Abstract
Pancreatic cancer (PC) remains a complex malignancy with the worst prognosis, lack of early diagnostic symptoms and resistance to conventional chemo- and radiotherapies. A better understanding of the etiology and early developmental events of PC requires profound attention. The evolution of fully blown PC from initial pancreatic injury is a multi-factorial phenomenon with a series of sequential events. The initial acute infection or tissue damage triggers inflammation that, in conjunction with innate immunity, establishes a state of homeostasis to limit harm to the body. Recurrent pancreatic injuries due to genetic susceptibility, smoking, unhealthy diet, and alcohol abuse induces a pro-inflammatory milieu, consisting of various types of immune cells, cytokines, chemokines, growth factors and restructured extracellular matrix, leading to prolonged inflammatory/chronic conditions. Cells having sustained DNA damage and/or mutagenic assault take advantage of this prolonged inflammatory response and aid in the initiation and development of neoplastic/fibrotic events. Eventually, many tumor-stromal interactions result in a chaotic environment accompanied by a loss of immune surveillance and repair response, thereby leading to PC. A better understanding of the inflammatory markers defining this “injury-inflammation-cancer” pathway would help to identify novel molecular targets for early screening and therapeutic intervention for this lethal malignancy.
Keywords: Neoplasms, Inflammation, Pancreatic neoplasms, Obesity, Smoking, Pancreatitis
Pancreatic cancer (PC) is the fourth and fifth leading causes of cancer-related deaths in the United States and world-wide respectively. As per National Cancer Institute (NCI) estimate, 43,920 new cases and 37,390 deaths will be caused by PC in 2012.1. The overall prognosis of PC is extremely poor due to the lack of early diagnostic symptoms, distant metastatic spread by the time of diagnosis as well as the intrinsic and acquired resistance to conventional therapeutic modalities.2 Surgical tumor resection is the only effective curative therapy but clinically >80% of patients present with an unresectable tumor with distant organ metastasis leading to a five-year survival rate of <6%.3 Unfortunately, post-surgically most PC patients succumb to recurrence and metastasis not withstanding adjuvant therapies.2 This highlights the importance of understanding the etiology of pancreatic cancer (PC).
Meta-analysis of various epidemiological studies has revealed the association of PC with several risk factors including chronic pancreatitis (CP), smoking, obesity, diabetes, age and family history,4 among which CP is the most considerate risk factor for PC.5 It has been reported that 40% of patients with hereditary pancreatitis develop PC in their lifetime.6 Cigarette-smoking causes DNA mutations,7 induces inflammatory markers, contributes to fibrosis and is known to double the risk of developing PC. Over time, data from various epidemiological studies and subsequent meta-analyses show a link between obesity and PC.8 Considerately, these meta-analyses revealed that both central and overall obesity are associated with a 1.4 to 2-fold increased risk of PC.9
The prolonged duration of chronic inflammation incurred during pancreatitis results in the development of PC in the long run.10 The mutations associated with pancreatitis are not found in sporadic pancreatic adenocarcinomas, suggesting that the effects are indirect via recurrent pancreatitis or chronic inflammation. Thus, numerous inflammatory mediators induced due to smoking, obesity, diabetes, alcohol abuse and CP are capable of causing genomic damage, altered gene expression and induction of oncogenic signaling pathways leading to the development of pancreatic intraepithelial neoplasias (PanINs) and further growth and progression of PC. Although genetic alterations associated with the progression of PC are well characterized, the exact mechanisms by which different risk factors contribute to these molecular alterations are still obscure. This timely review article highlights the potential role of the various risk factors in the process of oncogenic transformation in the pancreas, under the background of inflammation and it discusses the various mediators bridging the link between inflammation, pancreatitis and PC.
Inflammation and wound healing
In order to breach the complexity of inflammation–mediated carcinogenesis, it is important to understand its role in otherwise normal conditions. What is inflammation? Is it useful or harmful? How does it play a role in the evolution of cancer starting from a mild pancreatic injury, developing into a pancreatitis condition and consequentially resulting in initiation or the progression of cancer?
Under normal conditions, the architecture of the pancreas is preserved by equilibrium between ECM synthesis and degradation. The source of ECM synthesis is the fibroblast-like cell type, with a close resemblance to hepatic stellate cells, known as pancreatic stellate cells (PSCs).11 In the healthy pancreas, PSCs are located in the peri-acinar and interlobular area and have a low proliferation capacity. In this state, they hardly synthesize any ECM.12 Hence, the tissue maintains a defined integrity and homeostasis with considerable sophistication and subtlety until it encounters a perturbation such as an infection or tissue cell damage. This is when inflammation comes into the picture as an acute response in order to establish a homeostasis and to limit harm to the body. Without inflammation, wounds would never heal.
Upon tissue assault, inflammation organizes sequential catabolic and anabolic processes, first eliminating foreign pathogens and followed by tissue remodeling. Recruitment of the inflammatory cells to the sites of tissue injury and the extra-cellular matrix (ECM) includes the selectin family of adhesion molecules (L- P-, and E-selectin), α4β1 and α4β7 integrins, vascular cell-adhesion molecule-1 (VCAM-1) and extra-cellular proteases, such as matrix metalloproteinases (MMPs). The activation of immune responses under this pro-inflammatory milieu efficiently eliminates invading pathogens, damaged cells and extra-cellular matrix (ECM). Platelets are initially activated regulating vascular permeability, serum fibrinogen influx and fibrin clot formation. Endothelial cells migrate into the clot; they proliferate and form new blood vessels. Fibroblasts are then activated that migrate into the wound bed and secrete collagen type III, which is later replaced by collagen type I. Synthesis and deposition of collagen and fibrinogen by fibroblasts is stimulated by various factors including TGF-β, PDGF and IL-1s.13 A large percentage of the fibroblasts at the wound site differentiate into myofibroblasts, which are crucial for wound contraction. The final phase of the healing process is re-epithelialization and migration of the epithelial cells atop the wound bed provides protection for the new tissue. This requires both dissolution of the fibrin clot and collagen degradation.13
The different events that are involved in repair must be tightly regulated and synchronized, making inflammation a self-limiting process, with prompt and spontaneous production of anti-inflammatory cytokines, followed by the pro-inflammatory cytokines. During wound repair, collagen production and the degradation are under precise spatial and temporal control. Moreover, the reciprocal signaling between the epithelial and stromal cells in order to facilitate healing subsides right after the wound is healed.
Over healing hypothesis: unchecked inflammation leads to tumor
A gamut of studies provide evidence that chronic inflammatory process contributes to pathogenesis in chronic cases, including diabetes, asthma, Alzheimer’s disease, cardiovascular diseases and even cancer. Sir Alexander Haddow suggested that “tumor production is a possible overhealing”.14 The observation of leukocytes in neoplastic tissue, by Rudolf Virchow in 1863, postulated that chronic irritation and previous injuries are a precondition for the origin of tumorigenesis.15 In contrast to wound healing, the process is not self-limiting in cancer tissue.
