Abstract
An understanding of the diversity of cortical GABAergic interneurons is critical to understand the function of the cerebral cortex. Recent data suggest that neurons expressing three markers, the Ca2+-binding protein parvalbumin (PV), the neuropeptide somatostatin (SST), and the ionotropic serotonin receptor 5HT3a (5HT3aR) account for nearly 100% of neocortical interneurons. Interneurons expressing each of these markers have a different embryological origin. Each group includes several types of interneurons that differ in morphological and electrophysiological properties and likely have different functions in the cortical circuit. The PV group accounts for ~40% of GABAergic neurons and includes fast spiking basket cells and chandelier cells. The SST group, which represents ~30% of GABAergic neurons, includes the Martinotti cells and a set of neurons that specifically target layer IV. The 5HT3aR group, which also accounts for ~30% of the total interneuronal population, is heterogeneous and includes all of the neurons that express the neuropeptide VIP, as well as an equally numerous subgroup of neurons that do not express VIP and includes neurogliaform cells. The universal modulation of these neurons by serotonin and acetylcholine via ionotropic receptors suggests that they might be involved in shaping cortical circuits during specific brain states and behavioral contexts.
Keywords: GABAergic neuron, interneuron diversity, neocortex
INTRODUCTION
In neocortex, neurons releasing the neurotransmitter GABA are, throughout most part of the life of the animal, local inhibitory interneurons believed to play fundamental roles in shaping cortical circuits. In addition to releasing GABA, these cells are a source of neuropeptides that have important functions in modulating cortical function. The large diversity of GABAergic interneurons that have arisen through evolution has provided the means by which the cerebral cortex can perform complex operations. Interneuron subtypes differ in morphology, intrinsic membrane properties, connectivity, and the efficacy and dynamics of input and output synapses, and these differences are associated with the expression of specific molecular markers (Cauli et al., 1997; Kawaguchi and Shindou, 1998; Markram et al., 2004; Somogyi and Klausberger, 2005; Yuste, 2005; Ascoli et al., 2008). GABAergic interneuron subtypes also differ in their response to neuromodulators, actions that profoundly affect the function of neocortical circuits and are responsible for dynamic changes associated with changes in brain states and behavioral context (Kawaguchi, 1997; Xiang et al., 1998; Gao et al., 2003; Bacci et al., 2005; Fanselow et al., 2008; Kruglikov and Rudy, 2008). Although they represent a minority of all cortical neurons (10–20% in rodents), their axons ramify extensively, controlling the entire neuronal population of the cortex.
GABAergic interneurons control information flow in the cortex by targeting specific domains of the principal neurons and thereby controlling specific spatiotemporal aspects of their activity. GABAergic interneurons are believed to play important roles in controlling the timing of pyramidal cell firing, synchronizing network activity, and the generation of cortical rhythms; they seem to play an important role by responding to dynamic changes in excitation, increasing the dynamic range of cortical circuits, controlling sensory receptive fields and plasticity, and maintaining the excitatory and inhibitory balance necessary for the transfer of information while preventing runaway excitation (McBain and Fisahn, 2001; Pouille and Scanziani, 2001; Wehr and Zador, 2003; Markram et al., 2004; Trevelyan et al., 2006; Klausberger and Somogyi, 2008; Haider and McCormick, 2009). The required precision of these functions, both spatially and temporally, likely depend on the existence of a variety of interneuron types. Understanding interneuron diversity is thus critical to understand information processing within the cerebral cortex (Ascoli et al., 2008). Moreover, malfunction of these neurons has been implicated in a number of diseases ranging from epilepsy to schizophrenia, anxiety disorders and autism (Cossart et al., 2001; Noebels, 2003; Levitt et al., 2004; Cobos et al., 2005; Gonzalez-Burgos and Lewis, 2008).
This has resulted in major efforts to determine cortical interneuron diversity and characterize the anatomical and functional properties of interneuron subtypes (DeFelipe et al., 1993; Gonchar and Burkhalter, 1997; Markram et al., 2004; Ascoli et al., 2008).
In this article, we present a further contribution to this effort. It is largely based on the analysis of a population of GABAergic interneurons in mouse neocortex that express the ionotropic serotonin receptor 5HT3a (5HT3aR). This analysis has provided strong evidence that three major groups of neurons, those expressing the Ca2+-binding protein parvalbumin (PV), those expressing the neuropeptide somatostatin (SST), and those expressing 5HT3aRs account for nearly 100% of GABAergic neurons in somatosensory (S1) cortex.
5HT3aR-BACEGFP Mice Reliably Allow the Identification of 5HT3aR-Expressing Neurons in Neocortex
The analysis of 5HT3aR-expressing interneurons was facilitated by the availability of transgenic mouse lines expressing EGFP in these neurons (5HT3aR-BACEGFP mice). Two different 5HT3aR-BACEGFP mouse lines have been made, one by the Monyer lab at the University of Heidelberg (Inta et al., 2008) and one by the NINDS GENSAT project at Rockefeller University. The 5HT3aR-BACEGFP mouse lines were generated using a bacterial artificial chromosome (BAC) containing the promoter region of the 5HT3aR gene to control expression of EGFP. Using the GENSAT line, Lee et al. (2010) found a nearly perfect overlap between EGFP and 5HT3a receptor mRNA expression in the neocortex. There were no false positives, no neuron expressed EGFP that did not also contain 5HT3aR mRNA transcripts. Conversely, there were virtually no false negatives because 99.8 ± 0.3% of neocortical cells expressing the receptor were EGFP positive. Furthermore, it is likely that 5HT3aR mRNA expression reliably reports on the presence of functional receptors. Functional responses were detected to the specific 5HT3aR-agonist mCPBG in more than 40 EGFP-positive neurons recorded in the cortex of this transgenic line (Lee et al., 2010, also see below).
Three Major Groups of Neurons: (1) PV-expressing basket and chandelier neurons; (2) SST-expressing neurons; and (3) 5HT3a receptor-expressing neurons account for nearly 100% of GABAergic interneurons in somatosensory cortex
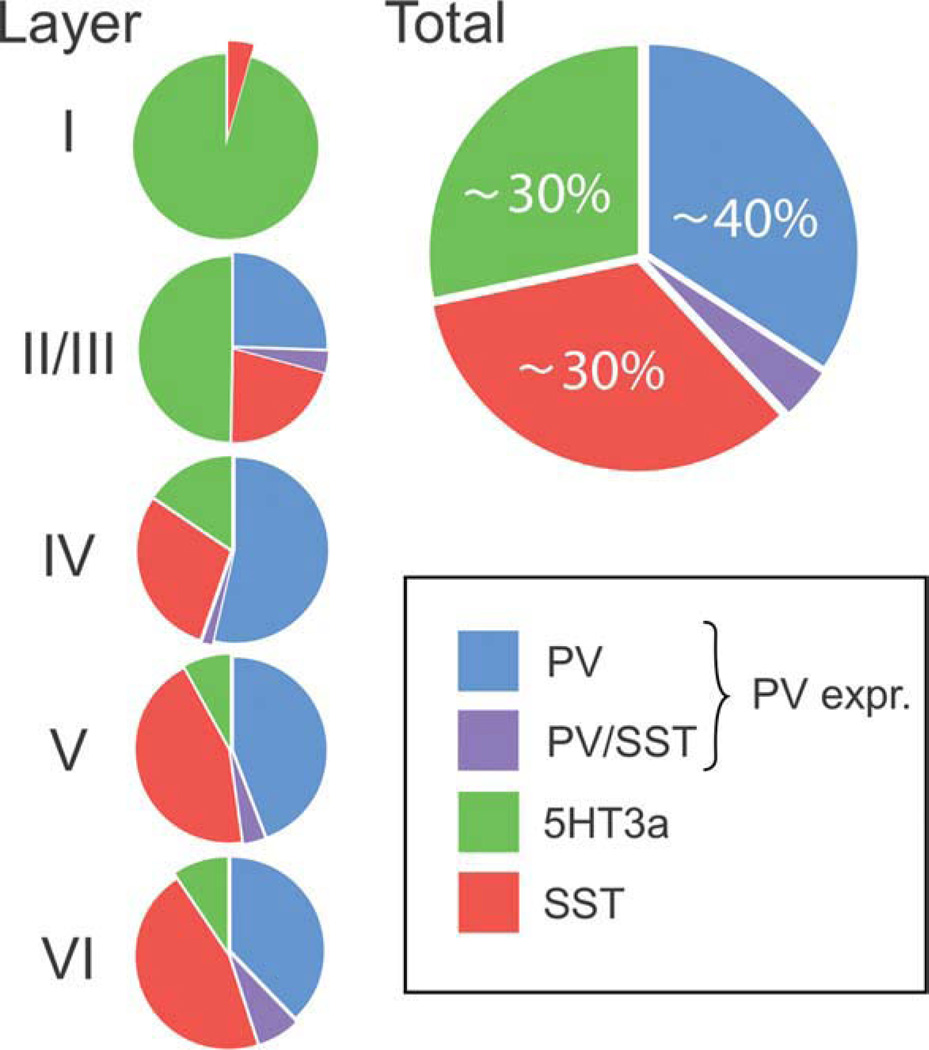
Calcium-binding proteins and neuropeptides have been used as markers that distinguish among cortical interneurons (Defelipe et al., 1989; Hendry et al., 1989; DeFelipe, 1993; Cauli et al., 1997; Gonchar and Burkhalter, 1997; Kawaguchi and Kubota, 1997; Markram et al., 2004; Somogyi and Klausberger, 2005; Ascoli et al., 2008). Numerous studies of rat and mouse brain tissue have suggested that among these, the Ca2+-binding protein PV and the neuropeptide SST, are particularly useful because they define nonoverlapping groups of interneurons in the neocortex (Gonchar and Burkhalter, 1997; Kawaguchi and Kubota, 1997; Ascoli et al., 2008). Our own studies in mouse have confirmed this conclusion; neurons expressing PV (as detected by immunofluorescence) do not express SST protein and vice versa (Lee et al., 2010). We do find that using in situ hybridization (ISH) some PV-containing neurons (<10%) in somatosensory cortex contain SST mRNA. The overlap is largest in layer VI where ~16.3% of PV cells express SST mRNA (Lee et al., 2010). Furthermore, RT-PCR of FACS purified PV-expressing neurons shows SST mRNA expression in a large percentage of the samples (Jeong and Rudy, unpublished observations). Together, the data suggest that although a fraction of PV neurons may express SST mRNA transcripts, SST protein levels are absent or are too low to be detected by immunohistochemistry (IHC). Thus, the data support the view that PV and SST protein expression defines two independent groups of GABAergic interneurons. These two groups of neurons account for 40 and 30% of GABAergic interneurons in somatosensory cortex, respectively (Miyoshi et al., 2010; Xu et al., 2010; Lee et al., 2010) (Fig. 1).
