Plants adjust growth to suit opportunities and limitations in their environment. Sugars from photosynthesis, the hormone auxin, and members of the PHYTOCHROME INTERACTING FACTOR (PIF) family of proteins have all been shown individually to regulate growth. This work shows that sugars regulate auxin biosynthesis via PIF proteins, indicating that the three in fact act together in growth regulation.
Abstract
Plants are necessarily highly competitive and have finely tuned mechanisms to adjust growth and development in accordance with opportunities and limitations in their environment. Sugars from photosynthesis form an integral part of this growth control process, acting as both an energy source and as signaling molecules in areas targeted for growth. The plant hormone auxin similarly functions as a signaling molecule and a driver of growth and developmental processes. Here, we show that not only do the two act in concert but that auxin metabolism is itself regulated by the availability of free sugars. The regulation of the biosynthesis and degradation of the main auxin, indole-3-acetic acid (IAA), by sugars requires changes in the expression of multiple genes and metabolites linked to several IAA biosynthetic pathways. The induction also involves members of the recently described central regulator PHYTOCHROME-INTERACTING FACTOR transcription factor family. Linking these three known regulators of growth provides a model for the dynamic coordination of responses to a changing environment.
INTRODUCTION
Auxin is a plant growth–regulating phytohormone that orchestrates a wide variety of developmental and environmental responses. Indole-3-acetic acid (IAA), the main auxin in plants, is synthesized from the amino acid precursor tryptophan TRP, and several parallel enzymatic pathways have been suggested to be involved in the process (Normanly, 2010; Mano and Nemoto, 2012; Novák et al., 2012). TRP-independent IAA biosynthesis has also been postulated, although no enzymes and genes have been identified in this pathway to date (Normanly, 2010; Mano and Nemoto, 2012). IAA mediates positional information in developing tissues and is an integral regulator of cell expansion, division, and differentiation. Both quantitatively and qualitatively different responses can be elicited, depending on the concentration of the hormone and the capacity of tissues to respond. Differences in concentration are established through localized biosynthesis and degradation of the hormone and its active polar transport (Tuominen et al., 1997; Bhalerao and Bennett, 2003; Petersson et al., 2009; Petrásek and Friml, 2009). Substantial evidence has shown that the regulation of IAA homeostasis is a central feature of numerous developmental and environmental responses (Normanly, 2010). Differential regulation of members of the YUCCA family of auxin biosynthetic genes, for example, has been shown to be involved in mediating environmental response mechanisms such as shade avoidance (Hornitschek et al., 2012) and adjustments to ambient temperature (Stavang et al., 2009; Sun et al., 2012). Shade avoidance involves physiological and morphological adaptations designed to optimize light capture and photosynthetic capacity, and TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1), another auxin biosynthetic gene, has been shown to be essential for these responses (Tao et al., 2008). Loss-of-function, taa1/sav3 mutant plants lack the capacity to respond to a shade avoidance–inducing reduction in the red:far-red light ratio, a process that involves phytochrome B (phyB) photoreceptors and members of the PIF family of transcriptional regulators (Hornitschek et al., 2012). In these and other cases (for example, see Halliday et al., 2009), regulation of auxin biosynthesis is integral to the plant’s capacity to respond appropriately to the prevailing environmental conditions.
Photosynthesis produces soluble sugars that influence plant growth in two ways: They serve as sources of reduced carbon, from which energy is derived in glycolysis and respiration, and act as signaling molecules through the action of receptor kinases (Rolland et al., 2006; Wind et al., 2010). Under ideal conditions, the rate of plant growth and biomass accumulation is directly related to photosynthetic efficiency. In elevated atmospheric carbon dioxide conditions, for example, the decrease in photorespiration and enhanced photoassimilation are often associated with accelerated growth and increased biomass production (Li et al., 2007). Glasshouse vegetable producers routinely use this effect to promote growth and productivity in their crops. Exogenous sugars applied in low concentrations have been shown to have growth-promoting effects in Arabidopsis (Roycewicz and Malamy, 2012). One manifestation of the sugar effect is in root growth, and glucose Glc-treated Arabidopsis thaliana seedlings have enhanced root growth rates and increased numbers of lateral roots (Mishra et al., 2009). Light levels and the concentration of endogenous sugars have also been shown to correlate positively with primary root growth and the density of lateral roots in Arabidopsis (Freixes et al., 2002; Kircher and Schopfer, 2012). It is well documented that low levels of applied auxin have similar effects on root growth (Jones and Ljung, 2012). Research has uncovered numerous examples of in situ links between Suc and IAA in growing organs. For example, auxin mediates altered root development after Arabidopsis plants are transferred from Suc-containing growth medium to Glc-supplemented medium (Mishra et al., 2009). It has also been shown that exogenous sugars can enhance the effects of applied auxin (e.g., IAA-induced coleoptile elongation in barley (Hordeum vulgare) was enhanced after supplementation of the media with Glc) (Takeda et al., 2010). In developing maize (Zea mays) kernels, there is a correlation between sugar content, IAA levels, and the expression of an auxin biosynthesis gene, the maize YUCCA homolog (LeClere et al., 2010). Although a link between sugars and IAA has been suggested, the mechanism has remained elusive.
