Abstract
Neighborhood disadvantage has consistently been linked to increased rates of morbidity and mortality, but the mechanisms through which neighborhood environments may get “under the skin” remain largely unknown. Differential exposure to chronic environmental stressors has been identified as a potential pathway linking neighborhood disadvantage and poor health, particularly through the dysregulation of stress-related biological pathways such as cortisol secretion, but the majority of existing observational studies on stress and neuroendocrine functioning have focused exclusively on individual-level stressors and psychosocial characteristics. This paper aims to fill that gap by examining the association between features of the neighborhood environment and the diurnal cortisol patterns of 308 individuals from Chicago, Illinois, USA. We found that respondents in neighborhoods with high levels of perceived and observed stressors or low levels of social support experienced a flatter rate of cortisol decline throughout the day. In addition, overall mean cortisol levels were found to be lower in higher stress, lower support neighborhoods. This study adds to the growing evidence of hypocortisolism among chronically stressed adult populations and suggests hypocortisolism rather than hypercortisolism as a potential mechanism linking social disadvantage to poor health.
Keywords: USA, cortisol, neighborhood effects, health inequalities, multi-level modeling, stress
BACKGROUND
Neighborhood disadvantage has consistently been linked to increased rates of morbidity and mortality (e.g. Diez-Roux & Mair 2010), but the physiological mechanisms through which neighborhood environments may get “under the skin” remain largely unknown. Differential exposure to chronic environmental stressors has been identified as a potential pathway linking neighborhood disadvantage and poor health, particularly through the dysregulation of stress-related biological pathways such as cortisol secretion.
The “ecology of stress” has emerged as an important area of research because it is able to integrate both environmental and biological mechanisms to explain health disparities (Matheson, Moineddin, Dunn, Creator, Gozdyra & Glazier, 2006). However, a large disconnect remains between the neighborhood literature on stressful environments and biomedical research on stress physiology. Thus far, few existing datasets have been able to collect both neighborhood and biological measures. While the majority of human studies of stress biology occur in controlled laboratory settings with individuals removed from their social and physical contexts, recent population health research has begun to integrate biomarkers into observational studies (McDade, Williams & Snodgrass 2007; Williams & McDade 2009). Despite this progress and the widespread recognition that disadvantaged neighborhoods are a potential source of chronic stress, the majority of existing observational studies on stress and neuroendocrine functioning have focused exclusively on individual-level stressors and psychosocial characteristics. This paper aims to fill that gap by examining the association between features of the neighborhood environment and the diurnal cortisol patterns of 308 individuals from Chicago, Illinois.
Stress, Cortisol and HPA dysfunction
Stress can generally be defined as the perception that the demands (or anticipated demands) of the environment exceed an individual s ability to cope (Lupien, King, Meaney & McEwen 2001). Two key systems are hypothesized to mediate the effects of stress exposure on disease outcomes: the sympathetic nervous system (SNS), which stimulates release of epinephrine (commonly referred to as adrenaline) from the adrenal gland, and the hypothalamic pituitary adrenal (HPA) axis, which produces cortisol (Fremont & Bird, 2000). During periods of stress, activity of the SNS and the HPA axis increase dramatically, resulting in increased levels of both cortisol and epinephrine, as well as numerous physiological changes (such as increased heart rate) that prepare the body for action (Fremont & Bird, 2000). Once released, cortisol has several important functions such as increasing access to energy stores, increasing protein and fat mobilization, as well as regulating the magnitude and duration of inflammatory responses (Sapolsky, 2005).
Of the stress hormones, cortisol has received the most attention because of the extensive regulatory role it plays in the central nervous system, the metabolic system and the immune system (Miller, Chen & Zhou, 2007). Cortisol follows a diurnal rhythm, with peak levels found 45-60 minutes after waking. Levels typically drop rapidly for the next couple of hours and then continue to decline slowly throughout the day and night, reaching a low point around midnight (Adam, Hawkley, Kudielka & Cacioppo, 2006). In addition to the diurnal pattern, considerable variation in cortisol secretion also exists across individuals, as well as within individuals across different days (Hruschka, Kohrt & Worthman, 2005). As a result, a number of different approaches to modeling cortisol have been examined in the literature: slope from highest to lowest point (diurnal slope), size of the cortisol awakening response (CAR), morning or evening levels, and total cortisol concentration over the day measured as area under the curve (AUC) of the diurnal pattern. (Dowd, Simanek & Aiello, 2009). Mean morning or evening levels have been most commonly used in existing population-based studies due to a relatively small number of cortisol collections per study. Recently, attention has turned away from mean levels to other aspects of diurnal cortisol secretion, specifically the relative steepness of the cortisol decline over the day.
As with other endocrine systems, the HPA axis is regulated by a negative feedback system, whereby receptors detect changes in cortisol levels and adjust production accordingly (McEwen, 2000). Cortisol secretion will be inhibited when circulating levels rise or stimulated when levels fall. While cortisol levels typically return to normal quickly after exposure to acute stressors due to negative feedback, long-term exposure to stressors, such as those associated with lower socioeconomic status, is thought to damage feedback loops, resulting in chronically elevated levels (McEwen, 1998). Elevated cortisol (hypercortisol) has also been linked to cognitive decline, immunosuppression, obesity and insulin resistance (Sapolsky, 2005).
Although theoretical work suggests that the key biological mechanism linking disadvantage and poor health is elevated cortisol, empirical evidence linking chronic stress to elevated cortisol has been mixed (Miller, et al 2007). While it is well known that cortisol increases in response to acute stressors in laboratory settings, there is inconsistent evidence concerning the effects of long-term exposure to chronically stressful environments. More recently, it has been suggested (Fries, Hesse, Hellhammer & Hellhammer, 2005; Dowd et al, 2009; Gunnar & Vazquez, 2001, Miller, et al 2007)) that long-term chronic activation of the HPA-axis might ultimately lead to a blunted, under-active HPA response (i.e. lower waking levels, smaller CAR, or flatter evening slopes). This blunted pattern- often resulting in lower overall cortisol production-represents a form of HPA-axis dysfunction termed “hypocortisolism”.
The Social Determinants of Cortisol
Biomedical research on cortisol has, until recently, focused on the responses to acute stressors such as taking exams, public speaking or parachute jumping (Lupien et al, 2001). Only recently have population-based studies attempted to understand the relationship between social position, chronic stress, and cortisol secretion. In a review of the literature on socioeconomic status (SES) and cortisol, Dowd et al. (2009) found highly inconsistent results across studies in association between SES and overall levels of cortisol. Because the dominant paradigm in the sociological literature presumes that disadvantage is associated with greater stress higher overall cortisol, these recent results from population-based studies have caused some confusion. However, among studies that were able to estimate the diurnal pattern, the results have been much more consistent. Dowd et al (2009) found that in the majority of studies lower SES was related to a blunted pattern of diurnal cortisol secretion, despite the fact that this inconsistently corresponded to overall mean levels of cortisol.
Specifically, Cohen et al. (2006) found that lower income and education were associated with flatter slopes and higher levels of cortisol during the evening and at bedtime in a sample of 781 middle-aged adults in the CARDIA study in the USA. Ranjit et al (2005) similarly found a flatter slope among those with lower SES; however, in this study the flatter slope resulted from a lower CAR and no difference in evening levels. DeSantis, Adam, Doane, Minkea, Zinbarg & Craske (2007) found in another U.S. study that African-American and Hispanic adolescents had flatter cortisol slopes across the waking day than their Caucasian counterparts. The difference in slopes was due both to higher bedtime cortisol levels in Hispanics and African-Americans and to lower morning levels in African-Americans. The authors argue that bedtime levels of cortisol may be more strongly influenced by social factors than waking levels or the CAR, as higher evening levels may suggest either continued stress exposure throughout the day or a failure to “turn off” the stress-response system in the evening. Moreover, the higher evening levels may be responsible for low morning levels via negative feedback over the night (DeSantis et al, 2007).