Figure 1.
Distribution of three groups of GABAergic interneurons in S1 cortex. Shown are the proportions of GAD-67 mRNA-expressing neurons also expressing mRNA for parvalbumin (PV), somatostatin (SST), or 5HT3aR based on the study by Lee et al. (2010). PV-expressing interneurons (including those expressing SST mRNA) account for ~40% of the total GABAergic population in the mouse S1 cortex. SST-expressing and 5HT3aR-expressing neurons account for ~30% each. The distribution of interneuron groups varies among different cortical layers. 5HT3aR neurons are concentrated in supragranular layers. Layer I, which has a much lower cell density, expresses mainly 5HT3aR neurons. In layer I and in layers II/III, the proportion of 5HT3aR neurons exceeds the proportion of PV cells. The proportion of PV cells is particularly large in layer IV. There are no PV neurons in layer I. SST interneurons are found in all layers, but are particularly abundant in infragranular layers.
It has been known for some time that 5HT3a receptors in neocortex are present exclusively in GABAergic neurons (Morales and Bloom, 1997; Ferezou et al., 2002; Puig et al., 2004) and that 5HT3aR-expressing cells do not express PV or SST (Ferezou et al., 2002), suggesting that the receptor is present in neuronal populations distinct from those expressing these two markers. There are no antibodies to the 5HT3a receptor available, precluding the use of IHC to determine the size of the 5HT3aR-expressing interneuron population and estimate its contribution to the total interneuron family. The availability of a GFP mouse line that reliably reports the presence of 5HT3a receptors allowed us to characterize this interneuron population (Lee et al., 2010). Consistent with the view that 5HT3aR interneurons are distinct from the PV- and SST-expressing populations, no overlap was detected between PV or SST IHC and GFP expression in the 5HT3aR-BACEGFP mouse. Qualitative observation of EGFP fluorescence in neocortical sections from the 5HT3aR-BACEGFP mouse suggests that the population of 5HT3aR neurons is large and of comparable size to the PV and STT groups (Lee et al., 2010; Vucurovic et al., 2010). Double ISH for the pan-GABAergic marker GAD67 and for PV, SST, or 5HT3aR was used to quantify the three interneuron populations and to determine the proportion and layer distribution of GAD67-expressing neurons that express each of these markers. In addition, double ISH for PV and SST was used to determine the overlap of these two markers. This analysis revealed that 5HT3aR neurons account for ~30% of the total population of GABAergic neurons, and that three group of neurons, those expressing PV, SST, and 5HT3aR, account for nearly all, if not all, GABAergic interneurons in primary somatosensory cortex (Fig. 1).
5HT3aR interneurons primarily populate superficial neocortical layers I–III (Fig. 1). In fact, these cells are the predominant population of interneurons (~60% of all interneurons) in these layers. Within layers I–III, the number of 5HT3aR-expressing neurons actually exceeds by two fold the number of PV-positive neurons, traditionally believed to represent the largest interneuron class. Almost all neurons in layer I, a layer that only contains GABAergic interneurons, are 5HT3aR-expressing.
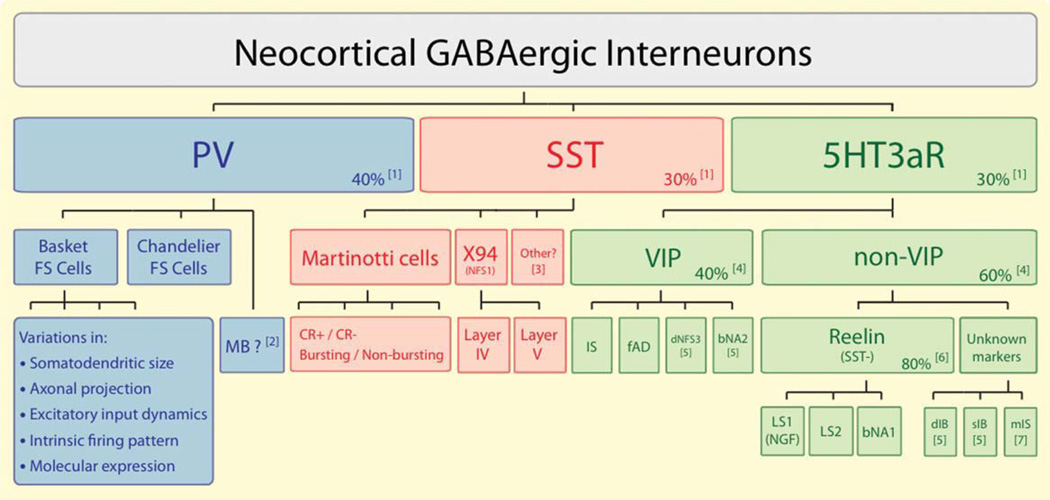
The conclusion that PV-, SST-, and 5HT3aR-expressing neurons account for nearly if not all GABAergic interneurons in mouse somatosensory cortex implies that other interneuron markers such as the neuropeptides VIP, NPY, and cholecystokinin (CCK) and the Ca2+-binding proteins calretinin and calbindin either label small interneuron populations or are expressed in one or more of the three groups defined above. Although further work is necessary to characterize in detail the PV, SST, and 5HT3aR neuronal groups, it is already clear that they are heterogenous, each consisting of more than one interneuron class. Of these, the 5HT3aR group might be particularly heterogenous. The challenge to understand interneuron diversity in neocortex now is in understanding the diversity within each of these three groups (Fig. 2).
Figure 2.