The PHYTOCHROME-INTERACTING FACTOR (PIF) family of transcriptional regulators has been shown to regulate a number of important aspects of growth and development (Leivar and Quail, 2011). In addition to their role in mediating the phyB light responses (Leivar et al., 2008; Lorrain et al., 2008), PIFs are also involved in environmental responses to changing light and temperature conditions. PIF4 and PIF5, for example, have been shown to be involved in light and temperature regulation of auxin biosynthesis and signaling (Franklin et al., 2011; Nozue et al., 2011; Hornitschek et al., 2012; Sun et al., 2012). The normal induction of IAA levels by high temperatures seen in wild-type seedlings is attenuated in the pif4 knockout line (Franklin et al., 2011; Sun et al., 2012), and the induction of IAA biosynthesis in response to a low red:far-red light ratio is abolished in the pif4 pif5 double mutant (Hornitschek et al., 2012). Curiously, the PIF5-overexpressing line also has considerably lower levels of IAA than the wild-type control under far-red enriched conditions (Hornitschek et al., 2012). However, in this line, PIF5 is under the control of the cauliflower mosaic virus 35S constitutive promoter, so the phenotype may be complicated by the ectopic expression of the gene. Overexpression of PIF4 leads to an increase in YUCCA8 expression and elevated IAA levels (Sun et al., 2012). Auxin sensitivity is also altered in the pif mutant lines. The pif4 pif5 double mutant has an increased responsiveness to low amounts of exogenous auxin and conversely shows less growth inhibition than the wild type to high levels of auxin (Nozue et al., 2011). Taken together, these data indicate that the PIF genes are intimately involved in the regulation of auxin metabolism and plant growth.
In this study, through a combination of techniques, we demonstrate that the rates of both de novo IAA biosynthesis and its metabolism are regulated by sugars and that diurnal levels of soluble sugars correspond to diurnal fluctuations in endogenous IAA levels. We also demonstrate that soluble sugars lead to the simultaneous upregulation of multiple IAA biosynthetic pathways and that this regulation involves members of the PIF family of transcriptional regulators. Together, our data establish a link between sugar levels, auxin metabolism, and PIF family proteins that provides a basis for understanding how the availability of photosynthates regulates growth.
