Abstract
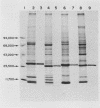
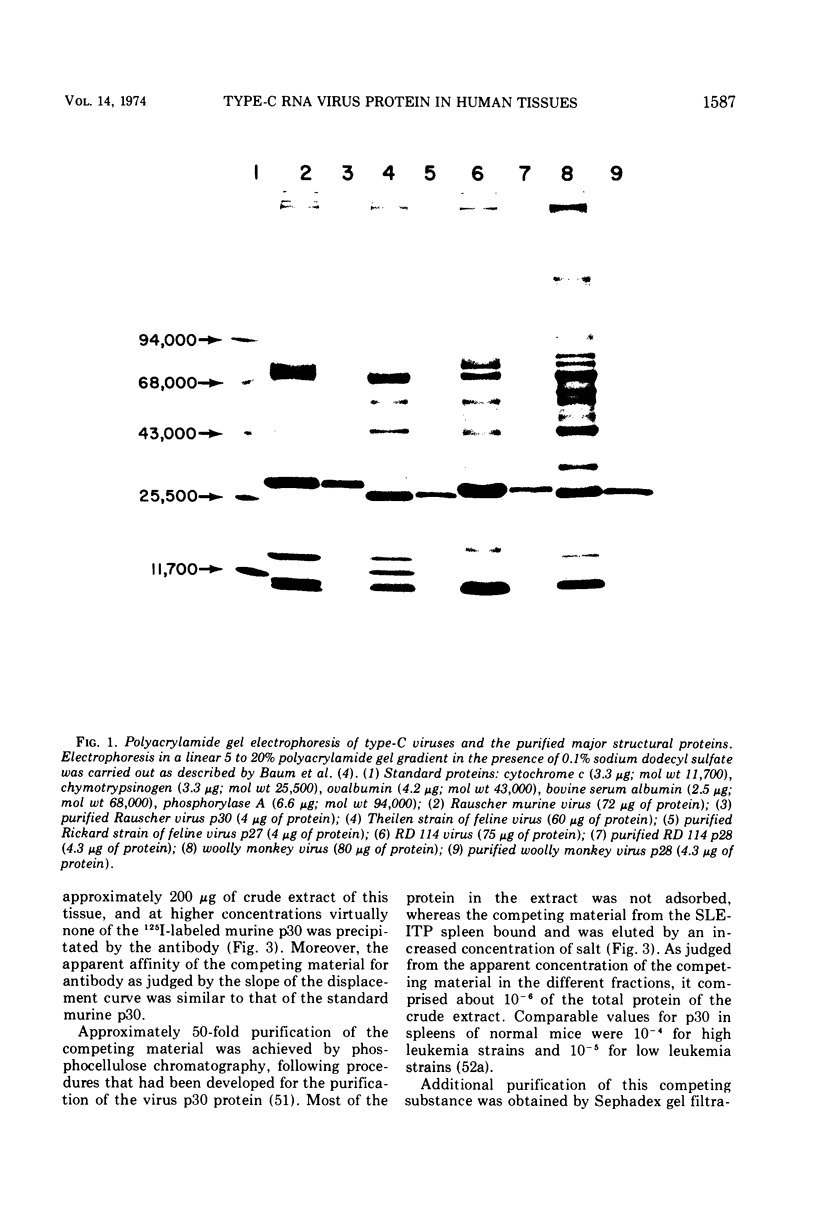
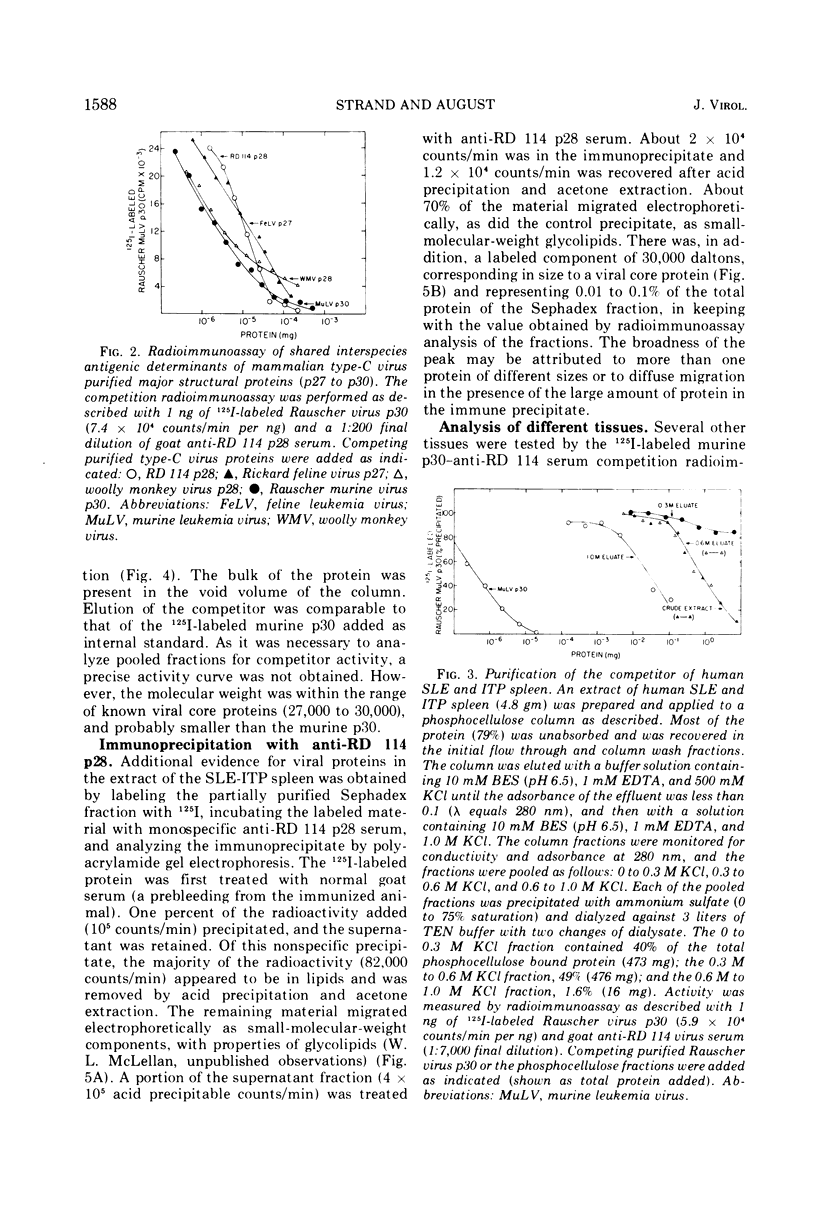
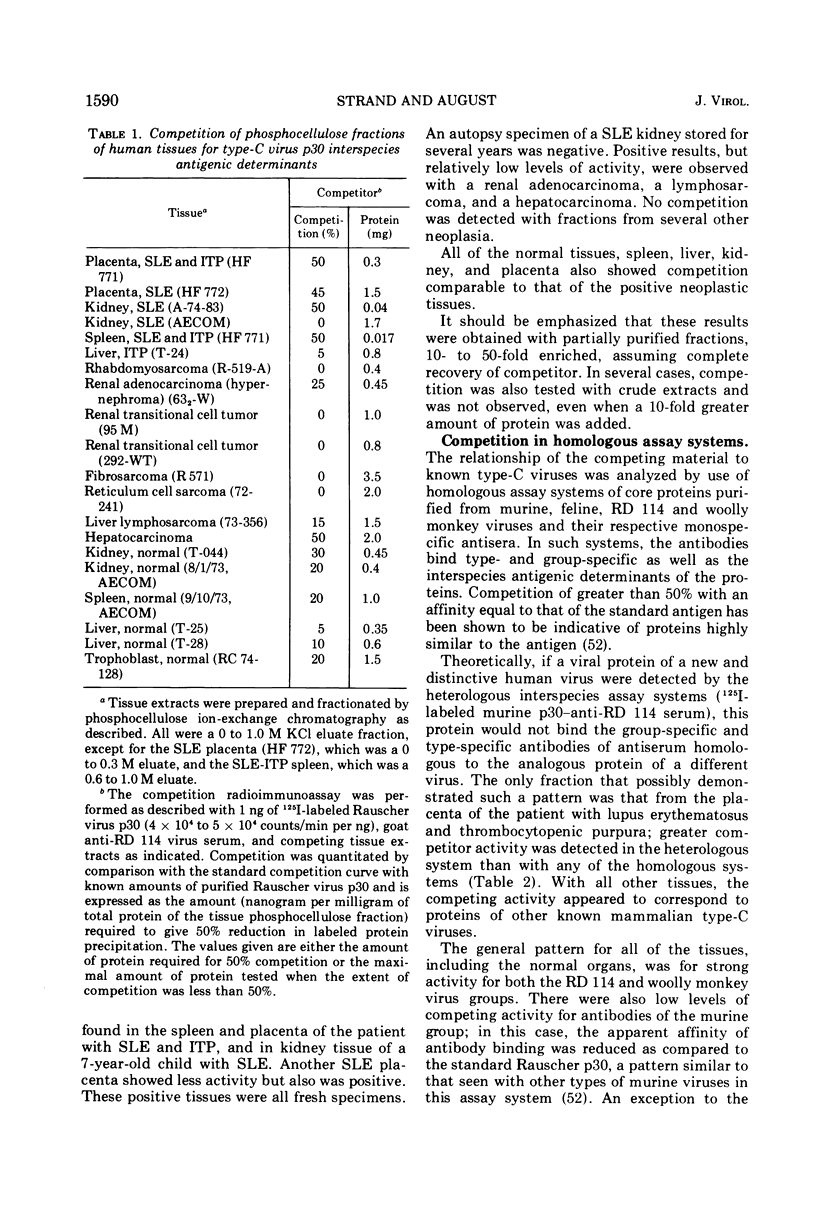
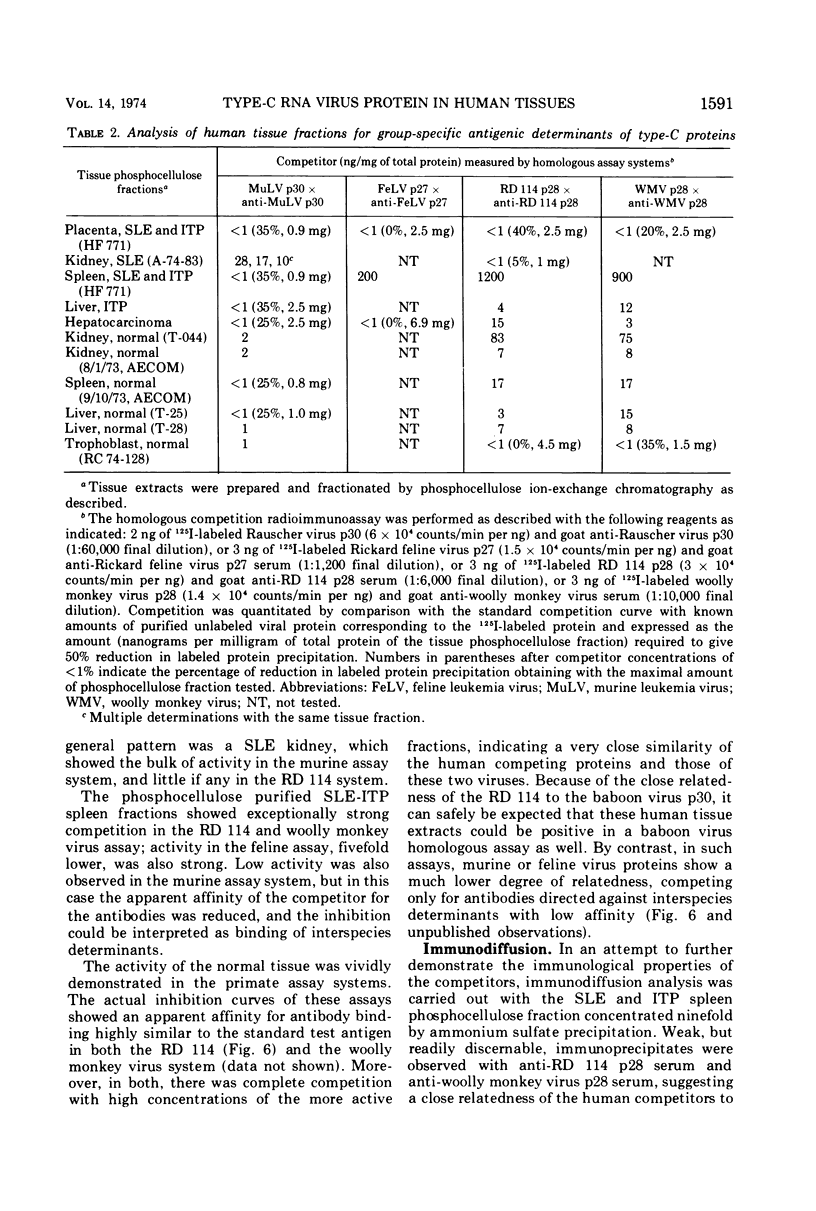
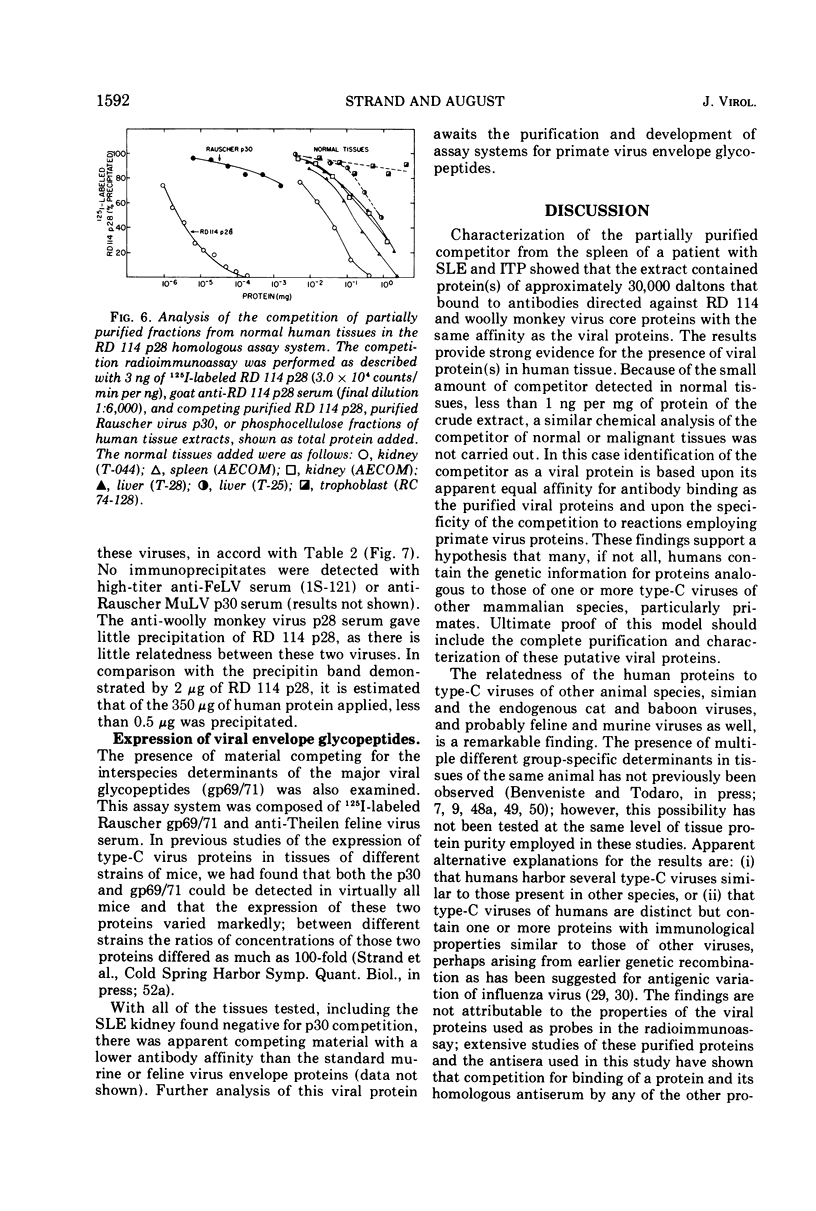
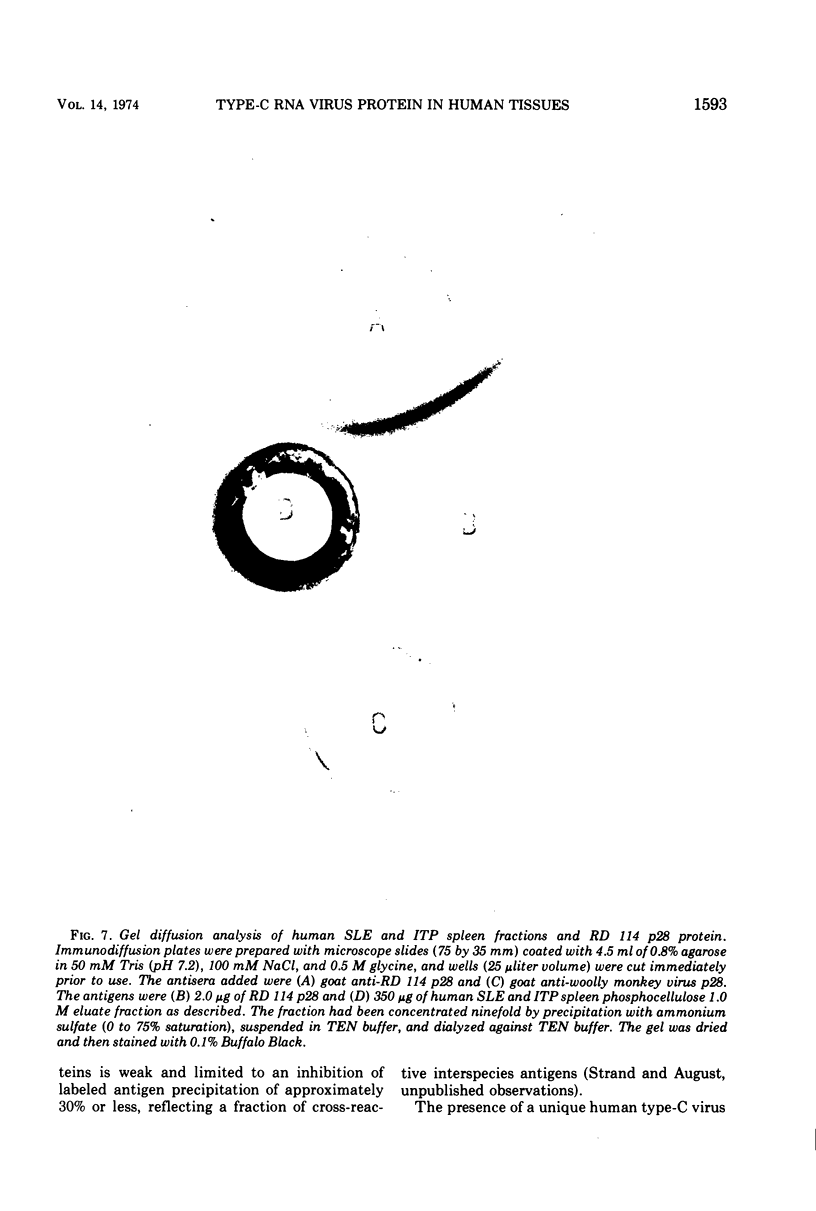
Partially purified fractions of human tissues have been analyzed by competition radioimmunoassay for the presence of two of the principle structural components of type-C RNA viruses, the major core protein (p27 to p30) and the major envelope glycopeptides (gp69/71). Screening of tissues was carried out by use of a heterologous assay system of 125I-labeled Rauscher murine virus p30 antigen and anti-RD 114 virus serum which was found to detect a class of interspecies determinants common to murine, feline, and primate viruses. A competitor with the same apparent affinity for antibody binding as that of purified viral core proteins was found in relatively high concentration in tissues from patients with systemic lupus erythematosus, in some neoplastic tissues, and also in normal human tissues. This competitor from a lupus spleen chromatographed on phosphocellulose and showed size fractionation during gel filtration similar to known p27 to p30 viral proteins. An immunologically reactive protein was also demonstrated by immunodiffusion and by immunoprecipitation of 125I-labeled human protein with anti-RD 114 p28 serum. Analysis of these human competitor proteins with homologous assay systems of viral core proteins and corresponding antisera showed that all, including the normal tissue extracts, appear similar to core proteins of known viruses, especially the RD 114 and woolly monkey species. A hypothesis