Why does it take decades for a subset of patients with chronic inflammatory diseases to develop cancer? The evolution of PC is a multifactorial phenomenon rather than a unique inflammation-mediated process. Interaction with environmental risk factors, and/or susceptible genetic mutations is likely to accelerate these phenomena (Figure 1A). The regeneration that occurs to replace damaged epithelium may increase the probability of somatic mutations in this abnormal microenvironment. These factors are known to lead to recurrent pancreatic injury (e.g., recurrent acute pancreatitis (RAP) leading to chronic inflammation and fibrosis.16 From an early age, patients often suffer from recurrent brief bouts of asymptomatic acute pancreatitis (AP). A study by Guerra et al. supports the hypothesis reporting that brief bouts of pancreatitis in adult mice lead to PC as long as the acinar cells express K-Ras oncogenes. Interestingly, K-Ras mutations occurring after pancreatitis also induced PC providing evidence that the inflammatory response has not subsided and that the permissiveness of adult acinar cells to malignant transformation by K-Ras oncogenes was restored.17 Another study demonstrates that in the presence of oncogenic Ras, inflammatory stimuli trigger a NF-κB-mediated positive feedback mechanism that in turn amplifies Ras activity to pathological levels.18
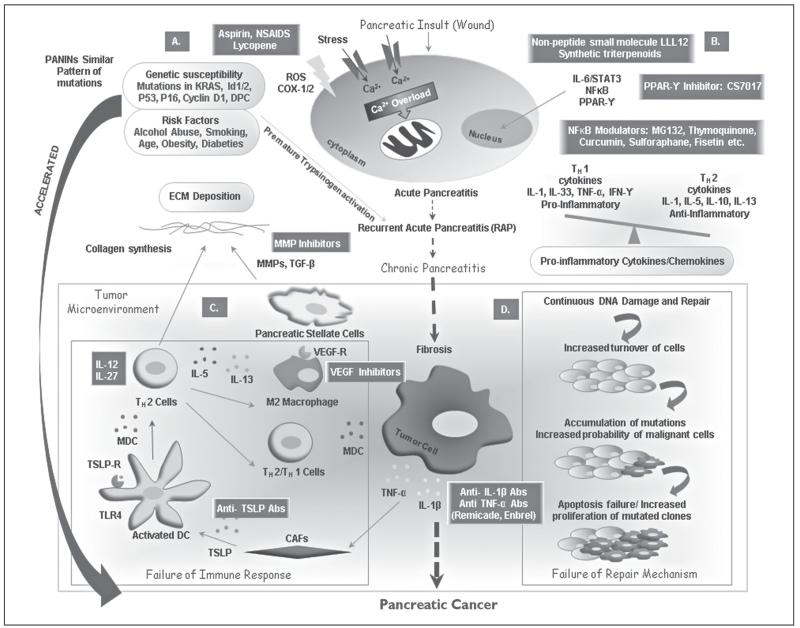
Figure 1.
Oncogenic transformation from pancreatic injury to pancreatic cancer under the inflammation background. A) Usually, it takes decades for a subset of patients with chronic inflammatory diseases to develop pancreatic cancer (PC). Genetic susceptibility to pancreatic injury (KRAS, Id1/2, P53, P16, Cyclin D1, and DPC) plus the necessary environmental factors (alcohol abuse, smoking, age, obesity, and diabeties) repeatedly triggering pancreatitis (recurrent acute pancreatitis) plus an immune response leads to chronic inflammation and fibrosis rather than healing. These mutations are responsible for premature activation of trypsinogen and thus intrapancreatic autodigestion and pancreatitis. This prolonged inflammatory response initiates chronic pancreatitis (CP) and appears to progress unrelentingly toward accelerated inflammatory destruction of the total organ and provides a milieu for the development of PC. Overproduction of ROS and an increased cation overload due to enhanced Ca2+ release from the internal stores lead to mitochondrial stress and cell death. B) Tumor cells produce various cytokines and chemokines that allure leukocytes. Abundance of proinflammatory cytokines over anti-inflammatory cytokines leads to a level of inflammation that favors the neoplastic outcome. The expression of these pro-inflammatory proteins is regulated primarily by the transcription various factors (TFs) such as STAT3, NFκB, PPAR-γ. C) It is postulated that extensive and prolonged inflammation may result in a secondary damage, enriching the surrounding microenvironment leading to neovascularization. This leads to activation of PSCs and over-production of ECM proteins in the inflamed pancreas leading to an imbalance in the homeostasis. As cells progress towards dysplasia, tumor cells coupled with its stroma exhibit immune evasion. Tumor cells release pro-inflammatory cytokines (TNFα and IL-1β) that induce CAFs-mediated release of thymic stromal lymphopoietin (TSLP). TSLP-mediated expression of TSLP-Receptor on resident DCs leads to its activation and migration to draining LNs where they prime Th2 cells. Cytokine pool leads to the docking of the Th2 cells on the tumor cells, further fostering fibrosis by increasing ECM and activating the M2-TAM. D) Intensified DNA damage induced by inflammatory cells creates an environment with a deficient response to DNA damage, increased turn-over of cells aggravating the accumulation of the potential oncogenic mutations, hence amplifying the probability of malignant cells accumulation. Further, the DNA damage, which is beyond repair, triggers apoptotic dearth in mutated clones, eventually leading to increased proliferation of malignant cells. These events lead to organ regeneration in an oxidative-species-rich environment and ultimately lead to PC. Various therapeutic modalities against specific inflammatory mediators are demonstrated in green boxes.
CAFs: cancer associated fibroblasts; DCs: dendritic cells; LNs: lymph nodes; MDC/CCL22: macrophage derived chemokine; PPAR-γ: peroxisome proliferator-activated receptor-γ; PSCs: pancreatic stellate cells; ROS: reactive oxygen species; STAT3: signal transducer and activator of transcription 3; TARC/CCL17: thymus and activation-regulated chemokine; TSLP: thymic stromal lymphopoietin; TSLPR: TSLP receptor.
Inactivation of a dozen of traditional tumor suppressor genes including p53, APC, Rb (“gatekeepers” of the genome) contributes directly to the neoplastic growth of the tumor. These traditional tumor suppressors are escorted by other susceptibility genes that indirectly suppress neoplasia (“care-takers” of the genome). A second class of indirectly acting cancer susceptibility genes includes factors causing an abnormal microenvironment due to inflammation, leading to neoplastic transformation.19 It has been postulated that the process driving oncogenesis in chronic inflammatory diseases is more of a “landscaper” defect than a germ line genetic “gatekeeper” or “caretaker” defect.20
Prolonged inflammatory response or over healing of the wound takes advantage of the cells that had sustained DNA damage and/or mutagenic assault even after the completion of repair. Under a favorable microenvironment rich in inflammatory cells, growth/survival factors and reactive oxygen species (ROS), these cells possess the potential to proliferate. In parallel many reciprocal interactions occur in a chaotic organization where neoplastic cells interact with other cell types (mesenchymal, hematopoietic, lymphoid, immune, fibroblasts and endothelial) and remodeled ECM. These factors potentiate tumor growth, induce fibroblast migration and maturation, stimulate angiogenesis, and enable metastatic spread. The journey from inflammation to cancer is comprised of several steps and converging risk factors including chronic pancreatitis (CP), diabetes, obesity, and age via various inflammatory mediators (Table I).