Gunnar & Vazquez (2001) argued that empirical evidence supporting hypocortisolism has often been downplayed because it challenges prevailing concepts on the neuroendocrinology of stress. In a review of the literature on stress and cortisol in children, Gunnar & Vazquez (2001) found that, more often than not, basal cortisol levels are actually lower for individuals experiencing higher levels of adversity and disadvantage. Broader evidence for this phenomenon has continued to accumulate, with evidence for low cortisol production in chronically stressed populations such as domestic violence victims and caregivers for ill family members (Miller, et al 2007).
Neighborhoods and Stress
In order to more fully understand the etiology of cortisol dysfunction, it is necessary to examine characteristics of the multiple environments in which individuals are embedded. Social structures determine, in part, the exposure of individuals to stressors as well as stress-buffering resources. Wheaton (1999) defines stressors as “conditions of threat, demands, or structural constraint that, by their very occurrence or existence, call into question the operating integrity of the organism.” Both quantitative and qualitative research indicates that socioeconomically disadvantaged neighborhoods may be highly stressful to their inhabitants (e.g. Latkin & Curry, 2003; Israel et al, 2006; Frohlich, Potvin, Chabot & Corin, 2002). Individuals in these neighborhoods are disproportionately exposed to psychosocial hazards such as crime and disorder. Moreover, the resources necessary for coping with chronic stress, such as social support, are unevenly distributed across socioeconomic status (Williams & Collins, 1995). The neighborhood environment-- although perhaps more distal that other sources of chronic stress, such as individual-level socioeconomic status, family functioning, or work-related stress-- is a potentially important component of the etiology of endocrine dysfunction.
Social disorganization, crime, and signs of physical deterioration (e.g. vacant housing, litter, graffiti) in a neighborhood can signal to residents that their immediate environment is unsafe. Canadian research indicates that individuals living in disadvantaged neighborhoods, ambient stressors are difficult to avoid and become integrated into daily living (Matheson et al, 2006). Israel et al (2006) used focus groups to examine perceptions of neighborhoods in Detroit, Michigan, USA and found that the language of “stress” emerged as meaningful across groups of participants. Common stressors reported by the focus groups included crime, deteriorated buildings, lack of trust in neighbors, gangs, inadequate services, discrimination, and job insecurity.
Gould, Mijanovich & Dillman (2001) outlined four potential ecological stressors that may operate as causal mechanisms linking neighborhood socioeconomic disadvantage and health: neighborhood institutions and resources (i.e. transportation, social services, accessibility to parks), physical stressors (i.e. housing stock, street maintenance, litter and graffiti), social stressors (i.e. crime, distrust), and neighborhood-based social networks (i.e. social support). Identifying the specific neighborhood features and processes that link neighborhoods to health is key to developing health promoting interventions targeted at neighborhood conditions (Diez Roux, 2010).
To our knowledge, only two existing studies, both from the U.S., have examined the association of neighborhood characteristics and cortisol. The first found that neighborhood SES as measured by Census data was associated with lower mean cortisol in adolescents (Chen & Patterson, 2006). A recently published article using data from the Multi-Ethnic Study of Atherosclerosis (MESA) found that greater neighborhood violence was associated with lower cortisol values at wakeup and a slower decline in cortisol over the earlier part of the day (Do, Diez Roux, Hajat, Auchincloss, Merkin, Ranjit, et al, 2011). The current study builds on this latter work by examining the association of multiple neighborhood characteristics on diurnal cortisol patterns and rates of change throughout the day in a sample from Chicago, Illinois, USA.
DATA
The individual-level data for this analysis come from the Chicago Community Adult Health Study (CCAHS), a multistage probability sample of 3,105 adults aged 18 or more years, living in the city of Chicago between 2001 and 2003. A representative subsample of 311 respondents provided up to four cortisol samples for each of two consecutive days. All subjects gave informed consent for both the interviews and the saliva collection, and the study was approved by the University of Michigan School of Public Health Institutional Review Board. The city of Chicago was stratified into 343 neighborhood clusters as previously defined by the Project on Human Development in Chicago Neighborhoods (PHDCN) as one or more geographically contiguous census tracts aggregated based on the demographic characteristics of the population, local knowledge of the city s neighborhoods and major ecological boundaries (Sampson et al. 1997). We draw upon multiple additional data sources to obtain measures of the neighborhood environment, including systematic social observation (SSO) of participant neighborhoods, Census measures, and Uniform Crime Reports.
MEASURES
Cortisol
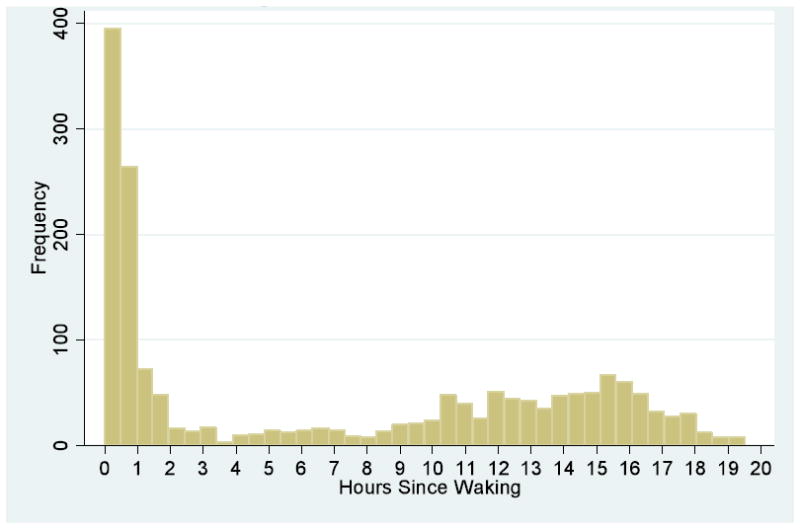
Participants were asked to collect saliva samples at waking, 30 minutes after waking, 45-60 minutes before dinner, and right before going to bed for two consecutive days. Respondents were asked to record information on waking and sleeping times as well as the time each sample was collected. Data from each respondent was combined over the two day collection period, giving a total of 8 possible samples. Saliva samples were returned by mail to the University of Michigan and frozen at -20C until assayed. Cortisol was measured in micrograms per deciliter (μg/dL). For the analysis, it was important that both the measure of cortisol and the collection time to be valid. 41% of respondents (n=127) collected all 8 samples. Another 39.7% of respondents (n=123) collected between 4 and 7 samples, and 19% (n=59) collected between 1-3 samples. Three participants were dropped from the analysis because of missing information on collection times and waking time. This resulted in a final sample of 308 participants representing 79 neighborhood clusters (average of 22.7 cortisol measures per neighborhood cluster). The average number of cortisol measures per respondent was 5.7, giving a total of 1,747 valid observations. Figure 2 reports a histogram of the number of cortisol measures by hours since waking. The mean cortisol level was .313μg/dL(SD=.269), with a range of .0002-1.95μg/dL. All models were adjusted for wake-up time.