suggested by these data is that many, if not all, humans harbor at least part of the genome of one or more type-C viruses, the properties of which are similar to those of viruses from other mammalian species, particularly primates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Andrews J. M., Gardner M. B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974 Apr;33(2):285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt W. G., Spiegelman S. Nuclear DNA sequences present in human leukemic cells and absent in normal leukocytes. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3737–3741. doi: 10.1073/pnas.69.12.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt W., Yates J. W., Wallace H. J., Jr, Holland J. F., Spiegelman S. Leukemia-specific DNA sequences in leukocytes of the leukemic member of identical twins. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2629–2632. doi: 10.1073/pnas.70.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese S. S., Jr Virus-like particles occurring in cultures of stable pig kidney cell lines. Brief report. Arch Gesamte Virusforsch. 1970;30(4):401–404. doi: 10.1007/BF01258369. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Peebles P. T., Nomura S., Haapala D. K. Isolation of RD-114-like oncornavirus from a cat cell line. J Virol. 1973 Jun;11(6):978–985. doi: 10.1128/jvi.11.6.978-985.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. E., Todaro G. J., Smith R. G., Livingston D. M., Gallo R. C. Relationship between RNA-directed DNA polymerase (reverse transcriptase) from human acute leukemic blood cells and primate type-C viruses. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1309–1313. doi: 10.1073/pnas.71.