Abstract
A novel method to prepare cyclosporin A encapsulated liposomes was introduced using supercritical fluid of carbon dioxide (SCF-CO2) as an antisolvent. To investigate the strength of the newly developed SCF-CO2 method compared with the modified conventional Bangham method, particle size, zeta potential, and polydispersity index (PDI) of both liposomal formulations were characterized and compared. In addition, entrapment efficiency (EE) and drug loading (DL) characteristics were analyzed by reversed-phase high-performance liquid chromatography. Significantly larger particle size and PDI were revealed from the conventional method, while EE (%) and DL (%) did not exhibit any significant differences. The SCF-CO2 liposomes were found to be relatively smaller, multilamellar, and spherical with a smoother surface as determined by transmission electron microscopy. SCF-CO2 liposomes showed no significant differences in their particle size and PDI after more than 3 months, whereas conventional liposomes exhibited significant changes in their particle size. The initial yield (%), EE (%), and DL (%) of SCF-CO2 liposomes and conventional liposomes were 90.98 ± 2.94, 92.20 ± 1.36, 20.99 ± 0.84 and 90.72 ± 2.83, 90.24 ± 1.37, 20.47 ± 0.94, respectively, which changed after 14 weeks to 86.65 ± 0.30, 87.63 ± 0.72, 18.98 ± 0.22 and 75.04 ± 8.80, 84.59 ± 5.13, 15.94 ± 2.80, respectively. Therefore, the newly developed SCF-CO2 method could be a better alternative compared with the conventional method and may provide a promising approach for large-scale production of liposomes.
Keywords: supercritical carbon dioxide, liposome, chemical stability, physical stability
Introduction
Cyclosporin A (CsA) is a neutral, hydrophobic, cyclic peptide of amino acids which contains four intramolecular hydrogen bonds that impart high rigidity to its cyclic structure.1 CsA is a powerful immunosuppressive drug which is used to prevent rejection of transplanted organs, and it is also used for the treatment of several autoimmune and some parasitic diseases.2,3 CsA is currently the second most commonly used agent in the treatment of immune-mediated ocular surface diseases, following corticosteroids, the side effects of which are well known.4 Presently there are various dosage forms – eg, capsules (oral), injectables (injection), solution (oral), and emulsion (ophthalmic) – of CsA reported in the market.5 The marketed CsA formulations Sandimmune® (Novartis International AG, Basel, Switzerland) and Neoral® (Novartis International AG) contain both surfactants and alcohol, which have limitations both in product safety for the parenteral dosage form and in shelf-life of oral products.2 Currently, CsA is commercially available in the form of oil-in-water emulsion eye drops (Restasis®; Allergan, Irvine, CA, USA). However, drawbacks of topical emulsions include poor ocular tolerance, low bioavailability, and instability.6 Considerable efforts have been made to improve the availability and the tolerance of topically applied CsA, but so far, none of the delivery systems have been fully satisfactory.7 Therefore, development of new formulations of CsA continues to be of great importance.
To overcome the problems associated with conventional delivery systems and to improve the efficiency of CsA, liposomes have been investigated as a delivery system for CsA.8 Over the past two decades, a number of studies9–15 have examined the potential application of liposomes as carriers for CsA. However, these studies are restricted to only small-scale manufacture of liposomal CsA.
In particular, the use of organic solvents is objectionable in the preparation of medicinal liposomes because of the unacceptable effects on the human body and environment that might be caused by residual solvent. Although various methods have been reported for preparation of liposomes, such as thin-film hydration,16 organic solvent injection, reverse-phase evaporation, and freeze/thaw method, almost all of these methods require a large quantity of organic solvents. The freeze/thaw method has been proposed as the only method to allow for organic solvent-free preparation of liposomes with a large inner aqueous phase;17 however, this method requires repeated freezing and thawing, thus consuming significant energy and time. Wagner et al18 presented a new scalable liposome production scheme for the ethanol injection method that enables a several-liter production, but there is still the issue of residual ethanol in the final product.19 An improved detergent depletion method (crossflow filtration) is also reported by Peschka et al for large-scale liposome production, but only preliminary studies have been carried out.20 Some other methods, eg, spray-drying,21 spray-freeze-drying,22 are also reported for liposome preparation. However, most of the currently available methods to generate liposomes are in particular suitable for the laboratory and less so for an industrial approach.19
Supercritical fluids (SCFs) exist as a single fluid phase above the critical temperature and pressure of the fluid.23 The solvent properties of SCFs can be adjusted by altering experimental conditions (temperature and pressure). In particular, SCF of CO2 (SCF-CO2) offers considerable potential as an environmentally friendly alternative solvent that can replace organic solvents because it has a low critical temperature (Tc = 31°C) and pressure (Pc = 7.38 MPa) and is nontoxic, noninflammable, and inexpensive.24,25 Due to the exceptional properties of these fluids, SCF-CO2 has attracted a great deal of attention for preparation of liposomes.24,26–28 There are several studies reporting on the preparation of liposomes using SCF-CO2.29 Frederiksen et al30 introduced the liposome preparation method in 1994 by injecting the SCF-CO2 containing lipid and cholesterol dissolved in organic solvent, into the aqueous phase, which produced small unilamellar vesicles 20–50 nm in size. This method utilized low consumption of organic solvent (ethanol), but low yield and entrapment efficiency (EE) and high capital cost is reported with this method.29 Supercritical reverse-phase evaporation method introduced by Otake et al24 is physically simpler than Frederiksen et al30 and requires less CO2; the mechanism of formation of liposome is more complicated.29 Later, Otake et al31 developed an improved supercritical reverse-phase evaporation technique to avoid the use of organic solvent in liposome formation and enhance the stability and drug-loading efficiency.29 The present SCF-CO2 method used in this study is a novel approach and is simpler compared with other methods reported.32 This method is based on the supercritical antisolvent (SAS) technique in which phospholipid is coated on the surface of lactose, forming a thin film, which on hydration gives multilamellar liposomes. Some related methods based on SAS technique are reported, but these methods generate fine and aggregated phospholipid particles, regardless of the thin film, and then liposomes are formed upon hydrolysis.27,33
According to the US Food and Drug Administration and the European Agency of the Evaluation of the Medical Products (EMA) guidelines for liposomal drug product, the physicochemical properties (eg, morphology, EE, size, size distribution, in-vitro release, phase transition temperature) of the liposome drug product are critical to ensuring drug product quality.19,34,35 The aim of the present study was the preparation of liposomal CsA using SCF-CO2 based on the SAS technique and comparison with conventionally prepared liposomes. Parameters of particle size, polydispersity index (PDI), zeta potential, morphology, EE, yield, drug loading (DL), and stability were compared between liposomes prepared by both methods. To minimize production costs, we used naturally derived phosphatidylcholine (PC) from soybean (Lipoid S100; Lipoid GmbH, Ludwigshafen, Germany) and egg yolk (Lipoid E80; Lipoid GmbH), respectively. The overall aim was to develop a liposomal CsA formulation using supercritical carbon dioxide, a simple and innovative method of preparing liposomes to facilitate large-scale production.
Materials and methods
Materials
CsA was purchased from Concord Drugs (Hyderabad, India). Soybean PC (Lipoid S100; PC > 96%) and egg PC (Lipoid E80; PC > 80%) were generously gifted from Lipoid GmbH. Cholesterol (USP) was obtained from Sigma-Aldrich (St Louis, MO, USA). Lactose anhydrous (DT NF 5X59009) was purchased from Quest International (Itaska, IL, USA). The CO2 with high purity of 99.99% was supplied by Hanmi Gas Co, Ltd (Seoul, South Korea). Ethyl alcohol (purity 99.9%) and methyl alcohol (purity 99.5%) were purchased from Samchun Chemicals (Gyeonggi-do, South Korea). All other chemicals and reagents used were of analytical grade. Purified water of Milli-Q quality (Milli-Q Reference, Millipore®, Molsheim, France) was used throughout the study.
