplasmic tail or where the ectodomains were removed, indicating that all these regions contributed substantively to cellular adhesion and the stabilization of cohesive cell contacts. Of note, dominant-negative mutants that retain the cytoplasmic tail often have an impact on adhesion mediated by a range of cadherins (171, 203).
Figure 3. Physiological role for cadherins in tissue integrity.
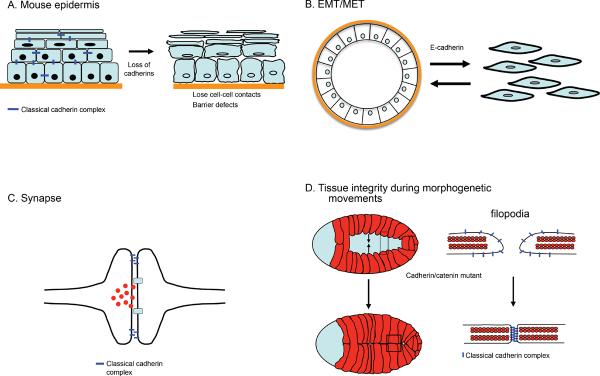
(A) Cadherin function in stratifying epithelium of the skin. Loss of all classical cadherins (E- and P-cadherin) in this tissue results in loosening of the intercellular contacts associated with loss of epidermal barrier function. (B) Loss of cadherin expression in epithelia results in loosening of contacts and the acquisition of more migratory behavior. This is a key feature of cells that undergo epithelial/mesenchymal transition. Vice versa, E-cadherin expression is induced during mesenchymal-epithelial transition resulting increased cellular adhesion and an epithelial appearance. EMT and MET are important processes not only during morphogenesis but also contribute to carcinogenesis. (C) Synapse formation. Loss of cadherins affects neuronal transmission and connectivity. Cadherin adhesive interactions are crucial for proper synapse formation between neurons as well as the neuro-muscular junctions. (D) Cadherins are required for the establishment of stable adherens junctions during morphogenetic movements. In C. elegans during ventral closure the embryonic epidermis spreads from the dorsal side and the leading cells of the two free edges meet at the ventral midline to enclose the embryo (left side). The leading edge cells extend filopodial protrusions that rapidly establish stable adherens junctions upon contact with an opposite filopodia, thereby rapidly increasing the contact sites between opposite cells resulting in sealing of the sheet. In the absence of the cadherin/catenin complex embryos are unable to form adherens junctions between opposite leading cells and thus cannot enclose the embryo.
Phenotypes as gross as these leave little doubt that cadherin function is necessary for cell-cell cohesion. However, in other contexts disrupting cadherins has more subtle effects on tissue integrity, for several reasons. For example, E-cadherin null mouse embryos fail to compact but do progress to implantation (195), likely because the preimplantation embryo is protected by a maternal pool of E-cadherin. In addition, other classical cadherins may compensate when specific cadherins are ablated. Thus, conditional disruption of E-cadherin in the mouse skin was associated with mild adhesion defects (370, 423) or defective tight junctions (379), but did not disrupt epidermal integrity. However, depletion of P-cadherin as well as E-cadherin disrupted cell-cell cohesion in the mouse epidermis (235, 371). Thus individual cadherin species may not be solely responsible for cell-cell cohesion, because of compensation by other classical cadherins or other adhesion molecules.
In other circumstances, cadherin dysfunction perturbs morphogenetic movements of tissues, without overt disruption of tissue integrity. For example, C. elegans embryo mutant for their sole cadherin most commonly display a hammerhead phenotype where the hypodermis, the covering layer, fails to envelope the embryo (62), rather than overt disruption of tissue integrity. Further, it is noteworthy that Xenopus embryos expressing weak dominant-negative cadherin mutants demonstrated tissue dissociation only when they underwent gastrulation (203), which is a process that is predicted to be distinguished by extensive forces exerted upon cells (167, 324). Finally, during Drosophila embryogenesis the phenotypic impact of DE-cadherin (Shotgun) mutant alleles is most pronounced in those tissues undergoing the greatest morphogenetic movements (e.g. neuroectoderm), and can be reduced by genetic maneuvers that decrease morphogenetic movements (367). Thus the demonstrable contribution of classical cadherins to tissue integrity also reflects the magnitude of disruptive forces that those tissues experience.
Here it should be noted that changes in cell-cell integrity or more subtle alterations in the morphology of contacts can occur without changes in cell surface adhesion (384). For example, hepatocyte growth factor (HGF, also known as scatter factor) induces colonies of MDCK cells to separate from one another (352). Despite the attractive inference that such scattering is due to loss of cell-cell adhesion, E-cadherin adhesiveness was not reduced (71). This disruption of epithelial integrity instead appears to reflect increased integrin-based contractility, which appears to mechanically pull the cells apart.
B. Cell sorting and cell-cell recognition
A fundamental developmental process is the capacity of cells with different cell fates to physically segregate from one another (Fig 4). This was first demonstrated by the classic experiments of Townes and Holtfreter (1955) who showed that when cells from dissociated gastrula stage embryos were allowed to re-aggregate, the cells would rearrange to reassociate with those from the same germ layer. In addition, the relative position of these cell populations within the reformed aggregate mirrored that found in the embryo (373). They proposed that this sorting behaviour was based on differential adhesion between different cell populations.
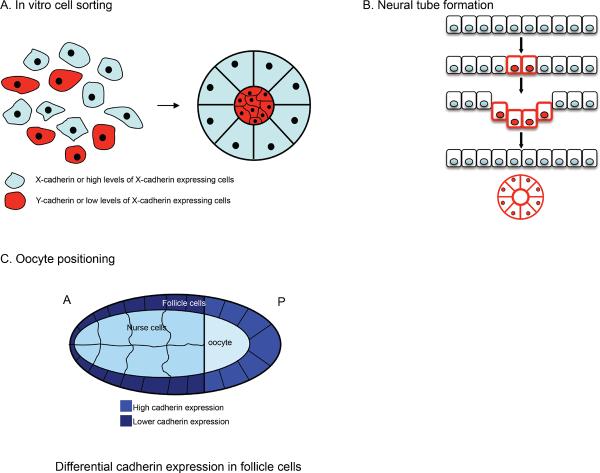
Figure 4. The role of cadherin in cell sorting and positioning.
(A) Differential type or levels of cadherin expression on cells drive cell sorting in vitro in cell (re)aggregation assays. (B) During the formation of the neural tube E-cadherin is switched off in a subset of ectodermal cells, whereas N-cadherin expression is turned on in these cells (red cell membranes) driving segregation of these cell populations. Other in vivo examples are neural crest cell migration and positioning and segregation of motor neuron cells. (C) Drosophila oocyte positioning where differential cadherin expression in the follicle cells is crucial to properly position the oocyte at the posterior end of the embryo. For detailed description see text.
There are three distinct components to this phenomenon of cell sorting: the ability of “like” cells to form discrete populations with one another; the ability of “unlike” cells to segregate away from one another; and the ability of these distinct cell populations to remain associated in a single aggregate. In principle, these phenomena could be accounted for in terms of relative surface adhesive energies between the surfaces of similar cells versus dissimilar cells. In the absence of factors that alter intrinsic properties of protein bonds, surface adhesion energies are determined by the identities of the adhesion proteins, i.e. their bond energies, and the number of such bonds formed between two cells, As discussed below, cells alter these parameters to regulate intercellular adhesion in the context of cadherin biology.
A causal role for cadherins in cell recognition and sorting was first suggested by key experiments that the Takeichi and Edelman labs performed using cell culture systems. Building on the demonstration that expression of E-cadherin in cadherin-null L-cells allowed cells to aggregate with one another in stirred suspensions (248), Nose et al (1988) found that mixed suspensions of cells expressing either E-cadherin or N-cadherin would form discrete aggregates of cells that expressed only one cadherin but not cells that expressed the other cadherin (268) (Fig 7). Similarly, cadherin-null murine S180 sarcoma cells engineered to express either L-CAM (the chick homologue of E-cadherin) or chick N-cadherin segregated away from one another (89). This indicated that the differential expression of classical cadherins, which would typically determine surface adhesive energies, was sufficient to recapitulate key elements of the cell sorting phenomenon observed in dissociated embryos (350, 373).
Figure 7. Role of the EC1 domain in cell sorting in vitro.
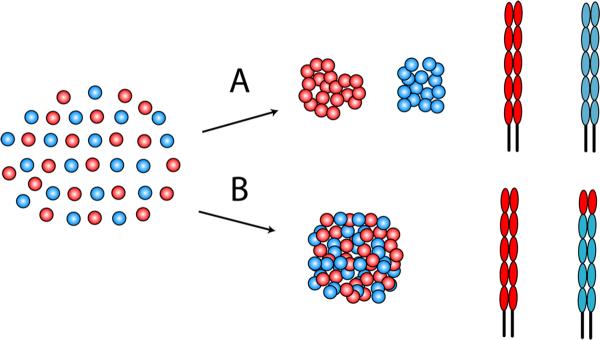
(A) Cells (red and blue circles) expressing E- or P-cadherin (red and blue ectodomains, respectively) sort out when mixed together. (B) If the EC1 domain of P-cadherin (blue) was replaced with the EC1 domain of E-cadherin (red), then cells expressing the E/P-cadherin chimera (blue) intermixed with cells expressing E-cadherin (red).
But does sorting occur in the intact organism? Indeed, changes in cadherin expression are commonly seen during developmental segregation events. A classic example is displayed by neural crest cells, which form over a long developmental time period from gastrulation through early organogenesis (319). The presumptive neural crest population is first induced at what becomes the border between the neural and non-neural ectoderm. During neurulation, these precursors become incorporated into the neural folds and the neural tube itself before eventually delaminating from the neuroepithelium and becoming migratory. A series of cadherin switches occur during this process (125): neural crest precursors down-regulate E-cadherin during their initial induction, express N-cadherin and cadherin-6b when they reside in the neuroepithelium, and then down-regulate the latter when they delaminate. The down-regulation of E-cadherin by transcriptional repression appears to be an essential early stage in the epithelial-mesenchymal transition (EMT) that this cell population undergoes, while N-cadherin and cadherin-6b are necessary at later stages (319). Such cadherin switching (typically from E-cadherin to N-cadherin) is commonly seen in many other forms of EMT (369).
As well as qualitative differences in cadherin expression, quantitative differences in the level of protein expressed on cells can also induce segregation behaviour. This was demonstrated in cultured L-cells transfected to express P-cadherin at different levels (76, 351) (Fig 4A) and strikingly confirmed by analysis of Drosophila oogenesis, in which a key step involves the oocyte coming into contact with somatic (follicle) cells at the posterior of the egg chamber (Fig 4C). DE-cadherin is found at all the cell-cell contacts in the egg chamber (103), and when either DE-cadherin (103) or armadillo (Drosophila β-catenin) (289) are disrupted oocytes become mispositioned in the egg chamber and lose polarity. Importantly, correct positioning of the oocyte required DE-cadherin to be expressed both in the germline cells as well as in the follicle cells (103, 105), implicating adhesive interactions between these two cell types in controlling oocyte patterning. But if cadherin adhesion is determinative, how does the oocyte consistently localize to the posterior of the egg chamber when DE-cadherin is expressed by all the germ cells and follicle cells that the oocyte comes in contact with? Here, the level of cadherin expressed appears to be critical (103). The posterior follicle cells, with which oocytes normally interact, have the highest level of cadherin expression of the somatic cells. Moreover, when posterior cells were genetically ablated, oocytes then preferentially interacted with the anterior follicle cells, the next most abundant sites of DE-cadherin expression. Positioning thus appeared to reflect a sorting process, where the oocyte preferentially interacted with follicle cells expressing the highest level of cadherin, independent of other morphogen or paracrine signaling events that might occur. Overall, this example illustrates the capacity for quantitative differences in cadherin expression, and by implication differences in adhesion, to have profound, long-lasting effects on developmental patterning. Consistent with this notion, flies bearing weak Shotgun alleles were infertile (367).
Adhesion energies may also influence cell shape. An intriguing example is the organization of cone cells in the retina of Drosophila (Fig 5) (128). The cell shapes are reminiscent of soap bubbles, whose geometry is determined entirely by surface tension. Indeed, a simple mechanical model was sufficient to predict cell geometries in vivo, for both different cluster sizes and for different mutants (136). Despite its appeal, this correlation is only apparent for small cell clusters. It is unlikely to be the predominant mechanism controlling cell morphology in more complex tissues where other forces likely play a much more greater role than adhesion energies (202).
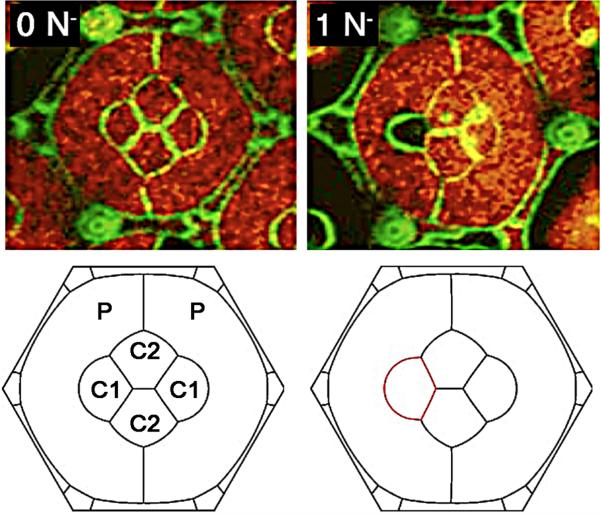
Figure 5. Cadherins and cell shape: illustration of the cell organization in the ommatidium of the Drosophila retina.
Each ommatidium in the compound eye comprises 20 cells. At the center are two anterior/posterior cone cells (C1) and two equatorial cone cells (C2). The cone cells are enclosed by two primary pigment cells P1. All cell boundaries express E-cadherin, but the boundaries between the cone cells express both N- and E-cadherin. The red indicates a genotypic marker for N-cadherin. The upper left panel shows the cell organization in a normal fly, and the lower panel is the cell organization predicted by a simple mechanical model that considered only adhesion energies and membrane elasticity. The right panels show the cell organization in a mutant fly in which the left cone cell (black) indicated by the red lines (lower panel) lacks N-cadherin. The effect of this deletion is accurately predicted by the mechanical model. (Adapted from (136))
C. Cadherins and morphogenetic movements
Finally, classical cadherins also contribute to morphogenetic movements that involve cell-cell rearrangements within tissues (115). Although less-well appreciated than EMT as a mode of cell locomotion, these movements are commonly found during early embryogenesis and take many forms. Classic examples during vertebrate gastrulation include epiboly, where radial intercalation of deeper cells into a more superficial cell layer allows the ectoderm to expand to cover the embryo; and convergent-extension in the mesoderm (Fig 6A), where intercalation of cells towards the midline of the animal causes the tissue to narrow and elongate (167, 205, 343). Of note, mutations in E-cadherin (half-baked) perturb epiboly in the zebrafish embryo (164), while during Xenopus gastrulation a regulated decrease in adhesion mediated by C-cadherin is necessary for convergent-extension to occur in response to mesoderm-inducing factors, such as activin (38, 426).
Figure 6. Cadherin in morphogenetic movements.
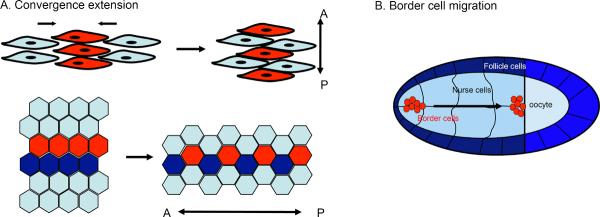
Cadherin function is essential for morphogenetic movements, such as cell-on-cell motility, epiboly, convergent extension movements or migration of intestinal epithelial cells along the crypt/villi axis. (A) Convergence extension movements in which cells align in the plane of the tissue and intercalate to e.g. drive anterior-posterior extension of tissues and/or embryos. Cadherins are crucial to establish contacts and through local modulation of the cytoskeleton provide the pulling force for intercalation. Either overexpression or loss of cadherins interferes with convergent extension movements in e.g. Xenopus or Zebrafish. (B) Drosophila border cells that through a complex signaling pathway switch on E-cadherin to migrate on E-cadherin expressing nurse cells. Loss of E-cadherin on either nurse or border cells prevents migration (see text for more details).
Border cell migration (Fig 6B), another well-characterized form of cadherin-dependent morphogenetic movement, also occurs in the Drosophila egg chamber (241). Here a small group of follicle cells emerge from the epithelium that covers the egg chamber and migrate through the nurse cells within the egg chamber to the anterior border of the oocyte. This form of invasive cell migration entails the movement of border cells upon the nurse cells and is subject to a hierarchy of regulatory signals. One key target of regulation is DE-cadherin, which is induced in the border cells at the time of migration (242). DE-cadherin is also necessary, in both the border cells and in their surrounding nurse cells, for the border cell cluster to migrate, indicating that it is a form of cadherin-dependent cell-upon-cell locomotion.
These examples of morphogenetic movements in the early embryo are likely to require the cells to use cadherins and other cell-cell adhesion receptors as the traction apparatus for intercalation and cell-upon-cell locomotion. An important challenge, then, is for cells to remodel their adhesive interactions with one another without disrupting the overall integrity of the tissue. Examples of predominantly cell-upon-cell locomotion are less common in post-developmental life, but many circumstances occur where cell-cell interactions must be dynamically remodeled during tissue turnover. A classic example is the gut epithelium, which displays constant and rapid turnover (19). Moreover, during their life cycle, gut epithelial cells move progressively and consistently up the crypt-villus axis before undergoing apoptosis and being shed at the tips of the villi. During this migration cells must preserve the intestinal epithelial barrier despite constant rearrangement. Importantly, expression of a dominant-negative cadherin disrupted the consistent patterning of migratory cells and disturbed the epithelial barrier (132), whereas overexpression of E-cadherin retarded the rate of cell migration (133). This indicated that cadherin was important both for epithelial barrier function and to regulate the rate of cell migration. Thus cadherins are likely to participate in morphogenetic cell-upon-cell rearrangements in post-developmental life as well as in the embryo.
III. Cellular and molecular effector mechanisms
We now turn to discuss the cellular and molecular mechanisms likely to support these morphogenetic effects of classical cadherins. We will focus on the following topics: 1) The adhesive binding characteristics of the cadherin ectodomain; 2) regulation of cadherin expression on the cell surface by turnover and membrane trafficking; 3) cadherins and the actin cytoskeleton; and 4) cell signaling and the regulation of cadherin biology.
A. The adhesive binding properties of cadherin ectodomains
The ability of classical cadherins to function as adhesion molecules and resist detachment force depends, ultimately, on the intrinsic binding properties of their ectodomains. Here we discuss recent progress in understanding how cadherin ectodomains mediate adhesion. Such analyses have been greatly facilitated because the isolated cadherin ectodomain retains adhesive binding capacity (32, 39). A number of cadherin ectodomains have now been made as recombinant proteins, typically expressed in mammalian cells to ensure their glycosylation (39, 97, 179). These recombinant proteins can bind to one another and immobilized ligands also support the adhesion of cells expressing cognate cadherin receptors. In combination with cell-based assays, the binding of isolated cadherin ectodomains has been subject to a range of analytic approaches, that include structure-function analysis, biophysical measurements of dynamic binding interactions, and structural examination of the binding interaction.
1. Characterizing the mechanisms of homophilic binding interactions
i) A central role for the EC1 domain
The functional importance of EC1 was first identified in a landmark study where Nose et al. (1990) demonstrated that substitution of EC1 domains between different cadherins could determine apparent binding specificity of cadherin proteins (269) (Fig 7). The authors used an in vitro cell sorting assay where cells expressing different cadherins segregated away from one another in agitated cell suspensions, but mixed randomly with cells expressing the same cadherin (268). They showed that cells expressing a chimeric protein, where the EC1 domain of P-cadherin was replaced by EC1 from E-cadherin, segregated away from cells expressing full-length P-cadherin, but intermixed with cells expressing full-length E-cadherin (269). Thus, substitution of the EC1 domain appeared to be sufficient to convert the binding selectivity of P-cadherin to that of E-cadherin. This finding focused attention on the functional significance of the EC1 domain.
Analysis of binding interactions using recombinant proteins also supported a key role for EC1 in cadherin adhesion, although additional domains were required for full adhesive activity (48, 328). Fragments containing the ectodomain of Xenopus C-cadherin supported adhesion, measured by the aggregation of protein-coated beads or binding of cells to protein-coated substrata so long as EC1 and EC2 were retained in the mutant molecules. Conversely, the expression in cells of an N-cadherin mutant lacking the EC3-5 region (i.e. presenting EC1-2 alone) supported weak cell-cell adhesion (328). A variety of structural studies also revealed molecular interfaces between EC1 domains, which constituted potential adhesive binding sites. Notably, N-terminal interfaces were observed in both the crystal structure of the EC1-2 fragment of N-cadherin (364) and that of the complete C-cadherin ectodomain (33); in each case a clear antiparallel alignment of molecules was identified, consistent with a trans interaction. Rotary shadowing electron micrographs of recombinant E-cadherin ectodomains also showed apparent association at the N-terminal tips of the molecules, suggesting that a molecular interface might occur in this region (293, 372).
The C-cadherin crystal structure (Fig 8B) further identified “strand dimer exchange” as a potential mechanism for interaction, where the side chain from Trp2 (W2) inserted into a complementary hydrophobic pocket on EC1 from the opposite protein (Fig 8B). Mutation of this conserved W2 residue substantially reduces cell adhesion in a variety of assays (293, 300, 328, 364), although W2A mutants still localize to cell-cell junctions (172, 364) and support bead aggregation (300). It should be noted that studies suggest that the strand dimer exchange through the W2 residue may also form cis-interactions between EC1 subunits of N-cadherin (330, 374) or docking to a hydrophobic cavity in its own molecule (for the EC1-2 fragment of E-cadherin) (293).
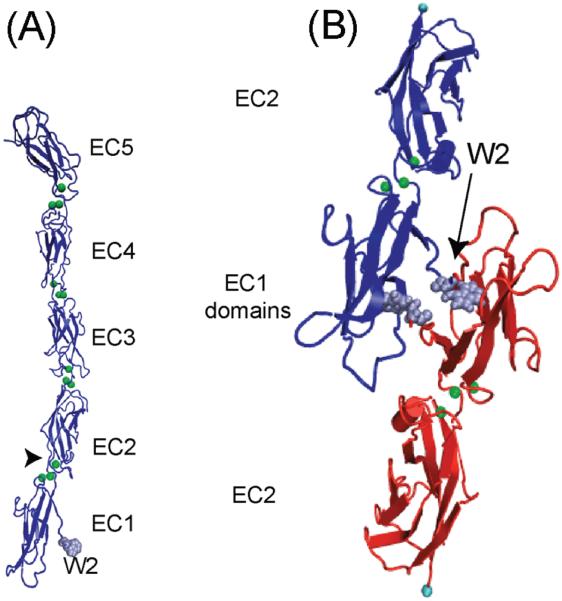
Figure 8. Classical Cadherin Structure and Model of Homophilic Binding.