A classification of neocortical GABAergic interneurons. Nearly 100% of all neocortical GABAergic neurons belong to one of three groups defined by the expression of parvalbumin (PV), somatostatin (SST), and the ionotropic serotonin receptor 5HT3a (5HT3aR). Each group consists of several subgroups. In turn, each subgroup consists of several functionally distinct types or classes of interneurons, most of which are still poorly defined. PV expression has been associated with the fast-spiking (FS) firing pattern. There are two anatomically distinct subgroups of FS neurons, basket cells, and chandelier cells. MB (multipolar bursting) neurons define a possible subgroup of non-FS, PV-expressing neurons. FS basket cells have been observed to vary in a number of properties, but it is not clear which or how many classes of FS basket cells are there in neocortex. Two subgroups of SST neurons are well characterized, Martinotti cells, which account for the majority of SST neurons, and a subgroup of neurons described in the X94 mouse line characterized by a profuse axonal projection to layer IV. Shown as “other” are SST neurons that do not seem to belong to any of these two categories (McGarry et al., 2010). Martinotti cells have been shown to vary in a number of morphological, electrophysiological, and molecular properties, but it is not possible yet to clearly define classes of Martinotti cells. For example, Martinotti cells can be subdivided into those that express calretinin (CR+) and those that do not (CR−), which vary in a number of properties (see text). X94 cells are present in layer IV and V, both of which project to layer IV. The subdivisions of the 5HT3aR group are still poorly defined. 5HT3aR neurons can be classified into two subgroups based on the expression of the neuropeptide VIP. Each of these subgroups of 5HT3aR neurons consists of several, still mostly undefined, types of interneurons. The most frequently observed types include, among the VIP neurons, the bipolar or bitufted neurons with an irregular spiking (IS) or a “fast adapting” (fAD) firing pattern, and among the non-VIP neurons, the neurogliaform (NGF) cells with a late spiking firing pattern (LS1) and multipolar neurons with an LS2 firing pattern. Note: (1) From Lee et al. (2010); (2) See Blatow et al. (2003); (3) See text for SST neurons described by McGarry et al. (2010); (4) Estimates based on Miyoshi et al. (2010) and Lee et al. (2010); (5) Neurons with a dFNS3, bNA2, dIB, and sIB firing patterns were more rarely sampled than other CGE-derived neurons by Miyoshi et al. (2010); (6) Reelin, which is also expressed by a fraction of SST neurons, was shown to be expressed in a major fraction of non-VIP CGE-derived (Miyoshi et al., 2010) and 5HT3aR (Lee et al., 2010) neurons; (7) A fraction of non-VIP 5HT3aR neurons (mIS) had multipolar morphology and an IS firing pattern (Lee et al., 2010) resembling neurons derived from the POA (see text).
PV Interneuron Group
PV expression has been associated with the fast-spiking firing pattern since the 1980s (Kawaguchi et al., 1987). This correlation between fast spiking and PV expression has been confirmed by many laboratories (Cauli et al., 1997; Kawaguchi and Kubota, 1997; Gibson et al., 1999; Ascoli et al., 2008; Xu and Callaway, 2009), including our own. We have recorded from several hundred PV-expressing neurons in several mouse lines expressing GFP in PV neurons and virtually all are fast-spiking. There are two types of PV neurons: basket cells, neurons that make synapses at the soma and proximal dendrite of target neurons and typically have multipolar morphology, and chandelier cells, which target the axon initial segment (AIS) of pyramidal cells (Kawaguchi and Kubota, 1997; Ascoli et al., 2008). FS cells fire sustained high-frequency trains of brief APs with a large and fast AHP and with little spike frequency adaptation. Near threshold, they fire abrupt episodes of nonadapting repetitive discharges. They have the lowest input resistance and the fastest membrane time constant of all interneurons, a feature that contributes to ensure fast synaptic responses (Connors and Gutnick, 1990; Cauli et al., 1997; Gibson et al., 1999; Kawaguchi and Kubota, 1997; Markram et al., 2004; Ascoli et al., 2008; Goldberg et al., 2008). Although both PV-expressing basket cells and chandelier cells are fast-spiking, their electrophysiological properties are not identical (Woodruff et al., 2009).
There might be additional diversification among these two types of FS cells, particularly among the basket cells. PV-expressing basket neurons have been shown to vary in size (“large and nest basket cells”), extent of dendritic and axonal projection (local, transcolumnar, or translaminar) (Markram et al., 2004; Uematsu et al., 2008; Helmstaedter et al., 2009; Tremblay et al., 2010), the expression of molecular markers such as substance P and its receptor NK1 (Vruwink et al., 2001; Jeong and Rudy, unpublished observations), the Ca2+-binding protein calbindin (Kawaguchi and Kubota, 1997), Kv3.2, and erg1 K+ channels (Chow et al., 1999; Saganich et al., 2001; Goldberg and Rudy, 2005), and firing properties (Clark et al., 2007; Goldberg et al., 2008; Puig et al., 2008; Tremblay et al., 2010). Some of these differences may represent specializations of FS cells associated with the layer or cortical area where they are located.
Excitatory synapses onto FS cells are typically strongly depressing. This includes thalamocortical inputs onto FS cells in layer IV and intracortical inputs onto FS cells in all layers. An exception to this is a population of FS neurons in layer VI that receives facilitating inputs, perhaps from corticothalamic neurons (Gil et al., 1999; Beierlein and Connors, 2002; Thomson and Lamy, 2007).
Fast spiking basket cells are likely the dominant inhibitory system in neocortex, and in layer IV, they are the main interneuron target of thalamocortical axons. They mediate fast, precise, and powerful inhibition of target neurons and have been shown to be involved in a number of functions, including mediating feedforward inhibition of several cortical circuits, including the thalamocortical input of sensory responses, which is important for creating a strict window for temporal summation of excitatory inputs and spike generation by principal cells (Pinto et al., 2000; Miller et al., 2001; Pouille and Scanziani, 2001; Pinto et al., 2003; Lawrence and McBain, 2003; Gabernet et al., 2005; Cruikshank et al., 2007). They have been implicated as well in the establishment and maintenance of fast (gamma frequency) cortical rhythms (Traub et al., 2004; Bartos et al., 2007; Cardin et al., 2009) and regulation of critical-period experience-dependent plasticity (Hensch, 2005). Given the preponderance and magnitude of FS basket cell responses, they are likely key contributors to the control of excitation/inhibition balance in the cortex (Hasenstaub et al., 2005; Haider and McCormick, 2009). The functions of chandelier cells are much less understood and are in fact controversial. It was recently shown that postsynaptic responses to GABA release from these neurons are depolarizing because of changes in Cl− concentration, and hence the reversal potential of GABAA receptors, at the pyramidal cell AIS, the target of chandelier cell axons, and may in fact be excitatory (Szabadics et al., 2006; Woodruff et al., 2009; but see Glickfeld et al., 2009).
In addition to basket cells and chandelier cells, which are the main types of PV neurons, Blatow et al. (2003) described a group of PV cells close to the border between layers I and II of mouse neocortex that differ from FS cells in their electrophysiological properties and connectivity. These neurons, which they called multipolar bursting (MB), had a firing pattern in response to depolarizing steps that differed significantly from that of FS cells and consisted of an initial burst of two or three APs followed by a pronounced afterhyperpolarizing gap and a subsequent train of tonic spiking at low frequency. MB cells also differ from FS cells in that their synapses with pyramidal cells or other MB cells showed paired-pulse facilitation, whereas FS cell synapses are typically strongly depressing. Moreover, MB neuron synaptic contacts were mostly on dendritic shafts. The preponderance of this class of PV neurons is not known and has not been described by other investigators; perhaps because Blatow et al. used 14-day-old mice, somewhat younger than what is typically used in the field and carried their recordings at room temperature.
SST Interneuron Group
SST expression in neocortex has typically been associated with Martinotti cells (MCs), GABAergic interneurons with ascending axons that arborize in layer I spreading horizontally to neighboring columns and making synapses on the dendritic tufts of pyramidal neurons. The ascending axon gives away collaterals that innervate the apical and basal dendrites of pyramidal cells. Synaptic contacts are found mainly on dendritic shafts and on spines. Martinotti cells are more abundant in layer V but are found throughout layers II–VI (Kawaguchi and Kubota, 1997; Wang et al., 2004; Uematsu et al., 2008). They display a regular adapting firing pattern (RSNP), but often, particularly in layer V, they initially fire bursts of two or more spikes on slow depolarizing humps when depolarized from hyperpolarized potentials (because of this property, these cells have also been called burst spiking nonpyramidal (BSNP), intrinsic bursting (IB), or low-threshold spike (LTS) cells).