Preparation of liposomes
Preparation of liposomes by SCF-CO2 method
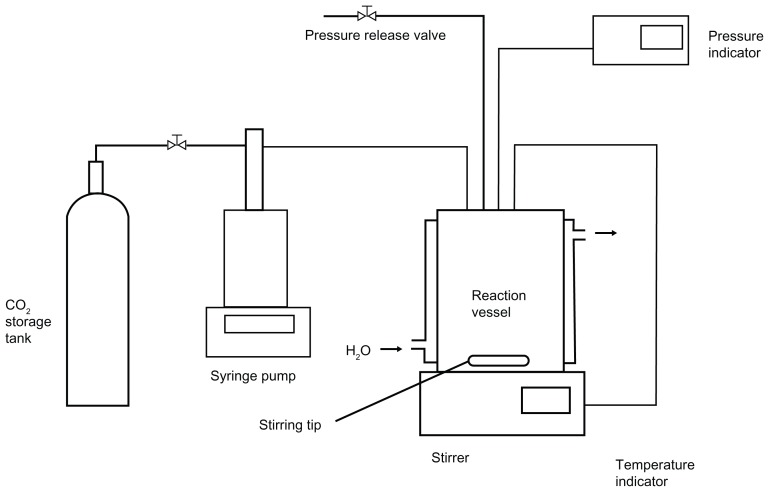
Multilamellar liposomal CsA was prepared using the method reported in a Korean patent.27,32,33 The experimental apparatus, as shown in Figure 1, was made up of the following components: CO2 syringe pump; circular and cooling lines for maintaining the CO2 pump head, and CO2 which flowed out of a storage tank (−7°C); and a reaction vessel (72 cm3) containing a magnetic stirrer, pressure indicator, and temperature indicator.
Figure 1.
Schematic representation of the experimental apparatus for liposome preparation by SCF-CO2 method.
Abbreviation: SCF-CO2, supercritical fluid of carbon dioxide.
For all preparations, 200 mg PC (Lipoid S100 or E80), 100 mg cholesterol, and 50 mg CsA were dissolved in approximately 2.5 mL of ethanol, followed by sonication (Ultrasonic Cleaner UC-20; Jeio Tech Co, Ltd, Seoul, South Korea) until a clear and homogenous solution was obtained. The CsA-lipid solution and 900 mg lactose were then sealed in the reaction vessel. The supercritical CO2 was pumped to the vessel by an ISCO syringe pump (Model 260D; ISCO Co, Louisville, KY, USA). The conditions of the reaction vessel were investigated at temperatures ranging from 35°C to 50°C and pressures ranging from 8 to 25 MPa. After approximately 30 minutes of stirring at equilibrium, additional supercritical CO2 continued to flow into the vessel for about 30 minutes to wash out any remaining solvent (ethanol). The vessel was then slowly depressurized to atmospheric pressure, and CsA-lipid coated the surface of lactose particles, forming a thin film. The resulting thin film was then hydrated with 10 mL of Milli-Q water in the reaction vessel at 50°C to form multilamellar liposomes. The liposomes obtained from this process were termed SCF-CO2 liposomes in this study.
Preparation of liposomes by the modified conventional Bangham method
The modified Bangham method16,36 was adopted as the conventional method used to prepare liposomes for comparison with CsA-liposomes prepared by the novel SCF-CO2 method. Briefly, 200 mg phospholipids (Lipoid S100 or E80), 100 mg cholesterol, and 50 mg CsA were dissolved in approximately 2.5 mL of ethanol, followed by sonication to obtain a clear and homogenous solution. Anhydrous lactose was then transferred to a round-bottom flask to which the drug-lipid solution was slowly poured. The flask was then connected to an EYELA rotary evaporator (N-1110V-W; EYELA, Shanghai, China) and water bath (SB-1200; EYELA), with the temperature maintained at 45°C with proper mixing. The organic solvent was then removed by reduced pressure and temperature to obtain a film on the wall of the vessel. The dry lipid film was hydrated with 10 mL Milli-Q water at 50°C to generate multilamellar liposomal suspensions. The obtained liposomes were referred to as conventional or film liposomes in this study.
Characterization of liposomes
Yield of CsA from liposomal CsA preparations
For determination of CsA in liposomes, 40 μL of liposomes were ruptured by methanol and the volume made up to 5.0 mL. The CsA content in the resulting solution was analyzed by an Agilent 1200 Series System (Agilent Technologies, Santa Clara, CA, USA). The chromatographic conditions were as follows: a C18 analytical column (Supelco™; Sigma-Aldrich), 4.6 mm × 150 mm, 3 μm, was used at 70°C. The mobile phase consisted of acetonitrile and distilled water (90%:10%, v/v) at a flow rate of 1.0 mL/min. Sample injection volumes were 10 μL, and CsA detection was performed using an ultraviolet detector (Agilent Technologies) at a wavelength of 210 nm. The relative retention time was found to be around 2.6 min. The percentage yield of CsA was calculated from equation 1.
| (1) |
Determination of EE and DL
For EE, 40 μL aliquots of liposomes were diluted with 960 μL of Milli-Q water. Resulting liposomal solutions were then placed in polycarbonate centrifuge tubes (Beckman Instruments, Inc, Fullerton, CA, USA) and centrifuged (Optima™ MAX-XP Ultracentrifuge; Beckman Instruments) at 50,000× g for 40 minutes at 4°C to separate the multilamellar vesicles from the aqueous solution containing free CsA. Free drug concentration in the aqueous solution was determined by high-performance liquid chromatography assay (Agilent 1200 Series; Agilent Technologies). Both the supernatant (clear solution) and residues (drug content) were dissolved in methanol, and analyses were performed. The EE and DL of CsA were calculated using equations 2 and 3, respectively.37
| (2) |
| (3) |
where Wfree is the analyzed weight of free drug in the supernatant, Wtotal is the analyzed weight of drug in the liposomal dispersions, and Wlipids is the theoretical weight of lipids used in the preparation.
Determination of particle size, PDI, and zeta potential of CsA-liposomes
The average diameter, polydispersity, and zeta potential of liposomes were measured by dynamic light scattering (DLS) with a particle size analyzer (ELS-Z; Otsuka Electronics, Hirakata, Japan) at room temperature. CsA-liposomal suspensions were properly diluted with Milli-Q water before measurements to adjust the intensity. To avoid interference from very large aggregates or dust particles, liposomes were filtered through polytetrafluoroethylene (PTFE) syringe filters with 1.0 μm pore size Whatman™ filters. The PDI was also determined as a measurement of the level of homogeneity of particle sizes. The PDI was calculated using the cumulants analysis method.38 A value of PDI less than 0.1 represented monodispersion, while values greater than 0.1 represented polydispersion of liposomal vesicles.
Transmission electron microscopy (TEM)
The morphology of SCF-CO2 liposomes was observed by TEM using a Philips CM200 (Koninklijke Philips Electronics NV, Amsterdam, Netherlands) at an accelerating voltage of 200 kV. For negative-staining, liposomes were suitably diluted with Milli-Q water and applied to a copper grid (200 mesh, hexagonal field). Further, samples were air-dried for 30 minutes at room temperature after removing the excessive sample with filter paper. After adhesion of liposomes, 10 μL of a 2% (w/v) uranyl acetate solution was dropped onto a grid as a staining solution. The excess staining solution was removed with filter paper over 30 seconds. To remove impurities, the uranyl acetate solution was filtered through 0.45 μm polycarbonate filters before deposition. Finally, samples were air-dried for about 10 minutes at room temperature, and TEM images were obtained.36,39
Stability studies of liposomes
Physical stability of liposomes
Liposomal size and PDI of both conventional and SCF-CO2 liposomes were the parameters chosen to indicate the physical stability of liposomes. Measurements were performed immediately after preparation of the liposomes and at different time intervals thereafter over a period of 3 months. Liposomes were stored in glass vials at 4°C. Filtered liposomes (1 μm PTFE filters) were used for this study. Particle sizes and PDIs were measured using the ELS-Z particle size analyzer (Otsuka Electronics).
Chemical stability of liposomes
For chemical stability, liposomes were kept refrigerated at 4°C, and at predetermined times over a period of 3 months, liposomal suspensions were subjected to analysis. The yield (%), EE (%), and DL (%) of CsA in liposomes were the parameters chosen to evaluate stability in this study.
Lyophilization of CsA-liposomes
The effect of lyophilization on the stability of liposomes was evaluated using a laboratory freeze-drier (FD 8508; Ilshin Laboratory Co, Ltd, Seoul, South Korea). Both conventional and SCF-CO2 liposomal formulations were lyophilized following two different strategies: with and without the use of an additional cryoprotectant (4% [w/v] trehalose and 6% [w/v] mannitol). An aliquot of 1.0 mL of liposomes without additional cryoprotectant and 100 μL of the liposomes diluted with Milli-Q water containing cryoprotectant as described above were kept in vials. Next, the samples were frozen at −70°C for 12 hours (Ultra-Low Temperature Chest, 700 Series; Thermo Scientific, OH, USA) followed by freeze-drying at −40°C for 72 hours under 10 Pa vacuum. Liposomes were rehydrated with normal saline water before measurement. Reconstituted liposomes were further characterized by determining particle size, PDI, yield, and EE.