(A) Crystal structure of the extracellular region (EC1-5) of Xenopus C-cadherin. (B) Binding between N-terminal domains of the C-cadherin extracellular region in which the Trp2 (W2) residues from opposing cadherins dock into the hydrophobic pocket of the opposed protein. Reproduced with permission from (199).
Finally, the potential significance of EC1 was also supported by the capacity of the prodomain to modulate adhesive function. Classical cadherins are synthesized containing prodomains, which are proteolytically cleaved to yield the mature form presented on the cell surface. Retention of the prodomain abolishes adhesion, potentially by modulating the dynamics of strand dimer exchange, so that the W2 residue cannot form a stable bond with adjacent molecules (126). The prodomain may therefore assist in preventing cadherins from interacting within intracellular compartments during the biosynthetic process.
Overall, then, these findings established the functional importance of the cadherin EC1 domain for cell adhesion and yielded an elegant model of trans adhesion through molecular interfaces between the tips of opposing cadherins (Fig 9). In the simplest form of this model EC1 domains present the binding interfaces and other regions of the ectodomain serve as necessary spacers (Fig 9B). Support for this model has also been inferred from ultrastructural examination of cell-cell contacts. The distance between neighbouring membranes at adherens junctions was measured at ~ 25 nm (239), sufficient to accommodate highly curved cadherin ectodomains that interact at their tips (33, 330) (Fig 8A). Moreover, surface projection densities of cadherins at 3.4nm resolution that appear to interact at their tips were observed by electron tomography of desmosomes (7, 130). These surface densities were assumed to represent the desmosomal cadherins, based in part on fitting of the classical C-cadherin crystal structure onto the electro-tomograms (7). However, definitive evidence that these represent cadherin ectodomains was lacking
Figure 9. Possible mechanism(s) of cadherin binding consistent with biophysical and structural data.
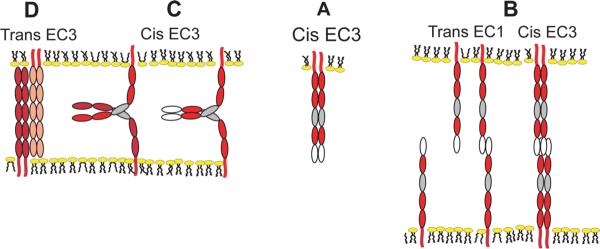
(A) Putative cis (lateral) bonds, possibly mediated by EC3 domains (grey ovals) stabilize lateral cadherin dimers. (B) The rapid, initial formation of trans bonds between EC1 domains (white ovals) facilitates cadherin accumulation at cell-cell junctions, and the subsequent slower lateral oligomerization via EC3 domains. (C) Cadherin flexibility could also enable the formation of EC3-dependent between cadherins on opposing membranes or between relatively unconstrained, opposing cadherins in force probe measurements. In (D) EC3 domains bind in an anti-parallel alignment between opposed membranes.
ii) Adhesive contributions by other regions of the cadherin ectodomain
Despite its elegance, a variety of functional data suggest that trans-binding of EC1 domains does not fully explain homophilic adhesion. Firstly, deletion analyses suggest that EC1 is necessary, but not sufficient, for cadherin adhesion. Thus, while the EC12 domains of C-cadherin were necessary for homophilic adhesion, adhesion to the EC12 fragment was shown to be much weaker than to the full ectodomain (48, 55). Moreover, the N-cadherin EC1 domain alone (i.e. deletion of EC2–5) could not support cell-cell adhesion (328). Secondly, genetic analyses of E-cadherin mutations associated with inherited gastric cancers identified clusters of mutations distributed over the entire extracellular domain, both within and distal to the EC1 domain (24, 30, 120, 121, 211). The most deleterious mutations appear to affect the EC2 and EC3 domains (121), whereas mutations at EC3–EC4 and EC4–EC5 junctions have little functional impact. Some of these mutations compromise, but do not abolish the adhesive function, and result in cadherin localization and adhesion defects (24, 30). The implication that these other cadherin ectodomain regions contribute to cadherin binding was further supported by biophysical studies (299).
Support for functional contributions of other regions of the cadherin ectodomain has also come from studies that probe different aspects of the dynamics and energetics of the binding interactions (described in greater technical detail elsewhere (199, 201). Firstly, analysis of intermembrane adhesion energies by surface force apparatus measurements identified multiple interactions between cadherin ectodomains (300, 427) and suggested that the EC3 domain was necessary for the strongest interaction (427). Secondly, kinetic analysis of homophilic binding by Xenopus C-cadherin on the cell membrane revealed a two stage process where an initial fast-forming state with a low probability of binding was then converted into a second, high probability state (55). The EC1–EC2 fragment displayed only the initial, low-probability state and comparison of deletion mutants indicated that EC3 was necessary for the transition to the high probability state.
Finally, direct measurement of binding between single cadherin ectodomains further suggested the existence of multiple different interactions (21, 22, 292, 333, 334, 377). Homophilic binding of EC1–EC2 fragments from C-cadherin or from E-cadherin displayed two weak bonds that dissociated rapidly (22, 292), whereas interactions between the full-length ectodomains also exhibited at least one other stronger bond with a lower dissociation rate. This stronger bond mapped to a region outside the EC1–EC2 domains. Moreover, the proportion of strong bonds increased with time (292, 333). Together, these findings emphasize that homophilic binding is a dynamic process and the results from these other approaches identify roles for regions outside EC1–2 in the transition between bound states.
How, then, do we reconcile a requirement for Trp2 in EC1 in adhesion with these contributions of other domains? One answer may lie in experimental evidence for allosteric cross-talk between different cadherin domains. Allostery is the ability of local structural perturbations to affect distal sites in a protein. The W2A mutation alters epitope accessibility more distally in EC1 of N-cadherin (124); alters epitope accessibility at several locations, including a site near EC4, in C-cadherin (334, 376); and substantially reduced the EC3-dependent bond strength in E-cadherin (300, 334). The cross-talk between EC1 and other regions of the protein demonstrated by these findings show that Trp2 docking both mediates strand exchange and regulates other domain interactions. These findings carry the important implication that EC1 cannot be treated as physically independent of other regions in the ectodomain.
Overall, these several lines of evidence suggest that understanding homophilic binding as a dynamic process may provide an opportunity to reconcile apparently disparate evidence (Fig 9). Thus, it is plausible to envisage a scenario where an initial weak interaction between EC1 domains is the essential precursor to a stronger interaction that requires EC3 and perhaps involves more extensive overlap between the ectodomains. This would be consistent with the demonstration that ectodomain interactions strengthen with duration of contact (292). Alternatively, slow lateral associations following fast, strand exchange could enhance the binding avidity or allosterically modify the intrinsic EC1 bond strength. Although many questions remain, these collective findings further highlight the notion that it is important to consider the dynamic and mechanical properties of possible cadherin interactions as well as their structural basis. Notably, as mechanisms to resist cell-cell detachment, the response of cadherin bonds to the forces they encounter during physiological cell-cell interactions is an open area that merits further investigation.
2. Lateral Organization of cadherins at the cell surface
Our discussion in the preceding section focused on understanding the intrinsic binding properties of the cadherin ectodomain. Substantial evidence indicates that the macroscopic adhesive behaviour of cadherins also reflects their lateral organization on the cell surface. This lateral organization takes two forms: the presentation of cadherins as lateral dimers and the organization of cadherins into larger scale lateral clusters and junctions (Fig 10).
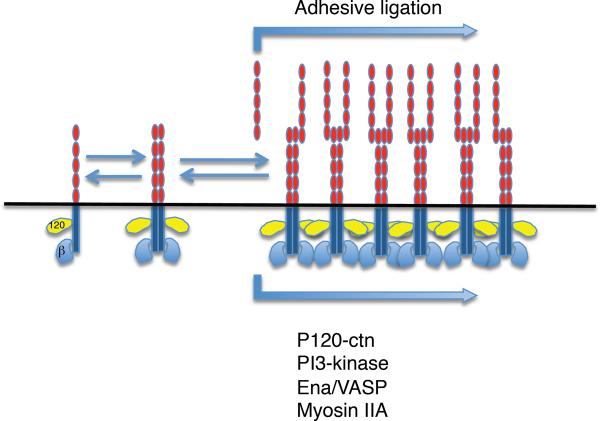
Figure 10. Surface organization of cadherins.
Monomers, dimers and oligomers of varying size are found on the cell surface and likely exist in dynamic equilibrium with one another. Adhesive ligation promotes oligomers and clusters, a process that also requires cytoplasmic factors including p120-ctn, signaling molecules, and elements of the acto-myosin cytoskeleton.
i) Cis-interactions and cadherin adhesion
The notion that classical cadherins might exist as lateral dimers was first suggested by the demonstration of cis-binding interfaces in the crystal structure of EC1 from N-cadherin (330) and EC1–EC2 from E-cadherin (251). Biochemical evidence for lateral dimers was then obtained for the full-length ectodomain of C-cadherin expressed as a recombinant protein as well as for full-length cadherins in cells (170, 173, 329, 362, 374). Additional evidence for cis-dimers has also been inferred crystal structures of other cadherin fragments and electron microscopy of recombinant ectodomains. Lateral dimerization is unlikely, however, to be a constitutive property of cadherins. Cis-dimers were not identified in biophysical studies of soluble E-cadherin ectodomains (425) and what appeared to be single cadherin molecules can be identified on cell surfaces (148). On balance, current evidence suggests the capacity for at least several cadherins to form cis-dimers, but the balance between cadherin monomers, dimers and higher-order oligomers on the cell surface is likely to be dynamic.
The functional significance of cis-dimers was first demonstrated by the observation that dimers of the C-cadherin ectodomain immobilized on beads supported stronger adhesion than did immobilized C-cadherin monomers (39). It should be noted that monomers retained adhesive capacity, but these data suggested that lateral dimerization was one mechanism to enhance adhesion. Recent single molecule force measurements, for example, suggest that cooperative interactions within cadherin dimers enhance the binding probability relative to cadherin monomers (425). How such adhesive enhancement might occur remains incompletely understood, but implies some synergistic interaction between the components of a lateral dimer.
The observation that lateral dimerization occurred with recombinant fragments of C-cadherin indicated that the ectodomain possesses the intrinsic capacity to dimerize (39). Identification of distinct binding interfaces that might mediate cis-dimerization has been more challenging. A putative cis binding interface between EC1 and an adjacent EC2 module seen in the crystal lattice of C-cadherin (33) was not confirmed by NMR measurements (127). Cross-linking and immunoprecipitation results suggest that lateral and adhesive interfaces are identical (374). Cadherin flexibility and the symmetry of the homophilic interaction could enable cadherins to use the same binding interface for either cis or trans interactions. The ectodomains are often portrayed as rigidly curved structures, but molecular dynamics simulations (344) and electron microscopy images (130, 177, 298) indicate that, under small forces and in the presence of calcium, cadherins can adopt configurations other than the curved structure in the crystal lattice (33). Other putative cis binding interactions may involve EC4, which is needed for the assembly of hexameric VE-cadherin ectodomains (134). Parallel E-cadherin EC1–2 fragments in the crystal lattice interacted via a calcium bridge at the interdomain junction (251). Although calcium site mutations at this junction disrupt adhesion (300), this may be due to compromised Trp2 docking (124, 127, 344). Despite several possibilities, a unique lateral binding interface(s) has yet to be identified.
ii) Lateral clustering and adhesive strengthening
An additional level of adhesive modulation can occur when cadherins organize into lateral clusters. Such cadherin clustering is observed at sites of homophilic adhesion between cells (14) as well as when cells adhere to cadherin-coated substrata (97, 325, 418). Analogous punctate structures have also been resolved by electron microscopy at the zonula adherens (ZA) of epithelial cells (139), leading to the inference that the ZA may arise from the local accumulation of cadherin clusters. The formation of these larger-scale lateral clusters often requires adhesion to cadherin ligands (179, 418), suggesting that it represents a mode of ligation-induced reorganization of surface cadherins. It should be noted, however, that structures thought to represent unliganded cadherin oligomers as well as monomers were also observed on the free surfaces of cells (148). This suggests that there may be a dynamic equilibrium between cadherin monomers, dimers and higher-order oligomers on the cell surface, with adhesive binding promoting oligomerization (Fig 10).
Ligation-induced cadherin clustering, resulting in the local accumulation of cadherin bonds, appears to be a mechanism to strengthen cadherin adhesion. This was first suggested by observed correlations between clustering and enhanced adhesion (14, 418). The conclusion was reinforced by the demonstration that adhesive strength was enhanced by forced lateral clustering of a chimeric cadherin, which retained the adhesive ectodomain but lacked the cadherin tail (418). This indicated that clustering of the adhesive receptor domains alone constituted a mechanism to strengthen adhesion. This strengthening can, in most cases, be attributed to increased local avidity, which has the simultaneous effect of increasing the probability of bonds rebinding after dissociation (389) and increasing the number of bonds resisting disruptive forces (395).
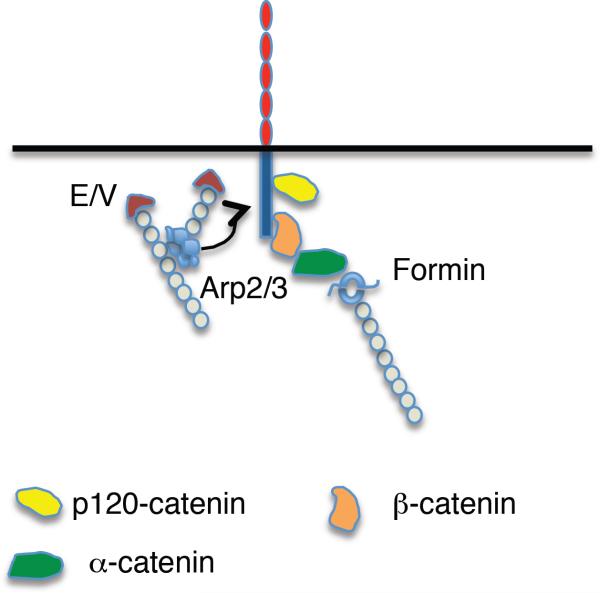
Although lateral clustering may enhance adhesion by controlling the local distribution of the ectodomain presented on the cell surface, the ectodomain alone is not sufficient to support clustering. Instead, multiple cytoplasmic factors contribute to cadherin clustering in cells. Clustering requires the juxtamembrane domain (JMD) of the cadherin cytoplasmic tail responsible for binding p120-ctn (418, 420). Intriguingly, the crystal structure of p120-ctn complexed with the JMD revealed oligomerization of the complexes, suggesting that binding of the JMD may induce oligomerization of p120-ctn, a possible mechanism for cadherin clustering (152). Additionally, clustering involves cytoskeletal effectors such as non-muscle Myosin II (332, 342), Ena/VASP proteins (325) as well as PI3-kinase signaling (97). This suggests that lateral clustering may arise from cadherin-activated cell signaling to the actin cytoskeleton, that enhances cell adhesion by controlling the distribution of adhesive binding sites presented on the cell surface (Fig 10).
B. Regulating the surface expression of classical cadherins
One fundamental determinant of cadherin biology is the amount of cadherin that is presented on the cell surface. Formally, then, regulated changes in surface cadherin levels constitute one potential way to modulate cell adhesion. Indeed, experimental manipulation of cadherin expression in cultured cells correlates well with changes in cell adhesiveness (14, 418).
Surface cadherin expression is, in turn, the product of a hierarchy of cellular processes. Ultimately the total level of cadherin expressed in cells must be determined by the balance between biosynthesis and degradation. Changes in transcription are the best-understood mechanisms that control cadherin biosynthesis; in contrast, a major site for cadherin degradation occurs in lysosomes. But between the birth and death of cadherin proteins, the proportion of total cellular cadherin that is presented on the cell surface reflects a complex trafficking itinerary that encompasses exocytic transport to the surface, internalization of cadherin, and then transfer for either recycling to the cell surface or transport towards lysosomal degradation (Fig 11). Importantly, these are not simply housekeeping pathways; instead, many trafficking steps have the potential to act as rate-limiting stages for the regulation of cadherin transport and fate. Indeed, junctional integrity can be compromised when membrane trafficking is disrupted (59, 100, 194, 313, 381). Additionally, cadherins can be cleaved whilst on the cell surface, providing an alternative regulated mechanism to rapidly alter the surface levels of cadherin.
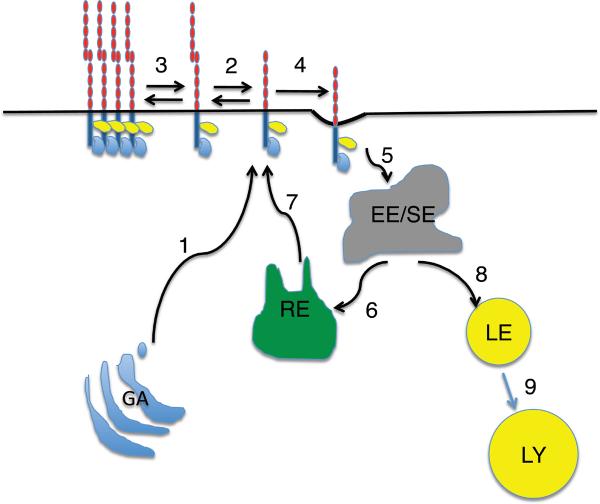
Figure 11. Itinerary of cadherins within the cell.
Newly synthesized cadherins are transported from the Golgi apparatus (GA) to the cell surface (1), where they are available to engage in cis-binding interactions (2) and form higher-order oligomers and clusters (3). Alternatively, free cadherin, that unable to engage in cis-interactions or which unbinds, is endocytosed (4). Following internalization (5), cadherins are trafficked to early sorting endosomes (EE/SE) from which they may transported back to the cell surface via Rab11-positive recycling endosomes (RE, 6,7), or trafficked through late endosomes/multivesciular bodies (LE,8) for degradation in lysosomes (LY, 9). For clarity, only β-catenin and 120-catenin are drawn in this diagram.
In this section we will discuss these individual processes that, collectively, control the surface expression of classical cadherins.
1. Cadherin Biosynthesis: Transcriptional regulation of cadherin expression
Classical cadherins undergo both inhibitory and stimulatory transcriptional regulation. Transcriptional down-regulation has been most intensively studied in the context of EMT, where E-cadherin expression is inhibited by a range of transcriptional repressors. These include the Snail/slug family of zinc-finger transcriptional regulators (258), the bHLH transcription factor, twist (417), and LEF1, a downstream mediator of the canonical Wnt signalling pathway (158). Both snail and twist repress transcription by binding to E-box sequences found in the E-cadherin promotor (258, 417), which also contains an independent LEF1-binding site (158). These transcriptional regulators presumably respond to extracellular signals or cellular cues. Indeed, snail family members form a common nexus for a range of growth factor signalling pathways, that include TGF-β1 and 2, Bone Morphogenetic Proteins (BMPs), and FGFs (258), suggesting that these transcriptional regulators may serve to integrate multiple cellular signals. Consistent with this, repression of E-cadherin transcription in the developing hair follicle required both a BMP signal (to induce LEF1) and a canonical Wnt signal (to activate β-catenin signaling) (158). Expression of these transcriptional repressors is also subject to inhibitory regulation by microRNAs, such as those of the miR200 family, that preserve E-cadherin expression by inhibiting ZEB-1 and ZEB-2 (109, 283).
Cadherin transcription has also been reported to be upregulated in a number of cell culture (273, 415) and developmental models (259). One of the most striking examples occurs during the previously-discussed process of Drosophila border cell migration (Fig 6B). Here DE-cadherin expression in border cells is upregulated during border cell migration in response to the transcription factor Slbo (242, 259). Moreover, this transcriptional up-regulation of DE-cadherin is necessary for this cadherin-dependent morphogenetic event to occur. The cues that activate Slbo are not fully known, but a range of developmental signals, including Wnt 7a (273) and WT1 (144) can stimulate cadherin transcription in cell culture.
It should be noted that it seems unlikely that transcriptional regulation alone contributes to rapid, dynamic changes in cadherin function. In particular, the metabolic half-life of cadherins (~5–10 hours for E-cadherin in cultured cells (226, 338)) suggests that delays of several hours would occur before transcriptional repression became manifest in altered protein expression. Nonetheless, many of these state changes have morphogenetic consequences, such as that exemplified by border cell migration. Moreover, several regulators of E-cadherin transcription are also implicated in tumor cell progression to invasion and metastasis, including the transcriptional repressors, Snail (258) and Twist (417), suggesting that cadherin dysregulation at the transcriptional level contributes to aberrant morphogenesis and disturbed homeostasis.
2. Cadherin exocytosis in the biosynthetic pathway: a mechanism for targeted delivery of cadherins
Like other transmembrane proteins (108, 244), newly synthesized cadherins are transported in membrane-bound carrier vesicles from the endoplasmic reticulum to the Golgi apparatus before subsequent transport to the plasma membrane (41, 52, 210, 338) (Fig 11). Most germane for our present discussion is the potential for processing through this exocytic pathway to influence the final surface distribution of cadherins. This selective regional distribution is best exemplified by the basolateral distribution of E-cadherin in simple polarized transporting epithelia, but likely pertains to some extent in other polarized cells, such as neurons.
A key question is whether exocytosis allows the targeted delivery of cadherin to specific regions of the cell surface, thereby supporting the regional expression of cadherins. An active role for sorting in the secretory pathway was first suggested by the observation that newly-synthesized E-cadherin was selectively delivered to the baso-lateral surfaces of polarized epithelial cells, but not to their apical surfaces (196). This indicated that, like other transmembrane proteins, the localization of cadherins to specific membrane domains might reflect the influence of trafficking processes such as protein sorting and selective directed delivery to the plasma membrane.
Following synthesis, the trans-Golgi network (TGN) is the first opportunity in the biosynthetic pathway for cadherins to be identified and sorted for transport to specific membrane domains (116, 346). It is commonly believed that membrane proteins destined for different regions of the plasma membrane are segregated in the TGN into distinct sets of carrier vesicles for transport to the cell surface (113, 232, 233). Such discrimination is achieved through specific signals consisting of conserved polypeptide sequences contained within the cargo proteins themselves (346). Such sorting signals are believed to mediate interactions with specific adaptor proteins that allow cargo proteins to be sorted into distinct transport vesicles, such as the μ1B adaptor subunit of adaptor protein complex 1 (AP-1) which is specialized for basolateral targeting.
