Abstract
Metastatic melanoma is one of the most challenging malignancies to treat and often has a poor outcome. Until recently, systemic treatment options were limited, with poor response rates and no survival advantage. However, the treatment of metastatic melanoma has been revolutionized by developments in targeted therapy and immunotherapy; the BRAF inhibitor, vemurafenib, and anticytotoxic T-lymphocyte antigen 4 antibody, ipilimumab, are the first agents to demonstrate a survival benefit. Despite the success of these treatments, most patients eventually progress, and research into response and resistance mechanisms, rationally designed combination therapies and evaluation of the role of these agents in the adjuvant setting is critically important.
Keywords: BRAF inhibitor, cutaneous, immunotherapy, ipilimumab, melanoma, mutations
Introduction
The incidence of malignant melanoma has increased steadily over recent decades. An estimated 76,250 new melanoma cases will be diagnosed in the United States in 2012 and 9180 are expected to die of their disease [Siegel et al. 2012]. Cutaneous melanoma which is detected at an early stage is usually cured by surgical excision, but a proportion of patients experience nodal or distant relapse. Metastatic melanoma is considered to be one of the most challenging malignancies to treat and is associated with a poor prognosis; median survival ranges from 8 to 18 months depending on substage [Balch et al. 2009].
Until recently, the only approved systemic treatment options for patients with metastatic melanoma were limited to dacarbazine (DTIC) and interleukin (IL-2). The response rates (RRs) to cytotoxic chemotherapy and cytokine therapy are low. Dacarbazine has a RR of about 10% with median response duration of 4–8 months [Chapman et al. 1999]; IL-2 has a similar RR of 16%, but up to 5% achieve a durable complete response [Atkins et al. 2000]. An analysis of 30 phase III trials of chemotherapy combined with hormone or immune-based therapies found that while the RR increased with combination treatment, so did toxicity, and there was no improvement in overall survival (OS) compared with DTIC alone [Eggermont and Kirkwood, 2004; Eggermont and Schadendorf, 2009]. In the last 2 years, two new agents, the anticytotoxic T-lymphocyte antigen 4 (CTLA-4) monoclonal antibody ipilimumab and the BRAF inhibitor vemurafenib, have improved survival in patients with metastatic melanoma in randomized controlled trials and have been approved for treatment [Chapman et al. 2011; Hodi et al. 2010; Robert et al. 2011]. These agents are undergoing further evaluation in a range of clinical settings as monotherapy or combination therapy. This article summarizes the clinical development of novel treatments for metastatic melanoma, including molecularly targeted therapy and immune-modulating agents, and discusses how their use might be optimized in clinical practice to improve outcomes in this challenging solid tumour type.
Targeted therapy in advanced melanoma
Oncogenic somatic gene mutations have now been identified, which, along with clinicopathologic features, define distinct subsets of patients with melanoma [Garnett et al. 2004; Curtin et al. 2005]. The relevant genes and signal transduction pathways include the RAS/RAF/MEK/ERK mitogen-activated protein (MAP) kinase pathway, c-KIT (CD117), the phosphoinositol 3 kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway and GNAQ/GNA11, and are discussed in the following paragraphs. The common oncogenic mutations in melanoma subtypes are summarized in Table 1.
Table 1.
Estimated frequency of common mutations in different melanoma subtypes.
| Melanoma type | Common genetic mutations |
|---|---|
| Cutaneous | BRAF ~ 50% [Davies et al. 2002; Cheng et al. 2011; Long et al. 2011] |
| V600E 80% | |
| V600K 16% | |
| V600G/R 3% | |
| NRAS 20% [Goel et al. 2006; Jakob et al. 2011] | |
| Acral/mucosal | c-KIT 10% [Curtin et al. 2006; Jiang et al. 2008] |
| BRAF/NRAS <10% [Wong et al. 2005] | |
| Uveal | GNAQ ~ 45% [Onken et al. 2008; Van Raamsdonk et al. 2010] |
| GNA11 ~ 60% [Onken et al. 2008; Van Raamsdonk et al. 2010] |
The RAS/RAF/MEK/ERK MAP kinase pathway is central to the pathogenesis of cutaneous melanoma. When constitutively active, it promotes cellular proliferation, survival and invasion [Hocker et al. 2008]. The major driver of this pathway in melanoma is mutant BRAF. The activated BRAF protein causes subsequent phosphorylation and activation of MEK, followed by ERK.
BRAF
BRAF mutations in malignant melanoma were first described in 2002 [Davies et al. 2002]. The discovery of a BRAF mutation in over half of malignant melanomas strongly suggested its oncogenic potential. Over 40 distinct BRAF mutations have been described in the literature [Garnett and Marais, 2004]. The most common somatic BRAF mutation occurs in exon-15 and involves a substitution of glutamate for valine at codon 600 (V600E) [Cheng et al. 2011; Davies et al. 2002; Long et al. 2011]. The V600K and V600G/R mutations are less common and represent about 16% and 3% of BRAF mutations respectively [Davies et al. 2002; Long et al. 2011].
Approximately 50% of all melanomas harbour an activating mutation in BRAF [Curtin et al. 2005; Lee et al. 2011; Maldonado et al. 2003]. Features of the primary melanoma that are significantly associated with a BRAF mutation include superficial spreading and nodular histopathological subtypes, presence of mitoses, presence of a single or occult primary, truncal location and younger age (less than 50 years) at the time of diagnosis [Long et al. 2011].
Compounds that inhibit BRAF have now been developed and are highly clinically effective. Two selective BRAF inhibitors have been tested in phase III studies against chemotherapy (DTIC), showing an improvement in OS. Vemurafenib (PLX4032, Zelboraf, Roche Pharma AG, Germany) is an oral, highly selective and competitive inhibitor of mutant BRAF. The BRAF in melanoma (BRIM-3) phase III study compared vemurafenib (960 mg twice daily orally) with DTIC (1000 mg/m2 every 3 weeks intravenously) as first-line treatment in patients with BRAF V600E-mutant metastatic melanoma [Chapman et al. 2011]. The RR was 48% for vemurafenib and 5% for DTIC with a median progression free survival (PFS) of 5.3 months in the vemurafenib arm compared with 1.6 months in the DTIC arm [hazard ratio (HR) 0.26; 95% confidence interval (CI) 0.20–0.33, p < 0.0001]. Recently, updated survival data were presented [Chapman et al. 2012]; after a median follow up of 12.5 months for patients treated with vemurafenib and 9.5 months for those randomized to DTIC, the OS was 13.6 months in the vemurafenib arm compared with 9.7 months in the DTIC arm (HR 0.70, 95% CI 0.57–0.87, p < 0.001). Around 25% of patients had crossed over to the vemurafenib arm at the time of the latest analysis, which could have underestimated the survival benefit with vemurafenib. The overall RR (ORR) with vemurafenib was higher (57%) compared with the initially published results in 2011 (48%) but not all patients were evaluable for response assessment at the time of initial publication. The complete response (CR) rate was also higher (5.6%) and about 51% of patients had a partial response (PR). The most frequent grade 2 and higher adverse events (AEs) associated with vemurafenib were arthralgia (21%), rash (18%), fatigue (13%), alopecia (8%), photosensitivity (12%), nausea (8%) and diarrhoea (5%). Cutaneous squamous-cell carcinoma (SCC), keratoacanthoma (KA) or both developed in 18% of patients, but in all cases, required simple excision only. Dose interruption and modification for toxicity were necessary in 38% of patients.