Statistical analysis
Statistical analysis was performed using SPSS (IBM Corporation, Armonk, NY, USA) software, version 18.0 for Windows. Paired t-tests were used to compare the mean values between the formulations. The level of significance was set as P < 0.05. All results were expressed as the mean ± standard deviation.
Results and discussion
Optimization of the liposomal CsA preparation process
The principle of liposome formation by SCF-CO2 is similar to that of the SAS process in which success depends on the solubility of the liquid solvent in the SAS, as well as the fact that the solute is not soluble in the antisolvent. In this method, it is necessary to find the optimum temperature and pressure for selective crystallization of the solute from its solution. Table 1 shows the mean diameter, yield, EE, and DL of liposomal CsA produced by the SCF-CO2 process using ethanol as an organic solvent at various conditions of temperature and pressure. The range of temperatures was between 35°C and 50°C, while the pressure was varied from 8 to 25 MPa. Ethanol was selected as the organic solvent for this study because it is considered suitable for contact with products for human consumption when present in moderate concentration. In all experiments, the dried lactose particles coated with the drug-lipid mixture were hydrated at 50°C.
Table 1.
Effects of pressure and temperature on the SCF-CO2 process of CsA-liposomes
| Temperature (°C) | Pressure (MPa)a | Mean diameter (nm) | Yield (%)b | EE (%) | DL (%) |
|---|---|---|---|---|---|
| 40 | 10.0 | 1232.98 ± 76.92 | 87.21 ± 2.97 | 90.84 ± 0.89 | 20.01 ± 1.31 |
| 45 | 10.0 | 1137.96 ± 131.39 | 89.34 ± 2.73 | 91.74 ± 1.23 | 20.49 ± 0.87 |
| 50 | 10.0 | 1217.74 ± 229.14 | 79.73 ± 4.28 | 90.26 ± 0.36 | 17.99 ± 0.34 |
| 45 | 12.5 | 1224.15 ± 263.39 | 70.37 ± 5.78 | 88.60 ± 1.85 | 15.57 ± 0.99 |
| 45 | 15.0 | 1092.34 ± 120.06 | 69.28 ± 2.96 | 90.28 ± 2.76 | 15.37 ± 0.90 |
| 45 | 17.5 | 1267.14 ± 69.24 | 60.99 ± 5.55 | 85.86 ± 1.79 | 13.10 ± 1.49 |
| 45 | 20.0 | 1126.02 ± 244.96 | 55.14 ± 8.88 | 85.13 ± 1.64 | 11.72 ± 1.81 |
| 45 | 25.0 | 1141.84 ± 298.11 | 42.15 ± 4.22 | 84.45 ± 2.13 | 9.35 ± 0.92 |
Notes: The lipid used in all preparations was Lipoid S100. Values denote the mean ± standard deviation of four separate sets of experiments.
A pressure of 8 MPa was unable to generate liposomes in this study;
the percentage yield of CsA in liposomes.
Abbreviations: SCF-CO2, supercritical fluid of carbon dioxide; CsA, cyclosporin A; EE, entrapment efficiency; DL, drug loading.
It was observed that pressures below 8 MPa and temperatures below 35°C were not appropriate for the liposomal CsA preparation because of residual solvent in the reaction vessel. This may have been due to the subcritical state of the mixtures of CO2 and organic solvents. Diffusion coefficients at subcritical temperatures were smaller than those at supercritical temperatures,40 resulting in insufficient removal of organic solvents. By increasing the temperature of the SCF-CO2 process, the percentage yield of CsA was significantly decreased. This can be explained by the increased solubility of the drug in supercritical CO2 at higher temperatures.
Liposomal CsA prepared by the SCF-CO2 process at pressures above 10 MPa was studied. Table 1 shows that the highest CsA yield of around 90% was obtained at 10 MPa. The antisolvent capacity of supercritical CO2 increases with increasing pressure at constant temperature, which means that the mutual solubility of supercritical CO2 and organic solvents for a given drug can also be increased with increasing pressure.41 If the solubility of the solute increases along with the pressure, then it follows that lower yields will be obtained from the amount of the solute dissolved in supercritical CO2. Consequently, there is competition between the antisolvent and solvent effects of the supercritical CO2 in the SAS process. Therefore, the reason that the highest CsA yield was found at 10 MPa might be that the solubility of CsA in supercritical CO2 was smallest among the experimental pressures. EEs were significantly decreased when the pressure exceeded 15 MPa; however, pressure did not seem to have a perceivable effect on particle size. These results were consistent with the findings of a previous study using the drug amphotericin B.32 Specifically, it is well known that pressure is the most relevant parameter in controlling particle size during the gas antisolvent process, but this is not the case for the SAS process.42
As shown in Table 1, among the pressure and temperature conditions evaluated, liposomes prepared at 10 MPa and 45°C exhibited the best results in all the parameters tested, with a relatively lower PDI (data not shown). Therefore, further experiments of liposomal CsA prepared by the SCF-CO2 process utilized a temperature of 45°C and pressure of 10 MPa.
This SCF-CO2 method of liposome preparations had some limitations. For preparing liposomes, carrier (eg, lactose) was required to coat, the rate of depressurization was manually controlled, and this method could not generate liposomes of much smaller size.
Particle size and zeta potential of CsA-liposomes
The results obtained with SCF and conventionally prepared liposomes are shown in Table 2. The mean diameter of the unfiltered liposomes prepared by the SCF-CO2 method was around 0.7–1.4 μm, whereas the unfiltered liposomes formed by the conventional method ranged from 1.4 to 2.5 μm as observed by the DLS method. The PDI of the SCF-CO2 liposomes was found to be relatively lower than that of conventional liposomes. The large PDIs of conventional liposomes suggested a heterogeneous vesicle population. The results of the particle size measurement by DLS indicated that liposomes prepared with SCF-CO2 were relatively much smaller (P < 0.02) and more homogenous in size. Similar results were also reported by Kadimi et al26 and Otake et al,31 where liposomes obtained by SCF-CO2 were much smaller in size compared to the Bangham method.
Table 2.
Particle size, polydispersity index, and zeta potential of different liposomes prepared by conventional and SCF-CO2 methods
| Liposome | Mean diameter (nm) | Polydispersity index | Zeta potential (mV) |
|---|---|---|---|
| Non-filtered | |||
| SCF-S100 | 1137.96 ± 131.39 | 0.34 ± 0.02 | −7.73 ± 3.38 |
| SCF-E80 | 747.04 ± 82.30 | 0.30 ± 0.03 | −30.77 ± 7.00 |
| Film-S100 | 2155.90 ± 373.55 | 0.61 ± 0.09 | −7.92 ± 5.55 |
| Film-E80 | 1543.01 ± 66.31 | 0.51 ± 0.10 | −22.4 ± 9.33 |
| Filtered | |||
| SCF-S100 | 188.93 ± 17.00 | 0.22 ± 0.02 | −6.82 ± 2.96 |
| SCF-E80 | 166.58 ± 9.60 | 0.18 ± 0.01 | −30.72 ± 1.92 |
| Film-S100 | 244.30 ± 44.15 | 0.22 ± 0.02 | −5.76 ± 0.56 |
| Film-E80 | 198.87 ± 26.24 | 0.20 ± 0.03 | −19.25 ± 6.12 |
Notes: “Filtered” represents liposomes passed through a 1.0 μm pore size PTFE syringe filter before analysis. Values denote the mean ± standard deviation of six separate sets of experiments.
Abbreviations: SCF-CO2, supercritical fluid of carbon dioxide; SCF-S100, supercritical fluid liposomes prepared using Lipoid S100; SCF-E80, supercritical fluid liposomes prepared using Lipoid E80; Film-S100, conventional liposomes prepared using Lipoid S100; Film-E80, conventional liposomes prepared using Lipoid E80; PTFE, polytetrafluoroethylene.