A range of potential peptide sorting signals can be identified in the cytoplasmic tails of classical cadherins (52, 238). While some possible motifs failed the experimental test (52), a highly-conserved di-leucine motif found in the juxtamembrane region of the cytoplasmic tail of many cadherins (Fig 12) does influence the basolateral expression of E-cadherin. Mutation of this motif resulted in the mis-sorting of mutant cadherin to the apical- as well as baso-lateral membranes when expressed in polarized epithelial cells, indicating that this signal is necessary for fidelity of baso-lateral localization (238). Interestingly, expression of this mis-targeted cadherin mutant also altered epithelial cell polarity and morphology, suggesting that selective sorting in the TGN can, indeed, influence the morphogenetic effect of the cadherin.
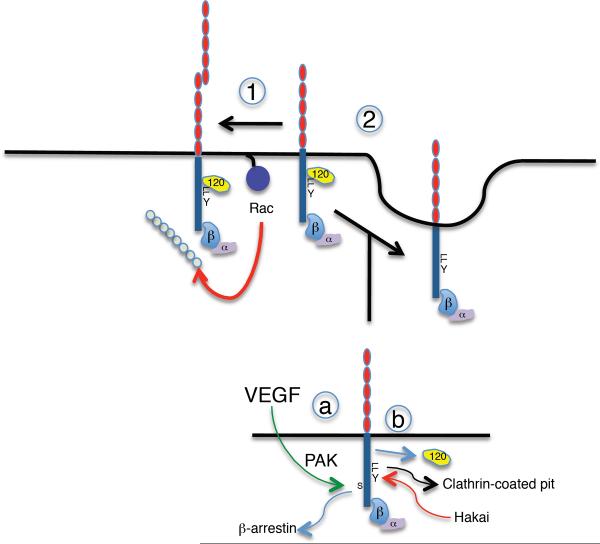
Figure 12. Regulation of cadherin endocytosis in control of surface expression.
Surface cadherins can be fated either for stabilization on the surface (1) or for endocytic uptake (2). (1) Stabilization, so that cadherins are not internalized, is promoted by cadherin ligation, masking of dileucine (LL) and tyrosine (Y) residues by p120-ctn, Rac/Cdc42 signaling and the actin cytoskeleton, involving proteins such as IQGAP. (2) Cadherin internalization can be promoted by multiple mechanisms: a) In endothelia, VEGF signaling induces phosphorylation of VE-cadherin ser 665 by a p21-activated kinase (PAK). This promotes binding of b-arrestin and targeting for endocytosis. b) Alternatively, displacement of p120-catenin unmasks the key dileucine and tyrosine residues that promote clathrin-coated uptake and Hakai binding, respectively.
Protein sorting in the TGN is unlikely to be sufficient to specify the basolateral delivery of cadherin. Instead, directed transport of post-Golgi vesicles is suggested to be necessary for fidelity of basolateral delivery. Cadherin-containing vesicles have been observed to move along microtubules towards cell-cell contacts (223, 234, 366). Furthermore, p120-ctn can interact with microtubules, both directly (88) as well as indirectly via binding to a kinesin (51, 416), thereby providing a potential mechanism to link cadherins to microtubules. Actin-based transport of cadherin may also occur, as VE-cadherin puncta were observed to be transported in filopodia of subconfluent endothelial cells by the actin-based motor, Myosin X (9).
Selection may also occur at the plasma membrane itself. In particular, the exocyst complex, which was first identified in the targeting patch that defines the site for secretory vesicle docking in budding yeast, also affects basolateral targeting in epithelia (243). Drosophila embryos mutant for the Sec 5 subunit of the exocyst complex accumulated DE-cadherin within intracellular vesicles (194), suggesting a defect in targeted delivery of cadherin to the cell surface. Moreover, the exocyst localizes to the apical junctional region in epithelia in an adhesion-dependent fashion (28, 111) that involves the tight junction scaffolding protein, PALS1 (394). This suggests a potential feedback mechanism, whereby localization of exocyst to the junctional complex promotes preferential docking of basolateral vesicles at those sites.
Together these observations suggest an attractive multi-stage model for targeted delivery of cadherins in the secretory pathway, which combines TGN selection via sorting signals, directed microtubule and actin tracks and target recognition at the plasma membrane itself. However, newly-synthesized cadherins may not be delivered directly from the TGN to the plasma membrane. Instead, in both polarized epithelial cells and non-polarized cells, E-cadherin was observed to be principally transported to an intermediary Rab11-positive compartment, consistent with recycling endosomes (210). Whether cadherins are then delivered directly to the plasma membrane or via other cellular compartments remains to be determined. Furthermore, whether the exocyst exclusively defines cortical targeting has yet to be directly tested for cadherins themselves, in contrast to other basolateral membrane markers (111). Indeed, the demonstration that cadherins at the lateral cell surface undergo cortical flow in a basal-to-apical direction (163) suggests that it is unlikely that cadherins at the lateral cell membrane are solely targeted to the apical junctional area by intracellular transport. Cadherins may be targeted to the lateral membrane more generally, then undergo regional surface redistribution.
3. Endocytosis and the post-internalization fate of cadherins
Membrane proteins can be internalised by diverse endocytic mechanisms (244), several of which are implicated in cadherin endocytosis. These include clathrin-dependent (54) and clathrin-independent uptake mechanisms (6, 284). Cadherins may internalise via different pathways, depending on cellular context. For example, constitutive uptake of E-cadherin in confluent epithelial monolayers appears to involve a clathrin-dependent process (154, 155, 198) but occurs principally by a clathrin-independent pathway in isolated cells (284).
Following internalization, cadherins enter a series of membrane-bound compartments that direct their traffic in the cell (233, 244) (Fig 11). These include early endosomes, which constitute one of the earliest way stations in the trafficking of many membrane proteins, capable of directing internalized proteins back to the plasma membrane or towards degradation. Transmembrane proteins are generally degraded in late endosomes or lysosomes, and this appears to also hold for cadherins. Thus lysosomal inhibitors can block cadherin turnover (67, 409) and cause surface-labelled cadherins to accumulate in late endosomes and lysosomes (409). However, inhibitor studies also suggest a potential role for proteasomes to participate in cadherin degradation (67). Whether this is through proteasomal turnover of proteins that generally regulate membrane transport to late endosomes or lysosomes, rather than a more specific effect on cadherin turnover, remains to be determined.
Internalized cadherins are not, however, obligatorily targeted for degradation (108). Instead, endocytosed E-cadherin can be recycled back to the cell surface (198) (Fig 11). Even confluent epithelial monolayers display a basal level of cadherin endocytosis and recycling, though this may be increased when cell contacts are broken (155, 198). In this regard, E-cadherin behaves like many cell surface receptors, such as the transferrin receptor, which can undergo many rounds of recycling before being degraded. Several endosomal compartments have been identified as sites to redirect membrane proteins back to the cell surface. In mammalian cells both endocytosed as well as newly synthesized E-cadherin enters a recycling endosomal compartment that can be identified by the GTPase Rab 11 (40, 210). Moreover, DE-cadherin trafficking is perturbed in Drosophila embryos mutant for either Rab5 (381) or Rab11 (59), key regulators of traffic at early endosomes and the recycling endosome, respectively. It is therefore possible that the recycling endosome serves as a common intermediary compartment for the sorting of both endocytosed and newly-synthesized cadherins for delivery to the cell surface.
Recycling of endocytosed cadherins may perform several potential functions. Firstly, recycling would provide a mechanism to protect endocytosed cadherins from degradation, thereby extending their metabolic lifetime. Secondly, recycling might contribute to the remodelling of adhesive interactions, allowing unbound cadherins to be redirected elsewhere on the cell surface (210). E-cadherin recycling occurs over time frames of minutes, which would allow it to participate in quite rapid remodelling of the cell surface. Such recycling then provides a mechanism by which the surface pool of cadherin can be sampled and sorted by cellular trafficking pathways. Finally, cadherin endocytosis may provide a mechanism for other proteins, such as growth factor receptor tyrosine kinases, that associate laterally with cadherins, to be coendocytosed for further processing (42). The precise biological impact of these potential scenarios remains to be thoroughly assessed.
An important open question is where the decision to recycle or target cadherins for degradation is made. One possibility is that internalized cadherins might immediately enter distinct pathways for recycling or degradation, as is reported to occur for the EGF receptor (340). The multiple potential pathways available for cadherin internalization would facilitate such a model. In this case, choice of endocytic entry becomes a critical decision point. Alternatively, endocytosed cadherin may enter a common compartment from which it is then distributed for either recycling or degradation. In this second scenario, which affects many membrane proteins, the regulation of distribution from a common endosomal compartment becomes critical. Noteworthy here is the observation that activation of a temperature-sensitive (ts) Src mutant biased E-cadherin transport to lysosomal degradation at several steps along the pathway, including GTP-loading of Rab5 and Rab7, key regulators in the endosomal system (277).
4. Regulation of cadherin internalisation: a mechanism to stabilize cadherin expression at the cell surface
While the itinerary of cadherin trafficking is likely to be regulated at several stages, as the first step into the endosomal system, internalization is an attractive point to regulate cadherin turnover. Indeed, an emerging theme is that the decision to endocytose critically determines the surface expression of cadherins. In the simplest form of this model, inhibition of endocytosis would stabilize cadherins at the cell surface, whereas an increase in cadherin endocytosis would be predicted both to decrease the surface pool of cadherin as well as facilitate degradation.
The potential for cadherin endocytosis to be rate limiting is most strikingly illustrated by the impact of p120-ctn (Fig 12). In vertebrates, cellular levels of p120-ctn appear to critically determine the steady-state levels of several classical cadherins. Thus, depletion of p120-ctn in mammalian cells (67, 151, 409), dramatically reduced steady-state cadherin levels which changed in approximate proportion to the reduction in p120-ctn. Conversely, over-expression of p120-ctn increased cellular cadherins (409). These p120-ctn-induced changes in cadherin levels were not accompanied by any changes in either cadherin mRNA levels or in the rate of protein biosynthesis. Instead, the reductions in cadherin levels induced by perturbing p120-ctn were effectively rescued by inhibitors of lysosomal activity (67, 409), suggesting strongly that loss of p120-ctn promoted cadherin degradation.
Formally, p120-ctn might influence cadherin degradation at any stage in the trafficking pathway from the cell surface to lysosomes. Interestingly, whereas newly-synthesized E-cadherin appeared to be transported to the cell surface normally in p120-ctn-depleted cells, its persistence at the surface was significantly reduced (67), suggesting that p120-ctn regulates the surface stability of the cadherin. Furthermore, perturbing p120-ctn activity in vascular endothelial cells appeared to promote endocytosis of VE-cadherin, while over-expression of p120-ctn reduced internalisation of the cadherin (409). Consistent with this, overexpression of p120-ctn prevented surface VE-cadherin chimeras from entering clathrin microdomains (54), suggesting a block in entry to presumptive clathrin-coated pits. Moreover, NMR analysis of purified p120-ctn bound to the JMD region of E-cadherin (152) suggested that a dynamic binding interaction allows p120-ctn to mask dileucine and tyrosine residues involved in clathrin-mediated internalization and association with Hakai, respectively (Fig 12).
Taken together, these findings suggest strongly that p120-ctn influences the surface stability, and ultimately the metabolic turnover, of classical cadherins by regulating their internalisation. In this model, p120-ctn would act to inhibit cadherin endocytosis, thus promoting its persistence at the cell surface and preventing its traffic to lysosomes for degradation. Conversely, loss of p120-ctn activity would promote endocytosis and ultimately traffic for degradation, thereby reducing the steady-state levels of cadherins within cells.
It should be noted that this impact of p120-ctn on cadherin turnover and function is most evident in mammalian systems (67, 68). In contrast, disruption of p120-ctn function in Drosophila and C. elegans has generally been reported to have a much weaker phenotypic impact (247, 276, 294), although exceptions do exist (214).
p120-ctn is not, however, the only signal that can determine cadherin endocytosis. The cbl-like protein, Hakai, can bind to and ubiquitylate the cytoplasmic tail of E-cadherin (91) in mammalian cells, thereby targeting it for internalisation (Fig 12), although this effect of Hakai was not apparent in Drosophila (161). Small GTPases of the Rho family, Rac and Cdc42, are also reported to inhibit cadherin internalisation through a process that requires actin filaments and the actin-binding protein, IQGAP (155). It is probable that many more such signals will be identified in the near future, but there is already interesting evidence that these signals may interact. Thus, both p120-ctn and Hakai may compete with one another to bind a very similar region of the E-cadherin cytoplasmic tail (91). Incorporation of p120-ctn also appears necessary for E-cadherin ligation to activate Rac signalling (107). Cadherin internalisation may thus be determined by a network of interacting cell signals.
If so, is it possible to identify dominant determinants of cadherin internalisation? Some evidence to date suggests that cadherin homophilic ligation itself play an important inhibitory role. Disruption of cadherin cell-cell contacts by chelation of extracellular calcium promoted cadherin endocytosis (198). More directly, recombinant cadherin ectodomains appeared to inhibit E-cadherin endocytosis in a cell-free assay system (155). Of note, these experiments used soluble recombinant cadherin ligands, suggesting that inhibition of endocytosis was not due to physical retention (kinetic trapping) of cellular cadherins bound to immobilized ligands. This implies that productive adhesive binding may act to inhibit cadherin endocytosis, thereby stabilizing the cadherin at the cell surface. One possibility is that ligand-activated cadherin signaling itself regulates endocytosis. As noted earlier, cadherin ligation can activate Rac signalling, which inhibited E-cadherin endocytosis in in vitro assays (155). Thus pathways activated by homophilic ligation may cooperate with kinetic trapping through ligation to stabilize cadherins at the cell surface.
Conversely, cadherin internalization may be acutely stimulated by cell signaling. This is exemplified by VEGF signaling in endothelia (96) (Fig 12), which activates a cascade including Rac and PAK that phosphorylates a specific serine residue in the cytoplasmic tail of VE-cadherin. This, in turn, recruits β-arrestin to drive the clathrin-dependent internalization of cadherin. Additionally, it has been suggested that the endocytic process may itself inhibit cadherin adhesion by affecting the assembly of trans-dimers (375). Cadherin internalization appears increasingly to constitute a key step that integrates many signals to influence the surface expression of this adhesion receptor.
5. Membrane trafficking and cadherin regulation
Overall, then, the surface expression of cadherins can potentially be regulated at multiple points in their itinerary for membrane trafficking. Junctional integrity can be perturbed when trafficking is disrupted at those various sites (59, 100, 194, 313, 381). Observations such as these suggest that regulation of cadherin trafficking may be a major mechanism to control its surface expression – and potentially to remodel contacts, especially in dynamic, developing tissues. However, it is important to note that current approaches to studying the functional impact of cadherin trafficking have targeted proteins such as dynamin (70), Rab 11 (210, 313) and Rab 5 (59, 381), which are not specific for cadherin trafficking. Indeed, Rab 5 can regulate signaling by GTPases, such as Rac (278), that may also affect cadherin function through regulation of the cortical actin cytoskeleton. Further efforts to characterize the molecular details of cadherin trafficking, and develop tools to specifically perturb these pathways if possible, will be important in future efforts to define the functional impact of cadherin trafficking.
6. Cadherin shedding: acute modulation of surface cadherin expression
In the previous section we discussed the capacity for cellular regulation to affect surface cadherin levels by biasing the movement of cadherins within their membrane trafficking itinerary within cells. Proteolytic cleavage is another mechanism that cells have at hand to rapidly and locally alter cadherins that are already on the cell surface (Fig 13). In essence, surface cadherins are cleaved at defined sites to release the ectodomain, a process called shedding. First observed in cultured breast cancer cells (402), cadherin shedding has been documented in a range of developmental contexts, such in the chick retina (281) and during various stages of Xenopus embryogenesis (227, 327). Moreover, ectodomain fragments are also found in the serum from cancer patients (72), indicating that this process may occur during tumor development. Shedding may also lead to a range of different cytoplasmic fragments being generated, which can also have biological effects.
Figure 13. Consequences of cadherin proteolytic cleavage.
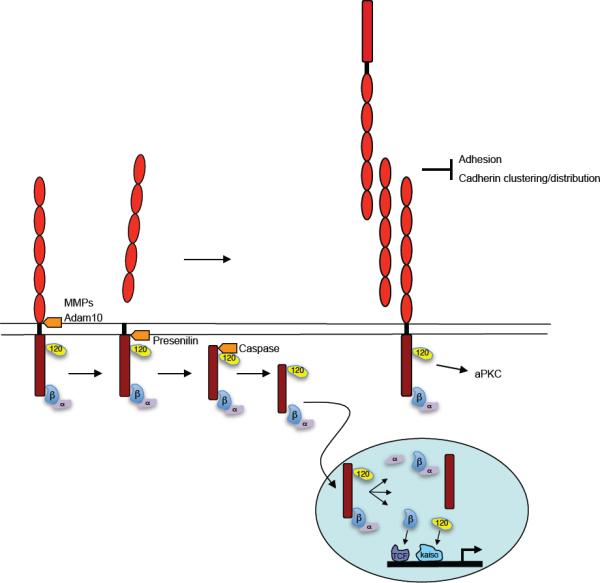
Ectodomain shedding by metalloproteases results in the release of the cadherin extracellular domain. This may result in reduced intercellular adhesion by directly reducing the number of functional adhesive complexes at the cell surface. In addition, the released cadherin extracellular domain may also interfere with adhesion. In addition, the extracellular domain can alter local signaling induced by the full length cadherin (e.g. aPKC activation), perhaps by altering clustering or local concentration of cadherins. Upon release of the cadherin extracellular domain, the cytoplasmic domain is subjected to cleavage by presenilins and caspase resulting in release from the membrane and translocation into the nucleus. Here it may modulate either directly or indirectly β-catenin/TCF dependent as well as p120/Kaiso dependent regulation of transcription. The function of the released cytoplasmic domain itself or of a-catenin in the nucleus is at present unclear.
Several different extracellular proteases (“sheddases”) have been implicated in cleaving the cadherin extracellular domain. A combination of overexpression, siRNA and knockout studies have provided compelling evidence that ADAM10 is a sheddase for E-cadherin and N-cadherin (221, 303). However, other studies reported roles for other metalloproteases such as Matrix Metallo Proteinases (228, 262), the serine protease family of kallikreins (174), and Meprinβ, a member of the astacin family (146). Although the precise sites of cleavage may differ, shedding most commonly generates a fragment of ~ 80 kD both in vitro and in vivo (327), a size consistent with that of the whole cadherin ectodomain. This implies that the sites of cleavage are likely to be close to the plasma membrane.
In addition to the loss of functional adhesive binding sites on the cell surface, shedding may release ectodomain fragments that are themselves biologically active. The soluble ectodomain fragment may serve as an adhesive substrate for other cells to attach to and/or migrate upon; it may further interfere with intercellular adhesion by competing with full length cadherin binding; and it may induce cell signals. For example, the soluble E-cadherin extracellular domain caused scattering of cells in culture (356, 402) and reduced cell aggregation associated with increased migration and invasion (262, 281). These results thus suggest a model in which the shed cadherin extracellular domain promotes migration and invasion by locally regulating cell adhesion. Interestingly, in early Xenopus embryos expression of C-cadherin ectodomain fragments interfered with gastrulation movements without affecting adhesion (327). This appeared to involve altered activity of aPKC, thus suggesting that planar polarity signaling was being affected. A recent study also found that the shed extracellular cadherin domain binds to and stimulates HER2/HER3 heterodimeric Erb receptors (252). Thus, shed ectodomain fragments may affect different forms of cell movement by different mechanisms.
Another immediate consequence of ectodomain cleavage is the generation of a membrane-associated fragment that contains the cytoplasmic domain, known as C-Terminal Fragment (CTF) 1 (219). This cytoplasmic fragment can then be further cleaved at different sites to release soluble polypeptides, CTF2 and CTF3, that are generally not stable (219). CTF2 is generated by cleavage close to the transmembrane domain and depends on the ε-protease activity of presenilin, whereas CTF3 generation requires caspases (219). Other intracellular proteases that have been implicated in cleavage of the cytoplasmic domain include calpain and the proteasome (310). While cleavage of the cadherin intracellular domain may occur independently of ectodomain shedding, in most cases cadherin shedding induces further cleavage and release of the cytoplasmic domain.
The cytoplasmic fragments generated by cadherin cleavage can also be biologically active. Both CTF2 and CTF3 have been reported to enter the nucleus (85). Due to their small size, this may be a passive process; however, overexpression studies showed that these fragments retain the ability to bind catenins (317), suggesting that active nuclear translocation may be involved in transport of a protein complex. The N-cadherin CTF2 fragment was shown to inhibit CRE dependent transcription by binding to CBP in the cytoplasm thereby excluding this transcription factor from the nucleus (220). On the other hand, BMP4 stimulates translocation of an N-cadherin cytoplasmic domain fragment into the nucleus where it activates cyclin D1 transcription (339). Along the same lines, E-cadherin CTF2 fragments translocate to the nucleus in a p120 dependent fashion where they modulate Kaiso dependent transcriptional activity (85). Such coordinated changes in adhesion and transcription may be functionally important. For example, BMP4-stimulated cleavage of N-cadherin by ADAM10 may promote neural crest delamination both by releasing cell-cell adhesion and also by regulating transcription through the nuclear translocation of cytoplasmic domain fragments (339). How these transcriptional events may be functionally coordinated with adhesive alterations due to ectodomain cleavage is an important issue to be addressed.
C. Cooperation between classical cadherins and the cytoskeleton
It has long been appreciated that the morphogenetic impact of classical cadherins entails a close functional and biochemical relationship between the adhesion receptors and elements of the cytoskeleton. Although the major focus in cadherin biology has been on the actin cytoskeleton, it is likely that all the cytoskeletal systems (microfilaments, microtubules, intermediate filaments) contribute to cadherin biology. There is increasing evidence that microtubules are recruited to cadherin adhesions and contribute to their function in diverse ways (5, 234, 347). Similarly, while intermediate filaments associate with desmosomal cadherins, they may also influence adherens junction organization (35). Nonetheless, actin microfilaments are the best understood cytoskeletal collaborators in cadherin biology and we will focus on what is know about their regulation and contribution to cadherin adhesive interactions.
1. Actin and cadherin biology
A role for actin in cadherin biology was first suggested by the observation that F-actin commonly localizes in proximity with cadherin adhesions (138); subsequent studies documented that dense perijunctional actin rings are found to localize with the ZA in many epithelia. Studies using light microscopy suggest that multiple pools of filaments may co-exist at cell-cell contacts (325, 424), including perijunctional filament bundles that terminate in cadherin adhesions (325, 421). Dynamic studies of actin turnover also suggest that multiple pools exist at cell-cell contacts in Drosophila tissues (45). However, the limited resolution of the light microscope makes it impossible to characterize three-dimensional filament organization more definitively, which is fundamental to understanding the functional impact of the cytoskeleton (341). Ultimately, advances that allow ultrastructural analysis of deep structures at cell-cell contacts will be needed to analyse the 3D structures of the perijunctional cytoskeleton as have been used to characterise filament organization in thin structures, such as the lamellipodia of migrating cells (178, 354).