Table I. Variables associated with pancreatic cancer risk via inflammatory pathways.
| Inflammatory mediators |
Regulation and Role | Association with PC risk factors and PC |
|---|---|---|
| Nuclear factor Kappa B (NF-κB) |
Transcriptional regulator activated by ROS, hypoxia, cytokines, bacterial/viral products, and UV radiation. Stimulates the expression of IL-1β, TNF-α, iNOS, COX-2 thus amplifying inflammatory response in tumor environment |
Activated during AP, CP, and MS and play central role during inflammation. By interacting with RAS oncogene, it is involved in initiation and progression of PC [18, 22, 30, 82-84] |
| Signal transducer and activator of transcription 3 (STAT3) |
Transcription factor, act as convergence point for various cytokine, growth factor pathways and mediates response to inflammation, cell proliferation and apoptosis |
During pancreatitis, STAT3 regulates increased expression of MMP7 in PanINs and PDACs causing increased proliferation, metastasis and decreased apoptosis [73, 74, 85, 86]. |
| Cyclooxygenase-2 (COX-2) |
Prostaglandin synthesizing inducible enzyme activated by cytokines/ growth factors, mediating immunologic surveillance, cell differentiation, proliferation, anti-apoptosis, and metastasis. |
Converts chemical carcinogens of tobacco smoke to mutagenic derivatives. Elevated during pancreatitis, obesity, IR and PC [53, 87-91]. |
| Neutrophil gelatinase associated lipocalin (NGAL) |
Acute phase secretory glycoprotein regulated by pro-inflammatory cytokines, interferons and retinoic acid. Elevated during type-2 diabetes, chronic and severe acute pancreatitis (SAP). |
Predicts multiorgan failure and fatal outcome in patients with SAP. Highly expressed in early dysplastic lesions in the pancreas [92, 93]. |
| Tumor necrosis factor-alpha (TNF-α) |
Pro-inflammatory cytokine secreted by activated macrophages, CD4+ lymphocytes, NK cells mediating acute phase reaction, acting as a potent chemo- attractant of neutrophils and promotes secretion of IL-1 oxidants and inflammatory prostaglandins from macrophages. |
Central point during inflammatory reaction. Activates NF-κB and c-JUN signaling, growth, differentiation, survival and apoptosis. Significantly increased TNF-α level are found in CP, obese individuals, and PC patients. Involved in development of IR and associated with activation of pancreatic stellate cells [12, 40, 59, 78]. |
| Interleukin 1β (IL-1β) |
Pro-inflammatory cytokine secreted by activated macrophages and malignant cells. Act as lymphocyte mitogen and mediates inflammatory response, cell proliferation, differentiation and apoptosis. |
Key contributor to the obesity-induced inflammation and subsequent IR, induced by fat necrosis in AP and results in NF-κB and JNK signaling mediated increased invasion and angiogenesis of PC cells [94, 95]. |
| Interleukin-6 (IL-6) |
Pro- and anti-inflammatory cytokine secreted by T-cells and macrophages, adipocytes and involved in mediating acute phase response and activating immune response. |
Activates JAK - STAT kinases. Elevated during smoking, obesity, diabetes, CP and PC [33, 38, 96, 97]. |
| Transforming growth factor-beta (TGF-β) |
Cytokine regulated by ROS, proteases and Integrin. Involved in recruitment of monocytes, macrophages, NK cells, neutrophils and T cells and mediates cell proliferation, differentiation, angiogenesis and wound healing. |
Elevated levels in chronic pancreatitis are associated with increased fibrosis in pancreas. Systemic blockade of TGF-β signaling protects mice from obesity and diabetes. Induces epithelial to mesenchymal transition (EMT) and angio- genesis to promote PC progression [13, 51, 98-100]. |
| Peroxisome proliferator- activated receptor-γ (PPAR-γ) |
Ligand dependent transcription factor involved in differentiation of immune cells towards anti-inflammatory phenotypes. Regulate adipocyte differentiation, fatty acid storage and glucose metabolism, and is a target of anti-diabetic drugs. |
Drugs of the thiazolidinedione family activate PPAR-γ to inhibit PC cell growth by inhibition of COX2, NF-κB and angiogenic factor VEGF [75, 101-104]. |
| Reactive oxygen species (ROS) |
Chemically reactive molecules containing oxygen with unpaired valence shell electrons. Imbalance of oxidants and antioxidants cause oxidative stress (OS) that results in DNA damage, inflammation by activating NF-κB. |
Increased ROS and lipid peroxidation is observed during MS, smoking, obesity, CP and PC [105-108]. |
AP: acute pancreatitis; CD4+,cluster of differentiation 4+; CP: chronic pancreatitis; EMT: epithelial mesenchymal transition; iNOS: inducible nitric oxide synthase; IR: insulin resistance; JNK: c-Jun N-terminal kinase; MMP7: matrix metalloproteinase7; MS: metabolic syndrome; NK cells: natural killer cells; OS: oxidative stress; PanIN: pancreatic intraepithelial neoplasia; PC: pancreatic cancer; PDAC: pancreatic ductal adenocarcinoma; ROS: reactive oxygen species; SAP: severe acute pancreatitis; UV: ultra violet; VEGF: vascular endothelial growth factor.
Chronic pancreatitis: smoking and alcohol abuse
Inflammation within the pancreas is commonly referred to as pancreatitis. CP is the chronic inflammatory disease of the exocrine pancreas known to increase the risk of developing PC by 10 to 20-folds.21 Importantly, similar inflammatory components and downstream effectors (S100A4, epidermal growth factor (EGF) receptor, cyclin E1, IL-8, NF-κB) are illustrated to be present in CP and PC.22 These findings suggest that a common pathway for PC development may be through a chronic inflammatory process. Subjects with CP usually have underlying genetic susceptibility gene mutations leading to RAP such as cationic trypsinogen (PRSS1) gene mutation (Hereditary Pancreatitis),23 pancreatic secretory trypsin inhibitor (SPINK1) gene mutation (Idiopathic CP, Familial CP) 24 and cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations (Alcoholic CP, Idiopathic CP, Cystic Fibrosis).25 All of these mutations subject the pancreas to auto-digestion due to premature trypsinogen activation and subsequent activation of other digestive enzymes. Interestingly, these mutation parallels the defined progressive pattern of mutation accumulation in the course of developing cancer through intraepithelial pancreatic neoplastic lesions (KRAS2-p16/CDKN2A-DPC4/MADH4-BRCA2).16.