Liposomes generally undergo further treatment, eg, sonication, homogenization, or extrusion, just after preparation in order to reduce the particle size to the nanometer range.19,29 Use of nanoliposomes has been reported in a wide variety of applications in drug-delivery systems.43
Two types of lecithin were used in this study. Lipoid S100 derived from soy lecithin contained PC > 95%, whereas Lipoid E80 derived from egg lecithin contained PC 80%–85%. These materials differed with respect to the content and properties of lipids, attributing to the difference in size of liposomes prepared by Lipoid S100 and Lipoid E80. To avoid interference by large aggregates that were nonrepresentative of the preparation as a whole, liposomes were passed through 1-μm PTFE (Whatman) syringe filters, which was considered to not affect the sub-1-μm liposome population.44,45 After filtration, SCF-CO2 liposomes had a mean diameter of 150–200 nm, with a PDI of not more than 0.25, whereas conventional liposomes had diameters of 200–250 nm, with a PDI around 0.25. Table 2 shows that there was a significant difference (P < 0.05) between the particle sizes of SCF-S100 and Film-S100, although none of the other liposome preparations exhibited similar differences.
The zeta potentials observed with all liposomal preparations showed that the negative surface charge ranged from −7 to −30 mV. This negative surface charge imparted by lecithin was mainly due to phosphatidic acid present in the phospholipid products.46
EE and DL of CsA-liposomes
The four different formulations of CsA-loaded conventional and SCF-CO2 liposomes are summarized in Table 3, together with their yields and DL capacities. Liposomes with high EEs were achieved. Specifically, the EEs for the SCF-S100, Film-S100, SCF-E80, and Film-E80 were 91.74 ± 1.73, 90.66 ± 1.53, 90.38 ± 0.96, and 89.11 ± 1.68, respectively. Similarly, Guo et al,14 Guan et al,15 and Shah et al36 have reported the high EE% of CsA in liposomes. Statistical analysis showed that EE of a drug is independent of preparation method, and that the differences between SCF-CO2 and conventional liposomes were not significant (P > 0.05). CsA is a highly lipophilic compound. Loading of lipophilic drugs in the liposome bilayer is a result of drug and phospholipid interactions, and EE is dependent on the solubility of the drug in bilayer membranes.47 The drug to phospholipid ratio is very important, because DL in liposomes for lipophilic drugs is restricted to the hydrophobic region of the bilayer. The EE of CsA in liposomes was also dependent on the cholesterol content.10 The ratio of phospholipid, drug, and cholesterol selected in this study resulted in a high EE together with enhanced yield and DL.
Table 3.
EE, yield, and DL of CsA-liposomes
| Liposome | Yield (%) | EE (%) | DL (%) |
|---|---|---|---|
| SCF-S100 | 89.34 ± 2.73 | 91.74 ± 1.73 | 20.49 ± 0.87 |
| Film-S100 | 90.23 ± 6.88 | 90.66 ± 1.53 | 20.45 ± 1.73 |
| SCF-E80 | 88.25 ± 2.69 | 90.38 ± 0.96 | 18.97 ± 1.56 |
| Film-E80 | 88.16 ± 6.10 | 89.11 ± 1.68 | 19.51 ± 1.03 |
Note: Values denote the mean ± standard deviation of five separate sets of experiments.
Abbreviations: EE, entrapment efficiency; DL, drug loading; CsA, cyclosporin A; SCF-S100, supercritical fluid liposomes prepared using Lipoid S100; Film-S100, conventional liposomes prepared using Lipoid S100; SCF-E80, supercritical fluid liposomes prepared using Lipoid E80; Film-E80, conventional liposomes prepared using Lipoid E80.
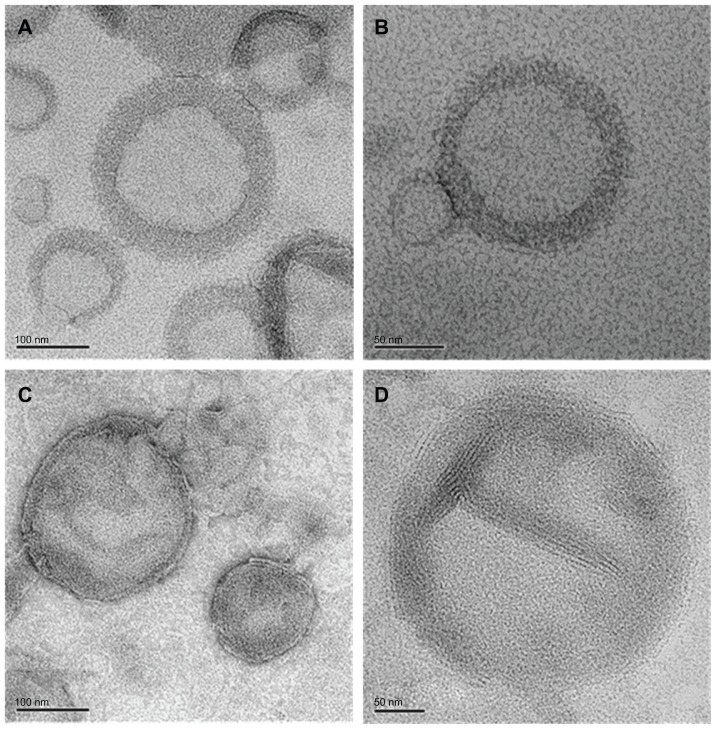
Negative-staining TEM of SCF-CO2 liposomes
The images from negative-staining TEM of SCF-CO2 liposomes prepared using Lipoid S100 (Figure 2A and B) and Lipoid E80 (Figure 2C and D) were multilamellar and spherical in shape. The sizes obtained ranged from 100 to 200 nm according to TEM analysis, which was consistent with the results obtained from the particle size measurement by DLS, shown in Table 2.
Figure 2.
Transmission electron microscopy image of SCF-S100 liposomes (A and B) and SCF-E80 liposomes (C and D).
Abbreviations: SCF-S100, supercritical fluid liposomes prepared using Lipoid S100; SCF-E80, supercritical fluid liposomes prepared using Lipoid E80.
Stabilities of liposomes
Physical stability
Stability is a critical factor that must be considered during formulation design and development.48 Physical instability of liposomal formulations might become apparent after an increase in particle size due to the aggregation or fusion of unstable liposomes during formulation processing and/or upon long-term storage. Consequently, this instability results in rapid uptake by the reticuloendothelial system with subsequent rapid clearance, resulting in a short half-life. Therefore, preparing liposomes at small and uniform sizes is the most important aspect for developing pharmaceutical products.