Several lines of evidence indicate that the actin cytoskeleton supports cadherin biology. Thus, drugs that disrupt the integrity of actin microfilaments (cytochalasins, latrunculin) compromise cell-cell and cadherin-based adhesions in cell culture models (14, 157). Moreover, genetic studies have identified roles for a range of actin regulators and effectors that contribute to many cadherin-based morphogenetic processes, ranging from cell-cell cohesion in the early embryo (61) to the cell-upon-cell locomotion of border cells in the fly egg chamber (99). The latter also highlight the notion that diverse effectors regulate the actin cytoskeleton to contribute to cadherin biology.
Just as the actin cytoskeleton contributes to cadherin function, so too do cadherin adhesive events themselves regulate the cytoskeleton. Actin organization changes morphologically as cells make and establish contacts with one another and many cytoskeletal regulators are recruited to the cortex when cadherin adhesions form between cells, potentially in response to homophilic cadherin ligation and cell signaling. As will be outlined below, these include proteins that affect many parameters of actin organization and dynamics. This yields a picture of cooperative functional interactions between cadherins and the actin cytoskeleton, in which cytoskeletal organization is modified at adhesive contacts in response to signaling and biological context; this, in turn, contributes to the functional properties of those intercellular contacts. Though more complex than earlier models that envisaged passive anchorage of cadherin to cortical actin filaments, this model potentially encompasses a wide functional repertoire to match the biological diversity of cadherin-based cell-cell interactions.
2. Actin regulators active in cadherin biology
The actin cytoskeleton is a dynamic polymer system that is capable of diverse mechanical effects, which include generating force, resisting force and providing a scaffold to anchor other molecules. The precise local function provided by actin filaments is guided by how intrinsic polymer dynamics are regulated to assemble and disassemble filaments; how those filaments are organized into meshworks and bundles; and by the force-generating motors that act upon the filaments. As such, the organization and dynamic activity of the cytoskeleton is determined by many classes of proteins, representatives of which are recruited to cadherin adhesions.
Indeed, it is important to emphasize that the mechanisms that regulate the actin cytoskeleton at cadherin adhesions are shared with many other processes in cells, such as cell locomotion, integrin adhesion, cell shape control and membrane traffic. This is consistent with the central role that the actin cytoskeleton plays in cellular biology. A key to understanding how such core actin regulators contribute to cadherin biology then lies in identifying how they are recruited to act locally at cadherin adhesions.
i) Regulators of actin filament assembly
Cadherin adhesions are sites of actin filament assembly (365) (Fig 14). Actin polymerization, identified by the localization of free barbed ends, can be readily identified at cadherin-based cell-cell contacts (383, 384) as well as at homophilic adhesions where cells interact with immobilized cadherin ligands (180, 182, 188). Thus cadherin adhesion appears to mark cortical sites that generate new actin filaments. While purified actin has the capacity to self-assemble, this intrinsic turnover is too slow to effectively remodel the cytoskeleton within biological contexts. Instead, many effector proteins exist to catalyse distinct steps in filament assembly, several of which have recently been found to interact with cadherin receptors.
Figure 14. Regulation of actin filament dynamics by cadherin adhesion complexes.
Cadherin adhesions may promote actin filament assembly by recruitment of actin nucleators, such as formins and the Arp2/3 complex. Filament growth following nucleation is regulated by other proteins, such as the Ena/VASP proteins, that promote growth of filaments at their barbed ends.
The rate-limiting step in de novo filament generation is the kinetically-unfavourable nucleation of filaments from monomers (53, 106, 135, 285). This is because actin dimers and trimers are unstable; additionally, cells typically contain large pools of monomer-buffering proteins (such as profilin and thymosin β4) that can sequester free actin monomer. Thus a number of molecular mechanisms exist to nucleate actin filaments, two of which are reported to interact with classical cadherins (Fig 14).
The first actin nucleator that was discovered, the Actin-Related Protein (Arp) 2/3 complex, consists of a stable complex of seven evolutionarily-conserved proteins (53, 135). When activated by nucleation-promoting factors (notably WASP-WAVE family proteins), Arp2/3 catalyses nucleation of filaments at their pointed (minus) ends, thereby allowing growth to occur at the barbed ends by intrinsic self-assembly. The purified Arp2/3 complex, however, has little intrinsic nucleating activity: instead, within cells it is activated by other proteins in response to a variety of cellular signals (135). Of note, Arp2/3 (180) and its nucleation-promotors, N-WASP and WAVE (153, 414), are found at cadherin-based cell-cell contacts. Arp2/3 can form a molecular complex with E-cadherin in response to homophilic ligation (180) and blocking Arp2/3 activity reduces actin assembly at cadherin adhesions (384).
Formins nucleate actin filaments by a different molecular mechanism. In contrast to Arp2/3, formins bind to the barbed ends of filaments and may promote nucleation by stabilizing spontaneously formed actin dimers and trimers (53). Moreover, formins have the remarkable capacity of processive association, where they remain bound to the barbed ends of growing filaments through many rounds of monomer addition (285). Many different formin family members exist in metazoa (106), several of which have been implicated at cadherin contacts. Formin-1, the founding member of the family, was recruited to keratinocyte cadherin contacts through association with α-catenin; disruption of the interation between these two proteins perturbed cell-cell integrity and the perijunctional actin cytoskeleton (176). Drosophila Diaphanous and its mammalian homolog, mDia1, also localize to cadherin-based cell-cell adhesions where they contribute to stabilizing the perijunctional actin ring and adherens junctions (44, 142, 318). The precise functional relationship between Arp2/3 and formins in actin regulation at cell-cell contacts remains to be elucidated.
Filament growth can also be initiated when new free barbed ends are generated from pre-existing filaments, either by uncapping barbed ends or severing filaments. This can potentially generate bursts of actin assembly in response to cell signaling (101). Gelsolin, a potent actin-severing protein is found in cadherin complexes at nascent cadherin-based cell-cell adhesions (46), where it supports actin assembly and cadherin adhesion (80). Gelsolin appears to be recruited to cadherin adhesions in response to locally-generated PIP2 signals (80). Of note, gelsolin will cap the barbed ends that it generates by severing, and these gelsolin caps must be removed for filament growth to occur (353). PIP2 also participates in uncapping gelsolin (353) and this uncapping ability appears to be essential for gelsolin to promote cadherin adhesion (80). It is likely that other proteins that sever and uncap filaments, like gelsolin, will be found to contribute to actin assembly at cadherin adhesions.
Once nucleation or fresh barbed ends initiate actin assembly, filament growth can potentially be driven by self-polymerization until actin monomer reserves are depleted. However, the degree of filament growth is antagonized by the existence of capping proteins that bind to barbed ends and prevent monomer addition (53). Persistent filament growth therefore requires the cooperation of proteins that protect the barbed ends. One class of anti-capping proteins (or “actin elongation factors”) are the formins themselves (53); thus cadherins may recruit formins to drive actin assembly both through nucleation and anti-capping. Moreover, formins can accelerate growth at barbed ends by recruiting profilin-actin complexes (53). Another class is the Enabled/Vasodilator-stimulated phosphoprotein (Ena/VASP) family, which preferentially bind to filament barbed ends, thereby protecting them from capping as well as accelerating barbed end growth (23, 53). Drosophila Ena and its mammalian homolog, Mena, are found at cell-cell contacts (110) and Mena can be recruited to the cortex in response to cadherin homophilic ligation. However, although Ena localizes to adherens junctions in the Drosophila embryo, its depletion does not affect junctional integrity, despite having morphogenetic effects (95). In contrast, the mammalian Ena homologs (Mena, VASP, EVL) support actin assembly at cell-cell contacts (94, 325) and the integrity of cell-cell contacts in cultured keratinocytes (383). Moreover, mice embryos depleted of all three Ena/VASP proteins were highly susceptible to disruption by mechanical stress (94).
ii) Actin filament-binding proteins
A number of proteins that share the ability to associate with F-actin have been implicated in cadherin biology (Fig 15), though their molecular actions are likely to differ.
Figure 15. Anchorage of cadherin adhesion complexes to the actin cytoskeleton.
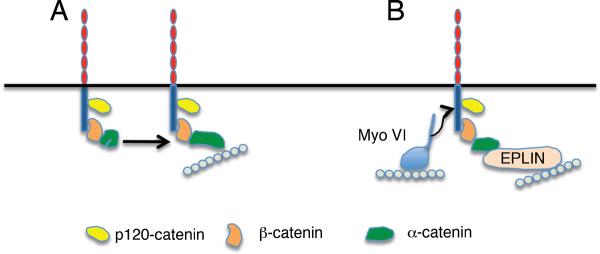
Potential models include:
A) Binding to cortical actin filaments directly via α-catenin. Although direct binding of cadherin-bound α-catenin to F-actin has not been confirmed in vitro conformational change induced by e.g. mechanical force may allow the cadherin-bound α-catenin to interact with actin filaments.
B. Alternative mechanisms to couple cadherin complexes to actin filaments. Potential other mechanisms to physically couple cadherin complexes to F-actin include binding proteins, such as EPLIN, which are recruited by α-catenin. Alternatively, but not exclusively, other actin binding proteins, such as Myosin VI, can interact with cadherins by as-yet-uncharacterized molecular mechanisms.
α-catenin
The most intensively-studied filament-binding protein in cadherin biology (326), α-catenin can interact with actin filaments in several ways. Recombinant α-catenin co-sediments with purified F-actin (74, 309), demonstrating that α-catenin can bind directly to actin filaments. It may also interact with other actin-binding proteins, including vinculin (399, 401), ZO-1 (149), α-actinin (175), afadin (297, 357) and EPLIN (2). Thus α-catenin has the direct and indirect potential to bind to actin filaments. α-catenin also inhibits Arp2/3-mediated actin assembly in vitro (74), suggesting its potential to regulate actin dynamics. The functional role of α-catenin in cadherin-actin cooperation is discussed at greater length below (Section C.3.i).
Vinculin
Although more commonly known as a component of integrin-based focal adhesions, vinculin is also found at cadherin-based cell-cell adhesions (98). It is identified in cadherin complexes (129, 213, 291, 399), an association that may depend on interactions with α-catenin (74, 399), β-catenin (291), and/or Myosin VI (213), depending on cell type and functional context. Vinculin can bind directly to actin filaments and can also associate with a variety of other actin-binding proteins, including Arp2/3, Ena/VASP proteins and α-actinin (428). Consistent with a functional impact at cell-cell adhesions, disruption of vinculin expression, its function or its interaction with the cadherin-catenin complex, perturbs cadherin-based cell-cell interactions, organization of the perijunctional actin cytoskeleton and the apical junctional complex (213, 399, 401).
Cortactin
This phosphoprotein is a versatile scaffolding molecule often found where the actin cytoskeleton interacts with the plasma membrane (11, 65). At cell-cell contacts cortactin can complex with both E-cadherin and N-cadherin (79, 131), an interaction that can be induced by cadherin homophilic ligation (131). Loss-of-function studies indicate that cortactin contributes to cadherin adhesion, junctional integrity and to the organization and dynamics of the perijunctional actin cytoskeleton (79, 305, 384). The molecular mechanism responsible for this diverse functional impact is likely to be complex (304). Cortactin can bind directly to F-actin and also interacts with a range of other actin-modulatory proteins, which include Arp2/3, N-WASP and WIP (11). One function may be to promote actin assembly by assembling a signaling complex that promotes Arp2/3 activity, but this is unlikely to be its only molecular action. Importantly, cortactin is a target for several cell signaling pathways (11, 65), functions as an effector of E-cadherin-activated Src signaling in epithelia (305), and is downstream of FER kinase at N-cadherin adhesions (78).
These three examples alone highlight the potential mechanistic diversity of cytoskeletal regulation that might be supported by these actin-binding proteins. Additional complexity is likely to be provided by other filament-binding proteins, such as KLEIP (122) and the cross-linker, α-actinin (175), that have also been identified at cadherin adhesions and implicated in modulating the perijunctional actin cytoskeleton.
ii) Actin-based motors
Myosin motors are mechanoenzymes that share two fundamental properties: the capacity to bind actin filaments and to move relative to those bound filaments. This latter property allows myosins to move on filaments and/or organize the cytoskeleton itself. The precise impact of an individual myosin will depend on its capacity to self-associate and the organization generated by such self-association; its kinetic and motor properties; and the properties of the local actin cytoskeleton where it is associated.
Four Myosins (II, VI, VII, X) have been implicated in cadherin biology. Non-muscle Myosin II is commonly found enriched with the perijunctional actin cytoskeleton at both invertebrate and mammalian epithelial contacts (29, 332, 342). Its junctional localization in cultured mammalian epithelia appeared to depend on a variety of upstream signals that and cadherin adhesion itself; indeed homophilic E-cadherin ligation can activate this motor (332). The precise regulation of its junctional localization is further influenced by the Myosin II isoform involved (342). Of the three isoforms found in mammals (386), both Myosin IIA and IIB are found at epithelial cell-cell junctions. Junctional recruitment of Myosin IIA appears to respond to signals that activate the motor (Rho, ROCK, MLCK) whereas junctional localization of Myosin IIB depends on Rap1 signaling (342). The biological impact of Myosin II on cadherin contacts seems to depend on cell type, functional context, and the Myosin II isoform involved. In some reports, especially where cell-cell contacts undergo dynamic reorganization, Myosin II appears to promote turnover of cadherin contacts (29) or disruption of junctions (71). In other contexts (61), notably where Myosin II may be activated in response to cadherin adhesion, it supports adhesive strengthening and maintains the ZA (332).
Myosin II isoforms also differ in their intrinsic motor properties and, while its contractile capacity is the most commonly known function of this motor, its capacity to anchor to filaments may also be important. Of note, cell-cell adhesive defects seen in the neural tube of mouse embryos deficient for Myosin IIB were rescued by a myosin mutant with defective motor (actin-sliding) capacity, but which retained the ability to bind filaments (212). Similarly, integrity of the ZA in Myosin IIA-deficient cells could be rescued with a poorly-contractile Myosin IIA mutant (342).
Myosin VI is unusual amongst myosins because it moves processively towards the minus-ends of actin filaments, whereas all other myosins are directed towards the plus ends (355). Myosin VI binds to E-cadherin at epithelial cell-cell contacts (213) and its recruitment coincides with the reshaping of nascent contacts into linear cadherin contacts during epithelial maturation. Myosin VI disruption perturbs junctional integrity in cultured mammalian cells and Drosophila embryos (213, 236), and in culture this coincides with reduced cadherin adhesion and disorganization of the perijunctional actin cytoskeleton. Myosin VI also cooperates functionally with DE-cadherin during border cell migration in the fly egg chamber, suggesting that it participates in several distinct forms of cadherin-based morphogenesis (99). Myosin VI contributes to the post-Golgi sorting of E-cadherin to the lateral cell surface (15), but its impact on junctional integrity appeared to occur without substantive changes in surface cadherin levels, suggesting that its impact on the perijunctional actin cytoskeleton is more significant for this effect (213). Interestingly, in established cell contacts Myosin VI was necessary for the junctional localization of vinculin, which cooperated as an apparent downstream effector of Myosin VI in preserving junctional integrity (213).
Finally, the role of Myosin X has been discussed earlier in the context of cadherin transport (Section B.2). Myosin VII is a plus-end directed motor that can interact indirectly with E-cadherin through the transmembrane protein verzatin (185). This interaction participates in cadherin-dependent internalization of Listeria bacteria (345), but its precise role in cadherin physiology is not certain.
3. Functional impact of cadherin-actin interactions
i) Anchoring cadherins to actin
Cadherin adhesions are sites where surface adhesion is mechanically coupled to the actin cytoskeleton. For example, N-cadherin molecules undergo actin-dependent retrograde flow on the dorsal surfaces of cells (188). This implies that cadherins can couple to the cortical actin cytoskeleton, as has been reported for other transmembrane proteins (84, 208), providing a mechanism for the actin cytoskeleton to mechanically influence the cadherin. Conversely, cadherin adhesions commonly appear to intersect with contractile actin cables (62, 325, 382, 424), suggesting that actin-based mechanical forces play on sites of adhesion. This is emphasized by recent evidence that contractile tugging force exerted on endothelial cell-cell contacts could increase the size of VE-cadherin junctions (209). Such observations imply that mechanisms must exist to physically couple cadherin adhesions to the actin cytoskeleton (Fig 15).
α-catenin has long been favoured to mediate cadherin anchorage to the cytoskeleton, a notion prompted by several observations. Firstly, the incorporation of α-catenin coincides with the cadherin-catenin complex becoming more Triton-insoluble (137, 254) and expression of α-catenin in tumor cells lacking this protein renders E-cadherin more detergent-resistant (398). Detergent-extractability has often been interpreted as evidence for cytoskeletal association, however, it is important to emphasize that many other factors influence the detergent sensitivity of membrane proteins (322). Secondly, α-catenin often localizes to sites where perijunctional actin cables intersect with cadherin adhesions, and loss of the catenin can lead to detachment of those cables from the cortex (62, 382), suggesting that α-catenin might couple these actin structures to adhesions. Finally, taken with the demonstration that purified α-catenin can directly bind F-actin in co-sedimentation assays (309), these findings supported a popular model whereby α-catenin directly couples the cadherin molecular complex to the cortical actin cytoskeleton.
In its purest form, this quaternary model of cadherin anchorage to F-actin via α-catenin has failed the empirical test. Using in vitro binding assays, Yamada et al. (2005) confirmed that recombinant α-catenin can bind actin filaments; however, an interaction with F-actin was not detectable when α-catenin was incorporated into a complex with recombinant β-catenin and the cadherin cytoplasmic tail (413). Instead, binding of α-catenin to β-catenin appeared to be mutually exclusive of its binding to actin filaments (74). Nor could F-actin binding be identified when the ternary cadherin-catenin complex was reconstituted on stripped plasma membranes and in protein exchange studies GFP-tagged actin at cell-cell contacts turned over much more rapidly than did E-cadherin, β-catenin or α-catenin itself (413). Although a simple quaternary complex linking E-cadherin to stable actin filaments through α-catenin could not be reconstituted in vitro, it is possible that in the cellular context other factors, such as mechanical force, may operate to activate its F-actin-binding capacity when α-catenin is part of the cadherin molecular complex (422) (Fig 15A).
Several other mechanisms exist that might physically couple cadherin adhesion to the cytoskeleton. One possibility is that the local lipid environment of the plasma membrane at adhesion sites might mediate binding to cortical filaments. Many actin-binding proteins, including vinculin, can bind to PIP2 (331), which is often enriched at intercellular contacts (222) and which mediates interactions between the plasma membrane and the cortical cytoskeleton (331). In an extreme form of this model, cortical filaments could interact with the plasma membrane in regions of adhesion without protein-protein linkages as well. However, lipid-based membrane-cytoskeleton interactions are relatively weak (200, 331, 404), it is difficult to envision even weaker polymer-membrane interactions supporting strong cell-cell adhesion.
Alternatively, other actin-binding proteins may couple cadherins to filaments at sites of adhesion (Fig 15B). The actin-binding protein, EPLIN, was recently identified as a junctional component that incorporates into the cadherin complex through a direct interaction with α-catenin. Moreover, EPLIN appeared capable of supporting the in vitro binding of actin filaments to beads coated with a reconstituted cadherin/β-catenin/α-catenin complex (2). Vinculin, cortactin and Myosin VI also have the potential to couple cadherins to filaments, insofar as they can interact biochemically with the cadherin complex and possess the intrinsic ability to bind F-actin. Depletion of these individual proteins also disturbs the organization of the perijunctional actin cytoskeleton (213, 305, 399, 401). Thus, there is the capacity for other proteins to confer both α-catenin-dependent and –independent anchorage to the perijunctional actin cytoskeleton. Moreover, these proteins may interact in a context-dependent fashion. Of note, α-catenin was recently reported to undergo conformational alteration in response to mechanical force, leading to vinculin-binding (422) (Fig 15A).
ii) Force generation and force resistance
Cadherin adhesions are subject to mechanical force. They are predicted to be sites where force is generated, sensed (197, 209), and resisted by cells. Force generation is most readily evident when cell contacts are being assembled or remodelled. For example, as cultured cells make contacts with one another, local protrusiveness of the membrane appears to bring cell surfaces together (4, 77, 383). Also, once initial contacts have been formed, such surface protrusiveness may promote the extension of contacts upon one another, thereby promoting more robust cell-cell interactions. Such “zippering”, which has often been described as part of the cell biology of cadherin interactions, requires contributions from the actin cytoskeleton and is not necessarily due to surface adhesion alone (384). Force generation at cadherin adhesions is also likely to participate in cadherin-dependent cell-upon-cell locomotion (115, 241), where cells must translocate upon one another, presumably making, as well as breaking, intercellular adhesive contacts in the process.
Cortical actin filament assembly has often been implicated in generating surface protrusive force. The Arp2/3 complex, in particular, participates in leading edge protrusion during cell locomotion (135), accumulates in newly-forming cadherin contacts (180) and participates in efficient assembly of adhesive contacts (384). Recruitment of Arp2/3 to E-cadherin complexes (180) could provide a mechanism that focuses actin assembly to promote surface protrusion and contact extension as cells first interact with one another. Though yet to be directly tested, formin-mediated actin assembly could provide an additional mechanism for force to be generated at sites of cadherin-based actin bundles.
Other molecular mechanisms can also complement and/or provide alternatives to filament assembly for force generation. These include the reorganization of filaments into bundles, a process that can involve Ena/VASP proteins (383), other bundling proteins, and non-muscle Myosin II (354, 388). Myosin II-based contractility itself is commonly invoked as a force-generator in many cellular contexts. One example is the apical constriction that mediates mesoderm invagination during Drosophila gastrulation (296). This process also requires cadherin-based adherens junctions (69), leading to the notion that Myosin II contractility against the adhesions drives the morphogenetic movement. This model implies some chain of physical linkage between Myosin II and cadherin receptors. It is then noteworthy that canoe (the fly homolog of afadin) can associate with DE-cadherin and in fly embryos mutant for canoe Myosin II appears to lose connection with adherens junctions and the cells fail to complete mesoderm invagination (320).
The precise direction of force that Myosin II will generate depends on its organization. Myosin II assembles into short bipolar arrays (“minifilaments”) (256), implying that motor force will be directed into the centers of the minifilaments. The exact organization of these minifilaments will determine the broader landscape of force supported by Myosin II. For example, isotropic distribution of Myosin II at the cell cortex would promote contraction of the whole cortex (63), whereas incorporation of Myosin II into actin filament bundles would tend to lead to shortening of the bundles.