Excitatory inputs onto Martinotti cells are generally strongly facilitating, which is a key feature that distinguishes these interneurons from FS neurons (Reyes et al., 1998; Beierlein et al., 2003; Kapfer et al., 2007; Silberberg and Markram, 2007; Fanselow et al., 2008). This is likely of considerable physiological interest. As a result of this feature, repetitive activity in a single pyramidal cell can drive a Martinotti cell to fire and provide feedback inhibition to pyramidal neurons across layers and columns (Kapfer et al., 2007; Silberberg and Markram, 2007). Given the facilitating dynamics of Martinotti cell excitatory inputs, the feedback inhibition mediated by these neurons will increase as a function of rate and duration of presynaptic discharge. These interneurons might be activated preferentially during periods of increased network activity in contrast to FS cells, which might be transiently activated preferentially when network activity is low. The differences in location of the output synapses of FS and Martinotti cells will also influence their effects on the cortical circuit. By forming proximal, peri-somatic strong synapses on target neurons, FS cells are ideally suited to quickly suppress the output of target neurons, as might be required during feedforward inhibition. Martinotti cell-mediated inhibition might be more graded and could be more selective by inhibiting more strongly excitatory inputs arriving at the dendrite in close proximity to the location of the interneuron inputs.
Diversity of SST Interneurons
Random insertion of a transgene encoding GFP under control of the GAD67 promoter resulted in the generation of a mouse line (X94) exhibiting specific expression of GFP in a population of SST-expressing interneurons that differed from Martinotti cells in a number of properties (Ma et al., 2006). X94 cells in S1 cortex were located in layer IV and V and had axonal projections that instead of reaching and targeting layer I, innervate profusely layer IV. The cells also differed from Martinotti cells in a number of electrophysiological properties. X94 cells had a much lower input resistance, approaching that of FS cells. They had spikes of shorter duration and often produced a stuttering firing pattern. They were capable of firing at higher frequencies than Martinotti cells but, in contrast to FS neurons, exhibited spike frequency adaptation (Ma et al., 2006). SST cells with similar intrinsic electrophysiological properties and named NFS1 were described in a fate mapping study of neurons derived from the medial ganglionic eminence (Miyoshi et al., 2007). On the other hand, as in the case of Martinotti cells, X94 cells receive strongly facilitating excitatory synapses. Both types of SST neuron also have in common powerful excitation by cholinergic agonists via muscarinic receptors (Fanselow et al., 2008; Xu and Rudy, unpublished observations). As a result of their lower input resistance, X94 cells are usually not driven to fire by repetitive activity in a single excitatory cell; however, the cells can be excited if the excitatory input is delivered in the presence of a muscarinic agonist (Xu and Rudy, unpublished observations).
There might be additional subpopulations of SST neurons in neocortex, given observed differences in intrinsic firing properties, the expression of molecular markers and connectivity (Xu et al., 2006; Gonchar et al., 2007; Miyoshi et al., 2007; McGarry et al., 2010). For example, about a third of SST interneurons in frontal, somatosensory (S1), and visual cortex (V1) contain calretinin (Xu et al., 2006). Some of this diversity within the SST population might be functionally significant. Although both SST/CR+ cells and SST/CR− cells exhibited similar Martinotti cell anatomical features and had similar adapting spike-firing patterns, they differed in the horizontal extension of their dendritic fields and number of primary processes (Xu et al., 2006). In addition, SST/CR− cells had narrower spikes with faster AHPs. Moreover, in layers II/III, where SST/CR+ cells were concentrated, the two subtypes of SST interneurons had different connectivity (Xu and Callaway, 2009). Although SOM+/CR− MCs received strong excitatory input from both layers II/III and IV, SOM+/CR+ MCs received excitatory input mainly from layers II/III and only weakly from layer IV. Another feature arguing for a true delineation between SOM+/CR− and SOM+/CR+ Martinotti cells is evidence suggesting that they are derived from different areas during development, with the SOM+/CR+ population mainly arising from the dorsal Nkx6-2-positive region of the MGE (Fogarty et al., 2007; Sousa et al., 2009).
Recently, McGarry et al. (2010) characterized the electrophysiological and anatomical properties of neocortical SST interneurons in GIN mice and used principal component analysis and cluster analysis to identify subtypes of SST neurons. The GIN mouse line, like the X94 line described earlier, is a transgenic mouse line expressing GFP under control of the GAD67 promoter that results in GFP expression in a subset of SST interneurons. In contrast to the X94 line, most GFP+ cells in this line are in layers II/III and have been characterized as Martinotti cells (Oliva et al., 2000; Ma et al., 2006). McGarry et al. (2010) detected, in addition to Martinotti cells, which were the majority (2/3) of studied neurons, two other types of SST neurons with a very sparse axonal arborization (McGarry et al., 2010). These cells also differed from those in the Martinotti cell group in a number of electrophysiological properties. Several investigators have used the GIN line to study Martinotti cells and have not reported the two other groups of SST neurons described by McGarry et al. (Ma et al., 2006; Xu et al., 2006; Fanselow et al., 2008; Xu and Callaway, 2009). McGarry et al. (2010) used mice with an average age of P14 (range P10–18), which is younger than what other investigators have used. FS cells mature significantly during an equivalent developmental period showing underdeveloped processes at early ages (Okaty et al., 2009; Goldberg et al., 2010). Some of the diversity observed in the study by McGarry et al. (2010) may reflect the presence of immature neurons.
5HT3aR Interneuron Group
As described earlier, Lee et al. (2010) found using double ISH and IHC that most, if not all, GABAergic neurons in S1 cortex that do not express PV or SST express the 5HT3a receptor; these 5HT3aR-positive interneurons compromise ~30% of GABAergic neurons in somatosensory cortex (Fig. 1). The group of interneurons defined by expression of the 5HT3a receptor is heterogeneous, more than the PV and SST groups, and remains to be fully characterized.
The neuropeptide VIP has been shown to be expressed in a set of neocortical interneurons that does not overlap with those that express PV or SST (Kawaguchi and Kubota, 1997; Xu and Callaway, 2009; Xu et al., 2010). Lee et al. (2010) found that all VIP-expressing neurons in somatosensory cortex express 5HT3a receptors. Based on coexpression of VIP and EGFP in 5Htr3aR-BACEGFP mice, it was estimated that VIP-expressing neurons account for ~40% of the 5HT3aR population in S1 (Lee et al., 2010). VIP cells are particularly enriched in layers II/III.
There seem to be several types of VIP interneurons, including neurons with bipolar, bitufted, and multipolar morphologies expressing different markers and exhibiting different intrinsic electrophysiological properties. In their characterization of the interneurons that originate in the caudal ganglionic eminence (CGE), where all VIP and at least 90% of 5HT3aR neurons originate (see below), Myoshi et al. (2010) described nine electrophysiologically different subtypes, of which six accounted for >93% of the CGE population. Among those expressing VIP, they described four types.
One type that has been described by several investigators has bitufted morphology with a vertically oriented, usually descending axon often reaching deep layers. These neurons have an irregular firing pattern characterized by APs elicited at irregular times during depolarizations near threshold, and a repetitive firing pattern with spike frequency and monotonic amplitude accommodation during larger depolarizations. They are often referred to as irregular-spiking (IS) cells (Cauli et al., 1997; Porter et al., 1998; Cauli et al., 2000; Ferezou et al., 2002; Galarreta et al., 2004; Lee et al., 2010; Miyoshi et al., 2010). A significant fraction of VIP neurons (40%) express CR, which, as described earlier, is also expressed in a fraction of SST neurons, as well as in interneurons that do not express VIP or SST (Cauli et al., 2000; Caputi et al., 2008; Lee et al., 2010; Miyoshi et al., 2010). The IS VIP cells have been reported to coexpress CR (Porter et al., 1998; Miyoshi et al., 2010). Future studies are needed to establish the proportion of the total VIP population that can be accounted by these neurons. In the study by Lee at al. (2010), IS bitufted cells accounted for a significant fraction of VIP neurons (>50%).
Another VIP-positive cell type described by various authors fired an initial train (often burst-like) of APs followed by spiking failure throughout the remaining period of a 500-ms depolarization and had bipolar/tripolar morphologies with axons that branched extensively locally as well as toward deeper layers. These cells have been named rapid-adapting (Butt et al., 2005), fast-adapting (Lee et al., 2010; Miyoshi et al., 2010), and IS2 (Porter et al., 1998) and seem to be the major VIP subtype that does not coexpress CR. It was the second most frequent VIP subtype observed by Lee et al. (2010). Two additional VIP+ cell types have been described, including a cell type identified as bursting nonadapting (bNA2) that exhibited bursts of two or three spikes followed after a delay by regular spiking and showed bipolar morphology (Lee et al., 2010; Miyoshi et al., 2010), and a more infrequent type with an “arcade” cell morphology called dNFS3 (for delayed non–fast-spiking 3), characterized by a delay to first spike at threshold and spikes with a sharp AHP and a regular firing pattern with monotonic fire frequency adaptation during larger depolarizations (Kawaguchi and Kubota, 1996; Butt et al., 2005; Miyoshi et al., 2010).