RESULTS
IAA Homeostasis Responds Rapidly to Alterations in Carbohydrate Levels in Arabidopsis Seedlings
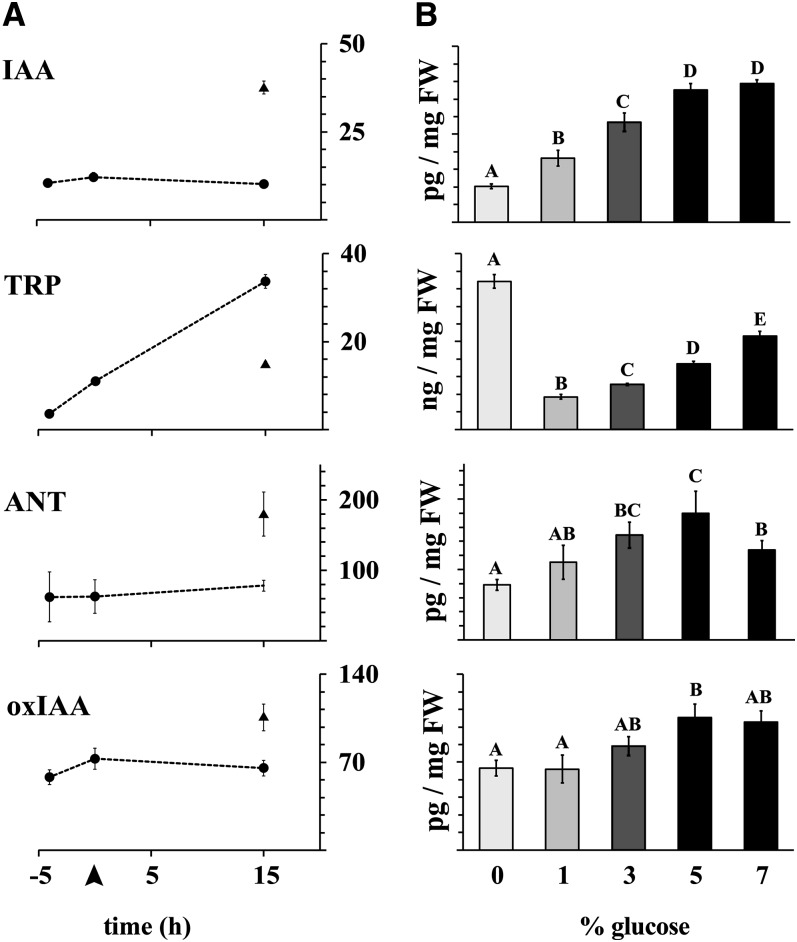
To study the effects of soluble sugars on auxin homeostasis, 10 d after germination (DAG) wild-type Arabidopsis seedlings were incubated with various concentrations of the hexose sugar Glc. To measure both biosynthesis and degradation of the hormone, the concentrations of IAA, its precursors, anthranilate (ANT) and tryptophan (TRP), and the major IAA catabolite, 2-oxoindole-3-acetic acid (oxIAA), were analyzed (Figure 1). Glc concentrations ranging from 1 to 7% increased the level of free IAA in a concentration-dependent manner. One percent Glc approximately doubled the level of endogenous IAA, and 5% more than tripled it compared with the nontreated control (Figure 1B). The concentration of two IAA precursors (ANT and TRP) and the IAA catabolite, oxIAA, increased in a similar manner (Figure 1B), indicating active regulation of IAA levels by Glc through biosynthesis and degradation of the hormone. Control experiments showed that IAA levels remained unaltered in the absence of exogenous Glc (Figure 1A), and when mannitol, a slowly metabolizable carbohydrate with similar osmotic properties to Glc, was substituted for Glc, no significant increase in IAA levels was observed (see Supplemental Figure 1A online), indicating the importance of the active sugar rather than osmotic potential in the response.
Figure 1.
IAA Metabolism Is Elevated after Glc Treatment.
IAA, TRP, ANT, and oxIAA concentrations increase after Glc treatment in a concentration-dependent manner. Before treatment, the seedlings were pretreated for 5 h in liquid medium.
(A) Time-series analysis of the levels of each compound. Dotted line connects data points with no Glc treatment, and the unconnected data point shows the metabolite concentration after 15 h of treatment with 5% Glc. Arrowhead at the horizontal axis indicates the start of the Glc treatment.
(B) Levels of each compound after 15 h of treatment with varying amounts of exogenous Glc. A one-way analysis of variance was used to compare the treatments. Error bars indicate sd (n = 3 or 4). Bars not connected with a letter indicate a statistical difference at 95% confidence level. FW, fresh weight.
Strikingly, during the 15-h control incubation in the absence of exogenous Glc, the level of TRP increased ninefold, while the IAA level remained unaltered (Figure 1A). Incubation with 1% exogenous Glc restored TRP to pretreatment levels, and increasing the level of exogenous Glc beyond 1% increased TRP levels in a concentration-dependent manner (Figure 1B). Together, these data suggest that Glc regulates the levels of this important IAA precursor and potentially also regulates its incorporation into downstream pathways. ANT and oxIAA levels remained constant throughout the control treatment (Figure 1A) but increased with the addition of exogenous Glc (Figure 1B). These experiments were performed in liquid medium. Control experiments, where seedlings were transferred from growth plates without sugars to treatment plates with added sugar (1 and 5% Glc) showed increases in IAA level in root tissues similar to those observed in the liquid medium (see Supplemental Figure 1B online).