As well as being sites where cells apply force to other cells, cadherin contacts are also sites where cells must resist forces that would tend to break contacts apart. This is exemplified by the impact of HGF, which stimulates integrin-based actomyosin contractility to mechanically disrupt epithelial cell-cell contacts (71). Similarly, such disruptive forces are seen during wound healing (58) and during morphogenesis in embryos (62). In all these examples, one envisages that forces generated elsewhere in cells, or from other tissues, act to pull against cell-cell contacts. More subtly, the generation of protrusive force by the cytoskeleton at cadherin contacts may also have the potential to distort or disrupt those contacts by exerting shear forces against the anchoring cadherin bonds. Such forces can disrupt cell-cell contacts, but in other cases force may act as a stimulus to reinforce intercellular junctions, through a tension-sensing mechanism that appears to involve α-catenin and vinculin (197, 422).
It is likely that multiple cytoskeletal mechanisms contribute to resisting force at cadherin adhesions. The organization of actin filaments into bundles provides a mechanism to distribute force across contacts. Potential bundling proteins, such as vinculin, may preserve junctional integrity in part by supporting perijunctional actin bundles. Motor proteins, such as Myosin II and Myosin VI, can reorganize actin filaments into bundles and networks, which would tend to resist forces (102, 263). As well, motors such as Myosin II and VI can respond to strain by “locking” and remaining bound to actin filaments (10, 181), providing a mechanism for anchorage. Such anchorage may explain the observation that adhesive defects seen in Myosin IIB null mouse embryos can be rescued with a myosin mutant that lacks filament sliding capacity, but retains actin binding (212). As well, though, the ease with which membrane tethers can be pulled away from the cell cortex (82, 140) argues that robust cytoskeletal attachments are necessary for cadherin complexes to resist force.
iii) Cortical membrane organization
As discussed earlier, cadherins display distinct patterns of distribution at cell-cell contacts, that include puncta (clusters) (14, 163, 332) as well as the apical ring structure in epithelial cells commonly thought to reflect the zonula adherens (34, 234). It was earlier suggested that cis-and trans-binding interactions between ectodomains might lead to the lateral assembly of cadherins into paracrystalline arrays (330). Despite this, cytoplasmic contributions of the cytoskeleton also appear to contribute to surface cadherin organization. Of note, Myosin II (332), and specifically the Myosin IIA isoform (342), promotes lateral cadherin clustering (Fig 10). Similarly, DE-cadherin puncta in Drosophila embryos co-localize with relatively stable F-actin plaques, whose lateral mobility is restricted by α-catenin (45). Thus cytoskeletal factors can restrict cadherin distribution within the plane of cell-cell contacts.
The cytoskeleton may also have other active roles in the surface distribution of cadherin. Contacts between motile cells display an actin-dependent cortical flow of cadherins from the basal surface to the apical contacts between cells (163). This also depended on myosin light chain kinase, suggesting that it represented a form of acto-myosin-driven cortical flow, akin to that which occurs on the free surfaces of motile cells (188, 208). Thus the apical cadherin ring that is characteristic of many epithelial cells may reflect multiple impacts of the actin cytoskeleton on cadherin distribution: both cortical flow that drives cadherin punta in an apical direction and Myosin II-dependent retention of cadherin in the apical ring. Moreover, while the heterogeneous distributions of cadherins are often most apparent at epithelial cell-cell contacts, the distinctive organizations of cadherins in other junctions, such as those in synapses, are likely to also involve cadherin-actin interactions (1).
D. The interplay between cadherins and cell signaling
In the earlier sections of this review we discussed the many ways in which membrane trafficking and the actin cytoskeleton may be coordinated with cadherin adhesion receptors in a variety of biological contexts. This implies that mechanisms must exist to regulate this coordination. Indeed, a host of signaling mechanisms are active at cadherin adhesive contacts. These include calcium signaling (46, 260) and regulators of β-catenin signaling (215). Within the scope of this review, we will focus on signals that have clearly defined morphogenetic impacts on the cytoskeleton and membrane trafficking: Rho family small GTPases and protein tyrosine kinases.
1. Cadherins as signaling receptors
It has often been attractive to postulate that the morphogenetic impact of cadherins might entail adhesion-activated cell signaling, where productive binding of the cadherins would alter intracellular signaling events that ultimately lead to changes in cell behavior. This gained support from evidence that the assembly of cell-cell contacts modulated a variety of signaling pathways in a cadherin-dependent fashion, including signaling by Rho family GTPases (31, 253), the lipid kinase PI3-kinase (286), and Src familiy kinases (229).
However, it was not possible in those experiments to determine whether signaling was altered as a direct response to the cadherin itself, or as a result of a juxtacrine signaling pathway (Fig 16). The first scenario envisages that ligand binding of the ectodomain initiates intracellular signalling events: the cadherin is both necessary and sufficient to activate cell signalling (Fig 16A). In the second scenario, cadherin adhesion brings together cell surfaces that bear contact-dependent ligands and their receptors (e.g. nectin Ig superfamily proteins (92)) or facilitates gap junction-assembly (114), which then initiate cell signalling (Fig 16B). In this second scenario cadherin adhesion is necessary for signalling, because it brings cell surfaces together; but it is not sufficient, because other receptors are more proximately responsible for altering signalling.
Figure 16. Models for cadherin-dependent cell signaling.
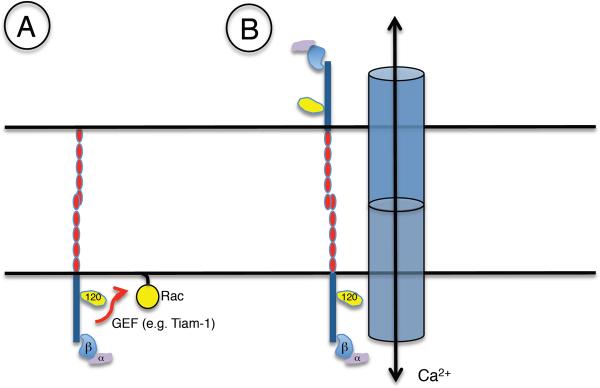
A) Direct cadherin-activated signaling: here adhesive ligation of the cadherin stimulates intracellular signal transduction processes, such as the small GTPase Rac, a process that involves intermediary Guanine nucleotide exchange factors (GEF) such as Tiam-1.
B) Juxtacrine signaling: here cadherin adhesion brings cell surfaces together, thereby allowing other contact-dependent signaling mechanisms (e.g. gap junction-mediated cell-cell communication) to be active.
Definitive evidence that these signals were being regulated in response to the cadherin receptor came with experiments that used recombinant adhesive ligands to ligate the cadherin without incurring effects due to juxtacrine signaling (419). Most commonly, cadherin ligation appears to stimulate intracellular signaling, although it is also capable of inhibiting signaling pathways (265, 267). Importantly, signaling events are modulated in response to productive ligation of the cadherin ectodomains, indicating that cadherins can function as adhesion-activated signaling receptors (403, 419).
2. Signaling molecules at cadherin adhesive contacts: Rho family GTPases
i) Rho GTPases and cadherin signaling
Given their well-established capacity to regulate the actin cytoskeleton (118, 156), much attention has focused on the potential for Rho family small GTPases to signal at cadherin-based cell-cell contacts. All three of the best-understood Rho family GTPases, Rho, Rac and Cdc42, have been identified at cell-cell contacts by immunofluorescence microscopy (49, 169, 184, 214, 253). Like other members of the Ras superfamily, Rho family GTPases act as molecular switches determined by their nucleotide-bound status: when bound to GTP they are capable of interacting with a range of effector molecules, thereby initiating signaling cascades, whereas they are unable to bind effectors (and are thus unable to signal) in their GDP-bound state (118). Biochemical studies have demonstrated that Rho (49), Rac (31, 191, 253) and Cdc42 (169) can be GTP-loaded when cells make contacts with one another or as cells grow to confluence. Moreover, studies that used biosensors to identify the GTP-loaded state identified active Rho, Rac (412) and Cdc42 (169) at cell-cell contacts. Thus cadherin contacts are sites for Rho GTPase signaling. Key issues, then, are how these signaling molecules are localized to, and activated at, cadherin-based cell-cell contacts.
Homophilic cadherin adhesion assays have further demonstrated that E-cadherin ligation acutely activates signaling by both Rac and Cdc42 (97, 107, 179, 182, 267). Characteristically, Rac is activated as an early, transient response to either cell-cell contact (253) or homophilic cadherin ligation (179, 267). Moreover, live cell imaging indicates that Rac tracks the margins where adhesions are being extended (77). Overall, these suggest that Rac is activated as an early-immediate response to cadherin ligation, perhaps preferentially at sites where contacts are being assembled.
The impact of cadherin adhesion on Rho signaling is more complex. In those studies that demonstrated acute activation of Rac by E-cadherin ligation, Rho was instead inhibited, through a process that involves p190 Rho-GAP, which activates the intrinsic GTPase activity of Rho to hydrolyse bound GTP to GDP (265, 267). As Rac can activate p190 Rho-GAP (261), it is possible that Rho was inhibited in response to cadherin-activated Rac signaling. In contrast, independent studies reported that homophilic ligation of N-cadherin in cultured C2C12 myoblasts activated Rho signalling without having any effect on Rac signalling (49). These discrepancies may reflect the different cadherins and cell types studied. Additionally, as cells first assemble contacts with one another, Rac appears to dominate at contacts to later be replaced by Rho (412). It is thus possible that the biological context of cell-cell interactions may critically influence which of these GTPases is active at contacts.
The active, GTP-loaded state of Rho family GTPases is determined by interactions with other regulatory proteins (156). Notably, Guanine nucleotide exchange factors (GEFs) catalyse the exchange of GDP for GTP, thereby acting as key activators of GTPase signaling. Over 60 Rho family GEFs are documented (321), with varying degrees of specificity for the various Rho family GTPases. Our knowledge of the GEFs that participate in cadherin signaling is still quite limited. Tiam-1 has been implicated in Rac activation: this GEF localizes in a cadherin-dependent fashion at E-cadherin- (143) and VE-cadherin-based cell-cell contacts (191), and is necessary for the integrity of those contacts (218). However, while GTP-Rac levels were reduced in VE-cadherin-null endothelial cells that fail to recruit Tiam-1 to contacts (191), it did not appear to be essential for E-cadherin to activate Rac signaling (182). It is very likely that several different GEFs may contribute to cadherin-activation of Rho, Rac and Cdc42. This may be due to redundancy, cell type-specific expression of GEFs or the use of different GEFs at different stages in the biogenesis, maturation and turnover of cell-cell interactions.
The location of GEFs is an important, but not the sole, factor that determines the activity and subcellular localization of Rho family GTPase signals (407). In this regard, p120-ctn has emerged as an interesting potential regulator of Rho GTPase signaling at cadherin contacts. When over-expressed in cadherin-deficient fibroblasts p120-ctn appears to coordinately inhibit signaling by Rho while stimulating Rac and Cdc42 (12, 112, 266). In vitro studies suggested that p120-ctn might inhibit Rho by acting like a Rho GDI, inhibiting the exchange on Rho of GDP for GTP (12). More recently, p120-ctn was demonstrated to inhibit Rho by forming a molecular complex with p190-Rho GAP thereby providing a scaffold to bring GAP and substrate together (405).
While the impact of p120-ctn in cadherin-deficient fibroblasts presumably reflects its action in the cytoplasm, p120-ctn can also influence Rho GTPase signaling when it is incorporated into the cadherin molecular complex. In Drosophila embryos Rho was identified in a complex with both p120-ctn and α-catenin, a biochemical interaction that might explain its tendency to concentrate in cadherin-enriched regions of cell-cell contact (214). Moreover, in vitro binding studies showed that Drosophila p120-ctn preferentially bound to GDP-loaded DRho (214), consistent with the notion that the catenin might sequester Rho in an inactive state (12, 13). Additionally, p120-ctn recruits p190-Rho-GAP to cell-cell contacts, providing an additional mechanism to inhibit Rho signaling at the cortex (405). The potential for cadherin-bound p120-ctn to influence signaling at cadherin contacts is supported by studies using cadherin mutants that cannot bind p120-ctn. Both deletion mutants lacking regions of the membrane-proximal region of the cytoplasmic tail and more limited point mutations in the p120-ctn-binding site fail to support Rac activation either in monolayer cultures or in cells acutely stimulated by cadherin adhesion (97, 107, 191). Though much remains to be established, these observations together suggest that when bound to cadherins p120-ctn may modulate Rho family signaling at adhesive contacts.
ii) Cellular targets of Rho family GTPases
Expression of dominant-negative GTPase mutants has identified roles for these signals, notably Rho and Rac, in supporting the integrity of cadherin-based cell-cell junctions. It should be noted, however, that there is also evidence that these signaling molecules may exert negative effects on cadherin biology. These discrepancies may reflect differences in the experimental systems used. However, it is possible that quantitative differences in signal strength or duration critically influence the impact of GTPase signaling on cadherin biology as appears to be the case for tyrosine kinase signaling (discussed below).
Ultimately, Rho family GTPases must alter cadherin biology by regulating processes such as cytoskeletal function and membrane trafficking. Indeed, there is ample evidence for such regulation in other contexts that are likely to be relevant for cadherin cell biology. In particular, Rho family GTPases are core regulators of the actin cytoskeleton that modulate many aspects of cytoskeletal organization and dynamics (118, 156). The latter include promotion of actin nucleation through nucleation promoting factors, such as N-WASP and WAVE (150), which respond to Cdc42 and Rac, respectively; control of Myosin II-dependent contractility through Rho (386); and regulating the recruitment of scaffolding proteins, such as cortactin which can respond to Rac (400).
Recent evidence is beginning to define the signaling pathways that link Rho family GTPases to specific aspects of cytoskeletal regulation at cadherin adhesions themselves. Thus, Rho and its downstream mediator, Rho kinase (ROCK), support Myosin II (332, 342) and formin (44) activity at cadherin contacts, while both Rac and Cdc42 can influence cadherin-activated actin assembly (182) and regulate the coupling of cadherins to the subcortical cytoskeleton (188). We anticipate that cytoskeletal regulation at cadherin contacts will involve a complex network of signals and effectors, as is found in other contexts. If so, how individual GTPase signals are expressed in space and time will constitute an important aspect of signaling at cell-cell contacts. For example, the recent observation that Rac and Rho are activated at different sites and phases during contact assembly (412) may provide a mechanism to activate different sets of cytoskeletal regulators at different stages of contact formation.
Similarly, Rho GTPases influence membrane traffic at many stages, that include endocytosis via both clathrin-dependent and -independent pathways (186, 316), and sorting at various intracellular sites (60, 246). Their potential impact for cadherin trafficking is beginning to be elucidated. Thus, Rac inhibited E-cadherin internalization in cell-free assay systems (155) and during post-Golgi transport (393). At least some of these effects of Rho GTPases on cadherin trafficking are likely to be mediated by local regulation of the actin cytoskeleton (100, 204).
3. Cadherin adhesions and phosphotyrosine signaling
i) Regulators of protein tyrosine phosphorylation at cadherin contacts
Cadherin-based adhesions are prominent sites for phosphotyrosine (pY) signaling within cells: they are highly enriched in tyrosine-phosphorylated proteins (104, 145, 187, 216, 217, 229, 360, 378, 380, 390) as well in the enzymes that control those phosphorylation events. The latter include both receptor tyrosine kinases (RTKs; e.g. EGF and VEGF receptors); non-receptor kinases (e.g. members of the Src family (SFKs) and Fer/FES kinases); transmembrane (receptor) protein tyrosine phosphatases (PTPs; e.g. RPTPμ and LAR); as well as cytoplasmic PTPs (e.g. PTP1B) (for references, see below).
Many of these proteins can interact, directly or indirectly, with the cadherin molecular complex itself, although many molecular details remain to be determined. For example, the EGF receptor (EGFR), an RTK, co-accumulates with E-cadherin at adhesive contacts and can be found in a protein complex with the cadherin (287, 302) or with β-catenin (270). Similarly, VEGF receptors interact with VE-cadherin in endothelia (187). In contrast, both Src family kinases and FER can associate directly with p120-ctn (168, 295, 314) and Src has also been found in E-cadherin and VE-cadherin protein complexes (27, 117, 187). Several PTPs are also reported to associate biochemically with cadherins, by several mechanisms (230, 245). Both the transmembrane proteins, RPTPμ and VE-PTP, interact laterally (in cis) with cadherins (36, 37); Nottebaum, 2008 #481}. However, RPTPm and E-cadherin appear to bind through their cytoplasmic domains (36, 37), whereas VE-PTP is reported to interact with VE-cadherin through its ectodomain (255). Amongst non-receptor PTPs, PTP1B was reported to bind directly to the cadherin cytoplasmic tail (17, 410), while SHP-1 was reported to bind to b- and/or p120-ctn (75, 166). Thus there are many potential molecular mechanisms for cadherins to interact with regulators of protein tyrosine phosphorylation.
Importantly, many of the targets for these tyrosine kinases and phosphatases are cadherins, their binding partners and/or proteins that interact functionally with the adhesion receptor complex. These include both β-catenin and p120-ctn, which are heavily tyrosine-phosphorylated in cells stimulated with growth factors or that express catalytically active kinases, such as v-Src (18, 25, 43, 119, 245, 335, 361). Indeed, both these catenins can be directly phosphorylated by a number of kinases in vitro, such as Src (315) and Fer (168, 295). Tyrosine phosphorylation on cadherin cytoplasmic tails has been clearly documented for VE-cadherin, where a number of tyrosine residues are targeted (3, 8, 104, 145, 187, 380). Other targets of tyrosine phosphorylation at cadherin adhesions include cytoskeletal regulators and signaling molecules (265, 405).
What then are the upstream receptors that trigger signaling to induce tyrosine phosphorylation of cadherins and their partner proteins? Most commonly, tyrosine phosphorylation at cell-cell contacts is envisaged to arise in response to growth factor receptor signaling (18, 81). These include the EGF- and HGF receptors in epithelial cells (90, 335, 337) and VEGF receptors in vascular endothelial cells (392). Stimulation of these receptors by ligand, or overexpression of receptors in cells, induces tyrosine phosphorylation of many proteins found at cell-cell contacts, including components of the cadherin molecular complex itself. In endothelial cells the cytoplasmic tail of VE-cadherin is tyrosine phosphorylated in response to a wide range of signals, that include growth factors (392), inflammatory mediators (104) and interaction with leukocyte adhesion molecules (8, 380). Such phosphorylation events may be direct, or mediated by downstream kinases that can include SFK and Abelson family kinases (8, 187, 308, 392). As discussed further below, such growth-factor-induced phosphorylation is commonly thought to perturb cadherin function.
However, cadherins can also influence tyrosine kinase signaling. One way is for tyrosine kinases to themselves transduce cadherin signals. In epithelial cells blocking cadherin function can reduce Src activity at cell-cell contacts, and homophilic ligation of E-cadherin activates Src (229), identifying Src as a downstream target in E-cadherin signaling. EGFR activity has also been implicated in E-cadherin-activated signaling to Rac and MAPK (31, 287), suggesting that it may mediate several downstream steps in cadherin signaling. Thus a variety of tyrosine kinases potentially mediate cadherin signaling. In addition, cadherins can also inhibit growth factor signaling through mechanisms that include altering receptor affinity for ligand and controlling the subcellular localization of growth factor receptors (190, 192, 302). Clearly, then, the precise functional relationship between cadherins and tyrosine kinases will depend on cell type and context.
ii) Functional impact
What then, are the functional consequences of tyrosine kinase signaling for cadherin biology? Though commonly regarded as negative regulators of cadherin function, there is increasing evidence for greater complexity. This is best illustrated by the example of the Src family kinases (SFK).
Many gain-of-function studies have clearly demonstrated that increased SFK signaling perturbs cadherin-based cell-cell interactions. For example, overexpression of v-Src or temperature-sensitive (ts) Src mutants disrupted the integrity of cadherin-based cell-cell contacts and reduced cadherin adhesion in a variety of cultured cells (25, 119, 224, 229, 361, 390, 396, 397). Such disruption manifested in a number of ways that include altered junctional morphology and reduced cadherin staining at cell-cell contacts. This negative effect of Src signaling has been attributed to destabilization of the cadherin-catenin complex and increased cadherin internalization and degradation (277). Moreover, Src is overexpressed or constitutively-activated by mutation in many epithelial tumors where cell-cell junctions are abnormal. Consistent with this, disruption of cell-cell interactions in cell culture models was accompanied by an apparent epithelial-to-mesenchymal transformation or a metastatic, invasive phenotype (16, 25, 224). This suggested that cadherin function might be targeted by SFK signaling as a key element in a broader change in cellular phenotype.
However, it should be noted that a negative impact of SFK signaling has generally been identified in gain-of-function studies. In contrast, loss of function analyses in animal and cell culture models have provided evidence for a positive contribution of SFK signaling to cadherin function. Both Src and fyn are found at cell-cell contacts in mouse keratinocytes and Src/Fyn −/− animals display loss of junctional integrity in the skin. Similarly, inhibiting SFK signaling reduced N-cadherin adhesion in cultured fibroblasts (79) and E-cadherin adhesion in epithelial cells (229). In addition to mammalian systems, the c-Src ortholog, DSrc-41, plays a vital role in the formation of cell-cell contacts in the developing Drosophila eye (358). Expression of a dominant negative DSrc-41 mutant perturbed adherens junctions, with both DE-cadherin and F-actin being lost from cell-cell contacts (358, 359). Together, these findings demonstrate that under certain circumstances SFK signaling can support cadherin adhesion and contact integrity.
One approach to reconcile these apparently disparate results is if differences in the strength of SFK signaling generate qualitatively different functional outcomes (387). In this model, physiological SFK signaling that promotes cadherin function would occur at lower levels than the pathologic forms of SFK signaling that inhibit adhesion and contact integrity. There is some experimental evidence for such a notion, from experiments that measured the degree to which cells spread upon cadherin-coated substrata as an index of cadherin function. Here, expression of constitutively-active Src had a bimodal effect on cell spreading, which was promoted at lower Src levels, then became inhibited as Src expression rose (229). While it will be necessary to test how generally applicable this model may be in other assays of cadherin biology, this observation suggests that SFK signaling will have complex effects on cadherin biology, depending on factors such as SFK expression and duration of activity. The extent to which other tyrosine kinases may have similar effects on cadherin biology remains to be tested.
iii) Functional targets of tyrosine kinase signaling in cadherin biology
Any comprehensive analysis of how tyrosine phosphorylation regulates cadherin biology will ultimately need to identify the target proteins and understand how their post-translational modification accounts for their biology. These issues remain poorly understood. The complex biological consequences of tyrosine kinase signaling, reviewed above, emphasize that there are likely to be many relevant target proteins, whose impact will depend on parameters such as the specific kinase and/or protein tyrosine phosphatase involved and the cellular context in which it acts. Here we highlight two examples of potential target proteins implicated in negative or positive contributions to cadherin biology.