The most frequent etiology of AP is cigarette-smoking and alcohol consumption. Smoking and alcohol abuse are likely to accelerate and exaggerate the series of events starting from RAP, potentiating chronic inflammation, leading to chronic pancreatitits (CP) and fibrosis, ultimately resulting in cancer. Alcohol abuse lowers the threshold for AP,26 and cigarette smoking potentiates the chronic inflammatory process.6, 26, 27 In subjects with hereditary pancreatitis, Lowenfels et al. reported a two-fold increased risk of developing PC in smokers as compared to non-smokers. Also, PC developed 20 years earlier in cases of tobacco smokers than in non-smokers.28 Also, individuals who consume high rate of alcohol are at an amplified risk for the development of CP.29
Alcohol consumption inclines the pancreas to inflammation. Alcohol metabolism involves ethanol esterification to form fatty acid ethyl esters that, upon accumulation into the pancreas, causes a release of endogenous hydrolases from pancreatic lysosomes. This is responsible for premature activation of trypsinogen and thus intrapancreatic autodigestion and pancreatitis.30 Alcohol metabolism by the oxidative enzyme system generates ROS resulting in pancreatic tissue injury, release of proinflammatory cytokines and activation of various potential transcriptional factors (TFs), including NF-κB, AP-1 and p38 MAP kinase.30, 31 Inflammation in the pancreatitis is tightly allied with the induction of necrosis/apoptosis. Overproduction of reactive oxygen species (ROS) and increased cation overload due to enhanced Ca2+ release from the internal stores leads to mitochondrial stress and cell death.32 Bhatia et al. reported parathyroid hormone-related protein (PTHrP) as a potential mediator of the inflammatory (increased IL-6 levels) and fibrogenic (increased ICAM-1 and procollagen-I levels) responses.33
In experimental models, nicotine, the major component of cigarette-smoke, has been observed to incite only an acute inflammatory reaction in the pancreas. However, repeated sessions of smoking-induced acute pancreatic inflammation may progress to CP.34 Nicotine plays a role in intra-pancreatic inflammation by increasing pancreatic protein synthesis in isolated acini, inducing cellular edema and cytoplasmic vacuolation in the exocrine pancreatic regions.7,35 Apart from nicotine, cigarette-smoke is composed of various carcinogens, some of which include N-nitrosamines, nicotine, tar, and arsenic. These carcinogens can induce DNA mutations forming DNA adducts 34 and ultimately leading to pancreatic fibrosis.36 Wittel et al. have also demonstrated an up-regulation of pro-collagen type 1, a known indicator for fibrotic tissue replacement, in pancreatic tissues of high-dose exposed rats.7
Elevated levels of systemic markers of inflammation, including myeloperoxidase, lysozyme and human neutrophil lipocalin and C-reactive protein have been demonstrated in cigarette smokers.29, 30 Smoking acts as a trigger for chronic inflammation also by enhancing the ethanol-induced pancreatic injury.37 These alterations may explain the increased incidence of PC in cigarette smokers, especially in at-risk cohorts, such as individuals with inherited PC-predisposing mutations as well as heavy drinkers.
Obesity and diabetes mellitus
Being overweight or obese, characterized by abnormal or excessive fat accumulation with a very high body mass index (BMI, 25 to >30 overweight and >30 is obese), are chronic health problems. As per a recent WHO report, being overweight or obese accounts for 44% of the diabetes cases, 23% of the ischemic heart disease problems and between 7-41% of various cancer cases (endometrial, breast, and colon). Over time, data from various epidemiological and meta-analyses show a link between obesity and PC.8 Recently, three large pooled analyses and two meta-analyses looked for an association between obesity/high BMI, the effect of long standing diabetes, smoking, age, as well as waist and hip circumference on pancreatic cancer risk.8 This study revealed that both central and overall obesity are associated with a 1.4 to 2-fold increased risk of PC.9
A plethora of studies have been carried out to explore the road from obesity to pancreatic cancer. Although the compete pathway remains obscure, the major players in the journey from obesity to PC include the development of a perturbed energy balance, chronic inflammation, insulin resistance, hyperinsulinemia, oxidative stress, altered secretion of adipokines, glucose intolerance and the development of diabetes. Inflammation, in conjunction with the immune system, plays a central role in the development of insulin resistance, diabetes and PC.
White adipose tissue (both subcutaneous and visceral forms) with its richness of triglycerides is the central energy store of the body. Additionally, it has an endocrine role and is involved in the secretion of a variety of cytokines and chemokines, including leptin, adiponectin, resistin, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1), angiotensinogen, visfatin, retinol-binding protein-4 and serum amyloid A (SAA), that manages the homeostatic balance of the body.38 First and foremost, the cause for obesity is an energy imbalance caused by an intake of a higher number of calories than calories being consumed. An accumulation of excess calories in adipocytes leads to an increase in their number (hyperplastic) and size (hypertrophic) along with dysregulation of their endocrine functioning. Enlarged adipocytes secretes chemotactic adipokines and chemokines (CCL5, CXCL12 and CCL20), leading to the recruitment of pro-inflammatory cells into an adipose tissue.39 Infiltration of adipose tissues especially visceral adipose tissue by inflammatory cells including alternatively polarized macrophages, Treg cells, neutrophils, eosinophils, CD8+ T cells and IL-17- secreting γδT leads to the development of insulin resistance (IR). Further free fatty acid present in circulation are implicated in development of insulin resistance. Lumneg et al. observed that adipose tissue macrophages are M2 polarized with IL-10 and arginase expression in lean insulin sensitive animals. These adipokines (especially IL-10) recruits alternatively polarized M1 macrophages with low IL-10 expression and increased inducible nitric oxide synthase (iNOS) and TNF-α production.40 These CCR2+ macrophages overpower the protective effects of M2 macrophages and produce an inflammatory environment governed by TNF-α and iNOS. Inflammatory cytokines, in conjunction with adipokines, induce the Jun N terminal kinase (JNK) and Ikappa-B kinase (IKK) signaling pathways and block and interfere with insulin signaling in adipose tissue and skeletal muscle. Additionally, the housekeeper of innate immunity i.e. TLR and advanced glycation end products directly activates Jun N terminal kinase (JNK) and Ikappa-B kinase (IKK) signaling leading to the development of inflammatory milleu.41, 42 TLR mediated activation of the NF-κβ pathway could be mediated by circulating fatty acid thus directly linking the aggravated immune system and an increased amount of circulating fatty acids observed in obese individuals.43 Once developed, insulin resistance (IR) leads to β-cell stress, excessive activation of insulin like growth factor-1 (IGF-1) and its receptor (IGF-1R) and subsequently β-cell apoptosis. Further, insulin resistance causes obligatory hyperinsulemia and diabetes.
Diabetes is a life-long chronic disease characterized by polyuria (frequent urination), polydipsia (increased thirst) and polyphagia (increased hunger) with insulin resistance being associated with type II diabetes. Lately, various epidemiological studies have revealed the bilateral association between diabetes (especially type II) and PC. Diabetes is associated with 1.8 fold increased risk of PC. Furthermore, newly diagnosed diabetes cases (<4 years) have a 50% greater risk of PC development as compared to individuals with long-standing diabetes (≥5 years) odd ratio (OR), 2.1 [95% CI, 1.9–2.3] vs. OR, 1.5 [95% confidence interval (CI), 1.3–1.8]; P=0.005).9, 44 Additionally, PC also acts as a causative agent for the development of diabetes.9, 45 Although the molecular mechanism from diabetes to PC is still unclear, the insulin resistance, inhibition of insulin secretion, increase in insulin and C-peptide levels to oral glucose challenge, hyperglycemia, altered metabolism in presence of genetic predisposition, unhealthy diet are considered as major player in further developments.46 Overall, these studies provide striking examples of the compounding effects of various risk factors. Coupled with inflammation, these risk factors contribute to the development of PC (Figure 1A).