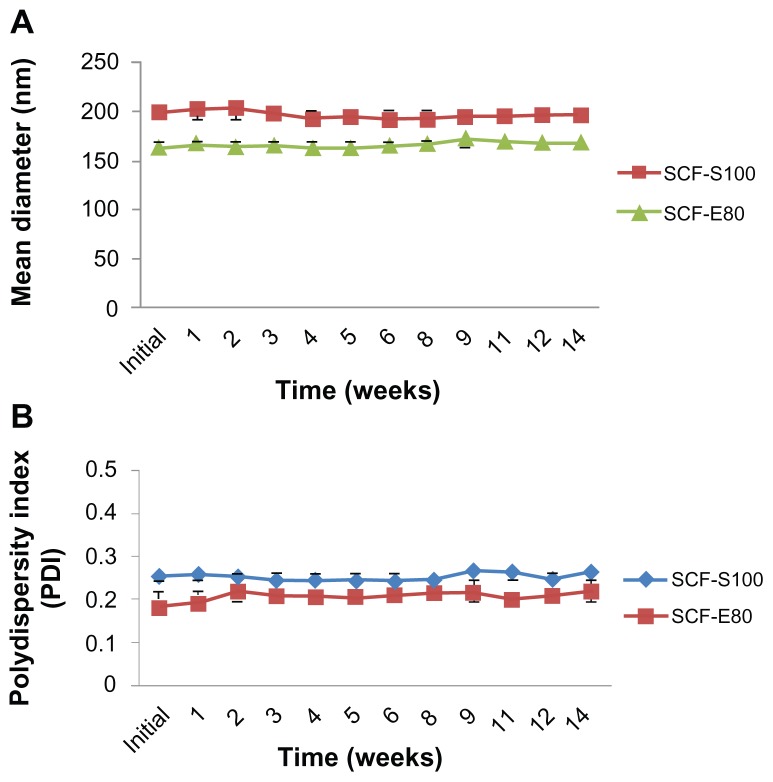
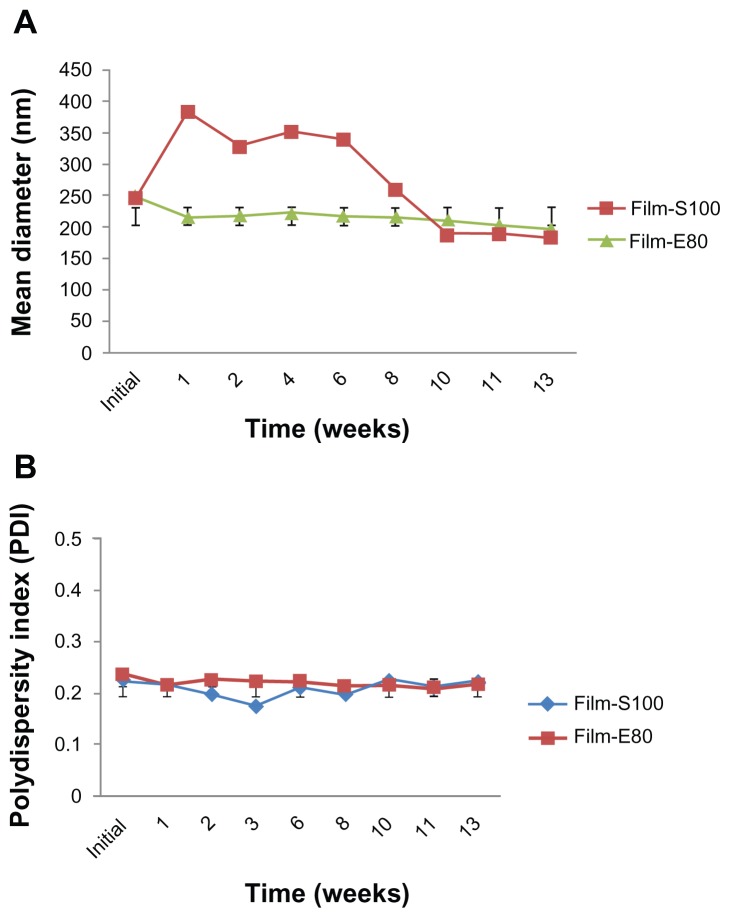
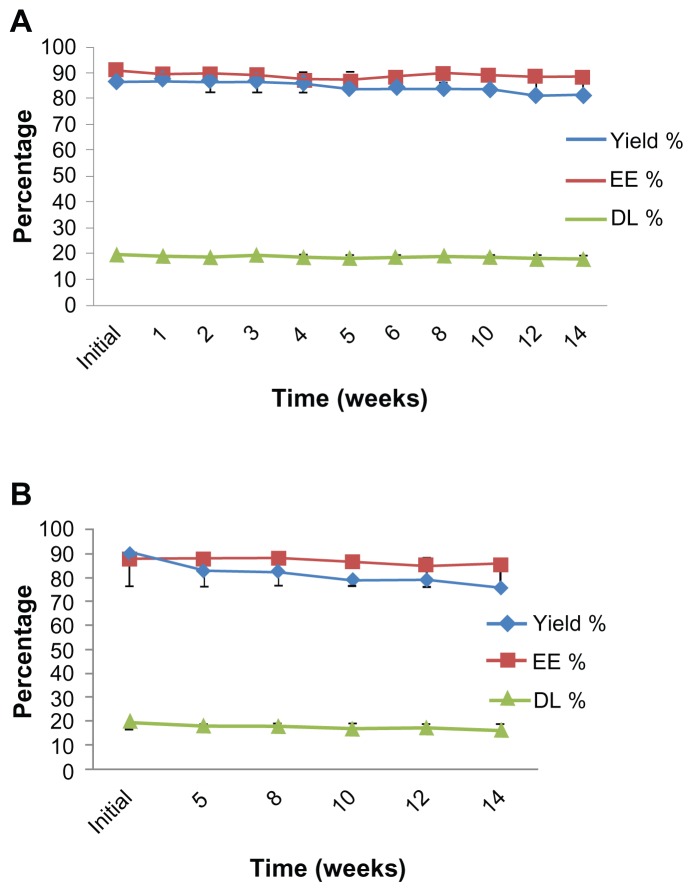
All liposomal preparations were stored at 4°C and protected from direct light for further stability studies. The filtered liposomes were used for this study. The initial size and PDI were 198.75 ± 10.57 nm and 0.25 ± 0.02 for SCF-S100 and 164.10 ± 8.47 nm and 0.18 ± 0.01 for SCF-E80, respectively. After 14 weeks of storage at 4°C, the particle size (Figure 3A) and PDI (Figure 3B) changed to 195.77 ± 9.02 nm and 0.26 ± 0.02 for SCF-S100 and 169.25 ± 4.05 nm and 0.18 ± 0.01 for SCF-E80, respectively. It was interesting to note that SCF-CO2 liposomes did not show significant changes in particle size (P > 0.05) or PDI (P > 0.05) during 14 weeks, indicating that there was no aggregation. On the contrary, Figure 4 shows the irregular behavior of particle sizes and PDI of conventional liposomes during 13 weeks of storage at 4°C.
Figure 3.
(A) Changes in particle size of SCF-CO2 liposomes stored at 4°C at different time intervals over 14 weeks. (B) Size distribution of SCF-CO2 liposomes stored at 4°C at different time intervals over 14 weeks.
Notes: Liposomes were passed through 1-μm PTFE filters. Values denote the mean ± standard deviation of three separate sets of experiments.
Abbreviations: SCF-CO2, supercritical fluid of carbon dioxide; SCF-S100, supercritical fluid liposomes prepared using Lipoid S100; SCF-E80, supercritical fluid liposomes prepared using Lipoid E80; PTFE, polytetrafluoroethylene.
Figure 4.
(A) Changes in particle size of conventional liposomes stored at 4°C at different time intervals over 13 weeks. (B) Size distribution of conventional liposomes stored at 4°C at different time intervals over 13 weeks.
Note: Liposomes were passed through 1-μm PTFE filters. Values denote the mean ± standard deviation of three separate sets of experiments.
Abbreviations: SCF-CO2, supercritical fluid of carbon dioxide; Film-S100, conventional liposomes prepared using Lipoid S100; Film-E80, conventional liposomes prepared using Lipoid E80; PTFE, polytetrafluoroethylene.
The above results, which suggest that liposomes prepared by the SCF-CO2 method would be stable for longer periods of time without lyophilization, are an indication that our newly developed liposomal formulations meet the requirements for an effective drug-delivery system.
Chemical stability
Chemical stability is one of the major barriers that limit the widespread use of liposomes as a pharmaceutical product.49 As a dry powder, CsA is very stable for at least two years under dark and refrigerated (2°C–8°C) conditions.50 However, liposomes encapsulated with drugs have several problems associated with storage, such as phospholipid hydrolysis and decomposition of encapsulated drug.13 Such chemical degradation processes might be due to hydrolysis of the ester bonds linking the fatty acids to the glycerol backbone and peroxidation of unsaturated acyl chains, if present.46
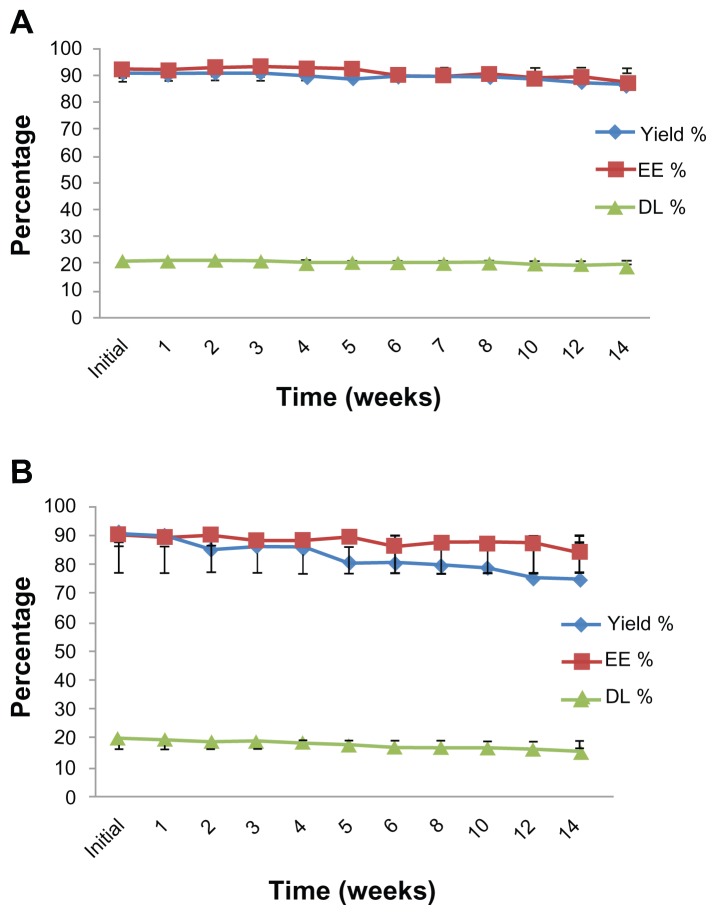
Figures 5 and 6 show the chemical stability of CsA-liposomes with respect to changes in yield (%), EE (%), and DL (%) during 14 weeks of storage at 4°C. The initial percentages of yield, EE, and DL were 90.98 ± 2.94, 92.20 ± 1.36, and 20.99 ± 0.84, respectively, for SCF-S100 liposomes. Even after 14 weeks of storage at 4°C, there were no significant changes (P > 0.05) in any of the parameters tested (Figure 5A). On the contrary, Film-S100 liposomes had significant reductions in yield (%) from 90.72 ± 2.83 to 75.04 ± 8.80, EE (%) from 90.24 ± 1.37 to 84.59 ± 5.13, and DL (%) from 20.47 ± 0.94 to 15.94 ± 2.80 after 14 weeks of storage at 4°C (Figure 5B). Statistically, there were significant differences (P < 0.05) between the two formulations, suggesting that SCF-CO2 liposomes prepared by Lipoid S100 were much more stable than the liposomes formed by the conventional method.