Figure 2.
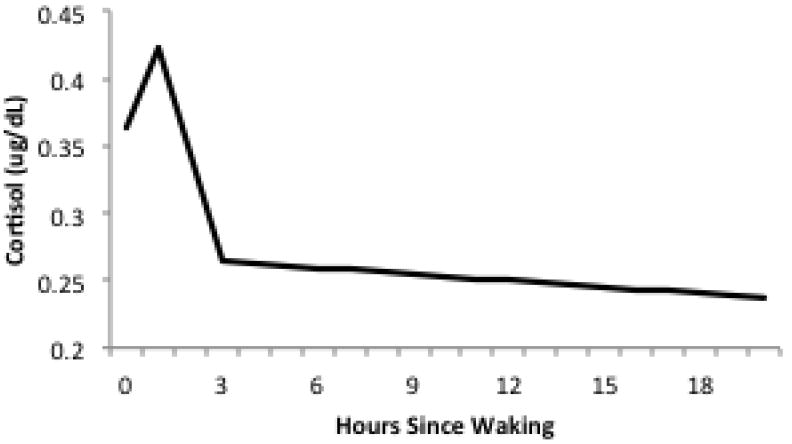
Unconditional Diurnal Cortisol Pattern
Individual-level Sociodemographic Measures
Individual-level characteristics (age, gender, race/ethnicity, and socioeconomic status) previously shown to be associated with cortisol patterns (Almeida, Piazza & Stawski, 2009; Hajat, et al., 2010), were included as potential confounders. Respondent age was included as a continuous measure. Race/ethnicity was based on respondent self-reports and categorized into four mutually exclusive categories: non-Latino white (reference), non-Latino black, Latino, and non-Latino other. Male was used as the reference category for gender. Educational attainment was dichotomized based on years of education: less than a high school degree (reference) and a high school degree or higher. Family income was dichotomized as less than $40,000 (reference) and greater than $40,000. Because there were significant numbers of respondents with missing data (13.2%) for income an additional missing income category was included to retain those individuals in the analysis. For both income and education, preliminary analyses suggested that initial categories (5 for income and 4 for education) could be collapsed into the above categories without loss of relevant information.
Additional Individual-Level Covariates
Several theoretically relevant individual-level characteristics and behaviors were examined in separate analyses to determine their effects on diurnal cortisol patterns. Two measures of stress at the individual-level were analyzed: financial stress and marital stress. Financial stress was a two-item scale that includes the following questions: “How satisfied are you with your and your family’s financial situation?” and “How difficult is it for you and your family to meet the monthly payments on your bills?” Marital stress was a four-item scale based on the following questions: “How often do you feel bothered or upset by your marriage/ relationship?” “there is a great deal of love and affection expressed in our relationship”, “my spouse/ partner doesn’t treat me as well as I deserve to be treated”, and “I sometimes think of divorcing or separating from my spouse/ partner”.
Depression was measured using an 11-item version of the Center for Epidemiologic Studies depression scale (CES-D). Each item in the shortened CES-D scale was scored from 1-4, with a higher score representing more depressive symptoms (Everson-Rose, House & Mero 2004). Previous work with the larger CCHAS dataset found significant associations between neighborhood stressors, social support, and depressive symptoms (Mair, Diez Roux & Morenoff, 2010), and there is evidence of a bidirectional relationship between depression and cortisol activity (Stetler & Miller, 2011). Anxiety was measured using a 5-item index. Respondents were asked how often (never, hardly ever, some of the time, most of the time) they felt the following: nervous, faint, hands trembling, had fear of the worst happening, had fear of dying. Hopelessness was a 4-item index based on whether respondent’s strongly agreed, agreed, disagreed, or strongly disagreed with the following statements: “I feel it is impossible for me to reach the goals that I would like to strive for”, “The future seems hopeless to me and I can’t believe things are changing for the better”, “I don’t expect to get what I really want”, and “There’s no use in really trying to get something I want because I probably won’t get it. Cynical hostility was measured using the Cook-Medley Hostility Scale. Respondents were asked whether they strongly agreed, agreed, disagreed, or strongly disagreed with the following statements: “Most people inwardly dislike putting themselves out to help other people”, “Most people will use somewhat unfair means to gain profit or an advantage rather than lose it”, “No one cares much what happens to you”, “I think most people would lie in order to get ahead”. Sleep difficulty used a three-item scale that measures how often (rarely or never, sometimes, often, almost every day) in the past 4 weeks the respondent had trouble falling asleep, woke up in the middle of the night and could not get back to sleep, and woke up very early and could not get back to sleep. Physical activity, which has been associated with lower reactivity of the sympathetic nervous system and the HPA axis to stressors (Rimmele, Seiler, Mart, Wirtz, Ehlert & Heinrich, 2009) was measured as a six category index: in bed or chair, never light/moderate and never vigorous exercise, light exercise, light-moderate exercise, moderate-heavy exercise, and heavy exercise. Measures of the number of drinks per day and whether the respondent was a current smoker were also included because of the known physiological effects of alcohol and nicotine on cortisol. Waist/hip ratio was included because of the link between cortisol and abdominal adiposity.
Neighborhood Measures
The neighborhood context measures included in the analysis were based on several sources: U.S. Census Data, Systematic Social Observation (SSO) of the neighborhood physical environment, neighborhood resident surveys, and Uniform Crime Reports (a nationwide collection of local police reports compiled monthly and annually by the FBI). The SSO component of the CCAHS involved trained raters that observed and rated neighborhood conditions on both sides of the streets enclosing the blocks of sampled residents. Neighborhood-cluster-level measures for each scale in the SSO were created using empirical Bayes estimation, which adjusts for missing items and improves neighborhood-level estimates by borrowing information across clusters (Mujahid, Diez Roux, Morenoff & Raghunathan 2007). Survey-based measures of resident perceptions were aggregated using the same estimation techniques.
A total of 21 measures were used to construct 4 theoretically distinct neighborhood dimensions: perceived stress, observed stress, social support, and participation. Confirmatory factor analysis was performed to justify the number of measures created and the grouping of variables (see Karb, 2010 for more information). All measures were standardized alpha scales with means of 0 and a standard deviation of 1. Perceived stress included 5 scales from neighborhood resident surveys: perceived disorder (5 items), perceived violence (5 items), neighborhood safety (2 items), physical hazards (4 items), and the quality of neighborhood services (2 items). Observed stress includes 8 measures from the SSO, Census, and Uniform Crime Reports: homicide rate, robbery rate, burglary rate, physical disorder (9 items), physical deterioration (5 items), vacant lots, percent vacant housing, and the condition of streets. Social support included 4 scales from neighborhood resident surveys: social cohesion (5 items), social control (5 items), intergenerational closure (5 items), and reciprocal exchange (5 items). Participation includes 4 scales from resident surveys: organizational participation (7 items), voting (2 items), civic activities (8 items), and contact with community officials (9 items).
U.S. Census data provide information on the socioeconomic composition of neighborhood clusters. A composite measure of neighborhood socioeconomic status was created using the following census variables: percent of families with income less than $10,000, percent of families with income greater than $50,000, percent of families below the poverty level, percent of families receiving public assistance, percent unemployed, percent of residents with 16 or more years of education, percent never married, percent female headed households, and percent in professional or managerial positions. The neighborhood SES measure was a standardized scale with a mean of 0 and a standard deviation of 1.