To get an overview of the metabolic status of the plants during our experiments, we performed gas chromatography–time-of-flight–mass-spectrometry based metabolite analyses on untreated seedlings, seedlings pretreated for 4 h in liquid medium, and on seedlings incubated with different concentrations of Glc (0, 1, 5, and 7%) for 15 h. Our method allowed us to detect ∼250 unique metabolites, of which 76 have known identities. Multivariate analysis was performed, with the control, nonsupplemented samples analyzed separately from the Glc treatment samples. In the control data set, the three sampling points arranged linearly to the horizontal component (component 2; see Supplemental Figure 2A online). This component separated carbohydrates (red) and amino acids (blue) into two distinct clusters. The increase in amino acids, combined with the noncorrelating patterns between ANT and TRP in Figure 1A, indicates that proteins are degraded when seedlings are incubated for longer periods in liquid medium without sugar. Comparison of the Glc treatments indicates that exogenous Glc supplementation effectively rescues the carbohydrate balance in these seedlings (see Supplemental Figure 2B online).
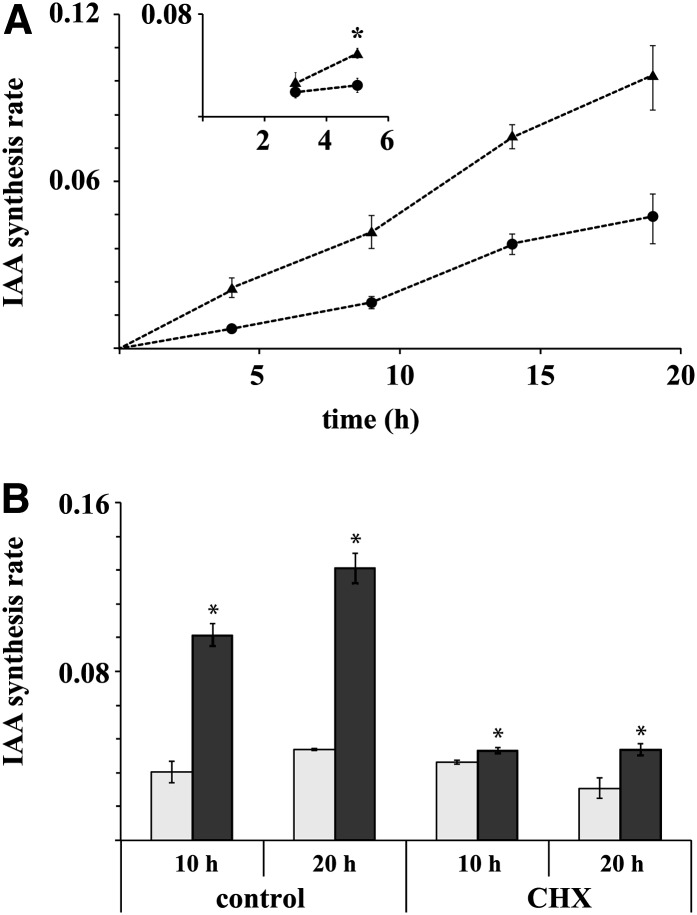
Next, we analyzed how Glc affected the rate of de novo IAA biosynthesis by measuring the incorporation of a heavy isotope label into the IAA pool after treatment with liquid medium containing 30% deuterated water. Five percent Glc dramatically and linearly elevated the rate of IAA biosynthesis over a 20-h incubation period, compared with incubation without Glc (Figure 2A). The earliest time where a significant difference in the synthesis rate could be observed between the Glc treatment and the control was after 5 h (Figure 2A, subset). Adding the protein synthesis inhibitor cycloheximide (CHX) to the reaction almost completely abolished the response, indicating that the Glc induction of IAA biosynthesis requires de novo protein synthesis (Figure 2B).
Figure 2.
CHX-Sensitive IAA Biosynthesis Is Rapidly Induced after Addition of 5% Glc.
(A) Incorporation of deuterium label into the IAA pool after treatment of 10 DAG seedlings with medium containing 30% deuterated water and 0% (circles) or 5% Glc (triangles). The seedlings were pretreated for 4 h in liquid medium. Increased IAA synthesis rates were observed up to 20 h after the beginning of treatment. Inset: Short incubation with 60% deuterated water. The asterisk denotes statistical difference at 95% confidence level.
(B) Inhibition of protein synthesis with CHX abolishes Glc induction of IAA synthesis. Gray bars indicate IAA biosynthesis rate without sugar treatment and dark bars after incubation in medium containing 5% Glc. Error bars denote se (n = 4).