Inflammation and microenvironment: revisiting the landscaper defect hypothesis
In the initial stages, both epithelial and stromal elements are likely to undergo alterations promoting epithelial cell mutations and deregulated proliferation. This unbalanced homeostasis can in turn cause an inflammatory response. It is postulated that extensive and prolonged inflammation may result in secondary damage, enriching the surrounding microenvironment with activated inflammatory mediators, cytokines, chemokines, O2 radicals, especially leading to neovascularization. Further, continued hyperplasia and dysplasia eventually leads to an invasive neoplastic state.
Damaged acinar cells are responsible for releasing the first inflammatory signals in response to pancreatic injury, leading to the activation of the immune system.47, 48 After the infiltration of white blood cells to the damaged acinar cells, the quiescent PSC gets converted into an “activated or myofibroblastic” state.12 With their inherent quality of communicating with inflammatory cells, acinar cells, and PC cells in a complicated network of interactions, PSCs might assume a coupling role in inflammation-associated carcinogenesis.12 Studies have validated specific PSC markers (Collagen type 11A1 (COL11A1), cytokine CCL2 and VCAM1) under inflammatory response.2
Studies provide insight into the primary events of tumor development, suggesting that inflammation-facilitated epithelial to mesenchymal transition (EMT) and entry into the circulation precedes pancreatic tumor initiation and progression.49 PSCs are known to produce huge levels of ECM proteins in the inflamed pancreas leading to an imbalance in the homeostasis.50 It expresses the regulatory cytokine TGFβ1 that, by inhibition of matrix metalloproteinase (MMP), contributes to loss of parenchymal barrier, resulting in exocrine/endocrine insufficiency, restricted collagen degradation, atrophy of acinar/islet tissue and ductal strictures that might lead to progressive fibrosis.51
Tumor cells produce various cytokines and chemokines that allure leukocytes (dendritic cells [DCs], neutrophils, eosinophils, macrophages, mast cells, lymphocytes, myeloid-derived suppressor cells [MDSCs]) that, in turn, secrete several cytokines, MMPs, TNFs, interleukins and interferon. The balance between pro-inflammatory and anti-inflammatory cytokines in any given tumor is crucial for regulating the type/extent of the inflammatory infiltrate (Figure 1B). An abundance of proinflammatory over anti-inflammatory cytokines lead to a level of inflammation that favors the neoplastic outcome.52 IL-1α sustains the expression of inflammatory factors including IL-6, CXCL8, VEGF-A, CCL20, and COX-2 in the human PC microenvironment, via a cross talk between the PC cells and cancer-associated fibroblasts (CAFs).53
As cells progress towards dysplasia, they should undergo repair or should be recognized/eliminated by the repair response or immune response respectively. Under inflammatory conditions, tumor cells coupled with their stroma encounter failure of both of these barriers. By recruiting various inflammatory cells and under enriched cytokine mileu, the tumor protects itself by recruiting the innate system to enhance its development (Figure 1C). Hirakona et. al. demonstrated immune surveillance via the synergistic effect of (C-X-C motif) ligand 17 (CXCL17) and intercellular adhesion molecule 2 (ICAM2) during the early stages of PC.54 Elevated MDSC levels have been demonstrated in the peripheral blood of PC compared to the controls, which has been correlated with an elevated risk of death.55 The recruitment of myeloid-derived spressor cells (MDSCs) by pancreatic tumors in genetically modified mice is complemented by increased T-regulatory cells (Tregs) and a lack of T-cells.56 Tumor-associated-macrophages (TAM) (M2-polarised macrophage) are also recruited to tumors via vascular endothelial growth factor (VEGF) receptor2,57 accelerating lymphatic metastasis and are associated with a poor prognosis.58 Tumor cells release pro-inflammatory cytokines (TNFα and IL-1β) that induce CAFs-mediated release of thymic stromal lymphopoietin (TSLP).59 TSLP-mediated expression of the TSLP-receptor on resident DCs leads to its activation 60 and migration to draining lymph nodes (LNs) where they prime Th2 cells. An elevated ratio of Th2/Th1 TH cells is associated with poor prognosis in pancreatic ductal adenocarcinoma (PDAC).59 A cytokine pool leads to the docking of the Th2 cells on the tumor cells, further fostering fibrosis by increasing ECM and activating the M2-TAM. Also, inflammation-induced inhibition of myeloid differentiation primary response gene (MyD88) elicits protumorigenic and fibro-inflammatory effects promoting the neoplastic transformation from pancreatitis to carcinoma by augmenting the DC-Th2 axis.61
Next, the dysplastic cells encounter DNA repair response failure (Figure 1D). A continuous damage/repair process leads to an increased turn-over of cells. Intensified DNA damage induced by inflammatory cells leads to the release of macrophage migration inhibitory factor (MIF) from macrophages and T lymphocytes. MIF-mediated suppression of p53 transcriptional activity in infiltrated tissues creates an environment with a deficient response to DNA damage,62 increasing the life span of cells and aggravating the accumulation of the potential oncogenic mutations, hence amplifying the probability of the accumulation of malignant cells. Further, the DNA damage that is beyond repair triggers apoptotic dearth in mutated clones, eventually leading to increased proliferation of malignant cells.63 These events lead to organ regeneration in an oxidative-species-rich environment and ultimately lead to PC.
Therapeutic standpoints and future perspectives
It is now evident that inflammation both drives and accelerates the pathogenesis of cancer, including PC. Clearly, the components responsible for initiation, promotion and expansion of PC are common with those functioning in the inflammatory response. Considering the relative chemoresistance of PC to traditional cytotoxic agents, appraising the use of both old and new drugs targeting the inflammatory mechanisms in combination with chemotherapy, is warranted to improve the survival rate of PC patients. Multiple links between inflammation and PC have inspired the development of a novel targeted therapy, which is under evaluation both in vivo and in vitro.
