Abstract
Neutrophils and other cells secrete pentraxin 3, which promotes innate immunity by binding to pathogens and activating complement. New work shows that pentraxin 3 also limits neutrophil recruitment by inhibiting rolling on P-selectin in inflamed venules.
During inflammation, flowing neutrophils tether to and roll on vascular surfaces by reversible binding of P-, L- and E-selectin to glycosylated ligands1, 2. One of the earliest events is mobilization of P-selectin from secretory granules to the surfaces of endothelial cells and platelets upon activation by thrombin, complement, and other mediators. P-selectin initiates rolling by interacting with the transmembrane mucin P-selectin glycoprotein ligand-1 (PSGL-1) on neutrophils. Rolling permits selectin ligands and chemokine receptors on neutrophils to signal integrin-dependent deceleration, arrest, and ultimately, emigration into infected or injured tissues3. Whereas the mediators that trigger inflammation have been extensively studied, less is known about the mechanisms that limit inflammation. In this issue of Nature Immunology, Mantovani and colleagues report that pentraxin 3 (PTX3), a protein known to augment innate immunity, also dampens neutrophil recruitment in vivo4. PTX3 binds to P-selectin and may limit inflammation by inhibiting neutrophil rolling on P-selectin.
PTX3 is an evolutionarily conserved, multimeric protein5. Cytokines and endotoxins induce endothelial cells, macrophages, and dendritic cells to synthesize PTX3, and neutrophils store PTX3 in specific granules. Like antibodies, PTX3 binds to pathogens, activates complement, and opsonizes particles. On the other hand, overexpression of PTX3 in mice increases resistance to lipopolysaccharide challenge6, and PTX3-deficient mice are more susceptible to ischemia-reperfusion injury7. The mechanism for these anti-inflammatory effects of PTX3 has remained unclear. The new work demonstrates that PTX3 reduces the number of neutrophils rolling on P-selectin. Furthermore, administration of PTX3 to mice inhibited acid-induced neutrophil recruitment to the lung or chemokine-induced neutrophil entry into the pleural cavity to the same extent as antibody blockade or genetic deficiency of P-selectin.
Each subunit of PTX3 has an N-terminal domain linked to a C-terminal domain related to those of other pentraxins5. The C-terminal domain binds to complement protein C1q and other known ligands of PTX3. It has a single sialylated, complex N-glycan attached to Asn220. Enzymatic removal of the glycan or site-directed mutagenesis of Asn220 to prevent attachment of the glycan enhances binding of the C-terminal domain to C1q and other known ligands5. This suggests that the glycan negatively regulates the conformation or accessibility of the ligand-binding surface of PTX3. Mantovani and colleagues show that the PTX3 C-terminal domain also interacts with P-selectin. Whereas removal of the N-glycan increased binding to other ligands, it decreased binding to P-selectin. The authors conclude that PTX3 uses this glycan to bind directly to P-selectin, but if so, the binding must be quite different than that of known glycan ligands for selectins. To mediate rolling, the N-terminal lectin domain of each selectin binds in a Ca2+-dependent manner to a small number of membrane glycoproteins. The essential, common recognition determinant on these glycoproteins is sialyl Lewis x, or sLex (NeuAcα2-3Galβ1-4[Fucα1-3]GlcNAcβ1-R), a terminal component of some N- and O-glycans2. A Ca2+ ion that coordinates residues in a loop in the lectin domain also makes contacts with the α1-3-linked fucose in the sLex determinant, explaining the Ca2+ dependency of binding8. Mantovani and colleagues did not examine the Ca2+ dependency of P-selectin binding to PTX3. For nearly all experiments, the authors used recombinant PTX3 expressed in Chinese hamster ovary cells. These cells lack the α1-3-fucosyltransferases that attach fucose to form the sLex determinant9. Indeed, previous structural analysis of the N-glycan on recombinant PTX3 reveals core α6-linked fucose but no terminal α3-linked fucose10.