METHODS
As a result of the diurnal pattern of cortisol excretion, a variety of modeling techniques have been employed in the literature. The most common approaches look at overall cortisol levels, the CAR or AUC; however, the most recent research on HPA dysregulation suggests that the diurnal pattern is most important, with dysregulated patterns of cortisol activity involving not only differences in overall levels but also in patterns of change (Van Ryzin, Chatham, Kryzer, Kertes & Gunnar 2009). The shape of the diurnal pattern is complex and not easily captured using a polynomial function. Therefore, we used multilevel linear splines with appropriately placed knots, an approach used in several recent papers (Dowd, Ranjit, Do, Young, House & Kaplan, 2011, Do, et al 2011, Hajat, et al 2010, Ranjit, et al 2009). In the three level hierarchical model, repeated cortisol measurements are considered nested with a person, who is in turn nested within a neighborhood. The splines represent segments of the daily cortisol pattern with different associations between cortisol and environmental factors, with the knots reflecting the time points during day when the strength of these associations change in magnitude. Visual examination of the data suggested that spline knots be placed at 1 and 3 hours after waking.
Linear splines can be used to build a piece-wise linear function. One can visualize splitting the data into three groups based on time since waking using the knots t1 and t2 and solving three separate regression problems. However, to ensure continuity, we can reduce the problem to straight linear regression using a simple linear combination of basis functions (Jo et al, 2007). We start with a linear function:
where xijk is the minute since waking for observation i for person j in neighborhood k. This function accounts for points before the first knot t1, in this case all points between waking and 1 hour after waking. Next, we add a second line equal to zero up until the first knot t1, that accounts for all points between t1 and t2 (3 hours after waking). Finally, we add a third line for zero up until the second knot k2, that accounts for all points after t2.
where
and
Figure 2 shows the unconditional three-level spline model. The intercept (.36) represents the expected cortisol level at waking. Slope 1 represents the expected change in cortisol between waking and 1 hour after waking, or the CAR. Cortisol increases .059 units by 1 hour after waking, and then begins to decrease sharply at a rate of .079 units per hour between 1 and 3 hours after waking (slope 2). Levels continue to decrease at a much slower rate (.002 units per hour) throughout the day (slope 3). We test the association of both individual-level and neighborhood-level covariates on diurnal cortisol patterns by including interaction terms between each of the slopes and the main effect of the covariate, where the main effect of the covariate is interpreted as the difference in cortisol level associated with the covariate at the time of waking.
Using the unconditional model, the total variance in cortisol can be decomposed into three components: between neighborhood, between individuals within neighborhoods, and within individuals. The proportion of variance due to differences between neighborhoods is referred to as the intra-class correlation (ICC) and represents the expected correlation between two individuals randomly selected within a neighborhood. The ICC of 12.2% is considered relatively large in neighborhood literature, which rarely finds ICCs greater than 10 percent for health outcomes (Morenoff, 2003).
Results
Table 1 summarizes descriptive statistics for the sociodemographic variables. The mean age was 45.7 (sd=16.9). Non-Latino whites comprised 23% of the sample, 37% were non-Latino black, 37% Latino, and 3% non-Latino other. The sample was 42.4 % male, and 28% overall had less than a high school education.
Table 1.
Sample Descriptive Statistics
| Mean/Proportion | SE | Min | Max | |
|---|---|---|---|---|
| Individual-Level Measures (n=308) | ||||
| Age | 45.7 | 0.97 | 18 | 89 |
| Sex | ||||
| Male | 42.4% | 0 | 1 | |
| Female | 57.6% | 0 | 1 | |
| Race | ||||
| White | 36.9% | 0 | 1 | |
| Black | 37.2% | 0 | 1 | |
| Latino | 23.0% | 0 | 1 | |
| Other | 2.9% | 0 | 1 | |
| Income | ||||
| <40K | 52.4% | 0 | 1 | |
| >40K | 34.3% | 0 | 1 | |
| Income Missing | 13.3% | 0 | 1 | |
| Education | ||||
| Less than High School | 27.8% | 0 | 1 | |
| High School or Higher | 72.2% | 0 | 1 | |
| Financial Stress | 2.60 | 0.06 | 1 | 5 |
| Marital Stress (n=125) | 1.91 | 0.07 | 1 | 4.67 |
| Sleep Difficulty | 1.76 | 0.04 | 1 | 4 |
| CESD | 1.91 | 0.03 | 1 | 3.82 |
| Anxiety | 1.59 | 0.03 | 1 | 4 |
| Hopelessness | 1.76 | 0.04 | 1 | 4 |
| Drinks per month | 12.90 | 1.9 | 0 | 180 |
| Current Smoker | 23.3% | 0 | 1 | |
| Physical Activity | 4.08 | 0.08 | 1 | 6 |
| Waist/Hip Ratio | 0.87 | 0.01 | 0.68 | 1.18 |
| Neighborhood Measures (n=79) | ||||
| Disadvantage | -0.10 | 0.04 | -2.06 | 0.99 |
| Perceived Stressors | 0.01 | 0.05 | -1.81 | 2.08 |
| Observed Stressors | -0.05 | 0.04 | -1.05 | 1.65 |
| Social Support | -0.06 | 0.05 | -1.71 | 2.54 |
| Participation | 0.04 | 0.04 | -1.56 | 1.27 |
Table 2 shows overall mean cortisol levels, mean morning levels (within the first hour after waking), mean evening levels (more than 12 hours after waking), and the AUCG for categories of independent variables (Table 2). The overall cortisol level averaged throughout the day for the full sample was .31 ug/dL (SD=.19). As expected, levels were higher in the morning (.37 ug/dL, SD=.26) and lower in the evening (.27 ug/dL, SD=.25). The AUCG is the area under the curve with respect to ground and represents the total cortisol output over the day. The AUCG was calculated using the trapezoid formula (Pruessner, Kirschbaum, Meinlschmid & Hellhammer, 2003). For the purposes of calculating AUCG, only respondents with at least three or four cortisol measures in a single day were included, giving a sample of 190. If a respondent had two AUCG values, the average of two was used. The overall AUCG was 267.7 ug*min/dL (SD=176.1).
Table 2.