We next investigated whether Suc, the main endogenous sugar found in the apoplast, has a similar capacity to induce IAA biosynthesis to Glc. The experiments showed that Suc has an even greater capacity to induce IAA biosynthesis than Glc (see Supplemental Figure 3A online). This was particularly evident in root tissues. Control assays with the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid, or abscisic acid (ABA) showed no increase in IAA synthesis rates (for the ABA treatment, there was even a small, but significant, decrease in the IAA synthesis rate), indicating the specificity of the response and that exogenous Glc does not elicit IAA through a stress response mechanism (see Supplemental Figure 3B online).
Taken together, our data provide strong evidence that TRP-dependent IAA biosynthesis is tightly controlled by Glc (and potentially other metabolizable sugars).
Glc Treatment Induces Genes in Parallel IAA Biosynthesis Pathways
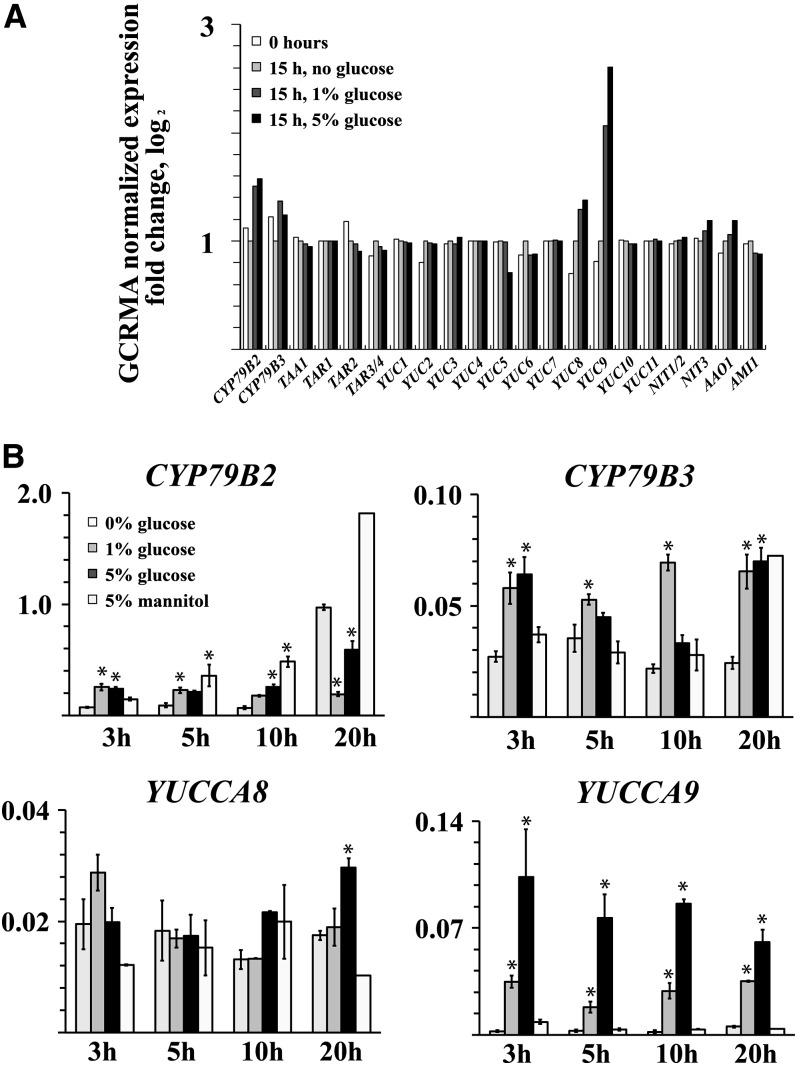
The amino acid TRP is a major IAA precursor in plants, and a number of discrete TRP-dependent IAA biosynthetic pathways have been identified (Normanly, 2010; Mano and Nemoto, 2012; Novák et al., 2012). To better define the mechanism by which Glc regulates IAA biosynthesis, we performed a transcript analysis on seedlings treated for 15 h with or without exogenous Glc. Control samples were also collected after the 4-h pretreatment. Microarray analyses indicated similarities in gene expression patterns between the treatments with different Glc concentrations (1 and 5%) (see Supplemental Figure 4 online). Four key IAA biosynthetic genes were clearly induced by the Glc treatment, CYP79B2, CYP79B3, YUCCA8, and YUCCA9 (Figure 3A), belonging to two separate IAA biosynthetic pathways (Novák et al., 2012). The Glc responsiveness of the four genes was confirmed in a time-course analysis using gene-specific primers. CYP79B2, CYP79B3, YUCCA8, and YUCCA9 all exhibited elevated expression after Glc treatment, with CYP79B2 also responding strongly to the 5% mannitol control (Figure 3B). The mannitol induction of CYP79B2 indicates that metabolism of the sugars is nonessential for the transcriptional response of this gene. In addition to being unresponsive to mannitol, YUCCA9 expression showed the most rapid and concentration-dependent response to exogenous Glc. The different response capacities indicate the existence of a number of sugar response mechanisms in terms of IAA inducibility. We examined whether single and double auxin biosynthetic gene mutants were altered for