Figure 5.
Changes in chemical properties of (A) SCF-S100 liposomes and (B) Film-S100 liposomes stored at 4°C at different time intervals over 14 weeks.
Note: Values denote the mean ± standard deviation of three separate sets of experiments.
Abbreviations: EE, entrapment efficiency; DL, drug loading; SCF-S100, supercritical fluid liposomes prepared using Lipoid S100; Film-S100, conventional liposomes prepared using Lipoid S100.
Figure 6.
Changes in the chemical properties of (A) SCF-E80 liposomes and (B) Film-E80 liposomes stored at 4°C at different time intervals over 14 weeks.
Note: Values denote the mean ± standard deviation of three separate sets of experiments.
Abbreviations: EE, entrapment efficiency; DL, drug loading; SCF-E80, supercritical fluid liposomes prepared using Lipoid E80; Film-E80, conventional liposomes prepared using Lipoid E80.
Figure 6 shows that both SCF and film liposomes prepared using Lipoid E80 had relatively lower percentage yield, and also there was less drug decomposition with SCF-CO2 liposomes during long-term storage. Unlike SCF-E80 liposomes (Figure 6A), conventional Film-E80 liposomes showed a remarkable reduction of yield (%) from 89.92 ± 2.71 to 75.71 ± 3.61 during 14 weeks of storage at 4°C (Figure 6B).
This study showed that, among the tested liposomes, SCF-S100 liposomes were suitable candidates for further liposomal formulation. This may have been due to the higher percentage of PC in the lecithin, which gave better solubility, as Lipoid S100 yielded better results than the Lipoid E80. Similar results were also reported by Badens et al42 and Karn et al,51 where Lipoid S100 produced better results than S20 and S75.
In summary, no significant changes in mean particle size, PDI, yield, EE, and DL were observed during the stability study of SCF-CO2 liposomes. These results indicated that liposomes prepared by the SCF-CO2 method were relatively more stable compared with those prepared by the conventional method, which was in accordance with the results by Otake et al,31 Aburai et al,52 and Kadimi et al.26 The stability of liposomes prepared with SCF-CO2 might be explained by the static repulsion of the carbonic acids incorporated into the bilayer membrane.53 On the other hand, the chemical instability of conventional liposomes might be caused by the hydrolysis of ester bonds and/or oxidation of polyunsaturated acyl chains of lipids.
Effect of lyophilization
Since the structural integrity of liposomes for a long period of time is one of the major objectives in optimizing a formulation, the effect of the lyophilization process was analyzed. It has been reported that liposomes containing drug molecules can be lyophilized and reconstituted with significant drug retention measured as encapsulated drug and without significant change in the mean particle size and PDI.54 Cryoprotection is needed to protect the liposomes during lyophilization, and disaccharides such as sucrose, lactose, and trehalose were mainly used to protect the liposomes during the freezing stage of the lyophilization cycle.55
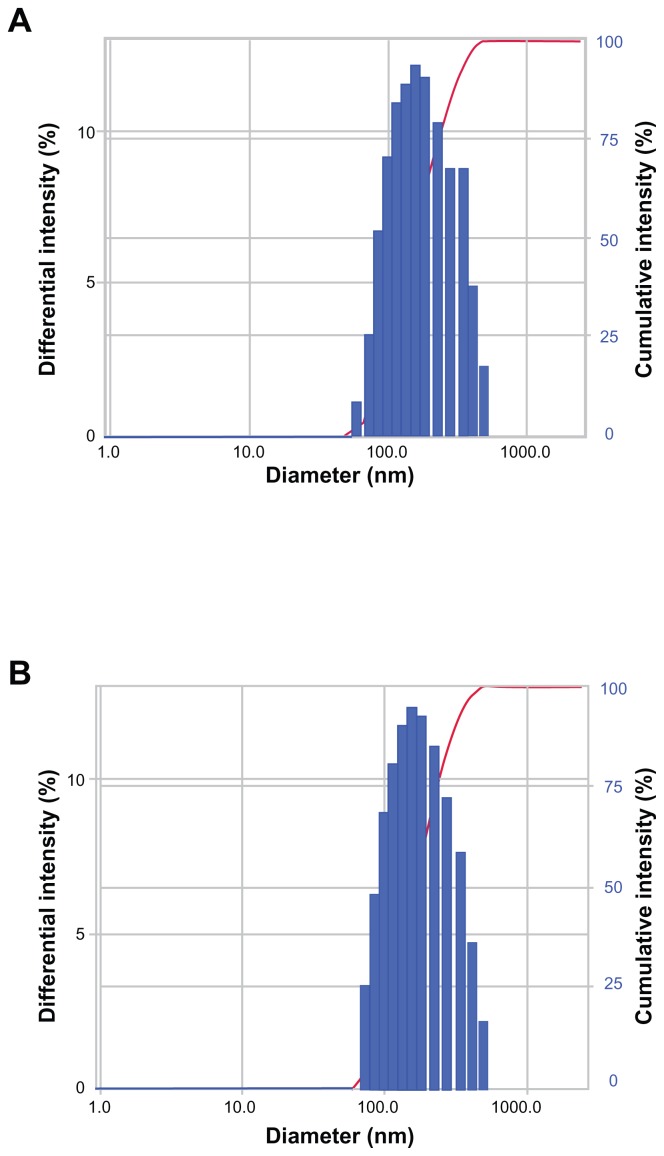
Two different lyophilization strategies were evaluated in the present study. Since our liposomal formulations contained 9% (w/v) lactose, which might play an important role as a cryoprotectant, liposomes were freeze-dried without any additional cryoprotectant. In the second set of samples, additional cryoprotectants (mannitol and trehalose) were used to prevent thermodynamic instability. All samples were evaluated according to changes in particle size, PDI, yield (%), and EE (%), and the results are listed in Table 4. Our data showed that the process of lyophilization did not affect the physical or chemical stability of liposomes. Indeed, neither SCF-CO2 nor conventional liposomes showed any significant changes (P > 0.05) with respect to particle size, PDI, yield (%), and EE (%) after freeze-drying, which was expected. The size distribution of SCF-CO2 liposomes prior to lyophilization and after reconstitution of dried liposomes showed mono-modal size distribution (Figure 7). Both strategies, with or without additional cryoprotectants, displayed similar behavior, supporting the possibility that liposomal formulations with 9% (w/v) lactose could be a promising strategy to provide a stable formulation.
Table 4.