Dabrafenib (GSK2118436) is a reversible selective inhibitor of mutant BRAF. Results of a phase III study (BREAK-3) were recently published [Hauschild et al. 2012]. Patients with previously untreated, unresectable stage III or IV BRAF V600E-mutated melanoma were randomized (3:1) to dabrafenib (150 mg orally twice daily) or DTIC (1000 mg/m2 intravenously every 3 weeks). Dabrafenib demonstrated a significant improvement in PFS and RR over DTIC with an acceptable safety profile. The median PFS was 5.1 months for dabrafenib and 2.7 for DTIC (HR 0.30 95%, CI 0.18–0.53, p < 0.0001). The ORR was 53% for dabrafenib (PR 50% and CR 3%) and 19% for DTIC. Survival data were not presented. Common grade 2 and higher AEs associated with dabrafenib were hyperkeratosis (12%), headache (5%), pyrexia (11%), arthralgia (5%), and skin papillomas (24%). Interestingly, despite being a drug of the same class as vemurafenib, the incidence of SCC/KA (6%) and photosensitivity (3%) seems to be less with dabrafenib compared with vemurafenib. However, dabrafenib caused pyrexia in 28% (all grade) of patients, a side effect not commonly observed with vemurafenib.
Two nonselective BRAF inhibitors have been evaluated in clinical trials. Sorafenib is an oral small-molecule multikinase inhibitor targeting BRAF, CRAF, vascular endothelial growth factor receptor, platelet-derived growth factor receptor (PDGFR), c-KIT, Flt-3 and RET. It has been tested in phase II/III studies as monotherapy or in combination with chemotherapy (carboplatin/paclitaxel and DTIC) but failed to show an improvement in OS [Eisen et al. 2011; Hauschild et al. 2009; McDermott et al. 2008]. RAF-265 is another small-molecule multikinase inhibitor of mutant/wild-type BRAF and VEGFR. In a phase I study, RAF-265 was associated with a RR of 16% in BRAF-mutant melanoma and 13% in BRAF wild-type melanoma [Sharfman et al. 2011]. Due to dose-limiting haematological toxicity, a modified dosing schedule will be evaluated [ClinicalTrials.gov identifier: NCT00304525].
MEK is another therapeutic target downstream of BRAF. Trametinib (GSK1120212) is an oral small molecule, selective inhibitor of MEK1 and MEK2 [Gilmartin et al. 2011]. The results of the first phase III study with the MEK inhibitor trametinib were recently reported [Flaherty et al. 2012]. Patients with advanced BRAF-mutant melanoma were randomized in a 2:1 ratio to receive either trametinib (2 mg orally) once daily or chemotherapy with DTIC (1000 mg/m2) or paclitaxel (175 mg/m2) every 3 weeks. Median PFS was 4.8 months in the trametinib group compared with 1.5 months in the chemotherapy group (HR 0.45, 95% CI 0.33–0.63, p < 0.001). The rate of OS at 6 months was 81% in the trametinib group and 67% in the chemotherapy group despite crossover (HR 0.54, 95% CI 0.32–0.92, p = 0.01). The most common grade 2 and higher side effects associated with trametinib were rash (27%), fatigue (9%), diarrhoea (6%) and peripheral oedema (5%) but were not serious for the majority of patients. In contrast to BRAF inhibitor therapy, secondary cutaneous neoplasms have not been reported with trametinib. Ocular toxicity (mostly grade 1 or 2) occurred in about 9% of patients in the trametinib group. Blurred vision was the most frequent ocular adverse event (4%); grade 3 chorioretinopathy occurred in only one patient but was reversible. Fourteen patients (7%) had reduced ejection fraction in the trametinib group and treatment was permanently discontinued in two patients due to grade 3 cardiotoxicity attributable to trametinib.
Selumetinib (AZD6244) is a selective non-ATP competitive inhibitor of MEK1 and MEK2 [Yeh et al. 2007]. A phase II study comparing selumetinib with temozolomide, failed to improve the PFS in patients with unresectable stage III/IV melanoma, but the study population was not selected for BRAF/NRAS mutations [Kirkwood et al. 2012]. In the overall population, an objective response was observed in six (5.8%) patients receiving selumetinib and nine (9.4%) patients in the temozolomide group. Among patients with BRAF mutations, objective responses were similar between selumetinib and temozolomide groups (11.1% and 10.7%, respectively). The time to death (TTD) analysis carried out after 130 deaths suggested an improved TTD with temozolomide compared with selumetinib (369 days versus 284 days respectively) but that was not statistically significant (HR 1.351, 95% CI 0.95–1.93, two-sided p = 0.099). Possible reasons for lack of improved survival with selumetinib were explored but no definite explanation was found. Despite a nonsignificant improvement in TTD with temozolomide compared with selumetinib, there were no differences in other efficacy endpoints (PFS and ORR) between the two groups. A high crossover rate between the two groups (61% temozolomide arm versus 25% selumetinib arm) and any subsequent therapy could also have impacted the results.
NRAS
The frequency of NRAS mutations in melanoma is about 20% [Goel et al. 2006; Jakob et al. 2011]. RAS is a difficult druggable target but there is emerging evidence for the role of MEK inhibitors in treatment of NRAS-mutant melanoma. Ascierto and colleagues presented the efficacy and safety data of MEK162 in patients with locally advanced and unresectable or metastatic cutaneous melanoma harbouring BRAFV600 or NRAS mutations at the American Society of Clinical Oncology (ASCO) 2012 meeting [Ascierto et al. 2012]. This is the first targeted agent to demonstrate any clinical activity in patients with NRAS-mutant melanoma. Out of 13 patients with NRAS-mutant melanoma evaluable for efficacy, three had a PR (two confirmed and one unconfirmed) and four had SD.
c-KIT
c-KIT (CD117) is a receptor tyrosine kinase that is mutated in up to 10% of melanomas arising in acral, mucosal or chronically sun damaged skin [Curtin et al. 2006; Jiang et al. 2008; Minor et al. 2012]. Mucosal and acral melanomas represent less than 5% of all melanomas in white people compared with over 70% in Asian populations [Kong et al. 2011]. Many oral tyrosine kinase inhibitors (TKIs) are currently under evaluation in advanced melanoma, for example, imatinib, dasatinib, nilotinib and sunitinib, and are discussed below:
Imatinib was evaluated in three phase II trials that did not show any clinical efficacy [Ugurel et al. 2005; Wyman et al. 2006; Kim et al. 2008]. However, patients with c-KIT mutations were not enrolled in these studies. Two subsequent phase II studies requiring c-KIT mutations for enrolment revealed different results. Carvajal and colleagues reported an RR of 16% with a median time to progression of 12 weeks and a median OS of 46.3 weeks [Carvajal et al. 2011]. The second study enrolled 43 patients with c-KIT-mutated metastatic melanoma and demonstrated a RR of 23.3% and median OS of 14 months, with 51% alive at 1 year [Guo et al. 2011].
A phase II study evaluating the predictive role of KIT activation for response to treatment with sunitinib was recently reported [Minor et al. 2012]. Of the four evaluable patients with KIT mutations, one had a complete remission for 15 months and two had PR lasting 1 and 7 months. In contrast, only one PR was seen in six patients with KIT amplification or overexpression. Responses were seen within 6 weeks of starting sunitinib. Two patients received less than 2 weeks of therapy with sunitinib either due to toxicity or rapid disease progression. In an exploratory overall survival analysis, presence of a KIT mutation was found to be an adverse prognostic factor for patients with advanced melanoma with a poor OS (p < 0.0001). The median OS was 6 months for patients with KIT mutation compared with 13 months for those with BRAF metastatic melanoma, 16 months for those with NRAS mutation and 17 months for patients with wild-type metastatic melanoma. Lack of central pathology review was a weakness of this study.