the capacity to respond to Glc. There were only minor differences from the wild-type response and some were even shown to have increased responses (see Supplemental Figures 5A and 5B online). The considerable redundancy in auxin biosynthetic pathways suggests that genes from multiple pathways may need to be combined before sizeable effects are observed. Because recent data have shown links between the TAA1/TAR family and growth responses (Stepanova et al., 2008; Tao et al., 2008), we also examined mutants for genes in this family. These mutants similarly showed an induction close to that of the wild type (see Supplemental Figure 5C online).
Figure 3.
Transcript Analysis Shows That Members of the Auxin Biosynthesis Gene Families CYP79B and YUCCA Are Induced by Exogenous Glc.
(A) Gene expression analysis of a subset of genes directly related to IAA biosynthesis using the ATH1 array. Whole seedlings were pretreated for 4 h in liquid medium and then treated for 15 h in medium containing 0, 1, or 5% Glc. Both pretreated (0 h) and treated samples are included in the analysis. Bars indicate fold change in logarithmic values compared with a 15-h treatment with no sugar.
(B) Quantitative PCR expression analysis for selected Glc-inducible genes using gene-specific primers. The transcript levels are expressed against the average expression of four reference genes. Treatment as in (A) with 15-h treatment in 5% mannitol added. Error bars indicate se (n = 3 or 4; in 20-h mannitol treatment, n = 1).
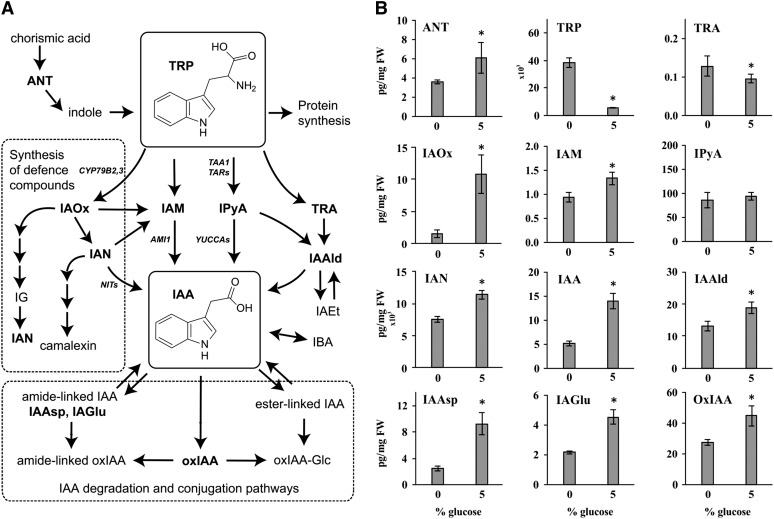
In a further attempt to unravel the mechanism by which Glc induces IAA biosynthesis, we analyzed the patterns of accumulation of IAA metabolites after the addition of Glc. Previously identified IAA metabolites (Figure 4A; Novák et al., 2012) were quantified after 20 h incubation with 0 or 5% Glc. Treatment with 5% Glc significantly increased the concentration of IAA and several IAA precursors, including ANT, indole-3-acetaldoxime (IAOx), indole-3-acetamide (IAM), indole-3-acetonitrile (IAN), and indole-3-acetaldehyde (IAAld) (Figure 4B). IAOx, IAM, IAN, and IAAld are all putative metabolites in different TRP-dependent IAA biosynthesis pathways (Figure 4A). IAA degradation was also induced, shown by the elevated levels of the major catabolite oxIAA and the conjugates IAA-aspartate (IAAsp) and IAA-glutamate (IAGlu). The levels of TRP and TRA were, by contrast, significantly higher in seedlings incubated with 0% Glc. Together, the data show the broad influence that Glc has on TRP and IAA biosynthetic mechanisms.