Influence of the lyophilization process on particle size, polydispersity index, yield, and EE of SCF-CO2 and conventional liposomes
| Liposome | Condition | Diameter (nm) | PDI | Yield (%) | EE (%) |
|---|---|---|---|---|---|
| SCF-CO2 | With CP | ||||
| Before FD | 175.77 ± 3.34 | 0.19 ± 0.00 | 87.71 ± 0.96 | 90.89 ± 0.86 | |
| After FD | 179.26 ± 8.17 | 0.24 ± 0.01 | 87.25 ± 0.32 | 89.91 ± 1.20 | |
| Without CP | |||||
| Before FD | 175.77 ± 3.34 | 0.19 ± 0.00 | 87.71 ± 0.96 | 90.89 ± 0.86 | |
| After FD | 178.57 ± 5.24 | 0.25 ± 0.01 | 86.15 ± 1.72 | 89.01 ± 1.04 | |
| Conventional | With CP | ||||
| Before FD | 235.31 ± 5.68 | 0.20 ± 0.01 | 89.03 ± 4.26 | 87.39 ± 2.79 | |
| After FD | 237.45 ± 23.488 | 0.23 ± 0.02 | 87.23 ± 5.13 | 84.66 ± 3.85 | |
| Without CP | |||||
| Before FD | 235.31 ± 5.68 | 0.20 ± 0.01 | 89.03 ± 4.26 | 87.39 ± 2.79 | |
| After FD | 240.18 ± 24.07 | 0.23 ± 0.02 | 86.40 ± 1.27 | 85.05 ± 2.15 |
Notes: The liposomes used in this study were SCF-S100 as SCF-CO2 liposome and Film-S100 as conventional liposome. Values represent the mean ± standard deviation of three separate sets of experiments.
Abbreviations: EE, entrapment efficiency; PDI, polydispersity index; CP, cryoprotectant; SCF-CO2, supercritical fluid of carbon dioxide; FD, freeze drying.
Figure 7.
Size distribution (by intensity) of SCF-CO2 liposomes obtained by DLS (ELS-Z; Otsuka Electronics, Hirakata, Japan) (A) prior to lyophilization and (B) after reconstitution of dried liposomes.
Abbreviations: SCF-CO2, supercritical fluid of carbon dioxide; DLS, dynamic light scattering.
SCF-CO2 liposomes versus conventional liposomes
In this study, we illustrated the difference between liposomes prepared using both SCF-CO2 and conventional modified Bangham methods. Compared with conventional liposomes, those produced using the SCF-CO2 method had promising features that can be summarized as follows:
Liposome preparation by conventional methods is limited only to laboratory scale; however, the SCF-CO2 method might be useful for the large-scale manufacture of liposomes that complies with good manufacturing practice restrictions.
Environmental friendliness is the best quality associated with the use of SCF-CO2, which is green, nontoxic, noninflammable, and inexpensive.
The particle size and PDI of SCF-CO2 liposomes were relatively smaller than those of the conventional liposomes.
Liposomes formed by SCF-CO2 were physically and chemically more stable. Therefore, SCF-CO2 liposomes might be useful for possible development of liposomal drug formulations.
By observing with the naked eye, conventional liposomes, if stored for a longer period of time, seemed to have some microbial contamination that manifested as changes in color and smell. In contrast, SCF-CO2 liposomes did not show any changes. This result suggests that SCF-CO2 preparation may have a sterilization effect.53
By varying pressure and temperature, the physiochemical properties of liposomes could easily be controlled using the SCF-CO2 method.
The whole operation took place under mild conditions (temperature, 45°C–50°C; pressure, 100–150 bar) and thus can avoid the phase transition of phospholipids and degradation of partial lipids or drug in liposomes, which has implications for large-scale production of liposomes.
A dry liposomal powder can be obtained directly, ie, liposomes need not undergo further processing, eg, freeze drying, spray-drying, or precipitation.
Conclusion
Liposomes have ample prospective uses in the pharmaceutical industry; however, the broad application of liposomes in drug delivery is still impeded due to scale-up issues. The current study revealed that the SCF-CO2 method provides a simpler and more efficient way to prepare liposomes with a characteristically smaller size and better morphology (uniform size and shape) together with improved EE and DL. In addition, this method was efficient in controlling the properties of liposomes. Most importantly, the physical and chemical stabilities of SCF-CO2 liposomes were much superior compared with conventional liposomes. Together, these results indicate that the SCF-CO2 method might serve as a potential alternative to the conventional Bangham method. Furthermore, SCF-CO2 CsA-liposomes may be a promising tool for the ophthalmic delivery of CsA. In addition, complete elucidation of this newly developed method, with a focus on the safety and in-vivo pharmacological efficacy of CsA-liposomes, is currently under investigation.
Acknowledgments
This research was financially supported by a grant of the Korean Health Technology R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (A092018). The work was also supported in part by the Yonsei University Research Fund of 2011.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. The authors alone are responsible for the content and writing of this paper.
References
- 1.Petcher TJ, Weber H, Ruegger A. Crystal and molecular structure of an iodo-derivative of the cyclic undecapeptide cyclosporin A. Helv Chim Acta. 1976;59(5):1480–1489. doi: 10.1002/hlca.19760590509. [DOI] [PubMed] [Google Scholar]
- 2.Klyashchitsky BA, Owen AJ. Drug delivery systems for cyclosporin: achievements and complications. J Drug Target. 1998;5(6):443–458. doi: 10.3109/10611869808997871. [DOI] [PubMed] [Google Scholar]
- 3.Malaekeh-Nikouei B, Jaafari MR, Tabassi SA, Samiei A. The enhancement of immunosuppressive effects of cyclosporin A on human T-cells using fusogenic liposomes. Colloids Surf B Biointerfaces. 2008;67(2):238–244. doi: 10.1016/j.colsurfb.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18(5):352–361. doi: 10.3109/09273948.2010.498657. [DOI] [PubMed] [Google Scholar]
- 5.Approved Drug Products With Therapeutic Equivalence Evaluations. 32nd ed. Section 3. US Department of Health and Human Resources, Food and Drug Administration; USA: 2012. pp. 115–116. [Google Scholar]
- 6.Gupta C, Chauhan A. Ophthalmic delivery of cyclosporin A by punctal plugs. J Control Release. 2011;15(1):70–76. doi: 10.1016/j.jconrel.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Lallemand F, Felt-Baeyens O, Besseghir K, et al. Cyclosporin A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. 2003;56(3):307–318. doi: 10.1016/s0939-6411(03)00138-3. [DOI] [PubMed] [Google Scholar]
- 8.Fahr A, Seelig J. Liposomal formulations of cyclosporin A: a biophysical approach to pharmacokinetics and pharmacodynamics. Crit Rev Ther Drug Carrier Syst. 2001;18(2):141–172. [PubMed] [Google Scholar]
- 9.Stuhne-Sekalec L, Stanacev NZ. Liposomes as carriers of cyclosporin A. J Microencapsul. 1991;8(4):441–446. doi: 10.3109/02652049109021867. [DOI] [PubMed] [Google Scholar]
- 10.Al-Angary AA, Bayomi MA, Khidr SH, Al-Meshal MA, Al-Dardiri M. Characterization, stability and in vivo targeting of liposomal formulations containing cyclosporin. Int J Pharm. 1995;114(2):221–225. [Google Scholar]
- 11.Ouyang C, Choice E, Holland J, Meloche M, Madden TD. Liposomal cyclosporin. Characterization of drug incorporation and interbilayer exchange. Transplantation. 1995;60(9):999–1006. [PubMed] [Google Scholar]
- 12.Al-Meshal MA, Khidr SH, Bayomi MA, Al-Angary AA. Oral administration of liposomes containing cyclosporin: a pharmacokinetic study. Int J Pharm. 1998;168(2):163–168. [Google Scholar]
- 13.Lee MK, Choi L, Kim MH, Kim CK. Pharmacokinetics and organ distribution of cyclosporin A incorporated in liposomes and mixed micelles. Int J Pharm. 1999;191(2):87–93. doi: 10.1016/s0378-5173(99)00260-4. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Ping Q, Chen Y. Pharmacokinetic behavior of cyclosporin A in rabbits by oral administration of lecithin vesicle and Sandimmun Neoral. Int J Pharm. 2001;216(1–2):17–21. doi: 10.1016/s0378-5173(00)00680-3. [DOI] [PubMed] [Google Scholar]
- 15.Guan P, Lu Y, Qi J, et al. Enhanced oral bioavailability of cyclosporin A by liposomes containing a bile salt. Int J Nanomedicine. 2011;6:965–974. doi: 10.2147/IJN.S19259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 17.Anzai K, Yoshida M, Kirino Y. Change in intravesicular volume of liposomes by freeze-thaw treatment as studied by the ESR stopped-flow technique. Biochim Biophys Acta. 1990;1021(1):21–26. [Google Scholar]