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Sarin P. S., Allen P. T., Newton W. A., Priori E. S., Bowen J. M., Dmochowski L. Reverse transcriptase in type C virus particles of human origin. Nat New Biol. 1971 Aug 4;232(31):140–142. doi: 10.1038/newbio232140a0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Yang S. S., Ting R. C. RNA dependent DNA polymerase of human acute leukaemic cells. Nature. 1970 Dec 5;228(5275):927–929. doi: 10.1038/228927a0. [DOI] [PubMed] [Google Scholar]
- Geering G., Hardy W. D., Jr, Old L. J., de Harven E., Brodey R. S. Shared group-specific antigen of murine and feline leukemia viruses. Virology. 1968 Dec;36(4):678–680. doi: 10.1016/0042-6822(68)90199-2. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S., Huebner R. J. Coexistence of intraspecies and interspecies specific antigenic determinants on the major structural polypeptide of mammalian C-type viruses. Nat New Biol. 1971 May 26;231(21):107–108. doi: 10.1038/newbio231107a0. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Parks W. P., Huebner R. J., Todaro G. J. Murine leukaemia virus group-specific antigen in the C-type virus-containing human cell line, ESP-1. Nature. 1971 Sep 10;233(5315):102–103. doi: 10.1038/233102a0. [DOI] [PubMed] [Google Scholar]
- Grrimley P. M., Decker J. L., Michelitch H. J., Frantz M. M. Abnormal structures in circulating lymphocytes from patients with systemic lupus erythematosus and related diseases. Arthritis Rheum. 1973 May-Jun;16(3):313–323. doi: 10.1002/art.1780160305. [DOI] [PubMed] [Google Scholar]