Cortisol Levels by Independent Measures
| Mean Cortisol (SD) (n=308) | Mean Morning (SD) <1 hour since waking (n=268) | Mean Evening (SD) >12 hours after waking (n=276) | AUCG (SD) (n=190) | |
|---|---|---|---|---|
| Total Sample | 0.31 (0.19) | 0.38 (0.26) | 0.27 (0.25) | 262.7 (176.1) |
| Individual Measures | ||||
| Sex | ||||
| Male (reference) | 0.33 (0.19) | 0.40 (0.27) | 0.30 (0.27) | 262.7 (163.2) |
| Female | 0.29 (0.18) * | 0.36 (0.24) | 0.25 (0.24) | 264.2 (186.2) |
| Race | ||||
| White (reference) | 0.33 (0.19) | 0.42 (0.28) | 0.25 (0.22) | 298.9 (205.9) |
| Black | 0.29 (0.19) | 0.34 (0.23) | 0.29 (0.29) | 249.4 (154.8) + |
| Latino | 0.30 (0.18) | 0.36 (0.24) | 0.27 (0.26) | 228.9 (155.0) * |
| Other | 0.34 (0.16) | 0.42 (0.21) | 0.28 (0.2) | 230.7 (32.6) |
| Income | ||||
| >40K (reference) | 0.30 (0.19) | 0.36 (0.23) | 0.27 (0.25) | 288.9 (166.7) |
| <40K | 0.33 (0.2) * | 0.42 (0.31) | 0.25 (0.22) | 249.7 (188.9) |
| Income Missing | 0.29 (0.16) | 0.30 (0.13) | 0.33 (0.38) | 240.1 (106.4) |
| Education | ||||
| High School or Higher (reference) | 0.33 (0.19) | 0.40 (0.26) | 0.27 (0.23) | 286.9 (184.9) |
| Less than High School | 0.26 (0.18) ** | 0.30 (0.22) ** | 0.28 (0.31) | 207.8 (207.8) ** |
| Neighborhood Measures | ||||
| Disadvantage | ||||
| Tertile 1 (reference) | 0.34 (0.19) | 0.44 (0.28) | 0.26 (0.24) | 294.8 (186.9) |
| Tertile 2 | 0.30 (0.2) + | 0.35 (0.26) * | 0.26 (0.23) | 244.5 (189.0) |
| Tertile 3 | 0.29 (0.17) + | 0.34 (0.22) * | 0.30 (0.29) | 247.4 (148.5) |
| Perceived Stressors | ||||
| Tertile 1 (reference) | 0.33 (0.2) | 0.40 (0.29) | 0.26 (0.22) | 285.4 (203.9) |
| Tertile 2 | 0.31 (0.2) | 0.40 (0.25) | 0.26 (0.25) | 265.7 (169.5) |
| Tertile 3 | 0.28 (0.16) + | 0.32 (0.21) * | 0.29 (0.28) + | 239.1 (152.2) |
| Observed Stressors | ||||
| Tertile 1 (reference) | 0.32 (0.2) | 0.40 (0.27) | 0.26 (0.22) | 291 (211.0) |
| Tertile 2 | 0.31 (0.2) | 0.39 (0.28) | 0.26 (0.25) | 246.5 (164.4) |
| Tertile 3 | 0.29 (0.17) | 0.34 (0.21) | 0.30 (0.29) | 255.3 (151.8) |
| Social Support | ||||
| Tertile 1 (reference) | 0.31 (0.18) | 0.37 (0.26) | 0.31 (0.28) | 263.9 (153.4) |
| Tertile 2 | 0.30 (0.17) | 0.37 (0.22) | 0.25 (0.2) | 246.8 (156.1) |
| Tertile 3 | 0.32 (0.21) | 0.39 (0.28) | 0.26 (0.21) | 280.1 (218.0) |
| Participation | ||||
| Tertile 1 (reference) | 0.32 (0.2) | 0.40 (0.28) | 0.27 (0.28) | 260.7 (178.4) |
| Tertile 2 | 0.31 (0.19) | 0.36 (0.25) | 0.28 (0.27) | 271.5 (192.9) |
| Tertile 3 | 0.29 (0.18) | 0.37 (0.24) | 0.26 (0.22) | 256.4 (159.2) |
p<.10,
p<.05,
p<.01
An interesting pattern emerged among the individual level socioeconomic measures. The overall mean cortisol levels and AUCG for objectively more disadvantaged categories were consistently lower than the overall mean levels for less disadvantaged groups. For example, the mean cortisol level for respondents with an income of less than $40,000 was .30 ug/dL (SD=.19) compared to .33 ug/dL (SD=.20) for respondents with an income greater than $40,000. Similarly, for respondent without a high school degree and respondents with a high school degree or higher, the mean cortisol levels were .26 (SD=.18) and .33(SD=.19) respectively.
The five continuous neighborhood measures were split into tertiles for the purposes of descriptive analyses. Again, overall cortisol levels and AUCG were slightly higher in less disadvantaged neighborhoods, with the exception of neighborhood participation. This pattern held true for morning cortisol levels; less disadvantaged groups had lower average cortisol levels in the first hour of waking. However, when looking at evening cortisol levels (more than 12 hours after waking), there was a suggestion that this pattern was reversed. Respondents in the most disadvantaged neighborhood tertiles had generally higher evening levels of cortisol, although these findings were only marginally significant for perceived stressors. Given the typical morning rise and gradual decline of cortisol, this pattern results in a smaller decline in cortisol among disadvantaged groups over the course of the day, suggesting a blunted diurnal pattern. These descriptive means underscore the importance of examining the patterns of change throughout the day.
Table 3 reports the results of spline models with individual-level characteristics interacted with each spline slope. There were no significant effects of age, race, or income on cortisol waking levels or on rates of change throughout the day. Respondents with a high school degree or higher had significantly higher waking levels of cortisol than respondents with less than a high school education; however, education did not affect the slope coefficients. Individual sociodemographics were kept in the subsequent neighborhood models as main effects, but were not interacted with the spline slopes.
Table 3.
Individual-Level Sociodemographics and Spline Slopes
| Waking | Slope 0-60 Min | Slope 60-180 Min | Slope 180+ Min | |
|---|---|---|---|---|
| Constant | 0.415 (0.045) ** | 0.051 (0.049) | -0.064 (0.031) * | -0.007 (0.004) |
| Age | 0.000 (0.001) | 0.001 (0.001) | -0.001 (0.001) | 0.000 (0.000) |
| Female | -0.035 (0.031) | 0.042 (0.046) | -0.041 (0.026) | 0.002 (0.004) |
| Race (White reference) | ||||
| Black | -0.018 (0.038) | -0.046 (0.057) | 0.018 (0.033) | 0.004 (0.004) |
| Latino | 0.004 (0.043) | -0.014 (0.062) | 0.020 (0.035) | -0.004 (0.005) |
| Other | 0.005 (0.089) | -0.020 (0.131) | 0.031 (0.097) | -0.009 (0.015) |
| Income (>40k Reference) | ||||
| <40K | -0.043 (0.034) | 0.015 (0.05) | -0.002 (0.029) | 0.004 (0.004) |
| Income Missing | -0.089 (0.055) | 0.070 (0.083) | 0.018 (0.047) | 0.001 (0.007) |
| Less than High School Education | -0.066 (0.038) + | -0.022 (0.054) | -0.006 (0.03) | 0.005 (0.004) |
p<.10,
p<.05,
p<.01
Table 4 shows the neighborhood measures and their slope interactions. There were no significant associations of any neighborhood measures with waking levels of cortisol. Neighborhood SES had no significant association with the rate of cortisol change throughout the day. Neighborhood perceived and observed stress was significantly associated with the slope from 3 hours onward, such that respondents who lived in neighborhoods characterized by high levels of both perceived and observed stress experienced a slight increase in cortisol throughout the day and into the evening compared to those with lower reported levels of stress. By contrast, respondents in lower stress neighborhoods experienced a decline in cortisol, consistent with the expected diurnal pattern. Figure 3 shows the predicted diurnal patterns from waking to 20 hours past waking for respondents with high (one standard deviation above the mean), medium (mean), and low (one standard deviation below the mean) levels of perceived neighborhood stress (for respondents of mean age and reference category of other covariates).
Table 4.