A phase II study of dasatinib in a molecularly unselected population of patients with advanced melanoma demonstrated two partial responses lasting 24 weeks or more out of 36 patients evaluable for response [Kluger et al. 2011]. Nilotinib is a second-generation TKI with a similar potency as imatinib against c-KIT. It is currently being evaluated in multiple phase II studies [ClinicalTrials.gov identifiers: NCT01395121, NCT01099514, NCT01168050, NCT00788775].
Results from the above-mentioned studies indicate that responses to treatment with different TKIs are highly variable and patients with specific KIT mutations, particularly exon 11 and exon 13, are more likely to benefit from treatment. Therefore, not all patients with KIT mutations (for example exon 9, 17 and 18) respond to treatment with TKIs, highlighting the heterogeneity of these mutations. This is in contrast to BRAF mutations which are more homogeneous with more than two-thirds being V600E type [Long et al. 2011].
GNAQ/GNA11
Uveal melanomas lack mutations in c-KIT, NRAS and BRAF. However, they harbour unique activating mutations in the G-protein GNAQ/GNA11. These mutations lead to constitutive activation of the MAP kinase pathway [Van Raamsdonk et al. 2009]. About 45 % of uveal melanomas exhibit GNAQ mutations and up to 60 % exhibit GNA11 mutations and these are mutually exclusive [Onken et al. 2008; Bauer et al. 2009; Van Raamsdonk et al. 2009, 2010]. Currently there are no therapeutic agents targeting these mutations.
Protein kinase C (PKC) is a component of the GNAQ to ERK pathway [Koivunen et al. 2006]. Therefore inhibition of PKC might regulate GNAQ mutation mediated MAP kinase activation. In a preclinical study, uveal melanoma cells carrying wild-type or mutant GNAQ were treated with the PKC inhibitor enzastaurin [Wu et al. 2012]. Enzastaurin demonstrated activity against uveal melanoma cells carrying GNAQ mutations through inhibition of the PKC/ERK pathway and induction of G1 arrest and apoptosis. A phase I study (AEB071) is currently evaluating the role of another PKC inhibitor (sotrastaurin) in the treatment of advanced uveal melanoma [ClinicalTrials.gov identifier: NCT01430416].
Monosomy 3 is one of the most powerful independent predictors of metastasis and poor outcome in uveal melanoma [Prescher et al. 1996]. Harbour and colleagues identified inactivating somatic mutations in the gene encoding BRCA1-associated protein 1 (BAP1) on chromosome 3p in 26 of 31 (84%) metastatic uveal melanoma tumours [Harbour et al. 2010]. Recent evidence suggests that germline BAP1 inactivating mutations are associated with both metastatic and hereditary uveal melanoma [Njauw et al. 2012].
In summary, aberrant activation of the MAP kinase pathway has been demonstrated in melanoma. Oncogenic mutations along the MAP kinase pathway have been discovered in varying frequencies in different melanoma subtypes. Drugs specifically targeting these mutations (BRAF, MEK/c-KIT) have been evaluated in phase II/III studies with encouraging results. Unfortunately most patients eventually become resistant to these agents and develop progressive disease.
Mechanisms of resistance
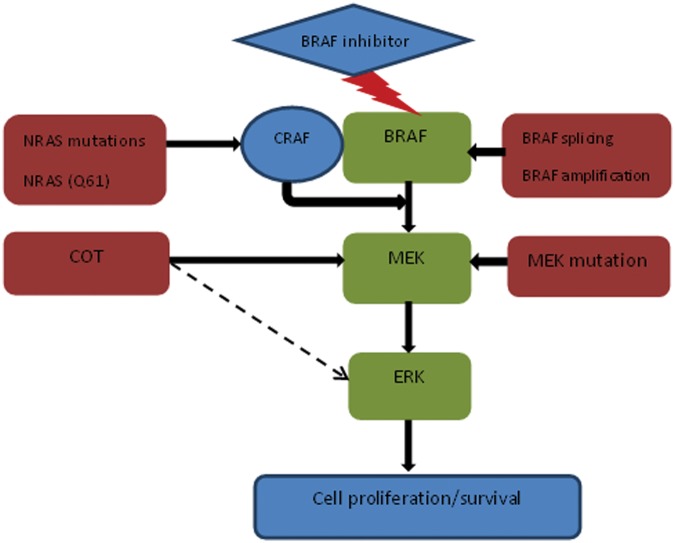
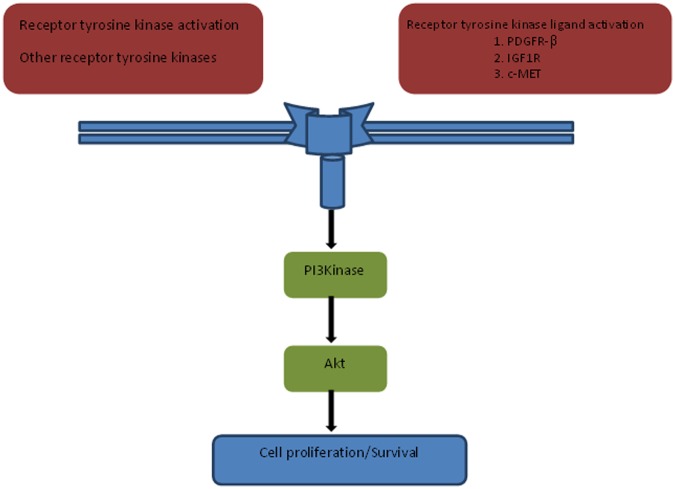
Clinical resistance to BRAF inhibitors is almost universal and can be primary or secondary. Primary resistance occurs in less than 15% of patients treated with BRAF inhibitors [Chapman et al. 2011; Flaherty et al. 2010; Kefford et al. 2010; Sosman et al. 2012]. However, secondary resistance, when the disease progresses after an initial response, occurs in the majority of patients [Flaherty et al. 2010]. Preclinical and clinical studies suggest multiple mechanisms of resistance that may occur upstream or downstream of BRAF along the MAP kinase pathway, bypass signalling pathways or through abnormalities in CDK4/cyclin D1 [Corcoran et al. 2011] (Figures 1 and 2). Data indicate that these resistance pathways are highly complex and are capable of both vertical and horizontal crosstalk.
Figure 1.
MEK-dependent resistance.
Figure 2.
MEK-independent resistance.
PDGFRβ, platelet-derived growth factor receptor β; PI3Kinase, phosphoinositol 3 kinase.
Upstream mutations in NRAS (Q61K) can cause secondary resistance to BRAF inhibitor therapy through increased signalling via other RAF isoforms [Nazarian et al. 2010]. This leads to phosphorylation of both MEK and ERK despite adequate BRAF inhibition. Alternatively, melanomas can escape BRAF inhibition via receptor tyrosine kinase mediated activation of alternate pathways such as PDGFRβ [Nazarian et al. 2010]. PDGFRβ overexpression can cause tumour cells to become less dependent on ERK signalling and develop resistance to the antiproliferative effect of BRAF inhibitor therapy.
Preclinical data appeared to indicate that gatekeeper mutations conferred resistance to BRAF inhibitor (PLX4032) [Whittaker et al. 2010]. However, secondary gate keeper mutations were not identified in any of 16 tumour biopsies from 12 patients with clinically acquired resistance [Nazarian et al. 2010]. This is in contrast to resistance to inhibitors of epidermal growth factor receptor (non-small cell lung cancer), KIT (gastrointestinal stromal tumour) and Abl (chronic myeloid leukaemia), where secondary gatekeeper mutations appear to play a dominant role [Solit and Rosen, 2011].