- Hehlmann R., Kufe D., Spiegelman S. RNA in human leukemic cells related to the RNA of a mouse leukemia virus (leukocytes-RNA-DNA hybridization-rauscher virus-polysomal RNA). Proc Natl Acad Sci U S A. 1972 Feb;69(2):435–439. doi: 10.1073/pnas.69.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman A., Peebles P. T., Strickland J. E., Fowler A. K., Kalter S. S., Oroszlan K. S., Gilden R. V. Baboon virus isolate M-7 with properties similar to feline virus RD-114. J Virol. 1974 Jul;14(1):133–138. doi: 10.1128/jvi.14.1.133-138.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie J. B., Helyer B. J. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T. G., Huff S. D., Buckley P. M., Dungworth D. L., Synder S. P., Gilden R. V. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972 Feb 9;235(58):170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- Kawano K., Miller L., Kimmelstiel P. Virus-like structures in lupus erythematosus. N Engl J Med. 1969 Nov 27;281(22):1228–1229. doi: 10.1056/NEJM196911272812207. [DOI] [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- Lambert P. H., Dixon F. J. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968 Mar 1;127(3):507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Downie J. C., Webster R. G. Studies on antigenic variation in influenza virus. Evidence for multiple antigenic determinants on the hemagglutinin subunits of A-Hong Kong-68 (H3 N2) virus and the A-England-72 strains. Virology. 1974 May;59(1):230–244. doi: 10.1016/0042-6822(74)90218-9. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Studies on the origin of pandemic influenza. 3. Evidence implicating duck and equine influenza viruses as possible progenitors of the Hong Kong strain of human influenza. Virology. 1973 Feb;51(2):383–391. doi: 10.1016/0042-6822(73)90437-6. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Benveniste R. E., Livingston D. M., Todaro G. J. Mammalian cells in culture frequently release type C viruses. Science. 1973 Oct 5;182(4107):56–59. doi: 10.1126/science.182.4107.56. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Todaro G. J. Endogenous type C virus from a cat cell clone with properties distinct from previously described feline type C virus. Virology. 1973 May;53(1):142–151. doi: 10.1016/0042-6822(73)90473-x. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rasheed S., Rongey R. W., Hardy W. D., Jr, Gilden R. V. RD-114 virus compared with feline and murine type-C viruses released from RD cells. Nat New Biol. 1973 Mar 21;242(116):75–78. doi: 10.1038/newbio242075a0. [DOI] [PubMed] [Google Scholar]
- McAllister R. M. Viruses in human carcinogenesis. Prog Med Virol. 1973;16:48–85. [PubMed] [Google Scholar]
- Mellors R. C. Autoimmune and immunoproliferative diseases of NZB/Bl mice and hybrids. Int Rev Exp Pathol. 1966;5:217–252. [PubMed] [Google Scholar]
- Moennig V., Frank H., Hunsmann G., Ohms P., Schwarz H., Schäfer W. C-type particles produced by a permanent cell line from a leukemic pig. II. Physical, chemical, and serological characterization of the particles. Virology. 1974 Jan;57(1):179–188. doi: 10.1016/0042-6822(74)90119-6. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Livingston D. M., Todaro G. J., Benveniste R. E., Scolnick E. M. Radioimmunoassay of mammalian type C viral proteins. 3. Detection of viral antigen in normal murine cells and tissues. J Exp Med. 1973 Mar 1;137(3):622–635. doi: 10.1084/jem.137.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Noon M. C., Watson C. J., Kawakami T. G. Radioimmunoassay of mammalian type-C polypeptides. IV. Characterization of woolly monkey and gibbon viral antigens. Int J Cancer. 