VIP-expressing basket cells with putative synaptic terminals surrounding the somata of other cells have been described but seem not to be abundant. Electrophysiologically these cells have been described as having regular (RSNP), bursting (BSNP), or IS firing patterns (Kawaguchi and Kubota, 1996, 1997; Xu and Callaway, 2009). Some of these neurons coexpress CCK and type 1 cannabinoid receptors (Galarreta et al., 2004; Sugino et al., 2006).
VIP neurons are among the interneurons with the highest input resistance and are therefore among the most excitable (Cauli et al., 2000; Lee et al., 2010; Miyoshi et al., 2010). Most VIP interneurons form synapses with dendrites. VIP-containing terminals have been observed on dendritic shafts and spines (Kawaguchi and Kubota, 1996; Kawaguchi and Kubota, 1997). In addition, VIP terminals have been observed around the somata of pyramidal cells, consistent with the observation that some VIP neurons are basket cells (Kawaguchi and Kubota, 1996). In addition, some VIP interneurons with bipolar/bitufted morphology have been shown to target mainly other interneurons (Acsady et al., 1996; David et al., 2007). The relationship between these patterns of connectivity and functional subtypes of VIP neurons remains to be established.
Non-VIP cells account for 60% of 5HT3aR neurons (Lee et al., 2010). More than 80% of these interneurons expressed reelin, which is also expressed by a fraction of SST neurons but not by VIP 5HT3aR neurons (Miyoshi et al., 2010). More than 90% of neurons in layer I belong to this group because only a small percentage of neurons in this layer expressed VIP. This reelin-positive, VIP-negative/SST-negative 5HT3aR group includes the neurogliaform cells. These cells, also called spiderweb cells, originally described by Ramon y Cajal, have a characteristic morphology consisting of a small, round soma from which multiple dendrites spread radially in all directions and have a wider round axonal plexus composed of fine branches (Kawaguchi and Kubota, 1997; Olah et al., 2007). Neurogliaform cells have a unique position among cortical interneurons because they establish electrical synapses not only with each other but also with other interneuron types, in contrast to all other tested interneuron types, which only make electrical contacts with homologous neurons. Thus, neurogliaform cells can link multiple networks of interneurons and were suggested to play a central role in generating and shaping synchronized activity of neuronal circuits (Price et al., 2005; Simon et al., 2005; Zsiros and Maccaferri, 2005). Neurogliaform cells are also unique in that they elicit slow, long-lasting IPSPs on pyramidal cells and other interneurons, through a combined activation of slow GABAA and GABAB receptors (Tamas et al., 2003; Olah et al., 2007). It was recently observed that neurogliaform axonal varicosities containing synaptic vesicles were often not associated with classical synaptic contacts, although neurogliaform cells produced hyperpolarizing responses in a large fraction of neighboring neurons. These results led to the suggestion that neurogliaform cells influence target neurons by volume release of GABA (Olah et al., 2009).
Neurogliaform cells typically have a late spiking (LS) firing pattern characterized by a slow ramp depolarization preceding firing at threshold. During suprathreshold depolarizations, the cells fire accommodating firing trains (Kawaguchi and Kubota, 1997; Tamas et al., 2003). Studies of CGE-derived and 5HT3aR interneurons have described two types of “late-spiking” cells, both of which are multipolar, express reelin but not VIP, and have been termed LS1 and LS2 (also described as “dense plexus cells” and initial adapting) (Butt et al., 2005; Miyoshi et al., 2007; Lee et al., 2010; Miyoshi et al., 2010). Electrophysiologically, LS1 and LS2 are distinguished by fact that the delay to first spike is more robust in LS1 neurons and can still be observed during depolarizations eliciting up to three to five spikes, whereas in LS2, the delay is only observed close to threshold when the cell responds with a single spike. Furthermore, LS2 neurons have significantly lower input resistance and maximum firing rate compared with LS1 cells. LS1 cells correspond to neurogliaform cells, whereas LS2 cells exhibit larger soma size and longer but less branching dendrites (Miyoshi et al., 2010).
In addition to LS1 and LS2, which were the most frequently encountered non-VIP 5HT3aR neurons, another population of non-VIP bursting nonadapting neurons (bNA) and a population of multipolar IS neurons have been observed (Gelman et al., 2009; Lee et al., 2010; Miyoshi et al., 2010). The bNA neurons electrophysiologically resemble the VIP-positive bNA2 neurons described earlier but have multipolar morphology and are found mainly in superficial layers I or I/II boundary. IS non-VIP multipolar cells resembled cells derived from an Nkx5-1-expressing progenitor population within the pre-optic area (POA) (Gelman et al., 2009).
Two rare populations, dIB (delayed intrinsic-bursting) and sIB (sigmoid intrisic-bursting), were detected in the analysis of CGE-derived interneurons (Miyoshi et al., 2010) but were not observed among 5HT3aR neurons (Lee et al., 2010). The dIB subtype shows intrinsic electrophysiological properties similar to the PV-expressing MB neurons described earlier (Blatow et al., 2003). Like these neurons, they have multipolar morphology and are observed mainly in superficial layers.
The lack of specific markers makes it difficult to estimate the frequencies of each of the subtypes described above. Although these studies have been performed by sampling randomly in the 5HT3aR mouse, the sample is not yet sufficiently large to definitely establish subtype proportions from the frequency of observing particular firing patterns.
Modulation of 5HT3aR Interneurons by Ionotropic Serotonin and ACh Receptors
Although 5HT3aR interneurons are heterogeneous, they are all potently modulated by ionotropic serotonin receptors (Lee et al., 2010). Local somatic application of mCPBG, a 5HT3aR-selective agonist, to EGFP-positive neurons evoked fast and robust depolarization in more than 40 cells tested throughout all layers and including all the subtypes identified electrophysiologically. In contrast, no response was observed after application of mCPBG to PV and SST-expressing neurons.
In addition to serotonin and glutamate, which mediate most local and long range excitation of interneurons, the other major neurotransmitter for which there are ionotropic receptors is acetylcholine (ACh). Interestingly, 5HT3aR are also excited by cholinergic agonists acting on nicotinic receptors. This also seems to be a common property of all 5HT3aR neurons independently of layer location or electrophysiological or morphological subtype (Ferezou et al., 2002; Lee et al., 2010).
Functional Significance of 5HT3aR Interneurons
Knowledge of the connectivity of 5HT3aR neurons is crucial to develop hypotheses about their functional roles. Some information on the source of inputs to VIP interneurons and neurogliaform cells in layers II/III was obtained by Xu and Callaway (2009) using glutamate uncaging to drive presynaptic sources (Xu and Callaway, 2009). All cells were found to receive strong excitatory and inhibitory input from layers II/III; however, inputs from other layers, mainly layer IV and Va, depended on the subtype of interneuron. Electrophysiological characterization of these inputs is still lacking. Furthermore, the correspondence between the subtypes of neurons studied by Xu and Callaway and the subtypes of 5HT3aR neurons described by other investigators is not easy to establish, emphasizing the need to develop better tools to classify 5HT3aR neurons. Furthermore, other than neurogliaform cells, little is known about the output connections and function of 5HT3aR interneurons. Overall, these cells are more poorly understood than PV and SST interneurons.
One important consideration is the physiological significance of the fact that all 5HT3aR neurons undergo fast modulation by ionotropic neuromodulatory receptors for ACh and serotonin. This provides insights into the possible functions of these neurons in cortical circuits. Perhaps, neuromodulatory systems rapidly engage these INs during specific brain states and behavioral contexts that are linked to these neuromodulators. Supporting this idea, a recent study by Gentet et al. (2010) demonstrate that FS versus non-FS GABAergic interneurons in superficial layers are differentially modulated depending on the brain state and behavioral context of the animal (Gentet et al., 2010). By recording intracellularly using GAD67-GFP mice, Gentet et al. showed that non-FS INs become more depolarized and exhibit enhanced firing rates during whisking periods, which presumably corresponds to an alert state. To our knowledge, this study provides the first direct evidence that different types of interneurons might be recruited differentially depending on a brain state and behavioral context of awake animals. Gentet et al. did not determine which types of non-FS interneurons were engaged during whisking; however, given their abundance relative to SST interneurons in superficial layers, it is likely that these were 5HT3aR neurons.
A critical issue that still needs to be studied is whether neuromodulators affect 5HT3aR interneurons differentially depending on their subtypes. Lee et al. (2010) showed that 5HT3aR neurons in deep layer III and in layer IV receive weak, but potentially significant, thalamocortical input. An intriguing possibility is that excitation by cholinergic or serotonergic inputs may act in synchrony with TC inputs to enhance activation of these interneurons.