Johannessen and colleagues identified COT/MAP3K8 as a MAP kinase pathway agonist that drives resistance to RAF inhibition in BRAF (V600E) mutant melanoma cell lines [Johannessen et al. 2010]. COT activates ERK through MEK phosphorylation in a RAF-independent manner. In addition, COT is believed to play a role in de novo resistance in BRAF-V600E-cultured cell lines and acquired resistance in melanoma cells and biopsies obtained from patients at progression after treatment with BRAF/MEK inhibitors [Johannessen et al. 2010]. There is also evidence to suggest that COT may directly activate ERK, bypassing MEK, explaining lack of sensitivity to MEK inhibitor therapy [Solit and Rosen, 2011].
A point mutation in MEK (MEK1), downstream of BRAF, was identified in a patient with resistant melanoma and conferred resistance against PLX4032 [Emery et al. 2009; Wagle et al. 2011]. However, different findings were recently reported by Shi and colleagues, who showed that MEK1-mutant expression in BRAF melanoma cell lines did not alter ERK1/2 levels or sensitivity to BRAF/MEK inhibitors [Shi et al. 2012]. MEK1/2 exon 3 in melanoma tumours was sequenced at baseline and upon disease progression; concurrent somatic BRAF/MEK1-activating mutations were found in 5(16%) out of 31 baseline BRAF (V600K/E) mutant melanomas, and no MEK1 mutation was identified in biopsy specimens taken from patients with melanoma at the time of disease progression, except when it was already identified at baseline.
Poulikakos and colleagues explained the first resistance mechanism that involves a structural change in BRAF [Poulikakos et al. 2011]. They identified a BRAF splicing variant [p61 BRAF (V600E)] in the tumours of 6 of 19 patients. This variant lacks the RAS-binding domain and exhibits enhanced dimerization of RAF despite low levels of RAS activation compared with full length BRAF (V600E). Secondary resistance to BRAF inhibitors mediated by their variants is due to insensitivity of the enzyme to these agents. The generation of splicing variants is believed to be due to a mutation or epigenetic phenomenon. Another interesting finding noted by the authors was that these tumours retained sensitivity to downstream inhibition of MEK. Hence a combination of BRAF and MEK inhibitors could prevent or delay the development of resistance by this mechanism. In an another recent study, gain in BRAF (V600E) copy number (BRAF amplification) was described as a mechanism of acquired resistance in 4 out of 20 (20%) patients treated with a BRAF inhibitor [Shi et al. 2012].
There are emerging data to indicate that acquired resistance to BRAF inhibitors is often associated with reactivation of the MAP kinase pathway. This renders BRAF-inhibitor-resistant melanoma cells susceptible to the combination of BRAF and MEK inhibition [Alcala and Flaherty, 2012]. Preclinical data suggest improved efficacy and a lower frequency of skin lesions with the combination of BRAF and MEK inhibitors compared with treatment with BRAF inhibitors alone. However, a recent study by Gowrishankar and colleagues evaluating combined BRAF/MEK-targeted therapy in resistant melanoma cell clones indicates that the resistance mechanism are heterogeneous and combination BRAF/MEK therapy might not prevent the emergence of acquired resistance [Gowrishankar et al. 2012]. Safety and efficacy results of dabrafenib combined with trametinib from a phase I/II study in patients with BRAF inhibitor naïve metastatic melanoma (n = 77) were recently presented at the ASCO 2012 meeting [Weber et al. 2012]. Four escalating dose levels of dabrafenib (twice daily)/trametinib (once daily) [75 mg/1 mg, 150 mg/1 mg, 150 mg/1.5 mg, 150 mg/2 mg] were evaluated. The ORR was 56% (95% CI 44.1%−67.2%), including 4 CR, 39 PR, 29 stable disease (SD) and 3 progressive disease. The overall PFS was 7.4 months (95% CI 5.5–9.2). The most common grade 3/4 AEs were pyrexia (5%), fatigue (5%) and dehydration (5%). Cutaneous SCC occurred in only 2% of patients. Two phase III trials are currently comparing a combination of dabrafenib and trametinib with dabrafenib [ClinicalTrials.gov identifier: NCT01584648] or vemurafenib [ClinicalTrials.gov identifier: NCT01597908] monotherapy.
Activated PI3K/AKT/mTOR pathway mediated bypass signalling can occur due to insulin like growth factor 1 upregulation [Gopal et al. 2010; Shao et al. 2010; Villanueva et al. 2010] or phosphatase and tensin homolog loss leading to disinhibition of this cascade [Paraiso et al. 2011].
In summary, the resistance mechanisms to BRAF-targeted therapy are multiple and complex, occurring at different levels along the MAP kinase pathway. The evidence regarding different mechanisms of resistance is predominantly based on preclinical studies. Further research into identifying and prospectively validating these resistance mechanisms through longitudinal tumour biopsies taken from patients with melanoma is extremely important to help guide the development of further targeted therapies. Despite the identification of oncogenic pathways involved in the pathogenesis of malignant melanoma and the development of therapeutic agents that target them, there remain a number of challenges. These include an improved understanding of the resistance mechanisms, evaluation of targeted agents in combination and in the adjuvant setting, minimizing drug toxicities and research into new treatment options for patients who lack these mutations.
Immunotherapy in advanced melanoma
Melanoma is believed to be an immunogenic tumour. This is supported by the identification of tumour-infiltrating lymphocytes in resected melanoma specimens [Hadrup et al. 2009; Cipponi et al. 2011], melanoma antigen-specific T cells in peripheral blood from patients with melanoma [Lee et al. 1999], downregulation of major histocompatibility complex class I expression [Ahmad et al. 2004] and release of cytokines such as transforming growth factor β [Krasagakis et al. 1998]. Profound clinical responses have been demonstrated with immune-modulating agents such as IL-2 and adoptive T-cell transfer in a small proportion of patients with metastatic melanoma [Rosenberg et al. 1994, 2011; Atkins et al. 1999, 2000; Hunder et al. 2008]. Spontaneous regression of metastatic disease has also been described in the literature, albeit rarely, and is speculated to be immune mediated [Kalialis et al. 2009]. There is renewed interest in immunotherapy with the discovery of immune checkpoint inhibitors and because of the historical observation that durable complete responses could be achieved with immunotherapy, as opposed to the short- to medium-term disease control achieved with targeted therapies.
CTLA4 is a member of the CD28:B7 immunoglobulin superfamily. It is normally expressed at low levels on the surface of effector and regulatory T cells [Alegre et al. 1996]. T cells autoregulate their activation through expression of CTLA4, which functions as a negative costimulatory molecule for the T cell [Harris et al. 1999; Melero et al. 2007]. Two anti-CTLA4 antibodies have been tested in phase III clinical trials: ipilimumab and tremelimumab.