1973 Jul 15;12(1):129–137. doi: 10.1002/ijc.2910120114. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori E. S., Dmochowski L., Myers B., Wilbur J. R. Constant production of type C virus particles in a continuous tissue culture derived from pleural effusion cells of a lymphoma patient. Nat New Biol. 1971 Jul 14;232(28):61–62. doi: 10.1038/newbio232061a0. [DOI] [PubMed] [Google Scholar]
- Rickard C. G., Post J. E., Noronha F., Barr L. M. A transmissible virus-induced lymphocytic leukemia of the cat. J Natl Cancer Inst. 1969 Jun;42(6):987–1014. [PubMed] [Google Scholar]
- Sarma P. S., Tseng J., Lee Y. K., Gilden R. V. Virus similar to RD114 virus in cat cells. Nat New Biol. 1973 Jul 11;244(132):56–59. doi: 10.1038/newbio244056a0. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Sarin P. S., Reitz M. S., Gallo R. C. Reverse transcriptase activity of human acute leukaemic cells: purification of the enzyme, response to AMV 70S RNA, and characterization of the DNA product. Nat New Biol. 1972 Nov 15;240(98):67–72. doi: 10.1038/newbio240067a0. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Fischinger P. J., Lange J., Pister L. Properties of mouse leukemia viruses. I. Characterization of various antisera and serological identification of viral components. Virology. 1972 Jan;47(1):197–209. doi: 10.1016/0042-6822(72)90252-8. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Todaro G. J., Aaronson S. A. Immunological characterization of primate C-type virus reverse transcriptases. Nat New Biol. 1972 Jan 12;235(54):35–40. doi: 10.1038/newbio235035a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Benveniste R. E., Todaro G. J. Type C viral expression in primate tissues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3721–3725. doi: 10.1073/pnas.71.