Abstract
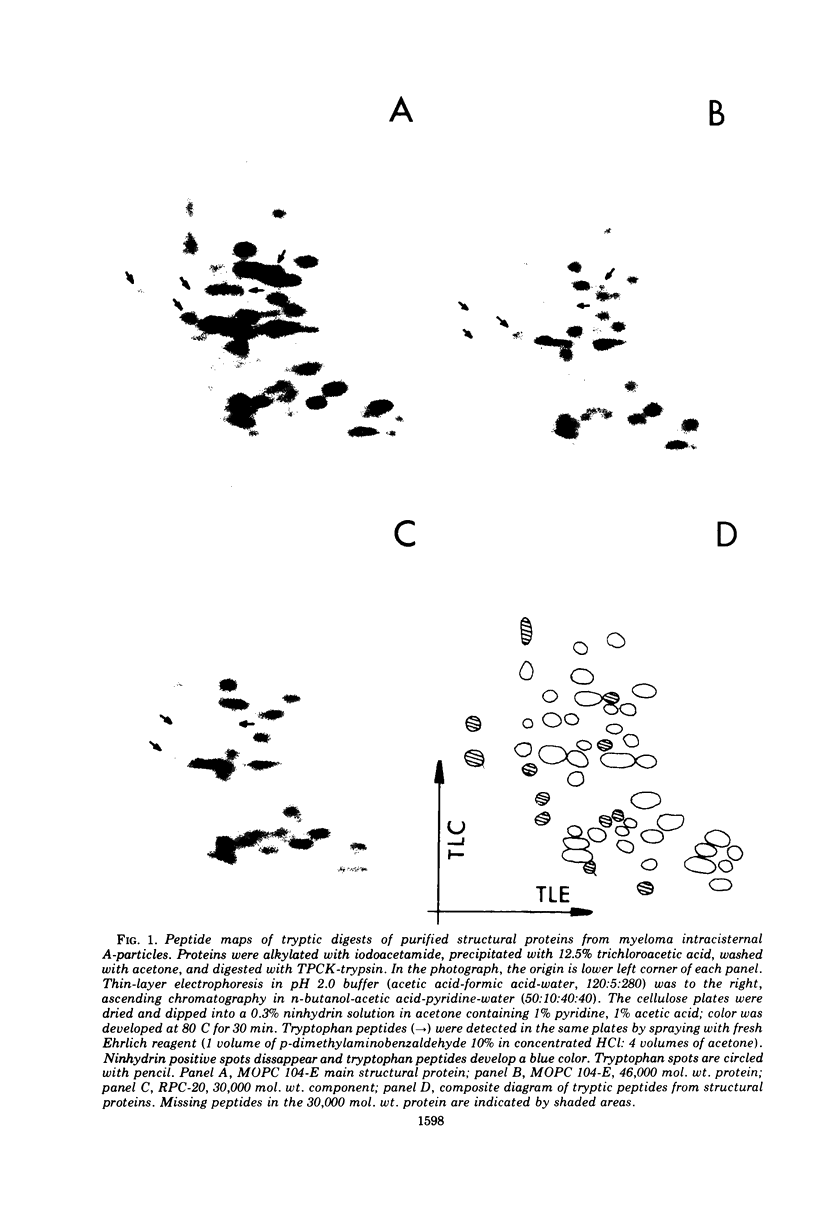
Peptide maps of tryptic digests of the structural proteins from inner shells of intracisternal A-particles have shown common peptides for all the proteins. The terminal amino group of the three different structural proteins was identified as arginine. The major protein revealed approximately half the number of peptides expected from the amino acid composition. Since evidence for a cross-link bond has not been found, the main structural protein may be a single polypeptide chain containing a total or partial duplication of sequence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN S. O. THE CROSS-LINKS IN RESILIN IDENTIFIED AS DITYROSINE AND TRITYROSINE. Biochim Biophys Acta. 1964 Oct 9;93:213–215. doi: 10.1016/0304-4165(64)90289-2. [DOI] [PubMed] [Google Scholar]
- Apte B. N., Zipser D. In vivo splicing of protein: one continuous polypeptide from two independently functioning operons. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2969–2973. doi: 10.1073/pnas.70.10.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein fusion: a novel reaction in bacteriophage lambda head assembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1451–1455. doi: 10.1073/pnas.71.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- Marciani D. J., Kuff E. L. Isolation and partial characterization of the internal structural proteins from murine intracisternal A particles. Biochemistry. 1973 Dec 4;12(25):5075–5083. doi: 10.1021/bi00749a008. [DOI] [PubMed] [Google Scholar]
- PAZ M. A., BLUMENFELD O. O., ROJKIND M., HENSON E., FURFINE C., GALLOP P. M. DETERMINATION OF CARBONYL COMPOUNDS WITH N-METHYL BENZOTHIAZOLONE HYDRAZONE. Arch Biochem Biophys. 1965 Mar;109:548–559. doi: 10.1016/0003-9861(65)90400-5. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. Chemical and enzymic detection of protein cross-links. Measurement of epsilon-(gamma-glutamyl)lysine in fibrin polymerized by factor XIII. Biochemistry. 1969 Mar;8(3):871–876. doi: 10.1021/bi00831a016. [DOI] [PubMed] [Google Scholar]
- Wivel N. A., Lueders K. K., Kuff E. L. Structural organization of murine intracisternal A particles. J Virol. 1973 Feb;11(2):329–334. doi: 10.1128/jvi.11.2.329-334.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetta J. P., Vincendon G., Mandel P., Gombos G. The utilisation of I-dimethylaminonaphthalene-5-sulphonyl chloride for quantitative determination of free amino acids and partial analysis of primary structure of proteins. J Chromatogr. 1970 Sep 23;51(3):441–458. doi: 10.1016/s0021-9673(01)96893-1. [DOI] [PubMed] [Google Scholar]