Ipilimumab
Ipilimumab is a fully human immunoglobulin G1 monoclonal antibody that targets CTLA4 and thereby leads to T-cell hyper responsiveness with disinhibition of antitumour immunity. Ipilimumab was the first drug in the management of metastatic melanoma to demonstrate a survival benefit. It has been tested both in treatment naïve and in pretreated patients. Hodi and colleagues conducted a randomized phase III study of ipilimumab in previously treated patients with unresectable stage III or IV melanoma and demonstrated an improved OS compared with glycoprotein 100 peptide vaccine (gp100) [Hodi et al. 2010]. The median OS was 10 months among patients receiving ipilimumab plus gp100 compared with 6.4 months among patients receiving gp100 alone (HR for death 0.68, p < 0.001). The median OS with ipilimumab alone was 10.1 months (HR for death in comparison with gp100 alone 0.66, = 0.003). There was no OS difference between the ipilimumab groups (HR with ipilimumab plus gp100 1.04, p = 0.76). The OS rates after 2 years for ipilimumab alone, ipilimumab with gp100 and gp100 alone were 23.5%, 21.6% and 13.7% respectively. There was no difference in median PFS in all groups at the time of first assessment of progression at 12 weeks but separation of the PFS curves was seen after that, with a 36% reduction in risk of progression with ipilimumab alone compared with gp100 alone (HR 0.64, p < 0.001). The best ORR was observed in the ipilimumab alone arm (10.9%). Grade 3 or 4 immune-related AEs (irAEs) occurred in 10–15% of patients treated with ipilimumab compared with only 3% in the gp100 alone group. There were 14 deaths related to the study drugs (2.1%), seven of which were attributable to irAEs. A criticism of this study was lack of standard treatment in the control arm given that gp100 monotherapy does not seem to have any meaningful activity in metastatic melanoma.
Robert and colleagues conducted a second phase III trial of ipilimumab plus DTIC in previously untreated patients with metastatic melanoma with standard chemotherapy (DTIC alone) as the control arm [Robert et al. 2011]. The median OS was significantly longer in the group receiving ipilimumab/DTIC than in the group receiving DTIC/placebo (11.2 months versus 9.1 months respectively, HR for death 0.72, p < 0.001). The survival rates were higher in the ipilimumab/DTIC arm at 1 year (47.3% versus 36.3%), 2 years (28.5% versus 17.9%) and 3 years (20.8% versus 12.2%) compared with DTIC/placebo. Grade 3 or 4 AEs occurred in 56.3% of patients treated with ipilimumab/DTIC compared with 27.5% treated with DTIC/placebo (p < 0.001). There was no treatment-related death in this study. In both the phase III studies with ipilimumab, the RR (CR/PR) was only 10–15%.
Monoclonal antibodies targeting CTLA4 can be associated with a variety of novel irAEs. They are not believed to be true autoimmune diseases as they typically resolve with cessation of ipilimumab and appropriate immunosuppressive therapy. The following major irAEs were reported in the second-line study: rash (43.5% any grade, 1.5% grade 3/4), diarrhoea/colitis (29.0% any grade, 7.6% grade 3/4), hepatitis (3.8% any grade, 0% grade 3/4), and the endocrine system (hypophysitis, thyroiditis, adrenal insufficiency; 7.6% any grade, 3.8% grade 3/4) [Robert et al. 2011]. These irAEs were slightly different when ipilimumab was given in conjunction with DTIC. There was a higher incidence of hepatitis (most likely related to the addition of DTIC) (31.6% grade 3/4) but a lower rate of colitis (4.9% grade 3/4).
Downey and colleagues investigated various prognostic factors related to clinical response in patients with metastatic melanoma treated by anti-CTLA4 monoclonal antibodies [Downey et al. 2007]. They found that better clinical responses were observed in patients with grade 3/4 irAEs. Development of any irAEs was significantly associated with likelihood of clinical response (p = 0.0004). All patients with a complete response had more severe irAEs. Attia and colleagues also reported that patients experiencing grade 3/4 irAEs with ipilimumab therapy had a significantly higher rate of tumour regression than those without any irAEs (36% versus 5% of patients) [Attia et al. 2005].
Corticosteroids are commonly used in the management of irAEs secondary to ipilimumab. However, concerns have been raised about the reduced efficacy of ipilimumab with the use of immunosuppressive corticosteroids. However, Downey and colleagues found that using corticosteroids to treat irAEs did not affect the duration of tumour response [Downey et al. 2007]. The efficacy of different doses of ipilimumab has also been evaluated. The initial phase III study used ipilimumab 3 mg/kg given every 3 weeks compared with a higher dose of ipilimumab 10 mg/kg used in combination with DTIC. Three different dosing schedules of ipilimumab were compared in a phase II trial [Wolchok et al. 2010]. A higher dose and an induction regimen consisting of 10 mg/kg every 3 weeks for 3 months along with a maintenance treatment of 10 mg/kg every 12 weeks starting at week 24 was compared with doses of 3 mg/kg and 0.3 mg/kg. The best ORR was 11.1% (95% CI 4.9–20.7) for 10 mg/kg, 4.2% (0.9–11.7) for 3 mg/kg, and 0% (0–4.9) for 0.3 mg/kg (p = 0.0015; trend test). A dose-dependent increase in irAEs of any grade was also seen with increasing dose of ipilimumab. A phase III study is currently underway to determine the best dosing schedule for ipilimumab [ClinicalTrials.gov identifier: NCT01515189].
Ipilimumab has been combined with other immune-modulating and targeted agents in attempts to improve its efficacy. A combination regimen of ipilimumab and high-dose IL-2 was evaluated in 36 patients [Prieto et al. 2010]. Six patients (17%) achieved a long-lasting CR (all over 5 years), and none of the patients relapsed. Moreover, there was no increase in side effects compared with high-dose IL-2 alone, which in itself is considered toxic and is usually offered only to young patients with good performance status and no major comorbidities.
A combination of ipilimumab and temozolomide was tested in a phase II trial and resulted in an overall disease control rate (CR/PR and SD) of 67%, higher than that observed in other studies of either agent alone [Patel et al. 2011]. A phase I study testing the combination of ipilimumab and bevacizumab resulted in a RR of 36% and overall disease control rate of 67% [Hodi et al. 2011]. However, the incidence of irAEs also increased with this combination. While these phase I/II studies possibly indicate improved efficacy of ipilimumab in combination with other agents, further randomized data will need to address the efficacy and safety with combination therapy.
Tremelimumab
Tremelimumab is a fully human IgG2 monoclonal antibody that also targets CTLA4. The half life of tremelimumab is longer than ipilimumab, so its dosing schedule is less frequent. The recommended phase II dose for tremelimumab is 15 mg/kg every 3 months based on the results from a phase I/II study [Camacho et al. 2009]. Despite durable responses in a minor subset of patients with metastatic melanoma in phase I/II trials, further development of tremelimumab has been halted as it failed to demonstrate an improvement in OS in a phase III study as a first-line treatment in patients with metastatic melanoma when compared with chemotherapy (DTIC or temozolomide) [Ribas et al. 2008]. However, these results may have been negatively influenced by the relatively short follow up in this study and by the unintentional crossover of patients in the control arm who may have had subsequent access to ipilimumab in other clinical trials. Updated interim results in 2010 demonstrated a nonsignificant trend in OS in favour of the tremelimumab arm (p = 0.14) [Marshall et al. 2010].
The clinical response patterns to immunotherapy differ to those of cytotoxic chemotherapy. In some cases after an initial apparent increase in tumour burden or the appearance of new lesions, a late response can be observed on the subsequent scans. This may be due to initial inflammatory infiltrate causing an apparent increase in the tumour volume. Response Evaluation Criteria In Solid Tumours or World Health Organization criteria designed to detect early effects of chemotherapy may therefore not be the ideal methods to assess response to immunotherapeutic agents. Immune-related response criteria (irRC) have been proposed to assess the response to immunotherapy with ipilimumab [Wolchok et al. 2009]. Four distinct response patterns can be seen and all have been associated with favourable survival:
Response in baseline target lesions without new lesions.
Response in baseline target lesions in presence of new lesions.
Response after an initial increase in the tumour burden.
Durable SD followed by a slow and steady decline in tumour burden.
Importantly, the irRC factors take into account the patient’s total tumour burden and require confirmation of suspected disease progression with a subsequent scan at least 4 weeks apart.