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Todaro G. J. Radioimmunoassay of the major group specific protein of endogenous baboon type C viruses: relation to the RD-114-CCC group and detection of antigen in normal baboon tissues. Virology. 1974 Sep;61(1):168–181. doi: 10.1016/0042-6822(74)90252-9. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Expression of endogenous RNA C-type virus group-specific antigens in mammalian cells. J Virol. 1973 Sep;12(3):564–569. doi: 10.1128/jvi.12.3.564-569.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Strand M., Lilly F., August J. T. Host control of endogenous murine leukemia virus gene expression: concentrations of viral proteins in high and low leukemia mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3682–3686. doi: 10.1073/pnas.71.9.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., Wilsnack R., August J. T. Structural proteins of mammalian oncogenic RNA viruses: immunological characterization of the p15 polypeptide of Rauscher murine virus. J Virol. 1974 Dec;14(6):1575–1583. doi: 10.1128/jvi.14.6.1575-1583.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandström H., Veijalainen P., Moennig V., Hunsmann G., Schwarz H., Schäfer W. C-type particles produced by a permanent cell line from a leukemic pig. I. Origin and properties of the host cells and some evidence for the occurrence of C-type-like particles. Virology. 1974 Jan;57(1):175–178. doi: 10.1016/0042-6822(74)90118-4. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The RNA tumor viruses--background and foreground. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1016–1020. doi: 10.1073/pnas.69.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Lieber M. M., Livingston D. M. Infectious type C viruses released by normal cat embryo cells. Virology. 1973 Oct;55(2):506–515. doi: 10.1016/0042-6822(73)90192-x. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Lieber M. M., Sherr C. J. Characterization of a type C virus released from the porcine cell line PK(15). Virology. 1974 Mar;58(1):65–74. doi: 10.1016/0042-6822(74)90141-x. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Gallo R. C. Immunological relationship of DNA polymerase from human acute leukaemia cells and primate and mouse leukaemia virus reverse transcriptase. Nature. 1973 Jul 27;244(5413):206–209. doi: 10.1038/244206a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E., Lieber M. M., Melnick J. L. Type C viruses of baboons: isolation from normal cell cultures. Cell. 1974 May;2(1):55–61. doi: 10.1016/0092-8674(74)90008-7. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Aaronson S. A. Comparative immunological studies of RNA C-type viruses: radioimmunoassay for a low molecular weight polypeptide of woolly monkey leukemia virus. Virology. 1974 Feb;57(2):347–356. doi: 10.1016/0042-6822(74)90174-3. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Friis R. R., Katz E., Vogt P. K. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971 Dec;46(3):920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Strand M., August J. T. The viral envelope glycoprotein of murine leukemia virus and the pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1974 Oct 1;140(4):1011–1027. doi: 10.1084/jem.140.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]