In one model, tyrosine phosphorylation of β-catenin perturbs cadherin-based cell-cell interactions through disassembly of the cadherin molecular complex (Fig 17A). This concept was first prompted by the demonstration that prominent tyrosine phosphorylation of β-catenin correlates with the disassembly of contacts between cells stimulated with growth factors or that express a variety of tyrosine kinases (90, 301, 335). Furthermore, in vitro tyrosine phosphorylation of β-catenin by Src, Fyn or FER reduced its ability to bind to the cadherin cytoplasmic tail or to α-catenin (295, 315). In vivo, broad-spectrum inhibition of protein tyrosine phosphatase activity with vanadate can reduce the interaction between α- and β-catenin (274), while decreased association of bβ-catenin with cadherins has been observed in reponse to growth factors (18).
Figure 17. Potential mechanisms for regulation of cadherin biology by tyrosine kinase signaling.
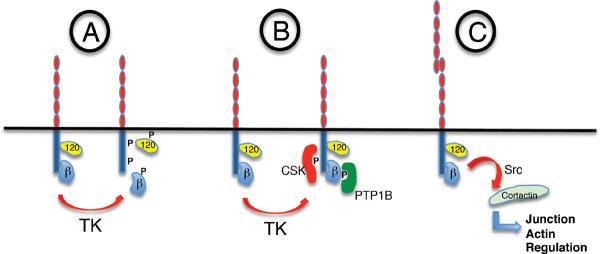
A) Uncoupling: tyrosine phosphorylation of the cadherin cytoplasmic tail and/or associated proteins dissociates catenins leading to disassembly of the cadherin molecular complex.
B) Scaffolding signaling complexes: tyrosine phosphorylation generates binding sites for association of signaling molecules such as C-terminal kinase (CSK, to VE-cadherin cytoplasmic tail) or PTP1B (to β-catenin).
C) Cadherin-based signaling: recruitment of tyrosine kinases as part of a cadherin signaling pathway mediates control of downstream effectors, such as the cytoskeletal regulator, cortactin.
This attractively elegant model is not, however, universally applicable. Tyrosine phosphorylation of β-catenin does not always perturb its interaction with the cadherin (295) and growth factor signaling can reduce cadherin adhesion without obvious changes in the cadherin-catenin complex (38). Moreover, the causal impact of β-catenin phosphorylation is not always clear-cut. This latter point is illustrated by an important experiment performed by the Tsukita laboratory, who took advantage of a chimeric protein where α-catenin was fused to the cadherin at its cytoplasmic tail (361). Of note, this chimeric protein lacked the region that binds β-catenin, and could thereby support strong cell-cell adhesion independently of β-catenin. Strikingly, they found that expression of constitutively-active Src inhibited adhesion mediated by this fusion protein as effectively as it inhibited adhesion by the wild-type cadherin. In this instance, then, Src inhibited cadherin adhesion independently of β–catenin.
Alternatively, tyrosine phosphorylation may mediate the assembly of signaling complexes at the plasma membrane (Fig 17B). In endothelia, VEGF signaling induces phosphorylation by Src of Tyr-685 in the cadherin cytoplasmic tail, which provides a potential docking site for the SH2 domain of C-terminal kinase (CSK) (392), that is known to complex with VE-cadherin (20, 117). This may modulate VEGF-activated Src signaling to influence mitogenesis (20, 117). Alternatively, tyrosine phosphorylation of catenins may indirectly regulate the interaction of signaling molecules with the cadherin complex. The FER tyrosine kinase associates with p120-catenin (295, 410), which can recruit FER to the cadherin complex in response to K-Ras signaling (295). Once recruited to the cadherin complex, FER potentially controls β-catenin phosphorylation by regulating association PTP1B (410) and/or directly phosphorylating β-catenin on Tyr-142 to influence binding of α-catenin (295).
Finally, the mechanisms by which tyrosine kinase signaling might support cadherin biology (Fig 17C) have been much less extensively investigated. Some insight comes from the example of cortactin, which is tyrosine phosphorylated in response to cadherin adhesion (78, 305). Furthermore, tyrosine-phosphorylated cortactin is necessary for the integrity of E-cadherin-based cell-cell contacts. As the interaction between cortactin and E-cadherin is not affected by tyrosine-phosphorylation, post-translational modification may influence interactions between cortactin and other proteins likely to ultimately affect the actin cytoskeleton (305). Cortactin is likely to be only one of many proteins that support cadherin biology in response to phosphotyrosine signaling (279, 311).
IV. Future challenges: Integrating cellular mechanisms and morphogenetic outcomes
In this review we have sought to discuss mechanisms of cadherin biology that are likely to contribute to their diverse morphogenetic impacts during development and in post-embryonic life. It is likely, however, that individual morphogenetic processes entail the integration of multiple distinct mechanisms of cadherin function. This is exemplified by ongoing efforts to understand the basis for cell segregation and sorting.
As noted earlier, the observation that cells expressing different cadherins sorted into distinct aggregates (89, 268) suggested a potential explanation for the classic results of Townes and Holtfreter. It was further interpreted as evidence that classical cadherins might exclusively form homophilic bonds with one another. This was reinforced by mapping of cell sorting specificity onto the EC1 domain of cadherins (269) and by evidence that cells engineered to express different cadherins also displayed differences in cellular adhesion. For example, heterotypic pairs of cadherin-null S180 cells that expressed either E- or N-cadherin failed to aggregate to one another (57). S180 cells expressing either classical (E- or N-) cadherin or Type II cadherins (cadherin 7- or 11) also displayed quite different adhesive strengths that mapped to the ectodomains (56). In its simplest form, the hypothesis of homophilic adhesion implied that cell segregation might arise because homophilic interactions would lead to productive adhesion, whereas heterophilic interactions would be ineffective.
However, a number of classical cadherins display the ability to engage in heterophilic interactions (257, 280, 329). Further, measurements done under different conditions or with controlled cadherin surface expression levels prompted a more nuanced view that differences between heterophilic and homophilic cell adhesion energies, rather than exclusive homophilic binding, might promote cell sorting (87). This built on Steinberg's Differential Adhesion Hypothesis, which postulated that the relative magnitudes of cell adhesion energies govern cell-sorting patterns (349). Both subtype and cell surface levels of cadherin could contribute to cell adhesion energies (348). In some cases, estimates of intercellular adhesion energies from analyses of shape deformations of cell aggregates in response to applied external centrifugal or mechanical forces appear to support this concept (87, 162, 406).
Several parameters can contribute to differences in cell adhesion energies including, but not limited to the intrinsic biophysical properties of the cadherin bonds and cadherin surface expression levels. A key question thus remains to what extent cell segregation can be explained solely in terms of the intrinsic properties of the ectodomain, or whether it is also necessary to incorporate cellular properties, including biomechanics and functional responses to cadherin ligation. Here it is noteworthy that molecular adhesion measurements with isolated ectodomains made using different techiques did not reveal clear, general correlations between quantitative bond energies and in vitro cell sorting (55, 300). This suggests that adhesion energies alone do not generally predict in vitro and, most likely in vivo, cell sorting.
The strong correlation between cadherin expression and sorting therefore begs the question as to what other cadherin-specific functions might contribute to cell sorting. One prime candidate is cortical tension that is controlled by the actomyosin cytoskeleton and will increase surface tension and thus reduce cell contacts. Studies of germ layer segregation in zebrafish embryos suggest that membrane tension, as characterized by the resistence to indentation, has a more pronounced effect on cell sorting than does cell surface adhesivity (183). This is consistent with increasing evidence that Myosin II defines a varity of tissue boundaries and sorting in embryos (86, 193). This raises the interesting possibility that disparate cadherin regulation of the cytoskeleton, especially Myosin II (332), may contribute to segregation of cells expressing different classical cadherins.
Overall, we propose that the morphogenetic impact of classical cadherins may be best understood to arise from the multiple mechanisms, including the biophysical properties of the ectodomain, as well as adhesion-regulated changes in cytoskeletal function and membrane trafficking, that are coordinated by cell signaling. This is consistent with the growing notion that three-dimensional tissues ultimately arise from the functional interactions between genes, the cellular machinery executes their orders, and the biomechanical environments in which cells find themselves. Providing a cohesive picture of these interactions will be an exciting challenge for the future.
Acknowledgements
We thank all the members of our laboratories for their input and support. CMN is supported by the Deutsche Krebshilfe and the DFG (SFB829 and SFB 832). DEL is supported by grants from the National Science Foundation (CBET 0853705) and the National Institutes of Health (R21 HD059002). ASY is supported by the National Health and Medical Research Council of Australia.
Footnotes
It is worth noting here that the term “adhesion” is used in a number of different ways throughout the cadherin literature, that often reflect how cadherin function has been assayed. However, these different operational definitions may not reflect the same cellular mechanisms. The well-established physical definition of “adhesion” is the resistance of bonds to detachment by force. This can encompass the microscopic behaviour of individual bonds as well as the macroscopic behaviour of multiple bonds distributed over the surfaces of cell-cell contacts. In biological systems, “adhesion” is often used to refer to the morphological characteristics of cell-cell contacts. In particular, cells that make extensive contacts with one another are often interpreted as engaging in strong adhesive interactions (315), While there is no doubt that cell adhesion is necessary for contacts to form, the morphology of interactions can be perturbed without demonstrable changes in cell resistance to detachment forces (314). Thus morphological changes may not always reflect changes in the actual cell surface adhesion.
REFERENCES
- 1.Abe K, Chisaka O, van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by aN-catenin. Nat Neurosci. 2004 doi: 10.1038/nn1212. [DOI] [PubMed] [Google Scholar]
- 2.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J Biol Chem. 2010;285:7045–7055. doi: 10.1074/jbc.M109.079277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams CL, Chen Y-T, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhmanova A, Stehbens SJ, Yap AS. Touch, grasp, deliver and control: functional cross-talk between microtubules and cell adhesions. Traffic. 2009;10:268–274. doi: 10.1111/j.1600-0854.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 6.Akhtar N, Hotchin NA. Rac1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell. 2001;12:847–862. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Amoudi A, Diez DC, Betts MJ, Frangakis AS. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832–837. doi: 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- 8.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 9.Almagro S, Durmort C, Chervin-Petinot A, Heyraud S, Dubois M, Lambert O, Maillefaud C, Hewat E, Schaal JP, Huber P, Gulino-Debrac D. The motor protein myosin-X transports VE-cadherin along filopodia to allow the formation of early endothelial cell-cell contacts. Mol Cell Biol. 2010;30:1703–1717. doi: 10.1128/MCB.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altman D, Sweeney HL, Spudich JA. The mechanism of Myosin VI translocation and its load-induced anchoring. Cell. 2004;116:737–749. doi: 10.1016/s0092-8674(04)00211-9. [DOI] [PubMed] [Google Scholar]
- 11.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 13.Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Op Cell Biol. 2001;13:604–610. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 14.Angres B, Barth A, Nelson WJ. Mechanism for transition from initial to stable cell-cell adhesion: kinetic analysis of E-cadherin-mediated adhesion using a quantitative adhesion assay. J Cell Biology. 1996;134:549–557. doi: 10.1083/jcb.134.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au JS, Puri C, Ihrke G, Kendrick-Jones J, Buss F. Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells. J Cell Biol. 2007;177:103–114. doi: 10.1083/jcb.200608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 17.Balsamo J, Arregui C, Leung T, Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Association of Csk to VE-cadherin and inhibition of cell proliferation. Embo J. 2005;24:1686–1695. doi: 10.1038/sj.emboj.7600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D. Cadherin interaction probed by atomic force microscopy. Proc Natl Acad Sci U S A. 2000;97:4005–4010. doi: 10.1073/pnas.070052697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayas MV, Leung A, Evans E, Leckband D. Lifetime measurements reveal kinetic differences between homophilic cadherin bonds. Biophys J. 2006;90:1385–1395. doi: 10.1529/biophysj.105.069583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122:1947–1953. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker KF, Kremmer E, Eulitz M, Becker I, Handschuh G, Schuhmacher C, Muller W, Gabbert HE, Ochiai A, Hirohashi S, Hofler H. Analysis of E-cadherin in diffuse-type gastric cancer using a mutation-specific monoclonal antibody. Am J Pathol. 1999;155:1803–1809. doi: 10.1016/S0002-9440(10)65497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamin JM, Kwiatkowski AV, Yang C, Korobova F, Pokutta S, Svitkina T, Weis WI, Nelson WJ. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J Cell Biol. 2010;189:339–352. doi: 10.1083/jcb.200910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett HL, Brummer T, Jeanes A, Yap AS, Daly RJ. Gab2 and Src co-operate in human mammary epithelial cells to promote growth factor independence and disruption of acinar morphogenesis. Oncogene. 2008;27:2693–2704. doi: 10.1038/sj.onc.1210928. [DOI] [PubMed] [Google Scholar]
- 28.Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol. 2005;169:635–646. doi: 10.1083/jcb.200410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 30.Berx G, Becker KF, Hofler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226–237. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Betson M, Lozano E, Zhang J, Braga VM. Rac activation upon cell-cell contact formation is dependent on signaling from the epidermal growth factor receptor. J Biol Chem. 2002;277:36962–36969. doi: 10.1074/jbc.M207358200. [DOI] [PubMed] [Google Scholar]
- 32.Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 34.Boller K, Vestweber D, Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985;100:327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornslaeger EA, Corcoran CM, Stappenbeck TS, Green KJ. Breaking the connection: displacement of the desmosomal plaque protein desmoplakin from cell-cell interfaces disrupts anchorage of intermediate filament bundles and alters intercellular junction assembly. J Cell Biol. 1996;134:985–1001. doi: 10.1083/jcb.134.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brady-Kalnay SM, Mourton T, Nixon JP, Pietz GE, Kinch M, Chen H, Brackenbury R, Rimm DL, Del Vecchio RL, Tonks NK. Dynamic interaction of PTPm with multiple cadherins in vivo. J Cell Biol. 1998;141:287–296. doi: 10.1083/jcb.141.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brady-Kalnay SM, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPm associates with cadherins and catenins in vivo. J Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brieher WM, Gumbiner BM. Regulation of cadherin function during activin induced morphogenesis of Xenopus animal caps. J Cell Biology. 1994;126:519–527. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biology. 1996;135:487–489. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci. 2007;120:1818–1828. doi: 10.1242/jcs.000653. [DOI] [PubMed] [Google Scholar]
- 41.Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Bryant DM, Wylie FG, Stow JL. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Mol Biol Cell. 2005;16:14–23. doi: 10.1091/mbc.E04-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Paolo Dotto G. Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion. J Cell Biol. 1998;141:1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci. 2007;120:3870–3882. doi: 10.1242/jcs.014365. [DOI] [PubMed] [Google Scholar]
- 45.Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 46.Chan MW, El Sayegh TY, Arora PD, Laschinger CA, Overall CM, Morrison C, McCulloch CA. Regulation of intercellular adhesion strength in fibroblasts. J Biol Chem. 2004;279:41047–41057. doi: 10.1074/jbc.M406631200. [DOI] [PubMed] [Google Scholar]
- 47.Chapman JA, Kirkness EF, Simakov O, Hampson SE, Mitros T, Weinmaier T, Rattei T, Balasubramanian PG, Borman J, Busam D, Disbennett K, Pfannkoch C, Sumin N, Sutton GG, Viswanathan LD, Walenz B, Goodstein DM, Hellsten U, Kawashima T, Prochnik SE, Putnam NH, Shu S, Blumberg B, Dana CE, Gee L, Kibler DF, Law L, Lindgens D, Martinez DE, Peng J, Wigge PA, Bertulat B, Guder C, Nakamura Y, Ozbek S, Watanabe H, Khalturin K, Hemmrich G, Franke A, Augustin R, Fraune S, Hayakawa E, Hayakawa S, Hirose M, Hwang JS, Ikeo K, Nishimiya-Fujisawa C, Ogura A, Takahashi T, Steinmetz PR, Zhang X, Aufschnaiter R, Eder MK, Gorny AK, Salvenmoser W, Heimberg AM, Wheeler BM, Peterson KJ, Bottger A, Tischler P, Wolf A, Gojobori T, Remington KA, Strausberg RL, Venter JC, Technau U, Hobmayer B, Bosch TC, Holstein TW, Fujisawa T, Bode HR, David CN, Rokhsar DS, Steele RE. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J Cell Biol. 2001;154:231–243. doi: 10.1083/jcb.200103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and {beta}-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y-H, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/b-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol. 1999;144:687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chesarone MA, Goode BL. Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol. 2009;21:28–37. doi: 10.1016/j.ceb.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. p120-Catenin Inhibits VE-cadherin Internalization through a Rho-Independent Mechanism. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-07-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chien YH, Jiang N, Li F, Zhang F, Zhu C, Leckband D. Two stage cadherin kinetics require multiple extracellular domains but not the cytoplasmic region. J Biol Chem. 2008;283:1848–1856. doi: 10.1074/jbc.M708044200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu YS, Eder O, Thomas WA, Simcha I, Pincet F, Ben-Ze'ev A, Perez E, Thiery JP, Dufour S. Prototypical type I E-cadherin and type II cadherin-7 mediate very distinct adhesiveness through their extracellular domains. J Biol Chem. 2006;281:2901–2910. doi: 10.1074/jbc.M506185200. [DOI] [PubMed] [Google Scholar]
- 57.Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark AG, Miller AL, Vaughan E, Yu HY, Penkert R, Bement WM. Integration of single and multicellular wound responses. Curr Biol. 2009;19:1389–1395. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 60.Cohen D, Musch A, Rodriguez-Boulan E. Selective control of basolateral membrane protein polarity by cdc42. Traffic. 2001;2:556–564. doi: 10.1034/j.1600-0854.2001.20805.x. [DOI] [PubMed] [Google Scholar]
- 61.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in Cell Adhesion and the Visceral Endoderm following Ablation of Nonmuscle Myosin Heavy Chain II-A in Mice. J Biol Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- 62.Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cowan CR, Hyman AA. Acto-myosin reorganization and PAR polarity in C. elegans. Development. 2007 doi: 10.1242/dev.000513. [DOI] [PubMed] [Google Scholar]
- 64.Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986;46:1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- 65.Daly RJ. Cortactin signalling and dynamic actin networks. Biochem J. 2004;382:13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or a-catenin. Mol Cell Biol. 1995;15:4819–4824. doi: 10.1128/mcb.15.9.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis MA, Reynolds AB. Blocked Acinar Development, E-Cadherin Reduction, and Intraepithelial Neoplasia upon Ablation of p120-Catenin in the Mouse Salivary Gland. Dev Cell. 2006;10:21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- 70.de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci U S A. 2009;106:7010–7015. doi: 10.1073/pnas.0811253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Wever O, Derycke L, Hendrix A, De Meerleer G, Godeau F, Depypere H, Bracke M. Soluble cadherins as cancer biomarkers. Clin Exp Metastasis. 2007;24:685–697. doi: 10.1007/s10585-007-9104-8. [DOI] [PubMed] [Google Scholar]
- 73.Diamond J. The epithelial junction: bridge, gate, and fence. Physiologist. 1977;20:10–18. [PubMed] [Google Scholar]
- 74.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duchesne C, Charland S, Asselin C, Nahmias C, Rivard N. Negative regulation of beta-catenin signaling by tyrosine phosphatase SHP-1 in intestinal epithelial cells. J Biol Chem. 2003;278:14274–14283. doi: 10.1074/jbc.M300425200. [DOI] [PubMed] [Google Scholar]
- 76.Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol. 2003;253:309–323. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 77.Ehrlich JS, Hansen MDH, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamelliodia dynamics during epithelial cell-cell adhesion. Develop Cell. 2002;3:259–270. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Sayegh TY, Arora PD, Fan L, Laschinger CA, Greer PA, McCulloch CA, Kapus A. Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol Biol Cell. 2005;16:5514–5527. doi: 10.1091/mbc.E05-05-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Sayegh TY, Arora PD, Laschinger CA, Lee W, Morrison C, Overall CM, Kapus A, McCulloch CA. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J Cell Sci. 2004;117:5117–5131. doi: 10.1242/jcs.01385. [DOI] [PubMed] [Google Scholar]
- 80.El Sayegh TY, Arora PD, Ling K, Laschinger C, Janmey PA, Anderson RA, McCulloch CA. Phosphatidylinositol-4,5 bisphosphate produced by PIP5KIg regulates gelsolin, actin assembly and adhesion strength of N-cadherin junctions. Mol Biol Cell. 2007;18:3026–3038. doi: 10.1091/mbc.E06-12-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Espada J, Perez-Moreno M, Braga VMM, Rodriguez-Viciana P, Cano A. H-Ras activation promotes cytoplasmic accumulation and phosphoinositide 3-OH kinase association of b-catenin in epidermal keratinocytes. J Cell Biol. 1999;146:967–980. doi: 10.1083/jcb.146.5.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans E, Heinrich V, Leung A, Kinoshita K. Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. I. Membrane separation from the cytoskeleton. Biophys J. 2005;88:2288–2298. doi: 10.1529/biophysj.104.051698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 84.Felsenfeld DP, Choquet D, Sheetz MP. Ligand binding regulates the directed movement of beta1 integrins on fibroblasts. Nature. 1996;383:438–440. doi: 10.1038/383438a0. [DOI] [PubMed] [Google Scholar]
- 85.Ferber EC, Kajita M, Wadlow A, Tobiansky L, Niessen C, Ariga H, Daniel J, Fujita Y. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem. 2008;283:12691–12700. doi: 10.1074/jbc.M708887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Foty R, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2004;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 88.Franz CM, Ridley AJ. p120 catenin associates with microtubules. Inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279:6588–6594. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 89.Friedlander DR, Mege RM, Cunningham BA, Edelman GM. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proc Natl Acad Sci U S A. 1989;86:7043–7047. doi: 10.1073/pnas.86.18.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fujii K, Furukawa F, Matsuyoshi N. Ligand activation of overexpressed epidermal growth factor receptor results in colony dissociation and disturbed E-cadherin function in HSC-1 human cutaneous squamous carcinoma cells. Exp Cell Res. 1996;223:50–62. doi: 10.1006/excr.1996.0057. [DOI] [PubMed] [Google Scholar]
- 91.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 92.Fukuhara T, Shimizu K, Kawakatsu T, Fukuyama T, Minami Y, Honda T, Hoshino T, Yamada T, Ogita H, Okada M, Takai Y. Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. J Cell Biol. 2004;166:393–405. doi: 10.1083/jcb.200401093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of b-catenin: evidence for intracellular signaling. J Cell Biology. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Furman C, Sieminski AL, Kwiatkowski AV, Rubinson DA, Vasile E, Bronson RT, Fassler R, Gertler FB. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol. 2007;179:761–775. doi: 10.1083/jcb.200705002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gates J, Mahaffey JP, Rogers SL, Emerson M, Rogers EM, Sottile SL, Van Vactor D, Gertler FB, Peifer M. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development. 2007;134:2027–2039. doi: 10.1242/dev.02849. [DOI] [PubMed] [Google Scholar]