Figure 4.
IAA Metabolite Profiling after Sugar Induction.
(A) Proposed TRP-dependent IAA biosynthesis and IAA degradation/conjugation pathways in Arabidopsis based on Östin et al. (1998), Normanly (2010), Won et al. (2011), Novák et al. (2012), and Mano and Nemoto (2012).
(B) Analysis of known IAA metabolites indicates that a 20-h treatment with 5% Glc significantly upregulates the level of IAA, several putative IAA precursors (ANT, IAOx, IAM, IAN, and IAAld), as well as three major IAA catabolites and conjugates (oxIAA, IAAsp, and IAGlu), while the levels of TRP and TRA are significantly downregulated. Error bars indicate sd (n = 5). FW, fresh weight.
IAA metabolites quantified (in bold in [A]): tryptamine (TRA), IAOx, IAM, indole-3-pyruvic acid (IPyA), IAN, IAAld, IAAsp, IAGlu, and oxIAA. IAEt, indole-3-ethanol; IBA, indole-3-butyric acid; IG, indoleglucosinolate.
IAA Biosynthesis Rates Correspond to Endogenous Hexose Levels
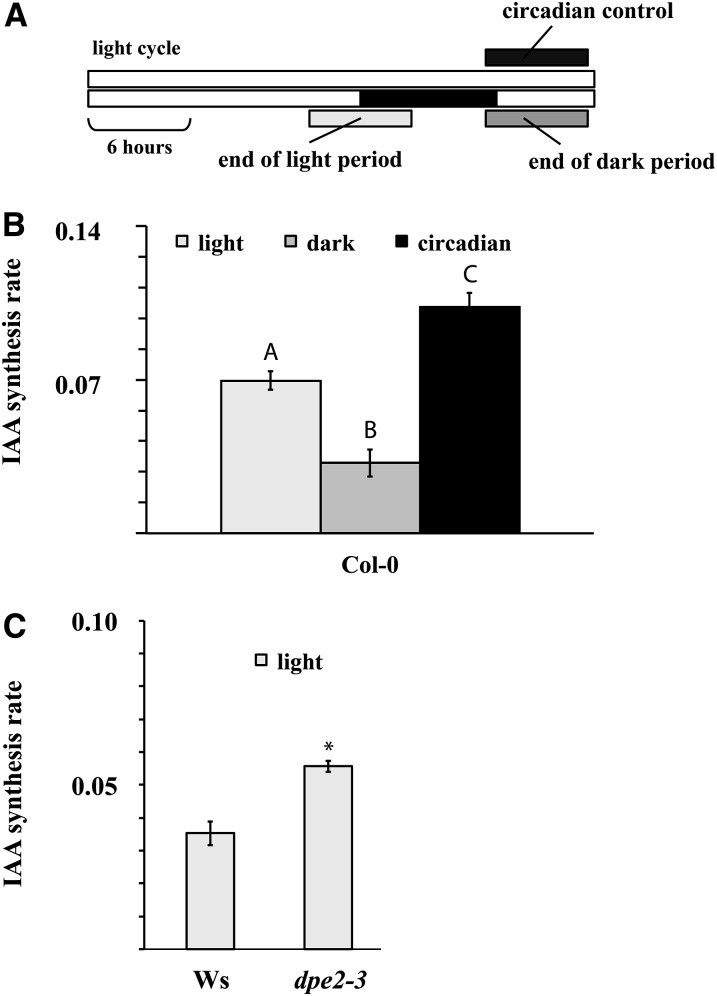
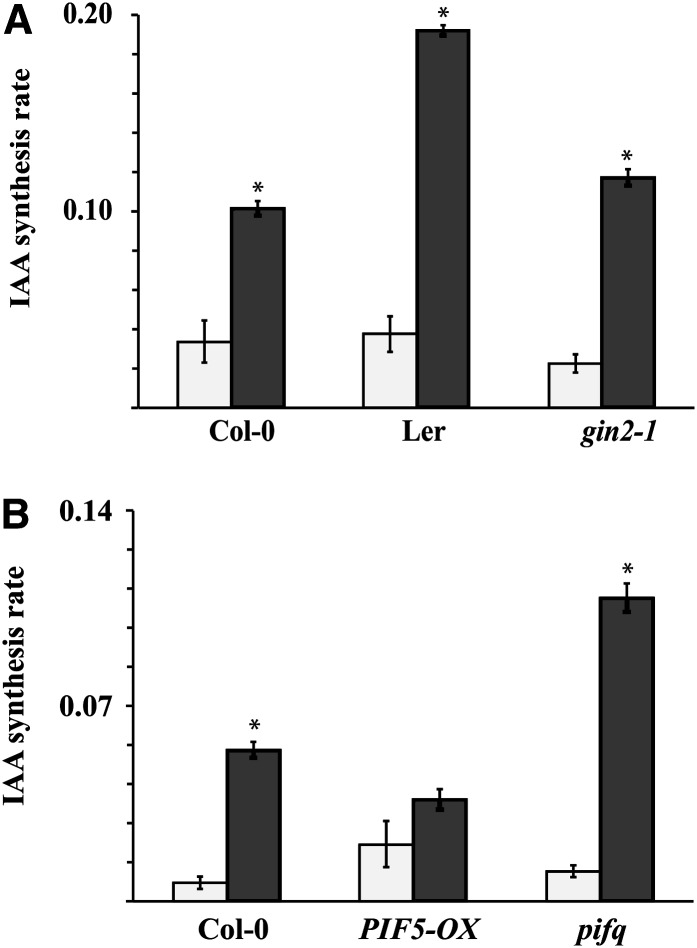
To understand the physiological significance of the Glc regulation of IAA metabolism, we investigated the relationship at the level of endogenous compounds. It is well established that the endogenous levels of soluble sugars rise and fall over a diurnal cycle (Smith and Stitt, 2007). A positive link between soluble sugar levels and IAA biosynthesis suggests that IAA biosynthesis rates should similarly rise and fall over the diurnal cycle. Sugar levels peak toward the end of the light period, and there is a corresponding minimum at the end of the dark period (Smith and Stitt, 2007). We measured the rate of IAA biosynthesis at the end of the light period and at the end of the dark period, and we also measured the effect of altering light regimes from the normal diurnal cycle on the rate of IAA biosynthesis (Figure 5A). Corresponding to the levels of sugars, the rate of IAA biosynthesis at the end of the light period was approximately double the rate at