Multilevel Spline Models Predicting Cortisol
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| Intercept | 0.405 (.058) ** | 0.404 (.057) ** | 0.405 (.057) ** | 0.409 (.057) ** | 0.406 (.056) ** | |
| Slope 1 (0-60 Min) | 0.063 (.023) ** | 0.061 (.022) ** | 0.060 (.022) ** | 0.060 (.022) ** | 0.062 (.022) ** | |
| Slope 2 (60-180 Min) | -0.077 (.013) ** | -0.074 (.013) ** | -0.075 (.013) ** | -0.077 (.013) ** | -0.078 (.013) ** | |
| Slope 3 (180+ Min) | -0.002 (.002) | -0.003 (.002) | -0.002 (.002) | -0.002 (.002) | -0.002 (.002) | |
| Wake Time | -0.001 (.005) | -0.001 (.005) | -0.001 (.005) | -0.001 (.005) | -0.001 (.005) | |
| Age | 0.000 (.001) | 0.000 (.001) | 0.000 (.001) | 0.000 (.001) | 0.000 (.001) | |
| Female | -0.042 (.023) + | -0.042 (.023) + | -0.041 (.023) + | -0.041 (.023) + | -0.041 (.023) + | |
| Race (White reference) | ||||||
| Latino | -0.003 (.034) | 0.004 (.034) | -0.001 (.033) | -0.010 (.025) | -0.007 (.033) | |
| Black | -0.013 (.031) | -0.006 (.029) | -0.008 (.031) | -0.011 (.039) | -0.003 (.028) | |
| Other | -0.023 (.069) | -0.022 (.069) | -0.021 (.069) | -0.061 (.027) | -0.019 (.069) | |
| Income (>40k Reference) | ||||||
| <40K | -0.012 (.026) | -0.010 (.026) | -0.012 (.025) | -0.011 (.025) | -0.013 (.025) | |
| Income Missing | -0.011 (.039) | -0.011 (.039) | -0.011 (.039) | -0.011 (.038) | -0.012 (.038) | |
| Less than High School Education | -0.062 (.027) * | -0.061 (.027) * | -0.061 (.027) * | -0.061 (.027) * | -0.066 (.027) * | |
| Neighborhood Measures | ||||||
| Disadvantage | -0.021 (.024) | |||||
| Disadvantage × Slope 1 | 0.019 (.029) | |||||
| Disadvantage × Slope 2 | -0.007 (.018) | |||||
| Disadvantage × Slope 3 | 0.004 (.003) | |||||
| Percieved Stress | -0.025 (.019) | |||||
| Percieved Stress × Slope 1 | 0.016 (.026) | |||||
| Percieved Stress × Slope 2 | -0.020 (.014) | |||||
| Percieved Stress × Slope 3 | 0.005 (.002) * | |||||
| Observed Stress | -0.013 (.028) | |||||
| Observed Stress × Slope 1 | -0.010 (.035) | |||||
| Observed Stress × Slope 2 | -0.010 (.02) | |||||
| Observed Stress × Slope 3 | 0.005 (.003) * | |||||
| Social Support | 0.011 (.017) | |||||
| Social Support × Slope 1 | -0.007 (.024) | |||||
| Social Support × Slope 2 | 0.021 (.013) | |||||
| Social Support × Slope 3 | -0.003 (.002) + | |||||
| Participation | -0.021 (.024) | |||||
| Participtation × Slope 1 | -0.054 (.032) + | |||||
| Participation × Slope 2 | 0.034 (.018) + | |||||
| Participation × Slope 3 | 0.000 (.002) | |||||
|
|
0.047 (.019) ** | 0.047 (.019) ** | 0.047 (.018) ** | 0.047 (.019) ** | 0.044 (.02) ** | |
|
|
0.161 (.01) ** | 0.161 (.01) ** | 0.161 (.01) ** | 0.161 (.01) ** | 0.162 (.01) ** | |
|
|
0.197 (.004) ** | 0.196 (.004) ** | 0.197 (.004) ** | 0.197 (.004) ** | 0.197 (.004) ** |
Standard Errors in parentheses:
p<.10,
p<.05,
p<.01
Figure 3.
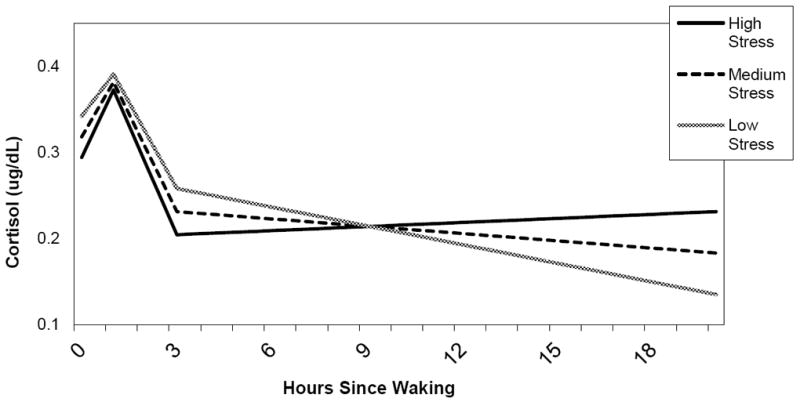
Diurnal Cortisol and Perceived Neighborhood Stressors
Similarly, neighborhood social support had a significant and negative effect on the slope from 3 hours onward. Respondents in neighborhoods with higher levels of social support experienced a steeper decline in cortisol than respondents in neighborhoods with lower social support. Similarly to perceived and observed stress, this pattern is reflected in a flatter cortisol profile for more disadvantaged neighborhoods. Figure 4 shows predicted diurnal cortisol patterns for respondents with high, medium and low levels of neighborhood social support (for respondents of mean age and reference category of other covariates).
Figure 4.
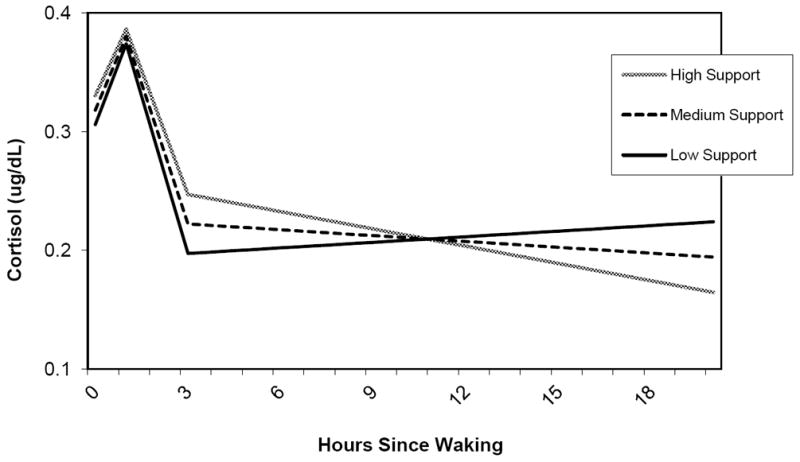
Diurnal Cortisol and Neighborhood Social Support
Neighborhood participation had a marginally significant effect on the slope of cortisol increase in the first hour after waking and the rate of decline from 1 to 3 hours after waking. Respondents in neighborhoods with higher levels of participation experienced a lower rise between hours 0 and 1 and, subsequently, a less steep decline from 1 to 3 hours. There was no significant effect of neighborhood participation on the rate of change into the evening, although the slope was flatter for respondents in neighborhoods with lower participation. Figure 5 shows predicted diurnal patterns for respondents with high, medium and low levels of neighborhood participation (for respondents of mean age and reference category of other covariates).
Figure 5.