- 96.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 97.Gavard J, Lambert M, Grosheva I, Marthiens V, Irinopoulou T, Riou J-F, Bershadsky A, Mege RM. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J Cell Sci. 2004;117:257–270. doi: 10.1242/jcs.00857. [DOI] [PubMed] [Google Scholar]
- 98.Geiger B, Tokuyasu KT, Dutton AH, Singer SJ. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980;77:4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geisbrecht ER, Montell DJ. Myosin VI is required for E-cadherin-mediated border cell migration. Nat Cell Biol. 2002;4:616–620. doi: 10.1038/ncb830. [DOI] [PubMed] [Google Scholar]
- 100.Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC Regulate Arp2/3-Mediated Endocytosis to Control Local Adherens Junction Stability. Curr Biol. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 101.Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- 102.Girard KD, Kuo SC, Robinson DN. Dictyostelium myosin II mechanochemistry promotes active behavior of the cortex on long time scales. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0508819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Godt D, Tepass U. Drosophila oocyte localization is mediated by differential cadherin- based adhesion. Nature. 1998;395:387–391. doi: 10.1038/26493. [DOI] [PubMed] [Google Scholar]
- 104.Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2008;283:13437–13449. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez-Reyes A, St Johnston D. The Drosophila AP axis is polarised by the cadherin-mediated positioning of the oocyte. Development. 1998;125:3635–3644. doi: 10.1242/dev.125.18.3635. [DOI] [PubMed] [Google Scholar]
- 106.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 107.Goodwin M, Kovacs EM, Thoreson MA, Reynolds AB, Yap AS. Minimal mutation of the cytoplasmic tail inhibits the ability of E-cadherin to activate Rac but not phosphatidylinositol 3-kinase: direct evidence of a role for cadherin-activated Rac signaling in adhesion and contact formation. J Biol Chem. 2003;278:20533–20539. doi: 10.1074/jbc.M213171200. [DOI] [PubMed] [Google Scholar]
- 108.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 110.Grevengoed EE, Loureiro JJ, Jesse TL, Peifer M. Abelson kinase regulates epithelial morphogenesis in Drosophila. J Cell Biol. 2001;155:1185–1198. doi: 10.1083/jcb.200105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu S-C, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 112.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 113.Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 114.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;197:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gumbiner BM. Epithelial morphogenesis. Cell. 1992;69:385–387. doi: 10.1016/0092-8674(92)90440-n. [DOI] [PubMed] [Google Scholar]
- 116.Gundelfinger ED, Kessels MM, Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat Rev Mol Cell Biol. 2003;4:127–139. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- 117.Ha CH, Bennett AM, Jin ZG. A novel role of vascular endothelial cadherin in modulating c-Src activation and downstream signaling of vascular endothelial growth factor. J Biol Chem. 2008;283:7261–7270. doi: 10.1074/jbc.M702881200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 119.Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherincatenin cell adhesion system. Embo J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Handschuh G, Candidus S, Luber B, Reich U, Schott C, Oswald S, Becke H, Hutzler P, Birchmeier W, Hofler H, Becker KF. Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene. 1999;18:4301–4312. doi: 10.1038/sj.onc.1202790. [DOI] [PubMed] [Google Scholar]
- 121.Handschuh G, Luber B, Hutzler P, Hofler H, Becker KF. Single amino acid substitutions in conserved extracellular domains of E-cadherin differ in their functional consequences. J Mol Biol. 2001;314:445–454. doi: 10.1006/jmbi.2001.5143. [DOI] [PubMed] [Google Scholar]
- 122.Hara T, Ishida H, Raziuddin R, Dorkhom S, Kamijo K, Miki T. Novel kelch-like protein, KLEIP, is involved in actin assembly at cell-cell contact sites of Madin-Darby canine kidney cells. Mol Biol Cell. 2004;15:1172–1184. doi: 10.1091/mbc.E03-07-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 124.Harrison OJ, Corps EM, Berge T, Kilshaw PJ. The mechanism of cell adhesion by classical cadherins: the role of domain 1. J Cell Sci. 2005;118:711–721. doi: 10.1242/jcs.01665. [DOI] [PubMed] [Google Scholar]
- 125.Hatta K, Takagi S, Fujisawa H, Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol. 1987;120:215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- 126.Haussinger D, Ahrens T, Aberle T, Engel J, Stetefeld J, Grzesiek S. Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography. EMBO J. 2004;23:1699–1708. doi: 10.1038/sj.emboj.7600192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haussinger D, Ahrens T, Sass HJ, Pertz O, Engel J, Grzesiek S. Calcium-dependent homoassociation of E-cadherin by NMR spectroscopy: changes in mobility, conformation and mapping of contact regions. J Mol Biol. 2002;324:823–839. doi: 10.1016/s0022-2836(02)01137-3. [DOI] [PubMed] [Google Scholar]
- 128.Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- 129.Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with the E-cadherin adhesion complex. J Biol Chem. 1997;272:32448–32453. doi: 10.1074/jbc.272.51.32448. [DOI] [PubMed] [Google Scholar]
- 130.He W, Cowin P, Stokes DL. Untangling desmosomal knots with electron tomography. Science. 2003;302:109–113. doi: 10.1126/science.1086957. [DOI] [PubMed] [Google Scholar]
- 131.Helwani FM, Kovacs EM, Paterson AD, Verma S, Ali RG, Fanning AS, Weed SA, Yap AS. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol. 1995;129:489–506. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hermiston ML, Wong MH, Gordon JI. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes and Development. 1996;10:985–996. doi: 10.1101/gad.10.8.985. [DOI] [PubMed] [Google Scholar]
- 134.Hewat EA, Durmort C, Jacquamet L, Concord E, Gulino-Debrac D. Architecture of the VE-cadherin hexamer. J Mol Biol. 2007;365:744–751. doi: 10.1016/j.jmb.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 135.Higgs HN, Pollard TD. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 136.Hilgenfeldt S, Erisken S, Carthew RW. Physical modeling of cell geometric order in an epithelial tissue. Proc Natl Acad Sci U S A. 2008;105:907–911. doi: 10.1073/pnas.0711077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biology. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biology. 1987;105:2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hirokawa N, Heuser JE. Quick-freeze, deep-etch visualization of the cytoskeleton beneath surface differentiations of intestinal epithelial cells. J Cell Biol. 1981;91:399–409. doi: 10.1083/jcb.91.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hochmuth FM, Shao JY, Dai J, Sheetz MP. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys J. 1996;70:358–369. doi: 10.1016/S0006-3495(96)79577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Homem CC, Peifer M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development. 2008;135:1005–1018. doi: 10.1242/dev.016337. [DOI] [PubMed] [Google Scholar]
- 143.Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 144.Hosono S, Gross I, English MA, Hajra KM, Fearon ER, Licht JD. E-cadherin is a WT1 target gene. J Biol Chem. 2000;275:10943–10953. doi: 10.1074/jbc.275.15.10943. [DOI] [PubMed] [Google Scholar]
- 145.Hudry-Clergeon H, Stengel D, Ninio E, Vilgrain I. Platelet-activating factor increases VE-cadherin tyrosine phosphorylation in mouse endothelial cells and its association with the PtdIns3'-kinase. Faseb J. 2005;19:512–520. doi: 10.1096/fj.04-2202com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huguenin M, Muller EJ, Trachsel-Rosmann S, Oneda B, Ambort D, Sterchi EE, Lottaz D. The metalloprotease meprinbeta processes E-cadherin and weakens intercellular adhesion. PLoS One. 2008;3:e2153. doi: 10.1371/journal.pone.0002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hyafil F, Babinet C, Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–454. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- 148.Iino R, Koyama I, Kusumi A. Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophysical J. 2001;80:2667–2677. doi: 10.1016/S0006-3495(01)76236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of a-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 151.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 153.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol. 2004;166:237–248. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 157.Jaffe SH, Friedlander DR, Matsuzaki F, Crossin KL, Cunningham BA, Edelman GM. Differential effects of the cytoplasmic domains of cell adhesion molecules on cell aggregation and sorting-out. Proc Natl Acad Sci U S A. 1990;87:3589–3593. doi: 10.1073/pnas.87.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jou T-S, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kaido M, Wada H, Shindo M, Hayashi S. Essential requirement for RING finger E3 ubiquitin ligase Hakai in early embryonic development of Drosophila. Genes Cells. 2009;14:1067–1077. doi: 10.1111/j.1365-2443.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 162.Kalantarian A, Ninomiya H, Saad SM, David R, Winklbauer R, Neumann AW. Axisymmetric drop shape analysis for estimating the surface tension of cell aggregates by centrifugation. Biophys J. 2009;96:1606–1616. doi: 10.1016/j.bpj.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kametani Y, Takeichi M. Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol. 2007;9:92–98. doi: 10.1038/ncb1520. [DOI] [PubMed] [Google Scholar]
- 164.Kane DA, McFarland KN, Warga RM. Mutations in half baked/E-cadherin block cell behaviors that are necessary for teleost epiboly. Development. 2005;132:1105–1116. doi: 10.1242/dev.01668. [DOI] [PubMed] [Google Scholar]
- 165.Kanner SB, Reynolds AB, Parsons JT. Tyrosine phosphorylation of a 120-kilodalton pp60src substrate upon epidermal growth factor and platelet-derived growth factor receptor stimulation and in polyomavirus middle-T-antigen-transformed cells. Mol Cell Biol. 1991;11:713–720. doi: 10.1128/mcb.11.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Keilhack H, Hellman U, van Hengel J, van Roy F, Godovac-Zimmermann J, Blhmer F-D. The protein-tyrosine phosphatase SHP-1 binds to and dephosphorylates p120 catenin. J Biol Chem. 2000;275:26376–26384. doi: 10.1074/jbc.M001315200. [DOI] [PubMed] [Google Scholar]
- 167.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 168.Kim L, Wong TW. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol Cell Endocrinol. 1995;15:4553–4561. doi: 10.1128/mcb.15.8.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim SH, Li Z, Sacks DB. E-cadherin-mediated cell-cell attachment activates Cdc42. J Biol Chem. 2000;275:36999–37005. doi: 10.1074/jbc.M003430200. [DOI] [PubMed] [Google Scholar]
- 170.Kim YJ, Johnson KR, Wheelock MJ. N-cadherin-mediated cell motility requires cis dimers. Cell Commun Adhes. 2005;12:23–39. doi: 10.1080/15419060500305971. [DOI] [PubMed] [Google Scholar]
- 171.Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- 172.Kitagawa M, Natori M, Murase S, Hirano S, Taketani S, Suzuki ST. Mutation analysis of cadherin-4 reveals amino acid residues of EC1 important for the structure and function. Biochem Biophys Res Commun. 2000;271:358–363. doi: 10.1006/bbrc.2000.2636. [DOI] [PubMed] [Google Scholar]
- 173.Klingelhofer J, Laur OY, Troyanovsky RB, Troyanovsky SM. Dynamic interplay between adhesive and lateral E-cadherin dimers. Mol Cell Biol. 2002;22:7449–7458. doi: 10.1128/MCB.22.21.7449-7458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Klucky B, Mueller R, Vogt I, Teurich S, Hartenstein B, Breuhahn K, Flechtenmacher C, Angel P, Hess J. Kallikrein 6 induces E-cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res. 2007;67:8198–8206. doi: 10.1158/0008-5472.CAN-07-0607. [DOI] [PubMed] [Google Scholar]
- 175.Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of a-actinin with the cadherin/catenin cell-cell adhesion complex via a-catenin. J Cell Biology. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Koch AW, Bozic D, Pertz O, Engel J. Homophilic adhesion by cadherins. Curr Opin Struct Biol. 1999;9:275–281. doi: 10.1016/S0959-440X(99)80038-4. [DOI] [PubMed] [Google Scholar]
- 178.Koestler SA, Auinger S, Vinzenz M, Rottner K, Small JV. Differentially oriented populations of actin filaments generated in lamellipodia collaborate in pushing and pausing at the cell front. Nat Cell Biol. 2008;10:306–313. doi: 10.1038/ncb1692. [DOI] [PubMed] [Google Scholar]
- 179.Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and PI3-kinase to regulate adhesive contacts. J Biol Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- 180.Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol. 2002;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- 181.Kovacs M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci U S A. 2007;104:9994–9999. doi: 10.1073/pnas.0701181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kraemer A, Goodwin M, Verma S, Yap AS, Ali RG. Rac is a dominant regulator of cadherin-directed actin assembly that is activated by adhesive ligation independently of Tiam1. Am J Physiol Cell Physiol. 2007;292:C1061–1069. doi: 10.1152/ajpcell.00073.2006. [DOI] [PubMed] [Google Scholar]
- 183.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 184.Kuroda S, Fukata M, Fujii K, Nakamura T, Izawa I, Kaibuchi K. Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem Biophys Res Comm. 1997;240:430–435. doi: 10.1006/bbrc.1997.7675. [DOI] [PubMed] [Google Scholar]
- 185.Kussel-Andermann P, El-Amraoui A, Safieddine S, Nouaille S, Perfettini I, Lecuit M, Cossart P, Wolfrum U, Petit C. Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex. EMBO J. 2000;19:6020–6029. doi: 10.1093/emboj/19.22.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lamaze C, Chuang T-H, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by rho and rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- 187.Lambeng N, Wallez Y, Rampon C, Cand F, Christe G, Gulino-Debrac D, Vilgrain I, Huber P. Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 2005;96:384–391. doi: 10.1161/01.RES.0000156652.99586.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lambert M, Choquet D, Mege RM. Dynamics of ligand-induced, Rac1-dependent anchoring of cadherins to the actin cytoskeleton. J Cell Biol. 2002;157:469–479. doi: 10.1083/jcb.200107104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lampugnani MG, Dejana E. Interendothelial junctions: structure, signalling and functional roles. Curr Opinion Cell Biol. 1997;9:674–682. doi: 10.1016/s0955-0674(97)80121-4. [DOI] [PubMed] [Google Scholar]
- 190.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-Cadherin Regulates Endothelial Actin Activating Rac and Increasing Membrane Association of Tiam. Mol Biol Cell. 2002;13:1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lampugnani MG, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Julicher F, Dahmann C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–1955. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 194.Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell. 2005;9:355–376. doi: 10.1016/j.devcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 195.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Le Bivic A, Sambuy Y, Mostov K, Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990;110:1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- 199.Leckband D. Beyond structure: mechanism and dynamics of intercellular adhesion. Biochem Soc Trans. 2008;36:213–220. doi: 10.1042/BST0360213. [DOI] [PubMed] [Google Scholar]
- 200.Leckband D, Muller W, Schmitt FJ, Ringsdorf H. Molecular mechanisms determining the strength of receptor-mediated intermembrane adhesion. Biophys J. 1995;69:1162–1169. doi: 10.1016/S0006-3495(95)79990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leckband D, Prakasam A. Mechanism and dynamics of cadherin adhesion. Annual review of biomedical engineering. 2006;8:259–287. doi: 10.1146/annurev.bioeng.8.061505.095753. [DOI] [PubMed] [Google Scholar]
- 202.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 203.Lee C-H, Gumbiner BM. Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Developmental Biology. 1995;171:363–373. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- 204.Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 205.Leptin M. Gastrulation movements: the logic and the nuts and bolts. Dev Cell. 2005;8:305–320. doi: 10.1016/j.devcel.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 206.Levine E, Lee CH, Kintner C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development. 1994;120:901–909. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- 207.Lien WH, Gelfand VI, Vasioukhin V. Alpha-E-catenin binds to dynamitin and regulates dynactin-mediated intracellular traffic. J Cell Biol. 2008;183:989–997. doi: 10.1083/jcb.200805041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- 209.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lock JG, Stow JL. Rab11 in Recycling Endosomes Regulates the Sorting and Basolateral Transport of E-Cadherin. Mol Biol Cell. 2005 doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Luber B, Candidus S, Handschuh G, Mentele E, Hutzler P, Feller S, Voss J, Hofler H, Becker KF. Tumor-derived mutated E-cadherin influences beta-catenin localization and increases susceptibility to actin cytoskeletal changes induced by pervanadate. Cell Adhes Commun. 2000;7:391–408. doi: 10.3109/15419060009109021. [DOI] [PubMed] [Google Scholar]
- 212.Ma X, Bao J, Adelstein RS. Loss of cell adhesion causes hydrocephalus in nonmuscle Myosin II-B-ablated and mutated mice. Mol Biol Cell. 2007;18:2305–2312. doi: 10.1091/mbc.E07-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Maddugoda MP, Crampton MS, Shewan AM, Yap AS. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell-cell contacts in mammalian epithelial cells. J Cell Biol. 2007;178:529–540. doi: 10.1083/jcb.200612042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and a-catenin, and regulates cadherin-based adherens junctions in Drosophia. Develop. 2002;129:3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- 215.Maher MT, Flozak AS, Stocker AM, Chenn A, Gottardi CJ. Activity of the beta-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J Cell Biol. 2009;186:219–228. doi: 10.1083/jcb.200811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Maher PA, Pasquale EB. Tyrosine phosphorylated proteins in different tissues during chick embryo development. J Cell Biol. 1988;106(5):1747–55. doi: 10.1083/jcb.106.5.1747. 106(5):1747-55: 1747-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maher PA, Pasquale EB, Wang JY, Singer SJ. Phosphotyrosine-containing proteins are concentrated in focal adhesions and intercellular junctions in normal cells. Proc Natl Acad Sci U S A. 1985;82:6576–6580. doi: 10.1073/pnas.82.19.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Malliri A, van Es S, Huveneers S, Collard JG. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem. 2004;279:30092–30098. doi: 10.1074/jbc.M401192200. [DOI] [PubMed] [Google Scholar]
- 219.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 221.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mary S, Charrasse S, Meriane M, Comunale F, Travo P, Blangy A, Gauthier-Rouviere C. Biogenesis of N-Cadherin-dependent Cell-Cell Contacts in Living Fibroblasts Is a Microtubule-dependent Kinesin-driven Mechanism. Mol Biol Cell. 2002;13:285–301. doi: 10.1091/mbc.01-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Matsuzaki F, Mege RM, Jaffe SH, Friedlander DR, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM. cDNAs of cell adhesion molecules of different specificity induce changes in cell shape and border formation in cultured S180 cells. J Cell Biol. 1990;110:1239–1252. doi: 10.1083/jcb.110.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.McCrea P, Gumbiner B. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin): characterization and extractability of the protein complex from the cell cytostructure. J Biological Chemistry. 1991;266:4514–4520. [PubMed] [Google Scholar]
- 227.McCusker C, Cousin H, Neuner R, Alfandari D. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol Biol Cell. 2009;20:78–89. doi: 10.1091/mbc.E08-05-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, Yap AS. E-cadherin adhesion activates c-Src signaling at cell-cell contacts. Mol Biol Cell. 2007;18:3214–3223. doi: 10.1091/mbc.E06-12-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.McLachlan RW, Yap AS. Not so simple: the complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med. 2007;85:545–554. doi: 10.1007/s00109-007-0198-x. [DOI] [PubMed] [Google Scholar]
- 231.Mege RM, Matsuzaki F, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci U S A. 1988;85:7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mellman I. Membranes and sorting. Curr Opin Cell Biol. 1996;8:497–498. doi: 10.1016/s0955-0674(96)80026-3. [DOI] [PubMed] [Google Scholar]
- 233.Mellman I, Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- 234.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacats. Cell. 2008;135:948–959. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 235.Michels C, Buchta T, Bloch W, Krieg T, Niessen CM. Classical cadherins regulate desmosome formation. J Invest Dermatol. 2009;129:2072–2075. doi: 10.1038/jid.2009.17. [DOI] [PubMed] [Google Scholar]
- 236.Millo H, Leaper K, Lazout V, Bownes M. Myosin VI plays a role in cell-cell adhesion during epithelial morphogenesis. Mech Dev. 2004;121:1335–1351. doi: 10.1016/j.mod.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 237.Miranda KC, Joseph SR, Yap AS, Teasdale RD, Stow JL. Contextual bnding of p120ctn to E-cadherin at the basolateral plasma membrane in polarized epithelia. J Biol Chem. 2003;278:43480–43488. doi: 10.1074/jbc.M305525200. [DOI] [PubMed] [Google Scholar]
- 238.Miranda KC, Khromykh T, Christy P, Le TL, Gottardi CJ, Yap AS, Stow JL, Teasdale RD. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem. 2001;276:22565–22572. doi: 10.1074/jbc.M101907200. [DOI] [PubMed] [Google Scholar]
- 239.Miyaguchi K. Ultrastructure of the zonula adherens revealed by rapid-freeze deep-etching. J Struct Biol. 2000;132:169–178. doi: 10.1006/jsbi.2000.4244. [DOI] [PubMed] [Google Scholar]
- 240.Miyatani S, Shimamura K, Hatta M, Nagafuchi A, Nose A, Matsunaga M, Hatta K, Takeichi M. Neural cadherin: role in selective cell-cell adhesion. Science. 1989;245:631–635. doi: 10.1126/science.2762814. [DOI] [PubMed] [Google Scholar]
- 241.Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- 242.Montell DJ, Rorth P, Spradling AC. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 1992;71:51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- 243.Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–293. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- 244.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. 759–803. [DOI] [PubMed] [Google Scholar]
- 245.Muller T, Choidas A, Reichmann E, Ullrich A. Phosphorylation and free pool of b-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem. 1999;274:10173–10183. doi: 10.1074/jbc.274.15.10173. [DOI] [PubMed] [Google Scholar]
- 246.Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J. 2001;20:2171–2179. doi: 10.1093/emboj/20.9.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol. 2003;160:433–449. doi: 10.1083/jcb.200211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329:341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- 249.Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nagafuchi A, Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regulation. 1989;1:37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- 252.Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem. 2008;283:18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nakagawa M, Fukata M, Yamagawa M, Itoh M, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- 254.Nathke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Niederman R, Pollard TD. Human platelet myosin. II. In vitro assembly and structure of myosin filaments. J Cell Biol. 1975;67:72–92. doi: 10.1083/jcb.67.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol. 2002;156:389–400. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 259.Niewiadomska P, Godt D, Tepass U. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 1999;144:533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nigam SK, Rodriguez-Boulan E, Silver RB. Changes in intracellular calcium during the development of epithelial polarity and junctions. Proc Natl Acad Sci USA. 1992;89:6162–6166. doi: 10.1073/pnas.89.13.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 262.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 263.Noguchi T, Lenartowska M, Miller KG. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol Biol Cell. 2006;17:2559–2571. doi: 10.1091/mbc.E06-01-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 265.Noren NK, Arthur WT, Burridge K. Cadherin Engagement Inhibits RhoA via p190RhoGAP. J Biol Chem. 2003;278:13615–13618. doi: 10.1074/jbc.C200657200. [DOI] [PubMed] [Google Scholar]