Importantly, P-selectin preferentially binds to PSGL-1 because it also recognizes non-glycan determinants. A broad surface of the lectin domain cooperatively interacts with sulfated tyrosines, adjacent amino acids, and an sLex-capped O-glycan, all clustered near the N-terminus of PSGL-18. PSGL-1 and PTX3 may bind to overlapping sites on P-selectin, as binding of PTX3 to P-selectin was inhibited by soluble PSGL-1 and by an anti-P-selectin monoclonal antibody that blocks binding to PSGL-1. Instead of directly binding to P-selectin, the N-glycan on recombinant PTX3 might positively regulate the conformation of the C-terminal domain to favor protein-protein interactions with P-selectin. Consistent with this possibility, heat treatment of recombinant PTX3 blocked its anti-inflammatory effects; heating typically alters protein rather than glycan conformation. A specific protein-protein interaction might explain the authors’ observation that recombinant PTX3 bound to P-selectin but not to L- or E-selectin. Less neutrophil-derived than recombinant PTX3 was required to detect binding to immobilized P-selectin, perhaps because neutrophils express an sLex-capped N-glycan on the C-terminal domain of PTX3. P-selectin might bind cooperatively to glycan and protein components of neutrophil-derived PTX3, as it does to PSGL-1. Alternatively, neutrophils might store PTX3 as larger multimers in specific granules, enabling it to bind with greater avidity to P-selectin. Mantovani and colleagues used surface plasmon resonance to measure binding of soluble P-selectin to immobilized recombinant PTX3. The data suggest that P-selectin binds with slower on- and off-rates to PTX3 than to PSGL-1. This conclusion should be considered tentative as soluble P-selectin was not confirmed to be monomeric and PTX3 was immobilized at high concentrations that could permit dissociating P-selectin to rebind.
In vitro, PTX3 reduced the number of neutrophils rolling on P-selectin in a concentration-dependent manner. Competitively inhibiting P-selectin-PSGL-1 bonds should increase the velocities of residual rolling cells by reducing the number of bonds. However, the effects of PTX3 on rolling velocities were not reported. In vivo, injection of PTX3 decreased the number of neutrophils rolling in thrombin-activated postcapillary venules in mesentery. Surprisingly, PTX3 had no effect on the velocity distributions of the residual rolling cells. This is difficult to reconcile with PTX3 acting by competitively inhibiting P-selectin. Anti-selectin antibodies were not used to define the rolling interactions. The discrepancy could be explained if neutrophils rolled on E- as well as P-selectin in the mesenteric venules. In TNF-α-activated venules of the cremaster muscle, neutrophils roll on both E- and P-selectin, and E-selectin primarily governs rolling velocities11.
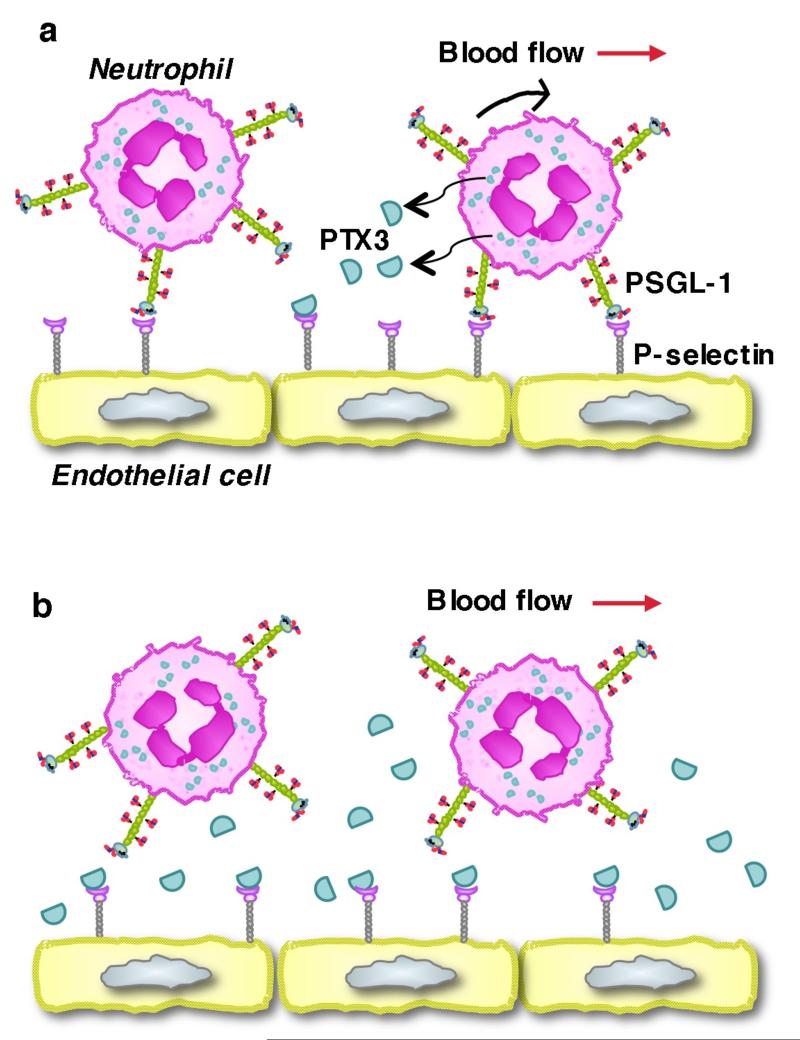
What is the source of the anti-inflammatory PTX3? By transplanting wild-type or PTX3-deficient bone marrow into irradiated wild-type or PTX3-deficient recipient mice, Mantovani and colleagues show that PTX3 from hematopoietic cells is required to suppress neutrophil recruitment into the pleural cavity in the first two hours after chemokine challenge. In this short time frame, neutrophils are the likely source of PTX3, as they are the only hematopoietic cells that store PTX3. The data suggest a testable model (Fig. 1). Neutrophils rolling on P-selectin in venules at sites of infection or injury receive signals that release PTX3 from specific granules. The released PTX3 binds to locally expressed P-selectin in a paracrine manner. The multimeric nature of PTX3 increases binding avidity that slows dissociation from P-selectin. As more neutrophils roll, they release more PTX3 that occupies more P-selectin molecules. This constitutes a local, negative feedback system that reduces neutrophil tethering, accelerates rolling, and enhances detachment. The magnitude of adhesion inhibition will depend on the number