Indeed, the studies suggesting high PC risk among long-term users of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) define the link between inflammation and neoplastic progression in a best possible manner. Such agents are under proficient usage in combination with radiation therapy64 and systemic chemotherapy for the management of PC.65 The ability of NSAIDs to inhibit COX enzymes defines their mechanisms of chemoprevention. COX enzymes, via several mechanisms including carcinogen activation, cell-cycle progression, interference with mitochondrial-mediated cell apoptosis and suppression of immune surveillance induce an inflammatory reaction in damaged tissues.66 Nonselective NSAIDs such as sulindac and indomethacin inhibit both COX-2 and COX-1.67 Also, aspirin inactivates both COX-1 and COX-2 by acetylation, thereby inhibiting the platelet function and prostaglandin synthesis. Flurbiprofen elicits strong antimetastatic effects via the inhibition of platelet aggregation.68 COX-2 produces prostaglandins that inhibit apoptosis and stimulate angiogenesis whereas COX-1 is known to be cytoprotective.67 Hence, nonselective NSAIDs might lead to platelet dysfunction, gastrointestinal ulceration and nephropathy. With these explanations, selective COX-2 inhibitors such as meloxicam, celecoxib, and rofecoxib are preferred over non-selective NSAIDS.69 Also, agents targeting the cytotoxic ROS have been in vogue for proficient treatment against PC, including ascorbate inducing cytotoxicity in PC 70 and lycopene eliciting protective effects on the oxidative stress-induced cell death of pancreatic acinar cells.71
The expression of various pro-inflammatory proteins is regulated primarily by the transcription factors (TFs). C/EBPδ has been demonstrated as a modulatory TF that inhibits the pro-apoptotic and pro-inflammatory gene networks activated by cytokines in pancreatic β-cells.72 Few agents affecting the ROS-responsive TF, NF-κB, include: a proteasome inhibitor; MG132, thymoquinone, a natural flavonoid; fisetin, sulforaphane and curcumin. Interestingly, synthetic tri-terpenoids prolonged survival in a PC mouse model by inhibiting the alliance between NF-κB and yet another TF, signal transducer and activator of transcription 3, STAT3.73 LLL12, a nonpeptide, cell-permeable small molecule, selectively blocked exogenous IL-6-induced STAT3 phosphorylation and its subsequent nuclear translocation, suggesting that the inhibition of IL-6/STAT3 signaling may be a potential therapeutic approach for PC.74 CS-7017 acts as an agonist for peroxisome proliferator-activated receptor-γ (PPAR-γ), a ligand-activated TF that has been widely implicated in PC. This novel thiazolidinediones (TZD) class of PPAR-γ agonist inhibited the proliferation of PC cells in vitro and is also known to act as insulin sensitizer, thereby decreasing the risk of PC.75
Cytokines, in particular TNF-α and IL-1β, represent attractive therapeutic targets for PC. Clinical studies have evaluated the usage of TNFerade, an adenovector for TNF-α gene delivery, in combination with radiation 76 and/or chemotherapy 77 for the treatment of PC, demonstrating significant anti-tumorigenic effects. Proinflammatory cytokine TNF-α is also a key downstream mediator in inflammation. Studies have established TNF neutralizing drugs infliximab (Remicade) and etanercept (Enbrel), demonstrating a beneficial role in PDAC treatment especially in the adjuvant setting after subtotal pancreatectomy.78 Moreover, blocking antibodies against TNF-α and IL-1β show significant inhibition of its expression levels in PC.
Keeping in mind the “landscaper” defect caused by inflammation in PC pathogenesis, it is very important to target the surrounding stroma along with the tumor itself. CD40 activation in PC, via targeting macrophages and re-establishing the tumor immune surveillance, plays a vital role in the destruction of the surrounding stroma.79 MMPs, which are produced both by the inflammatory cells and stromal cells, generate growth-promoting/cytostatic signals and activate angiogenesis, hence becoming attractive therapeutic targets against PC. Tumors are rich in both membrane-bound and secretory mucins with their glycosylation patterns acting as important tumor-associated antigens (TAAs) for various ligand commandeering. Interestingly, studies have suggested pro-inflammatory-cytokine-mediated modulation of the presentation of these TAAs influencing pancreatic tumor behavior.80 Ligands that may include the sialyl-Lewis X epitope are known to be recognized by selectins having a potential role in metastasis. Thus, inhibiting the selectin-tumor cell interaction by heparin might decrease the PC metastasis.81
Conclusions
In summary, pancreatic injury, coupled with common germ-line mutations diminishing the ability of pancreatic cells to protect themselves from environmental or metabolic stressors, resulting in a prolonged inflammatory response (chronic pancreatitis), followed by a series of events including fibrosis, tumor-stromal interaction, loss of immune surveillance and repair response, leads to the development of pancreatic tumor. Inflammatory markers defining this injury-inflammation-cancer pathway might act as attractive targets both for prevention and treatment of PC (Figure 1).
Acknowledgments
Funding.—The authors on this work, in part, are supported by grants from the National Institutes of Health (TMEN U54 CA163120, EDRN UO1 CA111294, RO1 CA131944, RO1 CA133774, SPORE P50 CA127297 and RO1 CA78590).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–67. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 3.Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148:21–3. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Wang DS, Wang ZQ, Zhang L, Qiu MZ, Luo HY, Ren C, et al. Are risk factors associated with outcomes in pancreatic cancer? PLoS One. 2012;7:e41984. doi: 10.1371/journal.pone.0041984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–58. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Whitcomb DC. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315–G319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 7.Wittel UA, Singh AP, Henley BJ, Andrianifahanana M, Akhter MP, Cullen DM, et al. Cigarette smoke-induced differential expression of the genes involved in exocrine function of the rat pancreas. Pancreas. 2006;33:364–70. doi: 10.1097/01.mpa.0000240601.80570.31. [DOI] [PubMed] [Google Scholar]
- 8.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog. 2012;51:53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebours V, Boutron-Ruault MC, Schnee M, Ferec C, Maire F, Hammel P, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111–9. doi: 10.1111/j.1572-0241.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 11.Peng JH, Zhang F, Zhang HX, Fan HY. Prepubertal octylphenol exposure up-regulate BRCA1 expression, down-regulate ERalpha expression and reduce rat mammary tumorigenesis. Cancer Epidemiol. 2009;33:51–5. doi: 10.1016/j.canep.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Algul H, Treiber M, Lesina M, Schmid RM. Mechanisms of disease: chronic inflammation and cancer in the pancreas--a potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol. 2007;4:454–62. doi: 10.1038/ncpgasthep0881. [DOI] [PubMed] [Google Scholar]
- 13.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031–7. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Haddow A. Addendum to “molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing”? Adv Cancer Res. 1974;20:343–66. doi: 10.1016/s0065-230x(08)60113-x. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Whitcomb DC, Pogue-Geile K. Pancreatitis as a risk for pancreatic cancer. Gastroenterol Clin North Am. 2002;31:663–78. doi: 10.1016/s0889-8553(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 17.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–39. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, et al. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–28. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grote VA, Kaaks R, Nieters A, Tjonneland A, Halkjaer J, Overvad K, et al. Inflammation marker and risk of pancreatic cancer: a nested case-control study within the EPIC cohort. Br J Cancer. 2012;106:1866–74. doi: 10.1038/bjc.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280:1036–7. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- 21.Dite P, Hermanova M, Trna J, Novotny I, Ruzicka M, Liberda M, et al. The role of chronic inflammation: chronic pancreatitis as a risk factor of pancreatic cancer. Dig Dis. 2012;30:277–83. doi: 10.1159/000336991. [DOI] [PubMed] [Google Scholar]