- 266.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via rho family GTPases. J Cell Biol. 2000;150:567–579. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 268.Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- 269.Nose A, Tsuji K, Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990;61:147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- 270.Ochiai A, Akimoto S, Kanai Y, Shibata T, Oyama T, Hirohashi S. c-erbB-2 gene product associates with catenins in human cancer cells. Biochem Biophys Res Comm. 1994;205:73–78. doi: 10.1006/bbrc.1994.2631. [DOI] [PubMed] [Google Scholar]
- 271.Oda H, Tagawa K, Akiyama-Oda Y. Diversification of epithelial adherens junctions with independent reductive changes in cadherin form: identification of potential molecular synapomorphies among bilaterians. Evol Dev. 2005;7:376–389. doi: 10.1111/j.1525-142X.2005.05043.x. [DOI] [PubMed] [Google Scholar]
- 272.Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- 273.Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, Zeng C, Baron A, Bemis L, Erickson P, Wilder E, Rustgi A, Kitajewski J, Gabrielson E, Bremnes R, Franklin W, Drabkin HA. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci U S A. 2003;100:10429–10434. doi: 10.1073/pnas.1734137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ozawa M, Kemler R. Altered cell adhesion activity by pervanadate due to the dissociation of a-catenin from the E-cadherin.catenin complex. J Biol Chem. 1998;273:6166–6170. doi: 10.1074/jbc.273.11.6166. [DOI] [PubMed] [Google Scholar]
- 275.Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Pacquelet A, Lin L, Rorth P. Binding site for p120/delta-catenin is not required for Drosophila E-cadherin function in vivo. J Cell Biol. 2003;160:313–319. doi: 10.1083/jcb.200207160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol. 2005;25:389–402. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 279.Pang JH, Kraemer A, Stehbens SJ, Frame MC, Yap AS. Recruitment of phosphoinositide 3-kinase defines a positive contribution of tyrosine kinase signalling to E-cadherin function. J Biol Chem. 2005;280:3043–3050. doi: 10.1074/jbc.M412148200. [DOI] [PubMed] [Google Scholar]
- 280.Panorchan P, Thompson MS, Davis KJ, Tseng Y, Konstantopoulos K, Wirtz D. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J Cell Sci. 2006;119:66–74. doi: 10.1242/jcs.02719. [DOI] [PubMed] [Google Scholar]
- 281.Paradies NE, Grunwald GB. Purification and characterization of NCAD90, a soluble endogenous form of N-cadherin, which is generated by proteolysis during retinal development and retains adhesive and neurite-promoting function. J Neurosci Res. 1993;36:33–45. doi: 10.1002/jnr.490360105. [DOI] [PubMed] [Google Scholar]
- 282.Park JI, Kim SW, Lyons JP, Ji H, Nguyen TT, Cho K, Barton MC, Deroo T, Vleminckx K, Moon RT, McCrea PD. Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell. 2005;8:843–854. doi: 10.1016/j.devcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 283.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J Biol Chem. 2003;278:21050–21057. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- 285.Paul AS, Pollard TD. Review of the mechanism of processive actin filament elongation by formins. Cell Motil Cytoskeleton. 2009;66:606–617. doi: 10.1002/cm.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. J Biol Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- 287.Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem. 2000;275:41227–41233. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- 288.Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM. The vertebrate adhesive junction proteins b-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biology. 1992;118:681–691. doi: 10.1083/jcb.118.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Peifer M, Orsulic S, Sweeton D, Wieschaus E. A role for the Drosophila segment polarity gene armadillo in cell adhesion and cytoskeletal integrity during oogenesis. Development. 1993;118:1191–1207. doi: 10.1242/dev.118.4.1191. [DOI] [PubMed] [Google Scholar]
- 290.Peifer M, Wieschaus E. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell. 1990;63:1167–1176. doi: 10.1016/0092-8674(90)90413-9. [DOI] [PubMed] [Google Scholar]
- 291.Peng X, Cuff LE, Lawton CD, DeMali KA. Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J Cell Sci. 2010;123:567–577. doi: 10.1242/jcs.056432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Perret E, Leung A, Feracci H, Evans E. Trans-bonded pairs of E-cadherin exhibit a remarkable hierarchy of mechanical strengths. Proc Natl Acad Sci U S A. 2004;101:16472–16477. doi: 10.1073/pnas.0402085101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pertz O, Bozic D, Koch AW, Fauser C, Brancaccio A, Engel J. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 1999;18:1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol. 2003;162:15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685–694. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- 297.Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem. 2002;277:18868–18874. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223:1019–1026. doi: 10.1111/j.1432-1033.1994.tb19080.x. [DOI] [PubMed] [Google Scholar]
- 299.Prakasam A, Chien YH, Maruthamuthu V, Leckband DE. Calcium site mutations in cadherin: impact on adhesion and evidence of cooperativity. Biochemistry. 2006;45:6930–6939. doi: 10.1021/bi060213m. [DOI] [PubMed] [Google Scholar]
- 300.Prakasam AK, Maruthamuthu V, Leckband DE. Similarities between heterophilic and homophilic cadherin adhesion. Proc Natl Acad Sci U S A. 2006;103:15434–15439. doi: 10.1073/pnas.0606701103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Qi J, Wang J, Romanyuk O, Siu CH. Involvement of Src family kinases in N-cadherin phosphorylation and beta-catenin dissociation during transendothelial migration of melanoma cells. Mol Biol Cell. 2006;17:1261–1272. doi: 10.1091/mbc.E05-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. Embo J. 2004;23:1739–1784. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. Embo J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Ren G, Crampton MS, Yap AS. Cortactin: Coordinating adhesion and the actin cytoskeleton at cellular protrusions. Cell Motil Cytoskeleton. 2009 doi: 10.1002/cm.20380. [DOI] [PubMed] [Google Scholar]
- 305.Ren G, Helwani FM, Verma S, McLachlan RW, Weed SA, Yap AS. Cortactin Is a Functional Target of E-cadherin-activated Src Family Kinases in MCF7 Epithelial Monolayers. J Biol Chem. 2009;284:18913–18922. doi: 10.1074/jbc.M109.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Reynolds AB, Kanner SB, Wang HC, Parsons JT. Stable association of activated pp60src with two tyrosine-phosphorylated cellular proteins. Mol Cell Biol. 1989;9:3951–3958. doi: 10.1128/mcb.9.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rhee J, Mahfooz NS, Arregui C, Lilien J, Balsamo J, VanBerkum MF. Activation of the repulsive receptor Roundabout inhibits N-cadherin-mediated cell adhesion. Nat Cell Biol. 2002;4:798–805. doi: 10.1038/ncb858. [DOI] [PubMed] [Google Scholar]
- 309.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. a1(E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rios-Doria J, Day KC, Kuefer R, Rashid MG, Chinnaiyan AM, Rubin MA, Day ML. The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J Biol Chem. 2003;278:1372–1379. doi: 10.1074/jbc.M208772200. [DOI] [PubMed] [Google Scholar]
- 311.Rodriguez AJ, Shenoy SM, Singer RH, Condeelis J. Visualization of mRNA translation in living cells. J Cell Biol. 2006;175:67–76. doi: 10.1083/jcb.200512137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 313.Roeth JF, Sawyer JK, Wilner DA, Peifer M. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS One. 2009;4:e7634. doi: 10.1371/journal.pone.0007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rosato R, Veltmaat JM, Groffen J, Heisterkamp N. Involvement of the tyrosine kinase Fer in cell adhesion. Mol Cell Biol. 1998;18:5762–5770. doi: 10.1128/mcb.18.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 316.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 317.Sadot E, Simcha I, Shtutman M, Ben-Ze'ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci U S A. 1998;95:15339–15344. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 319.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 320.Sawyer JK, Harris NJ, Slep KC, Gaul U, Peifer M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J Cell Biol. 2009;186:57–73. doi: 10.1083/jcb.200904001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 322.Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci U S A. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Schuman EM, Murase S. Cadherins and synaptic plasticity: activity-dependent cyclin-dependent kinase 5 regulation of synaptic beta-catenin-cadherin interactions. Philos Trans R Soc Lond B Biol Sci. 2003;358:749–756. doi: 10.1098/rstb.2002.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Scott JA, Shewan AM, den Elzen NR, Loureiro JJ, Gertler FB, Yap AS. Ena/VASP Proteins Can Regulate Distinct Modes of Actin Organization at Cadherin-adhesive Contacts. Mol Biol Cell. 2006;17:1085–1095. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Scott JA, Yap AS. Cinderella no longer: alpha-catenin steps out of cadherin's shadow. J Cell Sci. 2006;119:4599–4605. doi: 10.1242/jcs.03267. [DOI] [PubMed] [Google Scholar]
- 327.Seifert K, Ibrahim H, Stodtmeister T, Winklbauer R, Niessen CM. An adhesion-independent, aPKC-dependent function for cadherins in morphogenetic movements. J Cell Sci. 2009;122:2514–2523. doi: 10.1242/jcs.042796. [DOI] [PubMed] [Google Scholar]
- 328.Shan W, Yagita Y, Wang Z, Koch A, Fex Svenningsen A, Gruzglin E, Pedraza L, Colman DR. The minimal essential unit for cadherin-mediated intercellular adhesion comprises extracellular domains 1 and 2. J Biol Chem. 2004;279:55914–55923. doi: 10.1074/jbc.M407827200. [DOI] [PubMed] [Google Scholar]
- 329.Shan WS, Tanaka H, Phillips GR, Arndt K, Yoshida M, Colman DR, Shapiro L. Functional cis-heterodimers of N- and R-cadherins. J Cell Biol. 2000;148:579–590. doi: 10.1083/jcb.148.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand J-F, Als-Nielden J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 331.Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- 332.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 Is a Key Rho Kinase Target Necessary for the Local Concentration of E-Cadherin at Cell-Cell Contacts. Mol Biol Cell. 2005;16:4531–4532. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Shi Q, Chien YH, Leckband D. Biophysical properties of cadherin bonds do not predict cell sorting. J Biol Chem. 2008;283:28454–28463. doi: 10.1074/jbc.M802563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Shi Q, Maruthamuthu V, Li F, Leckband D. Allosteric cross talk between cadherin extracellular domains. Biophys J. 2010;99:95–104. doi: 10.1016/j.bpj.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of b-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhesion Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- 336.Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmemrbane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 337.Shiozaki H, Kadowaki T, Doki Y, Inoue M, Tamura S, Oka H, Iwazawa T, Matsui S, Shimaya K, Takeichi M, Mori T. Effect of epidermal growth factor on cadherin-mediated adhesion in a human oesophageal cancer cell line. Br J Cancer. 1995;71:250–258. doi: 10.1038/bjc.1995.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- 339.Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491–501. doi: 10.1242/dev.02742. [DOI] [PubMed] [Google Scholar]
- 340.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 341.Small JV, Rottner K, Kaverina I. Functional design in the actin cytoskeleton. Curr Opinion Cell Biol. 1999;11:54–60. doi: 10.1016/s0955-0674(99)80007-6. [DOI] [PubMed] [Google Scholar]
- 342.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 344.Sotomayor M, Schulten K. The allosteric role of the C6a2+ switch in adhesion and elasticity of C-cadherin. Biophys J. 2008;94:4621–4633. doi: 10.1529/biophysj.107.125591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sousa S, Cabanes D, El-Amraoui A, Petit C, Lecuit M, Cossart P. Unconventional myosin VIIa and vezatin, two proteins crucial for Listeria entry into epithelial cells. J Cell Sci. 2004;117:2121–2130. doi: 10.1242/jcs.01066. [DOI] [PubMed] [Google Scholar]
- 346.Spiliotis ET, Nelson WJ. Spatial control of exocytosis. Curr Op Cell Biol. 2003;15:430–437. doi: 10.1016/s0955-0674(03)00074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Stehbens SJ, Paterson AD, Crampton MS, Shewan AM, Ferguson C, Akhmanova A, Parton RG, Yap AS. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci. 2006;119:1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- 348.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 349.Steinberg MS. On the mechanisms of tissue reconstruction by dissociated cells. I. Population kinetics, the differential adhesiveness, and the absence of directed migration. Proc Natl Acad Sci. 1962;48:1577–1582. doi: 10.1073/pnas.48.9.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Steinberg MS, Gilbert SF. Townes and Holtfreter (1955): directed movements and selective adhesion of embryonic amphibian cells. J Exp Zoolog A Comp Exp Biol. 2004;301:701–706. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- 351.Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proceedings of the National Academy of Sciences, USA. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 353.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 354.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sweeney HL, Houdusse A. What can myosin VI do in cells? Curr Op Cell Biol. 2007;19:57–66. doi: 10.1016/j.ceb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 356.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 357.Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–1175. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Takahashi F, Endo S, Kojima T, Saigo K. Regulation of cell-cell contacts in developing Drosophila eyes by Dsrc41, a new, close relative of vertebrate c-src. Genes Dev. 1996;10:1645–1656. doi: 10.1101/gad.10.13.1645. [DOI] [PubMed] [Google Scholar]
- 359.Takahashi M, Takahashi F, Ui-Tei K, Kojima T, Saigo K. Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development. 2005;132:2547–2559. doi: 10.1242/dev.01850. [DOI] [PubMed] [Google Scholar]
- 360.Takata K, Singer SJ. Phosphotyrosine-modified proteins are concentrated at the membranes of epithelial and endothelial cells during tissue development in chick embryos. J Cell Biol. 1988;106:1757–1764. doi: 10.1083/jcb.106.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and b-catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Takeda H, Shimoyama Y, Nagafuchi A, Hirohashi S. E-cadherin functions as a cis-dimer at the cell-cell adhesive interface in vivo. Nat Struct Biol. 1999;6:310–312. doi: 10.1038/7542. [DOI] [PubMed] [Google Scholar]
- 363.Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biology. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Tamura K, Shan W-S, Hendrickson WA, Colman DR, Shapiro L. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 365.Tao Q, S. N, McCrea PD, Heasman J, Wylie C. G-protein-coupled signals control cortical actin assembly by controlling cadherin expression in the early Xenopus embryo. Development. 2007;134:2651–2661. doi: 10.1242/dev.002824. [DOI] [PubMed] [Google Scholar]
- 366.Teng J, Rai T, Tanaka Y, Takei Y, Nakata T, Hirasawa M, Kulkarni AB, Hirokawa N. The KIF3 motor transports N-cadherin and organizes the developing neuroepithelium. Nat Cell Biol. 2005;7:474–482. doi: 10.1038/ncb1249. [DOI] [PubMed] [Google Scholar]
- 367.Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Torok T, Hartenstein V. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10:672–685. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- 368.Tepass U, Troung K, Godt D, Ikura M, Peifer M. Cadherins in embyronic and neural morphogenesis. Nat Rev Mol Cell Biol. 2000;1:91–100. doi: 10.1038/35040042. [DOI] [PubMed] [Google Scholar]
- 369.Thiery J-P. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 370.Tinkle CL, Lechler T, Pasolli HA, Fuchs E. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc Natl Acad Sci U S A. 2004;101:552–557. doi: 10.1073/pnas.0307437100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci U S A. 2008;105:15405–15410. doi: 10.1073/pnas.0807374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Tomschy A, Fauser C, Landwehr R, Engel J. Homophilic adhesion of E-cadherin occurs by a co-operative two-step interaction of N-terminal domains. EMBO J. 1996;15:3507–3514. [PMC free article] [PubMed] [Google Scholar]
- 373.Townes PL, Holtfreter J. Directed movements and selective adhesion of embronic amphibian cells. J Experimental Zoology. 1955;128:53–120. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- 374.Troyanovsky RB, Sokolov E, Troyanovsky SM. Adhesive and lateral E-cadherin dimers are mediated by the same interface. Mol Cell Biol. 2003;23:7965–7972. doi: 10.1128/MCB.23.22.7965-7972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Troyanovsky RB, Sokolov EP, Troyanovsky SM. Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly. Mol Biol Cell. 2006;17:3484–3493. doi: 10.1091/mbc.E06-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Tsuiji H, Xu L, Schwartz K, Gumbiner BM. Cadherin conformations associated with dimerization and adhesion. J Biol Chem. 2007;282:12871–12882. doi: 10.1074/jbc.M611725200. [DOI] [PubMed] [Google Scholar]
- 377.Tsukasaki Y, Kitamura K, Shimizu K, Iwane AH, Takai Y, Yanagida T. Role of multiple bonds between the single cell adhesion molecules, nectin and cadherin, revealed by high sensitive force measurements. J Mol Biol. 2007;367:996–1006. doi: 10.1016/j.jmb.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 378.Tsukita S, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T. Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol. 1991;113:867–879. doi: 10.1083/jcb.113.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. Embo J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 382.Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and rho/rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 383.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 384.Verma S, Shewan AM, Scott JA, Helwani FM, Elzen NR, Miki H, Takenawa T, Yap AS. Arp2/3 Activity Is Necessary for Efficient Formation of E-cadherin Adhesive Contacts. J Biol Chem. 2004;279:34062–34070. doi: 10.1074/jbc.M404814200. [DOI] [PubMed] [Google Scholar]
- 385.Vestweber D, Kemler R. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp Cell Res. 1984;152:169–178. doi: 10.1016/0014-4827(84)90241-6. [DOI] [PubMed] [Google Scholar]
- 386.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Vidal M, Warner S, Read R, Cagan RL. Differing Src signaling levels have distinct outcomes in Drosophila. Cancer Res. 2007;67:10278–10285. doi: 10.1158/0008-5472.CAN-07-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Vignjevic D, Yarar D, Welch MD, Peloquin J, Svitkina T, Borisy GG. Formation of filopodia-like bundles in vitro from a dendritic network. J Cell Biol. 2003;160:951–962. doi: 10.1083/jcb.200208059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Vijayendran R, Hammer D, Leckband D. Simulations of the adhesion between molecularly bonded surfaces in direct force measurements. J Chem Phys. 1998;108:7783–7794. [Google Scholar]
- 390.Volberg T, Zick Y, Dror R, Sabanay I, Gilon C, Levitzki A, Geiger B. The effect of tyrosine-specific protein phosphorylation on the assembly of adherens-type junctions. EMBO J. 1992;11:1733–1742. doi: 10.1002/j.1460-2075.1992.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Wahl JK, 3rd, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem. 2003;278:17269–17276. doi: 10.1074/jbc.M211452200. [DOI] [PubMed] [Google Scholar]
- 392.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, Huber P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–1077. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 393.Wang B, Wylie FG, Teasdale RD, Stow JL. The polarized trafficking of E-cadherin is regulated by Rac1 and Cdc42 in MDCK cells. Am J Physiol Cell Physiol. 2005 doi: 10.1152/ajpcell.00533.2004. [DOI] [PubMed] [Google Scholar]
- 394.Wang Q, Chen X-W, Margolis B. PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol Biol Cell. 2007;18:874–885. doi: 10.1091/mbc.E06-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Ward MD, Dembo M, Hammer DA. Kinetics of cell detachment: peeling of discrete receptor clusters. Biophys J. 1994;67:2522–2534. doi: 10.1016/S0006-3495(94)80742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Warren SL, Handel LM, Nelson WJ. Elevated expression of pp60c-src alters a selective morphogenetic property of epithelial cells in vitro without a mitogenic effect. Mol Cell Biol. 1988;8:632–646. doi: 10.1128/mcb.8.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Warren SL, Nelson WJ. Nonmitogenic morphoregulatory action of pp60v-src on multicellular epithelial structures. Mol Cell Biol. 1987;7:1326–1337. doi: 10.1128/mcb.7.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biology. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Weed SA, Du Y, Parsons JT. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J Cell Sci. 1998;111:2433–2443. doi: 10.1242/jcs.111.16.2433. [DOI] [PubMed] [Google Scholar]
- 401.Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Wheelock MJ, Buck CA, Bechtol KB, Damsky CH. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987;34:187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- 403.Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–514. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 404.Wieland JA, Gewirth AA, Leckband DE. Single-molecule measurements of the impact of lipid phase behavior on anchor strengths. J Phys Chem B. 2005;109:5985–5993. doi: 10.1021/jp045461b. [DOI] [PubMed] [Google Scholar]
- 405.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 406.Winklbauer R. Cell adhesion in amphibian gastrulation. Int Rev Cell Mol Biol. 2009;278:215–275. doi: 10.1016/S1937-6448(09)78005-0. [DOI] [PubMed] [Google Scholar]
- 407.Wolfe BA, Glotzer M. Single cells (put a ring on it) Genes Dev. 2009;23:896–901. doi: 10.1101/gad.1801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009 doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Xu G, Arregui C, Lilien J, Balsamo J. PTP1B modulates the association of beta-catenin with N-cadherin through binding to an adjacent and partially overlapping target site. J Biol Chem. 2002;277:49989–49997. doi: 10.1074/jbc.M206454200. [DOI] [PubMed] [Google Scholar]
- 411.Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]
- 412.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell cell adhesion. J Cell Biol. 2007 doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yamazaki D, Oikawa T, Takenawa T. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J Cell Sci. 2007;120:86–100. doi: 10.1242/jcs.03311. [DOI] [PubMed] [Google Scholar]
- 415.Yanagawa S, Lee J-S, Haruna T, Oda H, Uemura T, Takeichi M, Ishimoto A. Accumulation of armadillo induced by Wingless, Dishevelled, and dominant-negative Zeste-white 3 leads to elevated DE-cadherin in Drosophila clone 8 wing disc cells. J Biol Chem. 1997;272:25243–25251. doi: 10.1074/jbc.272.40.25243. [DOI] [PubMed] [Google Scholar]
- 416.Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem. 2004;279:9512–9521. doi: 10.1074/jbc.M310895200. [DOI] [PubMed] [Google Scholar]
- 417.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 418.Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Current Biology. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- 419.Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Yap AS, Niessen C, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening and interaaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yonemura S, Itoh M, Nagafuchi A, Tsukita S. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci. 1995;108:127–142. doi: 10.1242/jcs.108.1.127. [DOI] [PubMed] [Google Scholar]
- 422.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 423.Young P, Boussadia O, Halfter H, Grose R, Berger P, Leone DP, Robenek H, Charnay P, Kemler R, Suter U. E-cadherin controls adherens junctions in the epidermis and the renewal of hair follicles. EMBO J. 2003;22:5723–5733. doi: 10.1093/emboj/cdg560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, Braga VM. Actin at cell-cell junctions is composed of two dynamic and functional populations. J Cell Sci. 2005;118:5549–5562. doi: 10.1242/jcs.02639. [DOI] [PubMed] [Google Scholar]
- 425.Zhang Y, Sivasankar S, Nelson WJ, Chu S. Resolving cadherin interactions and binding cooperativity at the single-molecule level. Proc Natl Acad Sci U S A. 2009;106:109–114. doi: 10.1073/pnas.0811350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Zhong Y, Brieher WM, Gumbiner BM. Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. J Cell Biol. 1999;144:351–359. doi: 10.1083/jcb.144.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Zhu B, Chappuis-Flament S, Wong E, Jensen IE, Gumbiner BM, Leckband D. Functional analysis of the structural basis of homophilic cadherin adhesion. Biophys J. 2003;84:4033–4042. doi: 10.1016/S0006-3495(03)75129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]