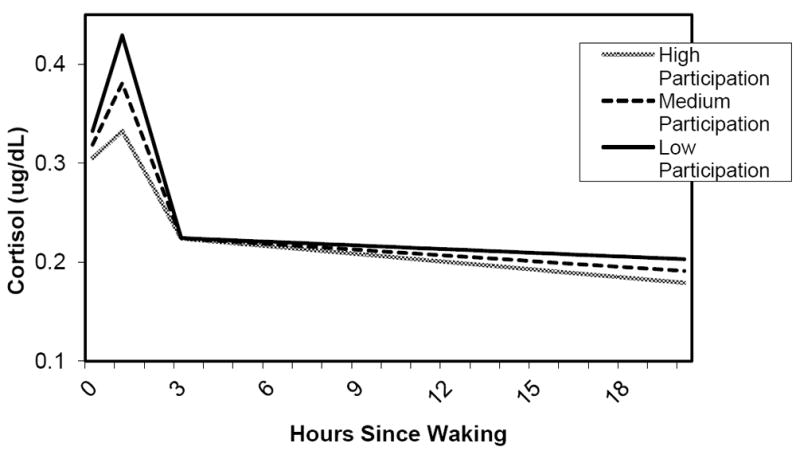
Diurnal Cortisol and Neighborhood Participation
Table 5 shows parameter estimates for the individual-level risk factors, adjusted for sociodemographics. Depression and drinks per day had a positive effect on cortisol waking levels. Major depression is consistently associated with elevated basal levels of cortisol in adults, typically with early morning cortisol levels that are elevated and remain high throughout the day (Gunnar and Vazquez 2001).
Table 5.
Individual Non-Sociodemographic Measures and Spline Slopes
| Waking | Slope 0-60 Min | Slope 60-180 Min | Slope 180+ Min | |
|---|---|---|---|---|
| Constant | 0.311 (0.052) | 0.104 (0.047) | -0.116 (0.029) | -0.001 (0.004) |
| Financial Stress | -0.025 (0.03) | 0.062 (0.045) | -0.045 (0.027) | 0.002 (0.004) |
| Marital Stress | -0.005 (0.037) | 0.032 (0.059) | 0.012 (0.036) | -0.002 (0.005) |
| Sleep Difficulty | -0.033 (0.041) | -0.034 (0.061) | 0.021 (0.037) | -0.001 (0.006) |
| CESD | 0.21 (0.076) ** | -0.043 (0.123) | -0.088 (0.068) | 0.009 (0.009) |
| Anxiety | -0.094 (0.065) | -0.142 (0.102) | 0.006 (0.056) | -0.002 (0.007) |
| Hopelessness | -0.048 (0.04) | 0.014 (0.056) | 0.015 (0.031) | 0.002 (0.004) |
| Drinks per day | 0.002 (0.001) * | -0.001 (0.001) | 0.001 (0.001) | 0.000 (0.001) |
| Current Smoker | -0.027 (0.075) | -0.076 (0.113) | 0.102 (0.065) | -0.001 (0.001) |
| Physical Activity | -0.007 (0.019) | 0.003 (0.031) | 0.026 (0.018) | -0.005 (0.002) * |
| Waist/Hip Ratio | 0.359 (0.369) | 0.41 (0.546) | -0.036 (0.347) | 0.025 (0.046) |
p<.10,
p<.05,
p<.01
The only behavioral measure that had a significant effect on the diurnal pattern was physical activity, with higher levels of physical activity resulting in a less steep decline from hours 1 to 3, and then a higher rate of decline from hour 3 onward. The mediating effect of physical activity on the neighborhood measured was tested in separate models (not shown), and physical activity did not attenuate the neighborhood effects.
DISCUSSION
This study identified a significant association between neighborhood social and physical characteristics and patterns of diurnal cortisol secretion. Respondents in neighborhoods with high levels of perceived and observed stressors or low levels of social support experienced a flatter rate of cortisol decline throughout the day. In addition, overall mean cortisol levels were found to be lower in higher stress, lower support neighborhoods.
This study adds to the growing evidence of hypocortisolism among chronically stressed adult populations and challenges the prevailing hypothesis that elevated cortisol levels are the critical mechanism linking social disadvantage to poor health (Dowd, Ranjit, Do, Young, House & Kaplan, 2011). This hypothesis typically posits that disadvantaged individuals are exposed to a greater number and intensity of stressful experiences, chronically activating the HPA axis. Long-term exposure to elevated cortisol in turn is believed to make the body more vulnerable to disease. A closer look at the biopsychology literature reveals a mixed picture regarding the effects of long-term exposure to stress on HPA functioning, as well as HPA functioning on health (Miller, et al 2007). Recently, critical features such as the time elapsed since stressor onset and the controllability of the stressor, among others, have been shown to impact the direction of the HPA activity-stress relationship. Early theoretical and animal models focused on chronic overexposure to cortisol as pathogenic, while recent evidence suggests that cortisol deviations in either direction are potentially harmful to health.
Low cortisol levels have received less theoretical attention and research because they challenge the dominant paradigm on the neuroendocrinology of stress. However, Heim et al (2000) argue that there is increasing evidence for hypocortisolism in individuals who have been exposed to severe stress or suffer from stress-related disorders. A chronic lack of cortisol has associated with post-traumatic stress disorder (Aardahl-Erickson, Erikckson & Thorell, 2001, Carrion, Weems, Ray, Glaser, Hessl & Reiss, 2002), aggression (McBurnett et al, 2000), behavior problems (Shoal, Giancola & Kirillova, 2003), and disengagement (Mason et al, 2001), though the direction of causality of these associations is not known. Fries et al (2005) finds that individuals with hypocortisolism report higher levels of fatigue, pain, and stress sensitivity. Contrary to expectations, levels of corticotropin-releasing hormone (CRH), a measure of HPA-axis activity in pregnancy, have been found to be lower rather than higher in disadvantaged women (Chen, Holzman, Chung, Senagore, Talge & Siler-Khodr 2010).
Further research is necessary to determine the etiology of the observed blunted cortisol profiles. It is possible that blunted patterns are adaptive, serving as a protective factor against chronic exposure to stressful environments. Alternatively, the observed blunted pattern could reflect the impact of stress exposure in early childhood. DeSantis et al (2007) argued that adverse early childhood experiences (and even prenatal stress exposure) may permanently alter physiological responses to subsequent stressors. Lupien et al (2000) posit that under conditions of chronic stress individuals can transition from hypercortisolism to hypocortisolism, and that this transition may often occur in infancy or childhood. Saridjan et al (2010) argue that early life events may modify the maturation of the HPA axis. This is in line with the model posited by Krieger (2001) and Diez Roux (2007) that social factors are not merely “downstream” from biological factor, but are themselves capable of shaping the development of biological systems. Meaney (2001) has demonstrated in animal models that early stress exposure is associated with permanent changes in gene expression in regions of the brain that participate in the stress response. Longitudinal studies from infancy onward are needed to understand the life course influences on the HPA axis and the best opportunities for intervention.
The current analysis has several limitations. The number of cortisol samples collected per person was relatively small given the complexity of the diurnal pattern. Future research should attempt to collect more samples across the day, which will permit more sophisticated modeling strategies, such as latent growth curve analysis (Muthen and Shedden, 1999) that can identify different patterns of dysregulation. Further, the cross-sectional nature of the current analysis makes it impossible to determine whether the observed blunted cortisol profile for residents of high stress/ low support neighborhoods reflected current stress response or the legacy of modified endocrine functioning from early stress exposure.
The current paper is one of the first to examine the association of neighborhood-level stressors on patterns of cortisol secretion. The results support the recent shift in focus to explaining deviations from normal HPA activity rather than elevations in overall cortisol levels. While the HPA axis has been hypothesized as a key mechanism in the relationship between disadvantage and health, a great deal of uncertainty exists concerning both the antecedents and consequences of HPA axis dysfunction. As such, the current emphasis on cortisol per se as a lynchpin mechanism seems premature.