More than two thirds of 5HT3aR interneurons reside in superficial layers, where they constitute the majority of GABAergic interneurons. Each cortical layer has a distinct input and output system, in addition to its own local circuit. Layers II/III in sensory cortex is central to the processing of sensory information. On arrival to thalamo-recipient layer IV, sensory information is transferred to layers II/III, where it is integrated with information from other columns and other cortical areas. Neurons in superficial layers are highly interconnected with other relevant cortical areas, thus mediating corticocortical communication. In primary somatosensory cortex, for example, long-range inputs to superficial layers originate predominantly from primary motor cortex and from secondary somatosensory cortex (Cauller et al., 1998; Zhang and Deschenes, 1998). One possible functional role of 5HT3aR neurons might be to provide feedforward or feedback inhibition in response to long-range corticocortical inputs. Although the functional connectivity of long-range cortical inputs to pyramidal neurons in superficial layers in primary S1 has been mapped (Petreanu et al., 2009), the long-range cortical projection to interneurons has not been studied. Future studies need to address the synaptic connectivity and dynamics of long-range intracortical inputs to different subtypes of 5HT3aR neurons. These neurons are ideally positioned to mediate rapid control of converging cortical activity depending on the brain state and behavioral context of animals. Furthermore, acetylcholine and serotonin are likely to be essential regulators of these neurons in information processing by modulating gain in the cortical network during task discrimination, plasticity, and learning.
In addition to releasing GABA, 5HT3aR neurons release vasoactive substances such as VIP. Moreover, neurogliaform cells have been shown to express NOS. These neurons have been shown to contact local microvessels. Evoked firing of single VIP- or NOS-expressing interneurons was sufficient to dilate neighboring microvessels (Cauli et al., 2004; Cauli and Hamel, 2010).
Embryological Origins of the Three Groups of Neocortical GABAergic Interneurons
Several studies have shown that the subtype identity of cortical interneurons can be predicted by its place and time of origin during development (Xu et al., 2004; Butt et al., 2005; Wonders and Anderson, 2006; Flames et al., 2007; Fogarty et al., 2007; Ghanem et al., 2007; Miyoshi et al., 2007; Gelman et al., 2009; Miyoshi et al., 2010). The majority of murine cortical interneurons are derived from progenitor zones of the ganglionic eminences in the ventral telencephalon. These zones can be divided into lateral, medial, and caudal (LGE, MGE, and CGE, respectively) based on morphological features as well as the expression of transcription factors. The ventral origin of cortical interneurons has been demonstrated using several techniques, such as dye labeling in cortical slices (Anderson et al., 1997), transplantation experiments (Wichterle et al., 2001; Nery et al., 2002; Xu et al., 2004; Butt et al., 2005; Flames et al., 2007), and most recently with a technique called genetic inducible fate mapping (GIFM) (Fogarty et al., 2007; Miyoshi et al., 2007; Gelman et al., 2009; Miyoshi et al., 2010). The latter technique uses the specific expression patterns of genes or transgenic elements in the different regions during development. By linking this expression to the expression of the enzyme cre-recombinase and a cre-dependent reporter, cells can be permanently labeled during development and then followed during their entire lifespan (Zinyk et al., 1998). This permanent labeling overcomes the problem associated with dynamic gene expression, which limits the use of direct EGFP-expressing alleles, such as the 5HT3aR-BACEGFP, in establishing the origin of the cells.
The two most studied groups of interneurons (PV and SST expressing) are derived exclusively from the MGE (Xu et al., 2004; Fogarty et al., 2007; Miyoshi et al., 2007). It has also been reported that different regions of the MGE give rise to different subtypes, with progenitors giving rise to SST and PV being biased to dorsal versus ventral MGE, respectively (Flames et al., 2007; Fogarty et al., 2007; Wonders et al., 2008). Until recently, less was known about the origin of the other interneurons, such the VIP- and/or the non-SST calretinin-expressing cells. In vitro and transplantation studies had suggested that among others, bipolar calretinin and neurogliaform cells are derived from the CGE (Xu et al., 2004; Butt et al., 2005; Wonders et al., 2008), and this was confirmed in a GIFM study using a Mash1-BACCreER mouse (whose expression is fortuitously restricted to the L/CGE-derived populations) crossed with an RCE (Rosa26-CAG-EGFP) reporter (Sousa et al. 2009; Miyoshi et al., 2010). We now know that the CGE gives rise to ~30% of all cortical interneurons and that this group not only contains the bipolar calretinin cells but also the entire VIP as well as the reelin-positive/SST-negative population that includes neurogliaform cells. Another marker often used in the field is neuropeptide-Y (NPY), which seems to label a variety of subtypes originating from both the MGE and the CGE. Furthermore, NPY expression is activity dependent and has been shown to be quickly upregulated in response to increased activity, which perhaps limits its use as a marker for specific interneuron populations.
Several studies have shown that EGFP-expression in the 5HT3aR-BACEGFP mouse can be detected early during embryogenesis (Inta et al., 2008; Chameau et al., 2009; Lee et al., 2010; Vucurovic et al., 2010). This expression is mainly confined to the CGE but also extends rostrally to parts of the LGE and ventrally to the POA and nearby areas. The EGFP expression excludes the VZ, suggesting that the expression is initiated after cells have excited the cell cycle at the point, when they have started their migration. Their active tangential migration into the cortex, coupled with the possibility that 5HT3aR-expression is actively up- or down-regulated, makes it difficult to know with certainty the origin of this population. This has been addressed partly by using two different GIFM strategies (Lee et al., 2010). One was by combining the Nkx2-1-BACcre and the cre-dependent red R26RtdRFP reporter with 5HT3aR-BACEGFP showing that there is no overlap between red and green cells. The Nkx2-1-BACcre line labels a majority of MGE-derived interneurons, which confirms the immunohistochemical findings of no or minimal overlap of 5HT3aR with PV or SST and excludes the MGE as a source. The second strategy used the L/CGE-specific Mash1-BACCreER; R26RtdRFP mouse line showing a nearly complete overlap with 5HT3aR cells confirming the CGE as the major source of this population. However, the finding that 5HT3aR is expressed in more than the populations reported to come from the CGE opens up for the possible contribution from other progenitor zones (Lee et al., 2010).
Several different sets of findings suggest that in addition to the ganglionic eminences, cortical interneurons might be derived from more ventral structures such as the septum and the POA. Recently, it was confirmed, using GIFM with an Nkx5-1cre line, that the POA also gives rise to a small and seemingly homogenous group of multipolar IS cortical interneurons mostly populating superficial layers (including layer I) that express NPY but not any of the interneuron markers PV, SST, CR, or VIP. The embryonic expression of 5HT3aR in the POA along with the fact that a proportion of the cells recorded in the 5HT3aR-BACEGFP animal exhibit electrophysiological and morphological characteristics of these POA-derived neurons argues for a contribution of POA-derived neurons to the 5HT3aR population. In the study by Gelman et al. (2009), however, they used the Nkx2-1cre together with electroporation of a cre-dependent reporter construct to label POA-derived cells. The fact that Nkx5-1 and Nkx2-1 are confined to subregions of the POA could help explain the seeming contradictive finding of no overlap between the Nkx2-1-BACcre;R26RtdRFP, and 5HT3aR-BACEGFP. It remains to be shown directly whether the Nkx5-1cre labeled neurons express 5HT3aR and to determine the size of the POA contribution to the 5HT3aR population.
Although neocortical neurogliaform cells seem to originate exclusively from progenitors in the CGE; in the hippocampus, both inducible fate-mapping and Nkx2.1 conditional loss of function experiments suggest that these interneurons can originate in both the MGE and the CGE (Tricoire et al., 2010). According to this study, neurogliaform cells expressing the neuronal isoform of nitric-oxidase synthetase (nNOS) and a related nNOS-expressing population known as Ivy cells (Fuentealba et al., 2008) are derived from the MGE, whereas nNOS-negative neurogliaform cells are CGE derived. These findings suggest the possibility that most neocortical neurogliaform cells lack nNOS and are equivalent to the nNOS-negative neurogliaform hippocampal population, and that the nNOS+ neurogliaform population in the neocortex is either small and has not been detected or does not exist. We are currently investigating these possibilities.