- 22.Farrow B, Sugiyama Y, Chen A, Uffort E, Nealon W, Mark EB. Inflammatory mechanisms contributing to pancreatic cancer development. Ann Surg. 2004;239:763–9. doi: 10.1097/01.sla.0000128681.76786.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 24.Pfutzer RH, Barmada MM, Brunskill AP, Finch R, Hart PS, Neoptolemos J, et al. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–23. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 25.Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653–8. doi: 10.1056/NEJM199809033391002. [DOI] [PubMed] [Google Scholar]
- 26.Deng X, Wang L, Elm MS, Gabazadeh D, Diorio GJ, Eagon PK, et al. Chronic alcohol consumption accelerates fibrosis in response to cerulein-induced pancreatitis in rats. Am J Pathol. 2005;166:93–106. doi: 10.1016/S0002-9440(10)62235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maisonneuve P, Lowenfels AB, Mullhaupt B, Cavallini G, Lankisch PG, Andersen JR, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510–4. doi: 10.1136/gut.2004.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, Dimagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–70. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 29.Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–45. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandol SJ, Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology. 2007;7:105–14. doi: 10.1159/000104235. [DOI] [PubMed] [Google Scholar]
- 31.Pandol SJ, Periskic S, Gukovsky I, Zaninovic V, Jung Y, Zong Y, et al. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117:706–16. doi: 10.1016/s0016-5085(99)70465-8. [DOI] [PubMed] [Google Scholar]
- 32.Gerasimenko OV, Gerasimenko JV. Mitochondrial function and malfunction in the pathophysiology of pancreatitis. Pflugers Arch. 2012;464:89–99. doi: 10.1007/s00424-012-1117-8. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia V, Kim SO, Aronson JF, Chao C, Hellmich MR, Falzon M. Role of parathyroid hormone-related protein in the pro-inflammatory and pro-fibrogenic response associated with acute pancreatitis. Regul Pept. 2012;175:49–60. doi: 10.1016/j.regpep.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Momi N, Kaur S, Ponnusamy MP, Kumar S, Wittel UA, Batra SK. Interplay between Smoking-induced Genotoxicity and Altered Signaling in Pancreatic Carcinogenesis. Carcinogenesis. 2012;33:1617–28. doi: 10.1093/carcin/bgs186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wittel UA, Pandey KK, Andrianifahanana M, Johansson SL, Cullen DM, Akhter MP, et al. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol. 2006;101:148–59. doi: 10.1111/j.1572-0241.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 36.van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Smoking is related to pancreatic fibrosis in humans. Am J Gastroenterol. 2011;106:1161–6. doi: 10.1038/ajg.2011.43. [DOI] [PubMed] [Google Scholar]
- 37.Malfertheiner P, Schutte K. Smoking--a trigger for chronic inflammation and cancer development in the pancreas. Am J Gastroenterol. 2006;101:160–2. doi: 10.1111/j.1572-0241.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 38.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 39.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012 doi: 10.1038/nrendo.2012.114. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–14. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 43.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 44.Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51:64–74. doi: 10.1002/mc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de AM, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Cao G, Ma Q, Liu H, Li W, Han L. The bidirectional interation between pancreatic cancer and diabetes. World J Surg Oncol. 2012;10:171. doi: 10.1186/1477-7819-10-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramudo L, Yubero S, Manso MA, Vicente S, De D. I. Signal transduction of MCP-1 expression induced by pancreatitis-associated ascitic fluid in pancreatic acinar cells. J Cell Mol Med. 2009;13:1314–20. doi: 10.1111/j.1582-4934.2008.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramnath RD, Sun J, Bhatia M. Involvement of SRC family kinases in substance P-induced chemokine production in mouse pancreatic acinar cells and its significance in acute pancreatitis. J Pharmacol Exp Ther. 2009;329:418–28. doi: 10.1124/jpet.108.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–98. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keane MP, Strieter RM. The importance of balanced pro-inflammatory and anti-inflammatory mechanisms in diffuse lung disease. Respir Res. 2002;3:5. doi: 10.1186/rr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tjomsland V, Spangeus A, Valila J, Sandstrom P, Borch K, Druid H, et al. Interleukin 1alpha sustains the expression of inflammatory factors in human pancreatic cancer microenvironment by targeting cancer-associated fibroblasts. Neoplasia. 2011;13:664–75. doi: 10.1593/neo.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiraoka N, Yamazaki-Itoh R, Ino Y, Mizuguchi Y, Yamada T, Hirohashi S, et al. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology. 2011;140:310–21. doi: 10.1053/j.gastro.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 57.Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, et al. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–6. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 58.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 59.De ML, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–78. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt RW, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 61.Ochi A, Nguyen AH, Bedrosian AS, Mushlin HM, Zarbakhsh S, Barilla R, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209:1671–87. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–82. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153–69. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 64.Blanquicett C, Saif MW, Buchsbaum DJ, Eloubeidi M, Vickers SM, Chhieng DC, et al. Antitumor efficacy of capecitabine and celecoxib in irradiated and lead-shielded, contralateral human BxPC-3 pancreatic cancer xenografts: clinical implications of abscopal effects. Clin Cancer Res. 2005;11:8773–81. doi: 10.1158/1078-0432.CCR-05-0627. [DOI] [PubMed] [Google Scholar]
- 65.Dragovich T, Burris H, III, Loehrer P, Von Hoff DD, Chow S, Stratton S, et al. Gemcitabine plus celecoxib in patients with advanced or metastatic pancreatic adenocarcinoma: results of a phase II trial. Am J Clin Oncol. 2008;31:157–62. doi: 10.1097/COC.0b013e31815878c9. [DOI] [PubMed] [Google Scholar]
- 66.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19:247–58. doi: 10.1023/a:1015587423262. [DOI] [PubMed] [Google Scholar]
- 67.Harris RE, Beebe-Donk J, Doss H, Burr DD. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–83. [PubMed] [Google Scholar]
- 68.Mamytbekova A, Rezabek K, Kacerovska H, Grimova J, Svobodova J. Antimetastatic effect of flurbiprofen and other platelet aggregation inhibitors. Neoplasma. 1986;33:417–21. [PubMed] [Google Scholar]
- 69.Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1–278. doi: 10.3310/hta12110. [DOI] [PubMed] [Google Scholar]
- 70.Du J, Martin SM, Levine M, Wagner BA, Buettner GR, Wang SH, et al. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin Cancer Res. 2010;16:509–20. doi: 10.1158/1078-0432.CCR-09-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seo JY, Masamune A, Shimosegawa T, Kim H. Protective effect of lycopene on oxidative stress-induced cell death of pancreatic acinar cells. Ann N Y Acad Sci. 2009;1171:570–5. doi: 10.1111/j.1749-6632.2009.04712.x. [DOI] [PubMed] [Google Scholar]