Novel agents
Programmed death-1 (PD-1) receptor is another negative immune checkpoint molecule and a promising therapeutic target currently under evaluation. PD-1 receptor is expressed on T cells after activation, and its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), are expressed on tumour cells, antigen-presenting cells, and other cells in the tumour microenvironment [Freeman et al. 2000; Hirano et al. 2005]. The interaction of PD-1 with its ligand is thought to induce T-cell apoptosis [Dong et al. 2002].
Anti-PD1 (BMS-936558) has shown encouraging results in phase I studies. In a recently reported phase I study, the RR was 28% among patients with melanoma (26 of 94 patients) [Topalian et al. 2012]. These responses were durable with 13 of 26 responses lasting 1 year or more. In another phase I study with anti-PD L1 antibody, response was observed in 9 of 52 patients with melanoma [Brahmer et al. 2012]. Responses lasted for 1 year or more in five of nine patients with melanoma who responded to treatment. Grade 3 or 4 toxic effects related to treatment occurred in 9% of patients. These agents are also associated with novel irAEs but their severity appears to be less compared with monoclonal antibodies targeting CTLA4.
Future research will focus on improving the risk–benefit of immunotherapy by understanding biomarkers predictive of response and irAEs, thereby enabling improved patient selection. Various combination therapies targeting different immune checkpoints and oncogenes are being evaluated in an effort to improve the efficacy. In addition, ipilimumab is being evaluated in high-risk early stage disease and in combination with radiotherapy.
Conclusion
Research into oncogenic pathways and immune checkpoints has greatly increased our understanding of the mechanisms involved in the pathogenesis of malignant melanoma, and importantly, has led to new therapies for advanced melanoma. Two novel therapies which target either signal transduction pathways or immune check points have, for the first time, led to an improvement in OS and provide significant palliative benefit for patients with baseline target lesions. Besides this, a wider spectrum of patients with melanoma, including those with brain metastases (with both ipilimumab and BRAF inhibitors) and poor performance status (with BRAF inhibitors only), benefit from these agents compared with conventional chemotherapy. Newer agents in each class are expected to produce further improvements in clinical outcomes in this challenging disease. Future research will focus on identifying the mechanisms of resistance to such treatments and how these might best be overcome, including the rational use of drug combinations, and the identification of biomarkers predictive of treatment response. Ultimately, it is hoped that the ability to select patients for treatment based on molecular targets, in both the advanced and adjuvant disease settings, might lead to long-term disease control or even cure of metastatic melanoma in a significant proportion of patients.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Muhammad Khattak, Rosalie Fisher and Samra Turajlic have no conflict of interest to declare. James Larkin received honoraria from Novartis, BMS and GSK.
Contributor Information
Muhammad Khattak, Royal Marsden NHS Trust, London, UK.
Rosalie Fisher, Royal Marsden NHS Trust, London, UK.
Samra Turajlic, Institute of Cancer Research, London, UK.
James Larkin, Medical Oncologist, Department of Medical Oncology, Royal Marsden NHS Trust, Fulham Road, London SW3 6JJ, UK.
References
- Ahmad M., Rees R.C., Ali S.A. (2004) Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother 53: 844–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala A., Flaherty K. (2012) BRAF inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin Cancer Res 18: 33–39 [DOI] [PubMed] [Google Scholar]
- Alegre M.L., Noel P.J., Eisfelder B.J., Chuang E., Clark M.R., Reiner S.L., et al. (1996) Regulation of surface and intracellular expression of Ctla4 on Mouse T Cells. J Immunol 157: 4762–4770 [PubMed] [Google Scholar]
- Ascierto P., Berking C., Agarwala S., Schadendorf D., Van Herpen C., Queirolo P., et al. (2012) Efficacy and safety of oral MEK162 in patients with locally advanced and unresectable or metastatic cutaneous melanoma harboring BRAFV600 or NRAS mutations. J Clin Oncol 30: abstract 8511. [Google Scholar]
- Atkins M., Kunkel L., Sznol M., Rosenberg S. (2000) High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 6(Suppl. 1): S11–S14 [PubMed] [Google Scholar]
- Atkins M.B., Lotze M.T., Dutcher J.P., Fisher R.I., Weiss G., Margolin K., et al. (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17: 2105–2116 [DOI] [PubMed] [Google Scholar]
- Attia P., Phan G., Maker A., Robinson M., Quezado M., Yang J., et al. (2005) Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 23: 6043–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C., Gershenwald J., Soong S., Thompson J., Atkins M., Byrd D., et al. (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27: 6199–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J., Kilic E., Vaarwater J., Bastian B.C., Garbe C., De Klein A. (2009) Oncogenic Gnaq mutations are not correlated with disease-free survival in Uveal Melanoma. Br J Cancer 101: 813–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Tykodi S., Chow L., Hwu W., Topalian S., Hwu P., et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L., Antonia S., Sosman J., Kirkwood J., Gajewski T., Redman B., et al. (2009) Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol 27: 1075–1081 [DOI] [PubMed] [Google Scholar]
- Carvajal R., Antonescu C., Wolchok J., Chapman P., Roman R., Teitcher J., et al. (2011) KIT as a therapeutic target in metastatic melanoma. JAMA 305: 2327–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P.B., Einhorn L.H., Meyers M.L., Saxman S., Destro A.N., Panageas K.S., et al. (1999) Phase iii multicenter randomized trial of the Dartmouth Regimen Versus Dacarbazine in patients with metastatic Melanoma. J Clin Oncol 17: 2745–2751 [DOI] [PubMed] [Google Scholar]
- Chapman P., Hauschild A., Robert C., Haanen J., Ascierto P., Larkin J., et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P., Hauschild A., Robert C., Larkin J., Haanen J., Ribas A., et al. (2012) Updated overall survival (OS) results for BRIM-3, a phase III randomized, open-label, multicenter trial comparing BRAF inhibitor vemurafenib (vem) with dacarbazine (DTIC) in previously untreated patients with BRAFV600E-mutated melanoma. J Clin Oncol 30: abstract 8502. [Google Scholar]
- Cheng S., Chu P., Hinshaw M., Smith K., Maize J., Sferruzza A. (2011) Frequency of mutations associated with targeted therapy in malignant melanoma patients J Clin Oncol 29: abstract 8597. [Google Scholar]
- Cipponi A., Wieers G., Van Baren N., Coulie P.G. (2011) Tumor-infiltrating lymphocytes: apparently good for Melanoma patients. But why? Cancer Immunol Immunother 60: 1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran R., Settleman J., Engelman J. (2011) Potential therapeutic strategies to overcome acquired resistance to BRAF or MEK inhibitors in BRAF mutant cancers. Oncotarget 2: 336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin J.A., Busam K., Pinkel D., Bastian B.C. (2006) Somatic activation of kit in distinct subtypes of Melanoma. J Clin Oncol 24: 4340–4346 [DOI] [PubMed] [Google Scholar]
- Curtin J., Fridlyand J., Kageshita T., Patel H., Busam K., Kutzner H., et al. (2005) Distinct sets of genetic alterations in melanoma. N Engl J Med 353: 2135–2147 [DOI] [PubMed] [Google Scholar]
- Davies H., Bignell G., Cox C., Stephens P., Edkins S., Clegg S., et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417: 949–954 [DOI] [PubMed] [Google Scholar]
- Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., et al. (2002) Tumor-associated B7-H1 promotes T-Cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800 [DOI] [PubMed] [Google Scholar]
- Downey S., Klapper J., Smith F., Yang J., Sherry R., Royal R., et al. (2007) Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 13: 6681–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont A., Kirkwood J. (2004) Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer 40: 1825–1836 [DOI] [PubMed] [Google Scholar]
- Eggermont A., Schadendorf D. (2009) Melanoma and immunotherapy. Hematol Oncol Clin North Am 23: 547–564 [DOI] [PubMed] [Google Scholar]
- Eisen T., Marais R., Affolter A., Lorigan P., Robert C., Corrie P., et al. (2011) Sorafenib and dacarbazine as first-line therapy for advanced melanoma: phase I and open-label phase II studies. Br J Cancer 105: 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery C.M., Vijayendran K.G., Zipser M.C., Sawyer A.M., Niu L., Kim J.J., et al. (2009) Mek1 mutations confer resistance to Mek and B-Raf inhibition. Proc Natl Acad Sci U S A 106: 20411–20416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K., Puzanov I., Kim K., Ribas A., McArthur G., Sosman J., et al. (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363: 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K., Robert C., Hersey P., Nathan P., Garbe C., Milhem M., et al. (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367: 107–114 [DOI] [PubMed] [Google Scholar]
- Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., et al. (2000) Engagement of the Pd-1 immunoinhibitory receptor by a Novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192: 1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett M., Marais R. (2004) Guilty as charged: B-RAF is a human oncogene. Cancer Cell 6: 313–319 [DOI] [PubMed] [Google Scholar]
- Gilmartin A., Bleam M., Groy A., Moss K., Minthorn E., Kulkarni S., et al. (2011) GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res 17: 989–1000 [DOI] [PubMed] [Google Scholar]
- Goel V., Lazar A., Warneke C., Redston M., Haluska F. (2006) Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol 126: 154–160 [DOI] [PubMed] [Google Scholar]
- Gopal Y.N., Deng W., Woodman S.E., Komurov K., Ram P., Smith P.D., et al. (2010) Basal and treatment-induced activation of Akt mediates resistance to cell death by Azd6244 (Arry-142886) in Braf-Mutant human Cutaneous Melanoma cells. Cancer Res 70: 8736–8747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar K., Snoyman S., Pupo G., Becker T., Kefford R., Rizos H. (2012) Acquired resistance to BRAF inhibition can confer cross-resistance to combined BRAF/MEK inhibition. J Invest Dermatol 132: 1850–1859 [DOI] [PubMed] [Google Scholar]
- Guo J., Si L., Kong Y., Flaherty K., Xu X., Zhu Y., et al. (2011) Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 29: 2904–2909 [DOI] [PubMed] [Google Scholar]
- Hadrup S.R., Bakker A.H., Shu C.J., Andersen R.S., Van Veluw J., Hombrink P., et al. (2009) Parallel detection of antigen-specific T-Cell responses by multidimensional encoding of Mhc multimers. Nat Methods 6: 520–526 [DOI] [PubMed] [Google Scholar]
- Harbour J., Onken M., Roberson E., Duan S., Cao L., Worley L., et al. (2010) Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330: 1410–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N.L., Ronchese F. (1999) The role of B7 costimulation in T-Cell immunity. Immunol Cell Biol 77: 304–311 [DOI] [PubMed] [Google Scholar]
- Hauschild A., Agarwala S., Trefzer U., Hogg D., Robert C., Hersey P., et al. (2009) Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 27: 2823–2830 [DOI] [PubMed] [Google Scholar]
- Hauschild A., Grob J., Demidov L., Jouary T., Gutzmer R., Millward M., et al. (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380: 358–365 [DOI] [PubMed] [Google Scholar]
- Hirano F., Kaneko K., Tamura H., Dong H., Wang S., Ichikawa M., et al. (2005) Blockade of B7-H1 and Pd-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 65: 1089–1096 [PubMed] [Google Scholar]
- Hocker T., Singh M., Tsao H. (2008) Melanoma genetics and therapeutic approaches in the 21st century: moving from the benchside to the bedside. J Invest Dermatol 128: 2575–2595 [DOI] [PubMed] [Google Scholar]
- Hodi F., Friedlander M., Atkins M., McDermott D., Lawrence D., Ibrahim N., et al. (2011) A phase I trial of ipilimumab plus bevacizumab in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 29: abstract 8511. [Google Scholar]
- Hodi F., O’Day S., McDermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunder N.N., Wallen H., Cao J., Hendricks D.W., Reilly J.Z., Rodmyre R., et al. (2008) Treatment of metastatic Melanoma with autologous Cd4+ T Cells against Ny-Eso-1. N Engl J Med 358: 2698–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob J., Bassett R., Ng C., Lazar A., Alvarado G., Rohlfs M. (2011) Clinical characteristics and outcomes associated with BRAF and NRAS mutations in metastatic melanoma. J Clin Oncol 29: abstract 8500. [Google Scholar]
- Jiang X., Zhou J., Yuen N.K., Corless C.L., Heinrich M.C., Fletcher J.A., et al. (2008) Imatinib targeting of Kit-Mutant Oncoprotein in Melanoma. Clin Cancer Res 14: 7726–7732 [DOI] [PubMed] [Google Scholar]
- Johannessen C.M., Boehm J.S., Kim S.Y., Thomas S.R., Wardwell L., Johnson L.A., et al. (2010) Cot drives resistance to Raf inhibition through map kinase pathway reactivation. Nature 468: 968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalialis L., Drzewiecki K., Klyver H. (2009) Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res 19: 275–282 [DOI] [PubMed] [Google Scholar]
- Kefford R., Arkenau H., Brown M., Millward M., Infante J., Long G., et al. (2010) Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol 28(15 Suppl.): abstract 8503. [Google Scholar]
- Kim K.B., Eton O., Davis D.W., Frazier M.L., Mcconkey D.J., Diwan A.H., et al. (2008) Phase ii trial of Imatinib Mesylate in patients with metastatic Melanoma. Br J Cancer 99: 734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood J., Bastholt L., Robert C., Sosman J., Larkin J., Hersey P., et al. (2012) Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res 18: 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger H.M., Dudek A.Z., Mccann C., Ritacco J., Southard N., Jilaveanu L.B., et al. (2011) A phase 2 trial of Dasatinib in advanced Melanoma. Cancer 117: 2202–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen J., Aaltonen V., Peltonen J. (2006) Protein kinase C (PKC) family in cancer progression. Cancer Lett 235: 1–10 [DOI] [PubMed] [Google Scholar]
- Kong Y., Si L., Zhu Y., Xu X., Corless C., Flaherty K., et al. (2011) Large-scale analysis of KIT aberrations in Chinese patients with melanoma. Clin Cancer Res 17: 1684–1691 [DOI] [PubMed] [Google Scholar]
- Krasagakis K., Tholke D., Farthmann B., Eberle J., Mansmann U., Orfanos C. (1998) Elevated plasma levels of transforming growth factor (TGF)-beta1 and TGF-beta2 in patients with disseminated malignant melanoma. Br J Cancer 77: 1492–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Choi J., Kim Y. (2011) Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol 164: 776–784 [DOI] [PubMed] [Google Scholar]
- Lee P.P., Yee C., Savage P.A., Fong L., Brockstedt D., Weber J.S., et al. (1999) Characterization of circulating T Cells specific for tumor-associated antigens in Melanoma patients. Nat Med 5: 677–685 [DOI] [PubMed] [Google Scholar]
- Long G., Menzies A., Nagrial A., Haydu L., Hamilton A., Mann G., et al. (2011) Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 29: 1239–1246 [DOI] [PubMed] [Google Scholar]
- Maldonado J., Fridlyand J., Patel H., Jain A., Busam K., Kageshita T., et al. (2003) Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst 95: 1878–1890 [DOI] [PubMed] [Google Scholar]