It is generally believed that the LGE does not give rise to a major cortical interneuron population (Wichterle et al., 2001; Wonders and Anderson, 2006; Miyoshi et al., 2010). There are reports contradicting this, but the issue is complicated by the fact that a proportion of MGE-derived cells, as well as CGE-derived cells, have been shown to migrate through the LGE on their way to the cortex (Wichterle et al., 2001). To definitely solve this issue, we would need a CGE or LGE-specific cre-driver line; until then, however, we hypothesize that the 5HT3aR-positive LGE-population does not significantly contribute to the populations of cortical interneurons.
SUMMARYAND PERSPECTIVES
The availability of a mouse line expressing EGFP in all neocortical 5HT3aR-expressing neurons and without ectopic expression allowed Lee et al. (2010) to conclude that these neurons account for virtually all the interneurons that do not express PV or SST, at least within S1 cortex, where the majority of the analysis has been done so far. Together these three groups of neurons account for nearly 100% of GAD67-expressing neurons.
A classification of neocortical interneurons that is useful to understand neocortical function must include, for all subtypes that serve different functional roles in the cortical circuit, good knowledge of their dendritic and axonal morphology, the sources, location and properties of their input synapses, the cell and cell domain distribution and properties of their output synapses, and their intrinsic electrophysiological properties.
Although it is likely that the major groups and subgroups of interneurons in the neocortex have been identified, most of the interneuron classes within each subgroup still need to be better defined (Fig. 2). As we discussed, PV basket cells have been shown to vary in a number of properties such as somatic shape, size and extent of dendritic field and axonal projections, expression of certain molecular markers, including functionally important molecules such as K+ channels and metabotropic receptors, the efficacy and dynamics of excitatory synaptic inputs, and firing properties. Similarly, Martinotti SST interneurons have been shown to vary in the expression of certain markers such as the Ca2+-binding protein calretinin and in their firing properties. However, although the potential significance of these differences is sometimes clear, in most cases, it is difficult to predict. Furthermore, often the anatomical, electrophysiological, and molecular data have been gathered independently. All these factors make it difficult to define interneuron subtypes.
The classes of interneurons in the VIP and non-VIP subgroups of 5HT3aR neurons also need to be much better defined. Further advance along these lines might be possible by increasing the sample of 5HT3aR neurons for which there is both anatomical and electrophysiological data.
Characterization of the input and output connections of interneurons is not only necessary to understand their roles, but is likely to be important in revealing which are important anatomical and electrophysiological differences that should be used for the purpose of defining functional interneuron classes. However, given that obtaining these data is time consuming, the sample of neocortical interneurons for which electrophysiological characterization is available is much larger than that for which information on connectivity is available. Somogyi and Klausberger (2005) classified the GABAergic interneurons in the CA1 area of the hippocampus into >16 classes, believed to reflect groups with distinct roles in the hippocampal circuit. Differences in the distribution of input and output synapses were key criteria for their classification. The assessment of differences in input and output connectivity is much easier in the CA1 area of the hippocampus, given the laminated pattern of the inputs and outputs in this structure.
One critical challenge, which applies to all the interneuron groups, is the need to distinguish incidental differences (even if useful as markers) from those that are important for the function of a given type of interneuron within neocortical circuits. For example, although it is clear that it will be important for a given interneuron whether its axon only makes local connections, or whether the axon extends to other layers or to other columns, it is more difficult to assess which differences in the firing properties observed in response to step depolarizations reflect significant differences in how the interneuron will respond to synaptic excitation. Does the degree of firing frequency adaptation during 500 ms depolarizations, or whether the neuron fires with a regular firing pattern or initially with closely spaced interspike intervals (“a burst”) represent important variables? One sure source of excessive variability is differences introduced by the use of different experimental conditions between laboratories.
One should also consider the possibility that cells with otherwise similar properties may, as an adaptation to the microcircuit in which they function, differ in the domain they innervate, their response to specific neuromodulators, or other specific properties. A recent study in olfactory cortex nicely illustrates the idea that although the intrinsic electrophysiological properties of a specific interneuron subtype must be important for its function, its role in a given brain structure will also depend on its connectivity, that is, the circuit in which it is incorporated. In both the hippocampus and neocortex, somatic targeting fast spiking basket cells mediate powerful feedforward inhibition of principal cells (Pouille and Scanziani, 2001; Gabernet et al., 2005; Cruikshank et al., 2007). In the neocortex, thalamocortical (TC) axons synapse on both FS cells and excitatory principal neurons in thalamo-recipient layers (mainly layer 4). Activation of both excitatory and inhibitory cells establishes a simple disynaptic circuit that provides powerful, local feedforward inhibition. The latency between the onset of the TC excitation of excitatory cells and the onset of feedforward inhibition from FS cells results in a brief time window during which the excitatory neurons can integrate TC inputs. This window is critical for the processing of sensory information in the neocortex (Miller et al., 2001; Swadlow, 2003; Wilent and Contreras, 2004, 2005; Gabernet et al., 2005; Cruikshank et al., 2007). A similar circuit exists in the CA1 area of the hippocampus, where basket cells mediate feedforward inhibition of CA1 pyramidal cells. As in the neocortex, the duration of the time window within which Schaffer collateral excitatory inputs on CA1 pyramidal cells can summate to reach spike threshold is determined by the basket cell-mediated feedforward inhibition (Pouille and Scanziani, 2001). FS cells in layers II/III in somatosensory cortex likely mediate feedforward inhibition of layer IV inputs unto layer II/III pyramidal cells (Helmstaedter et al., 2008). Moreover, FS cells have been shown to mediate powerful feedforward inhibition in many other structures, such as the neostriatum (Tepper and Bolam, 2004). Excitatory synapses on FS cells are usually strongly depressing and so are typically the output synapses of FS cells. This has led to the view of FS cells as mediators of fast and powerful, but transient feedforward inhibition in the brain, and to the idea that this early inhibition shifts to a late but more sustained inhibition of principal cells mediated by dendritic targeting interneurons that receive strongly facilitating excitatory inputs (Pouille and Scanziani, 2004; Silberberg and Markram, 2007; Kapfer et al., 2007; Tan et al., 2008).
However, in olfactory cortex, lateral olfactory tract afferents containing the axons of projecting mitral and tufted cells in the olfactory bulb target exclusively layer Ia. From the point of view of being the input layer, layer Ia in olfactory cortex is equivalent to layer IV in other sensory cortices; however, it lacks FS cells. Instead, mitral and tufted cell axons synapse on layer Ia interneurons, which target the layer Ia apical dendrites of pyramidal cells with cell bodies in deeper layers (Stokes and Isaacson, 2010). The same axons target the dendrites of the excitatory cells. As a result, in an homologous fashion to what is observed in neocortex and hippocampus, bursts of mitral and tufted cell activity mediate a short-latency feedforward disynaptic inhibition of the pyramidal cells, except that this inhibition occurs on the distal apical dendrite, instead of the soma, of the pyramidal cells. Fast-spiking cells in piriform cortex are localized mainly in layer III where they are recruited by recurrent excitation from the pyramidal cells. As in other structures, the FS cells in piriform cortex target the somatic domain of the pyramidal cells and their inputs are depressing. However, excitatory inputs on the pyramidal cells are facilitating. Hence, during bursts of inputs to the piriform cortex perisomatic targeting FS cells are recruited late and provide “feedback” somatic inhibition of the pyramidal cells. Thus, in olfactory cortex, inhibition shifts from the dendrite to the soma, the opposite of what is observed in neocortex and hippocampus, although the properties of the interneurons seem similar in the three structures.
Given the advances in mouse genetics, it is likely that an understanding of interneuron diversity will be obtained first in this species. A challenge will then be to determine how this applies to other species, particularly humans. We recently had the opportunity to record FS cells in human neocortex and found that their properties are remarkably similar to those in mice, but data exist to suggest that there might be important differences in interneuron populations between primates and rodents. For example, the total proportion of GABAergic neurons in the cortex might be much higher in primates (24–30%) compared with rodents ((15%) (Hendry et al., 1987; Beaulieu, 1993; Gabbott and Bacon, 1996; Gabbott et al., 1997). Also, it has been reported that 74% of GABAergic neurons in the primary visual cortex (V1) of macaques express PV, much higher than the values reported in rodents (Van Brederode et al., 1990).