- 72.Moore F, Santin I, Nogueira TC, Gurzov EN, Marselli L, Marchetti P, et al. The transcription factor C/EBP delta has anti-apoptotic and anti-inflammatory roles in pancreatic beta cells. PLoS One. 2012;7:e31062. doi: 10.1371/journal.pone.0031062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Surh YJ, Bode AM, Dong Z. Breaking the NF-kappaB and STAT3 alliance inhibits inflammation and pancreatic tumorigenesis. Cancer Prev Res (Phila) 2010;3:1379–81. doi: 10.1158/1940-6207.CAPR-10-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu A, Liu Y, Li PK, Li C, Lin J. LLL12 inhibits endogenous and exogenous interleukin-6-induced STAT3 phosphorylation in human pancreatic cancer cells. Anticancer Res. 2011;31:2029–35. [PMC free article] [PubMed] [Google Scholar]
- 75.Shimazaki N, Togashi N, Hanai M, Isoyama T, Wada K, Fujita T, et al. Anti-tumour activity of CS-7017, a selective peroxisome proliferator-activated receptor gamma agonist of thiazolidinedione class, in human tumour xenografts and a syngeneic tumour implant model. Eur J Cancer. 2008;44:1734–43. doi: 10.1016/j.ejca.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 76.Chadha MK, Litwin A, Levea C, Iyer R, Yang G, Javle M, et al. Surgical resection after TNFerade therapy for locally advanced pancreatic cancer. JOP. 2009;10:535–8. [PubMed] [Google Scholar]
- 77.Murugesan SR, King CR, Osborn R, Fairweather WR, O’Reilly EM, Thornton MO, et al. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16:841–7. doi: 10.1038/cgt.2009.32. [DOI] [PubMed] [Google Scholar]
- 78.Egberts JH, Cloosters V, Noack A, Schniewind B, Thon L, Klose S, et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008;68:1443–50. doi: 10.1158/0008-5472.CAN-07-5704. [DOI] [PubMed] [Google Scholar]
- 79.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu YM, Nowack DD, Omenn GS, Haab BB. Mucin glycosylation is altered by pro-inflammatory signaling in pancreatic-cancer cells. J Proteome Res. 2009;8:1876–86. doi: 10.1021/pr8008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A. 2001;98:3352–7. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crowley-Weber CL, Dvorakova K, Crowley C, Bernstein H, Bernstein C, Garewal H, et al. Nicotine increases oxidative stress, activates NF-kappaB and GRP78, induces apoptosis and sensitizes cells to genotoxic/xenobiotic stresses by a multiple stress inducer, deoxycholate: relevance to colon carcinogenesis. Chem Biol Interact. 2003;145:53–66. doi: 10.1016/s0009-2797(02)00162-x. [DOI] [PubMed] [Google Scholar]
- 83.Holcomb B, Yip-Schneider M, Schmidt CM. The role of nuclear factor kappaB in pancreatic cancer and the clinical applications of targeted therapy. Pancreas. 2008;36:225–35. doi: 10.1097/MPA.0b013e31815b3207. [DOI] [PubMed] [Google Scholar]
- 84.Kaidashev IP. [NF-kB activation as a molecular basis of pathological process by metabolic syndrome] Fiziol Zh. 2012;58:93–101. [PubMed] [Google Scholar]
- 85.Pini M, Rhodes DH, Castellanos KJ, Hall AR, Cabay RJ, Chennuri R, et al. Role of IL-6 in the resolution of pancreatitis in obese mice. J Leukoc Biol. 2012;91:957–66. doi: 10.1189/jlb.1211627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukuda A, Wang SC, Morris JP, Folias AE, Liou A, Kim GE, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Albazaz R, Verbeke CS, Rahman SH, McMahon MJ. Cyclooxygenase-2 expression associated with severity of PanIN lesions: a possible link between chronic pancreatitis and pancreatic cancer. Pancreatology. 2005;5:361–9. doi: 10.1159/000086536. [DOI] [PubMed] [Google Scholar]
- 88.Hermanova M, Trna J, Nenutil R, Dite P, Kala Z. Expression of COX-2 is associated with accumulation of p53 in pancreatic cancer: analysis of COX-2 and p53 expression in premalignant and malignant ductal pancreatic lesions. Eur J Gastroenterol Hepatol. 2008;20:732–9. doi: 10.1097/MEG.0b013e3282f945fb. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh PS, Jin JS, Chiang CF, Chan PC, Chen CH, Shih KC. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity (Silver Spring) 2009;17:1150–7. doi: 10.1038/oby.2008.674. [DOI] [PubMed] [Google Scholar]
- 90.Song Z, Bhagat G, Quante M, Baik GH, Marrache F, Tu SP, et al. Potential carcinogenic effects of cigarette smoke and Swedish moist snuff on pancreas: a study using a transgenic mouse model of chronic pancreatitis. Lab Invest. 2010;90:426–35. doi: 10.1038/labinvest.2009.145. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi M, Mutoh M, Ishigamori R, Fujii G, Imai T. Involvement of inflammatory factors in pancreatic carcinogenesis and preventive effects of anti-inflammatory agents. Semin Immunopathol. 2012 doi: 10.1007/s00281-012-0340-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 92.Kim SS, Song SH, Kim IJ, Yang JY, Lee JG, Kwak IS, et al. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:251–7. doi: 10.1016/j.diabres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 93.Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–69. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verma G, Bhatia H, Datta M. Gene expression profiling and pathway analysis identify the integrin signaling pathway to be altered by IL-1beta in human pancreatic cancer cells: role of JNK. Cancer Lett. 2012;320:86–95. doi: 10.1016/j.canlet.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 95.Maedler K, Dharmadhikari G, Schumann DM, Storling J. Interleukin-targeted therapy for metabolic syndrome and type 2 diabetes. Handb Exp Pharmacol. 2011:257–78. doi: 10.1007/978-3-642-17214-4_11. [DOI] [PubMed] [Google Scholar]
- 96.Sliwinska-Mosson M, Milnerowicz H, Jablonowska M, Milnerowicz S, Nabzdyk S, Rabczynski J. The effect of smoking on expression of IL-6 and antioxidants in pancreatic fluids and tissues in patients with chronic pancreatitis. Pancreatology. 2012;12:295–304. doi: 10.1016/j.pan.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 97.Tjomsland V, Niklasson L, Sandstrom P, Borch K, Druid H, Bratthall C, et al. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clin Dev Immunol. 2011;21:2810. doi: 10.1155/2011/212810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu Y, Colak T, Shenoy M, Liu L, Mehta K, Pai R, et al. Transforming growth factor beta induces sensory neuronal hyperexcitability, and contributes to pancreatic pain and hyperalgesia in rats with chronic pancreatitis. Mol Pain. 2012;8:65. doi: 10.1186/1744-8069-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang H, Wu J, Zhang Y, Xue X, Tang D, Yuan Z, et al. Transforming growth factor beta-induced epithelialmesenchymal transition increases cancer stem-like cells in the PANC-1 cell line. Oncol Lett. 2012;3:229–33. doi: 10.3892/ol.2011.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho N, Momose Y. Peroxisome proliferator-activated receptor gamma agonists as insulin sensitizers: from the discovery to recent progress. Curr Top Med Chem. 2008;8:1483–507. doi: 10.2174/156802608786413474. [DOI] [PubMed] [Google Scholar]
- 102.Eibl G. The Role of PPAR-gamma and Its Interaction with COX-2 in Pancreatic Cancer. PPAR Res. 2008;2008:326915. doi: 10.1155/2008/326915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fesinmeyer MD, Stanford JL, Brentnall TA, Mandel-son MT, Farin FM, Srinouanprachanh S, et al. Association between the peroxisome proliferator-activated receptor gamma Pro12Ala variant and haplotype and pancreatic cancer in a high-risk cohort of smokers: a pilot study. Pancreas. 2009;38:631–7. doi: 10.1097/MPA.0b013e3181a53ef9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakajima A, Tomimoto A, Fujita K, Sugiyama M, Takahashi H, Ikeda I, et al. Inhibition of peroxisome proliferator-activated receptor gamma activity suppresses pancreatic cancer cell motility. Cancer Sci. 2008;99:1892–900. doi: 10.1111/j.1349-7006.2008.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tandon RK, Garg PK. Oxidative stress in chronic pancreatitis: pathophysiological relevance and management. Antioxid Redox Signal. 2011;15:2757–66. doi: 10.1089/ars.2011.4115. [DOI] [PubMed] [Google Scholar]
- 106.Katiyar SK, Meeran SM. Obesity increases the risk of UV radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling. Free Radic Biol Med. 2007;42:299–310. doi: 10.1016/j.freeradbiomed.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol. 2011;165:1162–8. doi: 10.1111/j.1365-2133.2011.10526.x. [DOI] [PubMed] [Google Scholar]
- 108.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]