- Marshall M., Ribas A., Huang B. (2010) Evaluation of baseline serum C-reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first-line melanoma. J Clin Oncol 28: abstract 2609. [Google Scholar]
- McDermott D., Sosman J., Gonzalez R., Hodi F., Linette G., Richards J., et al. (2008) Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol 26: 2178–2185 [DOI] [PubMed] [Google Scholar]
- Melero I., Hervas-Stubbs S., Glennie M., Pardoll D.M., Chen L. (2007) Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 7: 95–106 [DOI] [PubMed] [Google Scholar]
- Minor D., Kashani-Sabet M., Garrido M., O’Day S., Hamid O., Bastian B. (2012) Sunitinib therapy for melanoma patients with KIT mutations. Clin Cancer Res 18: 1457–1463 [DOI] [PubMed] [Google Scholar]
- Nazarian R., Shi H., Wang Q., Kong X., Koya R., Lee H., et al. (2010) Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468: 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njauw C., Kim I., Piris A., Gabree M., Taylor M., Lane A., et al. (2012) Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PLoS One 7: e35295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken M.D., Worley L.A., Long M.D., Duan S., Council M.L., Bowcock A.M., et al. (2008) Oncogenic mutations in Gnaq occur early in Uveal Melanoma. Invest Ophthalmol Vis Sci 49: 5230-5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso K.H., Fedorenko I.V., Cantini L.P., Munko A.C., Hall M., Sondak V.K., et al. (2010) Recovery of Phospho-Erk activity allows Melanoma cells to escape from Braf inhibitor therapy. Br J Cancer 102: 1724-1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Bedikian A., Papadopoulos N., Hwu W., Kim K., Homsi J., et al. (2011) Ipilimumab plus temozolomide in metastatic melanoma. J Clin Oncol 29: abstract 8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos P.I., Persaud Y., Janakiraman M., Kong X., Ng C., Moriceau G., et al. (2011) Raf inhibitor resistance is mediated by dimerization of aberrantly spliced Braf(V600e). Nature 480: 387–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescher G., Bornfeld N., Hirche H., Horsthemke B., Jockel K., Becher R. (1996) Prognostic implications of monosomy 3 in uveal melanoma. Lancet 347: 1222–1225 [DOI] [PubMed] [Google Scholar]
- Prieto P., Yang J., Sherry R., Hughes M., Kammula U., White D., et al. (2010) Cytotoxic T lymphocyte-associated antigen 4 blockage with ipilimumab: long-term follow-up of 179 patients with metastatic melanoma. J Clin Oncol 28(15 Suppl.): abstract 8544. [Google Scholar]
- Ribas A., Hauschild A., Kefford R., Punt C., Haanen J., Marmol M., et al. (2008) Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide or dacarbazine) in patients with advanced melanoma. J Clin Oncol 26: abstract LBA9011. [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’Day S., Weber J., Garbe C., et al. (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364: 2517–2526 [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., et al. (2011) Durable complete responses in heavily pretreated patients with metastatic Melanoma using T-Cell transfer immunotherapy. Clin Cancer Res 17: 4550–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A., Yang J.C., Topalian S.L., Schwartzentruber D.J., Weber J.S., Parkinson D.R., et al. (1994) Treatment of 283 consecutive patients with metastatic Melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 271: 907–913 [PubMed] [Google Scholar]
- Shao Y., Aplin A.E. (2010) Akt3-mediated resistance to apoptosis in B-Raf-targeted Melanoma cells. Cancer Res 70: 6670–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharfman W., Hodi S., Lawrence D., Flaherty K., Amaravadi R., Kim K., et al. (2011) Results from the first-in-human (FIH) phase I study of the oral RAF inhibitor RAF265 administered daily to patients with advanced cutaneous melanoma. J Clin Oncol 29: abstract 8508. [Google Scholar]
- Shi H., Moriceau G., Kong X., Koya R.C., Nazarian R., Pupo G.M., et al. (2012) Preexisting Mek1 Exon 3 mutations in V600e/Kbraf Melanomas do not confer resistance to Braf inhibitors. Cancer Discov 2: 414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Naishadham D., Jemal A. (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10–29 [DOI] [PubMed] [Google Scholar]
- Solit D., Rosen N. (2011) Resistance to BRAF inhibition in melanomas. N Engl J Med 364: 772–774 [DOI] [PubMed] [Google Scholar]
- Sosman J., Kim K., Schuchter L., Gonzalez R., Pavlick A., Weber J., et al. (2012) Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366: 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Hodi F., Brahmer J., Gettinger S., Smith D., McDermott D., et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurel S., Hildenbrand R., Zimpfer A., La Rosee P., Paschka P., Sucker A., et al. (2005) Lack of clinical efficacy of Imatinib in metastatic Melanoma. Br J Cancer 92: 1398–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk C., Bezrookove V., Green G., Bauer J., Gaugler L., O’Brien J., et al. (2009) Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457: 599–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk C.D., Griewank K.G., Crosby M.B., Garrido M.C., Vemula S., Wiesner T., et al. (2010) Mutations in Gna11 in Uveal Melanoma. N Engl J Med 363: 2191–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J., Vultur A., Lee J.T., Somasundaram R., Fukunaga-Kalabis M., Cipolla A.K., et al. (2010) Acquired resistance to Braf inhibitors mediated by a Raf Kinase switch in Melanoma can be overcome by Cotargeting Mek and Igf-1r/Pi3k. Cancer Cell 18: 683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle N., Emery C., Berger M.F., Davis M.J., Sawyer A., Pochanard P., et al. (2011) Dissecting therapeutic resistance to Raf inhibition in Melanoma by tumor genomic profiling. J Clin Oncol 29: 3085–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Flaherty K., Infante J., Falchook G., Kefford R., Daud A., et al. (2012) Updated safety and efficacy results from a phase I/II study of the oral BRAF inhibitor dabrafenib (GSK2118436) combined with the oral MEK 1/2 inhibitor trametinib (GSK1120212) in patients with BRAFi-naive metastatic melanoma. J Clin Oncol 30: abstract 8510. [Google Scholar]
- Whittaker S., Kirk R., Hayward R., Zambon A., Viros A., Cantarino N., et al. (2010) Gatekeeper mutations mediate resistance to Braf-targeted therapies. Sci Transl Med 2: 35ra41. [DOI] [PubMed] [Google Scholar]
- Wolchok J.D., Hoos A., O’day S., Weber J.S., Hamid O., Lebbe C., et al. (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15: 7412–7420 [DOI] [PubMed] [Google Scholar]
- Wolchok J.D., Neyns B., Linette G., Negrier S., Lutzky J., Thomas L., et al. (2010) Ipilimumab monotherapy in patients with pretreated advanced Melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11: 155–164 [DOI] [PubMed] [Google Scholar]
- Wong C.W., Fan Y.S., Chan T.L., Chan A.S., Ho L.C., Ma T.K., et al. (2005) Braf and Nras mutations are uncommon in Melanomas arising in diverse internal organs. J Clin Pathol 58: 640–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhu M., Fletcher J., Giobbie-Hurder A., Hodi F. (2012) The protein kinase C inhibitor enzastaurin exhibits antitumor activity against uveal melanoma. PLoS One 7: e29622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman K., Atkins M.B., Prieto V., Eton O., Mcdermott D.F., Hubbard F., et al. (2006) Multicenter phase ii trial of high-dose Imatinib mesylate in metastatic Melanoma: significant toxicity with no clinical efficacy. Cancer 106: 2005–2011 [DOI] [PubMed] [Google Scholar]
- Yeh T., Marsh V., Bernat B., Ballard J., Colwell H., Evans R., et al. (2007) Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res 13: 1576–1583 [DOI] [PubMed] [Google Scholar]