of P-selectin molecules that PTX3 occupies. Variants of the model are possible. For example, release of PTX3 might require that neutrophils engage chemokine receptors or receive outside-in signals through integrins after they arrest or begin to crawl toward endothelial cell junctions. Neutrophils interacting with P-selectin on activated platelets might deploy PTX3 in a similar paracrine loop. Mantovani and colleagues suggest that widespread inflammation such as sepsis could generate high levels of circulating PTX3 with systemic anti-inflammatory effects. In this scenario, activated endothelial cells, dendritic cells, and/or macrophages may be major sources of PTX3. The new work demonstrates that PTX3 inhibits P-selectin-dependent adhesion, but other still-undefined mechanisms could contribute to its anti-inflammatory properties in vivo.
Figure 1. Model for PTX3 paracrine regulation of neutrophil rolling on P-selectin.
(a) Interactions of PSGL-1 on neutrophils with P-selectin on activated endothelial cells mediate rolling in venules at sites of infection or injury. Rolling neutrophils release PTX3 from specific granules. PTX3 binds to locally expressed P-selectin, preventing subsequent interactions with PSGL-1. (b) As PTX occupies more P-selectin molecules, neutrophils tether less frequently, roll faster with less stability, and detach rather than arrest. See text for discussion.
PTX3 is the second described endogenous inhibitor of leukocyte adhesion to vascular surfaces. The first is developmental endothelial locus-1 (Del-1), a glycoprotein secreted by endothelial cells that associates with the cell surface12. Del-1 binds to the leukocyte integrin αLβ2. But rather than promoting αLβ2-dependent adhesion, Del-1 inhibits adhesion by competitively inhibiting binding of αLβ2 to intercellular adhesion molecule-1, a well-characterized integrin ligand on endothelial cells12. Thus, distinct endogenous proteins limit inflammation by inhibiting either selectin- or integrin-dependent leukocyte adhesion. These proteins may cooperate with other anti-inflammatory systems, for example, activation of protein C, a serine protease, by the thrombin/thrombomodulin complex on endothelial cell surfaces13. The findings of Mantovani and colleagues underscore the delicate balance between initiating inflammation to contain microbial invasion and dampening inflammation to prevent untoward tissue injury. Defining natural mechanisms that check neutrophil recruitment may offer new approaches to treat inflammatory diseases.
References
- 1.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 2.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Deban L, et al. Binding to P-selectin and regulation of leukocyte recruitment by the long pentraxin PTX3, a key component of humoral innate immunity. Nat Immunol. 2010 doi: 10.1038/ni.1854. in press. [DOI] [PubMed] [Google Scholar]
- 5.Bottazzi B, et al. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol Rev. 2009;227:9–18. doi: 10.1111/j.1600-065X.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 6.Dias AA, et al. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol. 2001;69:928–936. [PubMed] [Google Scholar]
- 7.Salio M, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 8.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, et al. The selectin GMP-140 binds to sialylated, fucosylated lactosaminoglycans on both myeloid and nonmyeloid cells. J Cell Biol. 1991;115:557–564. doi: 10.1083/jcb.115.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inforzato A, et al. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry. 2006;45:11540–11551. doi: 10.1021/bi0607453. [DOI] [PubMed] [Google Scholar]
- 11.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice – E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 12.Choi EY, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esmon CT, Glass JD. The APCs of neuroprotection. J Clin Invest. 2009;119:3205–3207. doi: 10.1172/JCI40682. [DOI] [PMC free article] [PubMed] [Google Scholar]