- 18.Wagner A, Platzgummer M, Kreismayr G, et al. GMP production of liposomes-a new industrial approach. J Liposome Res. 2006;16(3):311–319. doi: 10.1080/08982100600851086. [DOI] [PubMed] [Google Scholar]
- 19.Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011:591325. doi: 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peschka R, Purmann T, Schubert R. Cross-flow filtration – an improved detergent removal technique for the preparation of liposomes. Int J Pharm. 1998;162(2):177–183. [Google Scholar]
- 21.Payne NI, Salmon JR, inventors. ER Squibb and Sons Inc, assignee. United States patent US 4830858. Spray-drying method for preparing liposomes and products produced thereby. 1989 May 16;
- 22.Wang Z, Orszanska H, Finlay WH, inventors. University of Alberta, assignee. United States patent US 2006/0280691A1. Spray freeze dried liposomal ciprofloxacin powder aerosol drug delivery. 2006 Dec 14;
- 23.Kim MS, Kim JS, Park HJ, Cho WK, Cha KH, Hwang SJ. Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process. Int J Nanomedicine. 2011;6:2997–3009. doi: 10.2147/IJN.S26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otake K, Imura T, Sakai H, Abe M. Development of a new preparation method of liposomes using supercritical carbon dioxide. Langmuir. 2001;17(13):3898–3901. [Google Scholar]
- 25.Kim MS, Jin SJ, Kim JS, et al. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur J Pharm Biopharm. 2008;69(2):454–465. doi: 10.1016/j.ejpb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Kadimi US, Balasubramanian DR, Ganni UR, Balaraman M, Govindarajulu V. In vitro studies on liposomal amphotericin B obtained by supercritical carbon dioxide-mediated process. Nanomedicine. 2007;3(4):273–280. doi: 10.1016/j.nano.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Naik S, Patel D, Surti N, Misra A. Preparation of PEGylated liposomes of docetaxel using supercritical fluid technology. J Supercrit Fluids. 2010;54(1):110–119. [Google Scholar]
- 28.Zhong J, Dai LC. Liposomal preparation by supercritical fluids technology. Afr J Biotechnol. 2011;10(73):16406–16413. [Google Scholar]
- 29.Meure LA, Foster NR, Dehghani F. Conventional and dense gas techniques for the production of liposomes: a review. AAPS Pharm Sci Tech. 2008;9(3):798–809. doi: 10.1208/s12249-008-9097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frederiksen L, Anton K, Barratt BJ, van Hoogevest P, Keller HR, Leuenberger H. Use of supercritical carbon dioxide for preparation of pharmaceutical formulations. Proceedings of the 3rd International Symposium on Supercritical Fluids; October 17–19, 1994; Strasbourg, France. 1994. pp. 235–240. [Google Scholar]
- 31.Otake K, Shimomura T, Goto T, et al. Preparation of liposomes using an improved supercritical reverse phase evaporation method. Langmuir. 2006;22(6):2543–2550. doi: 10.1021/la051654u. [DOI] [PubMed] [Google Scholar]
- 32.Hwang SJ, Park HJ, Cho W, et al., inventors. Foundation of University-Industry Research Collaboration of Chungnam National University, assignee. South Korean patent KR 2011-0096962. New methods and apparatus for preparing liposomes. 2011 Aug 31;
- 33.Lesion L, Crampon C, Boutin O, Badens E. Preparation of liposomes using the supercritical anti-solvent (SAS) process and comparison with a conventional method. J Supercrit Fluids. 2011;57(2):162–174. [Google Scholar]
- 34.US Department of Health and Human Services, Food and Drug Administration. Guidance for Industry. Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation (draft guidance) 2002. [Accessed October 31, 2012]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070570.pdf.
- 35.European Medicines Agency. Reflection Paper on the Data Requirements for Intravenous Liposomal Products Developed with Reference to an Innovator Liposomal Product (draft) London, UK: European Medicines Agency; 2011. [Accessed October 31, 2012]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500109479.pdf. [Google Scholar]
- 36.Shah NM, Parikh J, Namdeo A, Subramanian N, Bhowmick S. Preparation, characterization and in vivo studies of proliposomes containing cyclosporin A. J Nanosci Nanotechnol. 2006;6(9–10):2967–2973. doi: 10.1166/jnn.2006.403. [DOI] [PubMed] [Google Scholar]
- 37.Du B, Li Y, Li X, AY, Chen C, Zhang Z. Preparation, characterization and in vivo evaluation of 2-methoxyestradiol-loaded liposomes. Int J Pharm. 2010;384(1–2):140–147. doi: 10.1016/j.ijpharm.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 38.Frisken BJ. Revisiting the method of cumulants for the analysis of dynamic light-scattering data. Appl Opt. 2001;40(24):4087–4091. doi: 10.1364/ao.40.004087. [DOI] [PubMed] [Google Scholar]
- 39.Yang T, Cui FD, Choi MK, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338(1–2):317–326. doi: 10.1016/j.ijpharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Wang W. Adsorption and diffusion of supercritical carbon dioxide in slit pores. Langmuir. 2000;16(21):8063–8070. [Google Scholar]
- 41.Xia F, Jin H, Guo X. Preparation of coenzyme Q10 liposomes using supercritical anti-solvent technique. J Microencapsul. 2012;29(1):21–29. doi: 10.3109/02652048.2011.629742. [DOI] [PubMed] [Google Scholar]
- 42.Badens E, Magnan C, Charbit G. Microparticles of soy lecithin formed by supercritical processes. Biotechnol Bioeng. 2000;72(2):194–204. doi: 10.1002/1097-0290(20000120)72:2<194::aid-bit8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Park JB, Noh HG, Jung JH, Kim JM, Kang CY. Enhanced transdermal delivery and optimization of nano-liposome preparation using hydrophilic drug. J Pharm Invest. 2012;42:57–63. [Google Scholar]
- 44.New RRC. Characterization of liposomes. In: New RRC, editor. Liposomes: A Practical Approach. New York, NY: Oxford University Press; 1990. pp. 157–158. [Google Scholar]
- 45.Zuidam NJ, de Vrueh R, Crommelin DJA. Characterization of liposomes. In: Torchilin V, Weissig V, editors. Liposomes: A Practical Approach. New York, NY: Oxford University Press; 2003. pp. 70–71. [Google Scholar]
- 46.Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24:4283–4300. doi: 10.1016/s0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- 47.Schote U, Ganz P, Fahr A, Seelig J. Interactions of cyclosporins with lipid membranes as studied by solid-state nuclear magnetic resonance spectroscopy and high-sensitivity titration calorimetry. J Pharm Sci. 2002;91(3):856–867. doi: 10.1002/jps.10071. [DOI] [PubMed] [Google Scholar]
- 48.Zalba S, Navarro I, Troconiz IF, Tros de Ilarduya C, Garrido MJ. Application of different methods to formulate PEG-liposomes of oxaliplatin: evaluation in vitro and in vivo. Eur J Pharm Biopharm. 2012;81(2):273–280. doi: 10.1016/j.ejpb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Park SJ, Choi SG, Davaa E, Park JS. Encapsulation enhancement and stabilization of insulin in cationic liposomes. Int J Pharm. 2011;415(1–2):267–272. doi: 10.1016/j.ijpharm.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 50.Beauchesne PR, Chung NSC, Wasan KM. Cyclosporin A: a review of current oral and intravenous delivery systems. Drug Dev Ind Pharm. 2007;33(3):211–220. doi: 10.1080/03639040601155665. [DOI] [PubMed] [Google Scholar]
- 51.Karn PR, Vanic Z, Pepic I, Skalko-Basnet N. Mucoadhesive liposomal delivery systems: the choice of coating material. Drug Dev Ind Pharm. 2011;37(4):482–488. doi: 10.3109/03639045.2010.523425. [DOI] [PubMed] [Google Scholar]
- 52.Aburai K, Yagi N, Yokoyama Y, et al. Preparation of liposomes modified with lipopeptides using a supercritical carbon dioxide reverse-phase evaporation method. J Oleo Sci. 2011;60(5):209–215. doi: 10.5650/jos.60.209. [DOI] [PubMed] [Google Scholar]
- 53.Bothun GD, Knutson BL, Strobel HJ, Nokes SE. Liposome fluidization and melting point depression by pressurized CO2 determined by fluorescence anisotropy. Langmuir. 2005;21(2):530–536. doi: 10.1021/la0496542. [DOI] [PubMed] [Google Scholar]
- 54.Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142(3):299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 55.Vemuri S, Rhodes CT. Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharm Acta Helv. 1995;70(2):95–111. doi: 10.1016/0031-6865(95)00010-7. [DOI] [PubMed] [Google Scholar]