Figure 1.
Histogram of Cortisol Collection Times
Highlights.
Chronic environmental stressors may link neighborhood disadvantage to poor health.
Existing studies on stress and cortisol have focused exclusively on individual-level stressors and psychosocial characteristics.
This study tests the association of neighborhood characteristics and diurnal cortisol patterns in 308 adults from Chicago, IL.
Those from neighborhoods with more stressors and low social support had lower levels and flatter patterns of cortisol.
This adds to growing evidence of hypocortisolism among chronically stressed adult populations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aardahl-Eriksson E, Eriksson TE, Thorell LH. Salivary cortisol, post-traumatic stress symptoms and general health in the acute phase and during 9-months follow-up. Biological Psychiatry. 2001;50:986–993. doi: 10.1016/s0006-3223(01)01253-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and wor demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Interindividual differences and intraindividual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging. 2009;24(4):819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal Salivary Cortisol in Pediatric Posttraumatic Stress Disorder. Biological Psychiatry. 2002;51:575–83. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Holzman C, Chung H, Senagore P, Talge NM, Siler-Khodr T. Levels of maternal serum corticotropin-releasing hormone (CRH) at mid-pregnancy in relation to maternal characteristics. Psychoneuroendocrinology. 2010;35(6):820–32. doi: 10.1016/j.psyneuen.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: how do they relate to adolescent health? Health Psychology. 2006;25:704–14. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosomatic Medicine. 2006;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbard RE, Craske MG. Racial/Ethnic Differences in Cortisol Diurnal Rhythms in a Community Sample of Adolescents. Journal of Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV. Integrating social and biologic factors in health research: A systems view. Annals of Epidemiology. 2007;17(7):569–574. doi: 10.1016/j.annepidem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Do DP, Diez Roux AV, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, Shea S, Seeman T. Circadian rhythm of cortisol and neighborhood characteristics in a population-based sample: The Multi-Ethnic Study of Atherosclerosis. Health and Place. 2011;17(2):625–632. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. International Journal of Epidemiology. 2009;38:1297–1409. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Ranjit N, Do D, Young EA, House JS, Kaplan GA. Education and levels of salivary cortisol over the day in US adults. Annals of Behavioral Medicine. 2011;41(1):13–20. doi: 10.1007/s12160-010-9224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, House JS, Mero RP. Depressive symptoms and mortality risk in a national sample: confounding effects of health status. Psychosomatic Medicine. 2004;66:823–30. doi: 10.1097/01.psy.0000145903.75432.1f. [DOI] [PubMed] [Google Scholar]
- Fremont AM, Bird CE. Handbook of Medical Sociology. Prentice-Hall, Inc.; New Jersey: 2000. Social and psychological factors, physiological processes, and physical health. [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Frolich KL, Potvin L, Chabot P, Corin E. A theoretical and empirical analysis of context: neighbourhoods, smoking, and youth. Social Science and Medicine. 2002;54(9):1401–17. doi: 10.1016/s0277-9536(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Gould Ellen I, Mijanovich T, Dillman KN. Neighborhood effects on health: Exploring the links and assessing the evidence. Journal of Urban Affairs. 2001;23(3-4):391–408. [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert E, Hellhammer H. The potential role of hypcortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Israel BA, Schulz AJ, Estrada-Martinez L, Zenk SN, Viruell-Fuentes E, Villarruel AM, Stokes C. Engaging urban residents in assessing neighborhood environments and their implications for health. Journal of Urban Health. 2006;83(3):523–39. doi: 10.1007/s11524-006-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo CH, Gossett J, Simpson P. SAS Global Forum; 2007. Regression splines with longitudinal data. www2.sas.com/proceedings/forum2007/143-2007.pdf. [Google Scholar]
- Karb R. Ph D dissertation. University of Michigan; United States -- Michigan: Neighborhood social and physical environments and health: Examining sources of stress and support in neighborhoods and their relationship with self-rated health, cortisol and obesity in Chicago. Retrieved November 17, 2011, from Dissertations & Theses @ CIC Institutions.(Publication No. AAT 3441200) [Google Scholar]
- Krieger N. Theories for social epidemiology in the 21st century: an ecosocial perspective. International Journal of Epidemiology. 2001;30:668–677. doi: 10.1093/ije/30.4.668. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Curry AD. Stressful neighborhoods and depression: a prospective study of the impact of neighborhood disorder. Journal of Health and Social Behavior. 2003;44(1):34–44. [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother s socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–80. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–76. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Mair C, Diez Roux AV, Morenoff JD. Neighborhood stressors and social support as predictors of depressive symptoms in the Chicago Community Adult Health Study. Health and Place. 2010;16(5):811–819. doi: 10.1016/j.healthplace.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JW, Wang S, Yehuda R, Riney S, Charney DS, Southwick SM. Psychogenic lowering of urinary cortisol levels linked to increased emotional numbing and a shame-depressive syndrome in combat-related post- traumatic stress disorder. Psychosomatic Medicine. 2001;24:387–401. doi: 10.1097/00006842-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Matheson FI, Moineddin R, Dunn JR, Creatore MI, Gozdyra P, Glazier RH. Urban neighborhoods, chronic, stress, gender and depression. Social Science and Medicine. 2006;63:2604–2616. doi: 10.1016/j.socscimed.2006.07.001. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low Salivary Cortisol and Persistent Aggression in Boys Referred for Disruptive Behavior. Archives of General Psychiatry. 2000;57:38–44. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- McEwen B. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338(3):171–180. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Meany MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Morenoff JD. Neighborhood mechanisms and the spatial dynamics of birthweight. American Journal of Sociology. 2003;108(5):976–1017. doi: 10.1086/374405. [DOI] [PubMed] [Google Scholar]
- Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. American Journal of Epidemiology. 2007;165:858–67. doi: 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- Muthen B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Kaplan GA. Material hardship alters the diurnal rhythm of salivary cortisol. International Journal of Epidemiology. 2005;34:1138–43. doi: 10.1093/ije/dyi120. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Seiler R, Marti B, Wirtz PH, Ehlert U, Heinrich M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 2009;34:190–8. doi: 10.1016/j.psyneuen.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, Kirschbaum C, Verhulst FC, Tiemeier H. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? Hormones and Behavior. 2010;57:247–254. doi: 10.1016/j.yhbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR, Kirillova GP. Salivary cortisol, personality, and aggressive behavior in adolescent boys: A 5-year longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(9):1101–7. doi: 10.1097/01.CHI.0000070246.24125.6D. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosomatic Medicine. 2011;73(2):144–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Van Ryzin MJ, Chatham M, Kryzer E, Kertes DA, Gunnar MR. Social regulation of cortisol levels in children: Paper 1: Identifying atypical cortisol patterns in young children: the benefits of group-based trajectory modeling. Psychoneuroendocrinology. 2009;34(1):50–61. doi: 10.1016/j.psyneuen.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton B. Social Stress. In: Aneshensel CS, Phelan JC, editors. Handbook of the Sociology of Mental Health. New York: Plenum; 1999. pp. 277–300. [Google Scholar]
- Williams DR, Collins C. US socioeconomic and racial differences in health: patterns and explanations. Annual Review of Sociology. 1995;21:349–86. [Google Scholar]