REFERENCES
- Acsady L, Gorcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996;73:317–334. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrio-nuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, et al. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat rev. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Modulation of neocortical interneurons: Extrinsic influences and exercises in self-control. Trends Neurosci. 2005;28:602–610. doi: 10.1016/j.tins.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain res. 1993;609:284–292. doi: 10.1016/0006-8993(93)90884-p. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Connors BW. Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. J Neurophysiol. 2002;88:1924–1932. doi: 10.1152/jn.2002.88.4.1924. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, et al. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Caputi A, Rozov A, Blatow M, Monyer H. Two Calretinin-Positive GABAergic Cell Types in Layer 2/3 of the Mouse Neocortex Provide Different Forms of Inhibition. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn175. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, et al. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci USA. 2000;97:6144–6149. doi: 10.1073/pnas.97.11.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller LJ, Clancy B, Connors BW. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J Comp Neurol. 1998;390:297–310. [PubMed] [Google Scholar]
- Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci USA. 2009;106:7227–7232. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Erisir A, Farb C, Nadal MS, Ozaita A, Lau D, Welker E, Rudy B. K(+) channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J Neurosci. 1999;19:9332–9345. doi: 10.1523/JNEUROSCI.19-21-09332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BD, Goldberg EM, Rudy B. Diversity of nearthreshold discharge properties among fast-spiking interneurons of mouse somatosensory cortex. Soc Neurosci. 2007 Abstract. [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, et al. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feed-forward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- David C, Schleicher A, Zuschratter W, Staiger JF. The innervation of parvalbumin-containing interneurons by VIP-immunopositive interneurons in the primary somatosensory cortex of the adult rat. Eur J Neurosci. 2007;25:2329–2340. doi: 10.1111/j.1460-9568.2007.05496.x. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Neocortical neuronal diversity: Chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb Cortex. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Garcia Sola R, Marco P, del Rio MR, Pulido P, Ramon y Cajal S. Selective changes in the micro-organization of the human epileptogenic neocortex revealed by parvalbumin immunoreactivity. Cereb Cortex. 1993;3:39–48. doi: 10.1093/cercor/3.1.39. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SHC, Jones EG. Visualization of Chandelier Cell Axons by Parvalbumin Immunoreactivity in Monkey Cerebral-Cortex. Proc Natl Acad Sci USA. 1989;86:2093–2097. doi: 10.1073/pnas.86.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Márton LF, Csicsvari J, Thomson A, et al. Ivy cells: A population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a, b,c, 25 and 32) in the monkey. II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: Morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Erdelyi F, Szabo G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci. 2004;24:9770–9778. doi: 10.1523/JNEUROSCI.3027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J Neurosci. 2003;23:1622–1630. doi: 10.1523/JNEUROSCI.23-05-01622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nobrega-Pereira S, Pierani A, Kessaris N, Marin O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Ghanem N, Yu M, Long J, Hatch G, Rubenstein JL, Ekker M. Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J Neurosci. 2007;27:5012–5022. doi: 10.1523/JNEUROSCI.4725-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortical and intracortical synaptic connections: Quanta, innervation, and reliability. Neuron. 1999;23:385–397. doi: 10.1016/s0896-6273(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12:21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Jeong HY, Kruglikov I, Tremblay R, Lazarenko RM, Rudy B. Rapid Developmental Maturation of Neocortical FS Cell Intrinsic Excitability. Cereb Cortex. 2010 Aug 12; doi: 10.1093/cercor/bhq138. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Rudy B. Molecular contributions to the subthreshold properties of FS cells in mouse medial prefrontal cortex. Soc Neurosci. 2005 Abstract. [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat. 2007;1:3. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, McCormick DA. Rapid neocortical dynamics: Cellular and network mechanisms. Neuron. 2009;62:171–189. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Sakmann B, Feldmeyer D. Neuronal correlates of local, lateral, and translaminar inhibition with reference to cortical columns. Cereb Cortex. 2009;19:926–937. doi: 10.1093/cercor/bhn141. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Emson PC, Lawson DE, Heizmann CW, Streit P. Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res. 1989;76:467–472. doi: 10.1007/BF00247904. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Schwark HD, Jones EG, Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci USA. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78:1743–1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Katsumaru H, Kosaka T, Heizmann CW, Hama K. Fast spiking cells in rat hippocampus (CA1 region) contain the calcium-binding protein parvalbumin. Brain res. 1987;416:369–374. doi: 10.1016/0006-8993(87)90921-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: Containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.1869-10.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–406. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- McGarry LM, Packer AM, Fino E, Nikolenko V, Sippy T, Yuste R. Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front Neural Circuits. 2010;4:12. doi: 10.3389/fncir.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Escabi MA, Schreiner CE. Feature selectivity and interneuronal cooperation in the thalamocortical system. J Neurosci. 2001;21:8136–8144. doi: 10.1523/JNEUROSCI.21-20-08136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–7052. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah S, Komlosi G, Szabadics J, Varga C, Toth E, Barzo P, Tamas G. Output of neurogliaform cells to various neuron types in the human and rat cerebral cortex. Front Neural Circuits. 2007;1:4. doi: 10.3389/neuro.04.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol. 2000;83:1158–1166. doi: 10.1152/jn.2000.83.3.1158. [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Hartings JA, Brumberg JC, Simons DJ. Cortical damping: Analysis of thalamocortical response transformations in rodent barrel cortex. Cereb Cortex. 2003;13:33–44. doi: 10.1093/cercor/13.1.33. [DOI] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Staiger JF, Lambolez B, Rossier J, Audinat E. Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur J Neurosci. 1998;10:3617–3628. doi: 10.1046/j.1460-9568.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25:6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Santana N, Celada P, Mengod G, Artigas F. In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb Cortex. 2004;14:1365–1375. doi: 10.1093/cercor/bhh097. [DOI] [PubMed] [Google Scholar]
- Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking interneurons during neocortical UP states. Proc Natl Acad Sci USA. 2008;105:8428–8433. doi: 10.1073/pnas.0712219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21:4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Simon A, Olah S, Molnar G, Szabadics J, Tamas G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci. 2005;25:6278–6285. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6–2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–i10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes CC, Isaacson JS. From dendrite to soma: Dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron. 2010;67:452–465. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- Tan Z, Hu H, Huang ZJ, Agmon A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc Natl Acad Sci USA. 2008;105:2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front Neurosci. 2007;1:19–42. doi: 10.3389/neuro.01.1.1.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Clark BD, Rudy B. Layer-specific organization within the fast-spiking interneuron population of mouse barrel cortex. Soc Neurosci. 2010 Abstract. [Google Scholar]
- Trevelyan AJ, Sussillo D, Watson BO, Yuste R. Modular propagation of epileptiform activity: Evidence for an inhibitory veto in neocortex. J Neurosci. 2006;26:12447–12455. doi: 10.1523/JNEUROSCI.2787-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Daw MI, Sousa VH, Miyoshi G, Jeffries B, et al. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30:2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, Obata K, et al. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex. 2008;18:315–330. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- Van Brederode JF, Mulligan KA, Hendrickson AE. Calcium-binding proteins as markers for subpopulations of GABAergic neurons in monkey striate cortex. J Comp Neurol. 1990;298:1–22. doi: 10.1002/cne.902980102. [DOI] [PubMed] [Google Scholar]
- Vruwink M, Schmidt HH, Weinberg RJ, Burette A. Substance P and nitric oxide signaling in cerebral cortex: Anatomical evidence for reciprocal signaling between two classes of interneurons. J Comp Neurol. 2001;441:288–301. doi: 10.1002/cne.1413. [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, et al. Serotonin 3A Receptor Subtype as an Early and Protracted Marker of Cortical Interneuron Subpopulations. Cereb Cortex. 2010 doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat rev. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Callaway EM. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci. 2009;29:70–85. doi: 10.1523/JNEUROSCI.4104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. Origin and classification of neocortical interneurons. Neuron. 2005;48:524–527. doi: 10.1016/j.neuron.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Deschenes M. Projections to layer VI of the posteromedial barrel field in the rat: A reappraisal of the role of corticothalamic pathways. Cereb Cortex. 1998;8:428–436. doi: 10.1093/cercor/8.5.428. [DOI] [PubMed] [Google Scholar]
- Zinyk DL, Mercer EH, Harris E, Anderson DJ, Joyner AL. Fate mapping of the mouse midbrain-hindbrain constriction using a site-specific recombination system. Curr Biol. 1998;8:665–668. doi: 10.1016/s0960-9822(98)70255-6. [DOI] [PubMed] [Google Scholar]
- Zsiros V, Maccaferri G. Electrical coupling between interneurons with different excitable properties in the stratum lacunosum-moleculare of the juvenile CA1 rat hippocampus. J Neurosci. 2005;25:8686–8695. doi: 10.1523/JNEUROSCI.2810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]