the end of the dark period (Figure 5B). Maintaining illumination throughout the night period restored IAA biosynthesis to daytime levels (Figure 5B). Endogenous hexose levels are also known to be elevated in the glucosyltransferase mutant line dpe2-3 (Chia et al., 2004). The rate of IAA biosynthesis was elevated in this mutant compared with the Wassilewskija (Ws) wild-type control (Figure 5C). We also observed a difference in the Glc induction of IAA synthesis between the ecotypes Landsberg erecta (Ler) and Columbia 0 (Col-0) (Figure 6A). The Ler ecotype is known to have low β-amylase activity compared with Col-0 and to accumulate hexoses during exogenous sugar feeding (Mita et al., 1997). These data provide considerable further evidence that IAA biosynthesis is regulated by endogenous sugar levels. The phosphorylation status of hexoses is also known to contribute to their activity (Rolland et al., 2006). To ascertain whether the Glc regulation of IAA metabolism is dependent on hexose phosphorylation, we tested whether IAA induction is altered in the gin2-1 mutant line, which has a reduced capacity for hexose phosphorylation and is severely impaired in Glc signaling because of a mutation in the HEXOKINASE1 (HXK1) gene (Moore et al., 2003). Both the basal IAA level and the Glc-induced rate of IAA biosynthesis were reduced in gin2-1 compared with Ler, its corresponding wild type (Figure 6A).
Figure 5.
IAA Synthesis Rates Correlate with Cytosolic Carbon Metabolism.
(A) Schematic representation of the experimental setup. Two 6-h dark incubations were made, presented as gray bars, one at the end of the light period, and the other at the end of the dark period. Plants illuminated through the dark period (circadian control) were incubated in the same way.
(B) IAA synthesis rates in wild-type Col-0 seedlings after incubation in medium containing deuterated water as described in (A). Bars not connected with a letter indicate a statistical difference at 95% confidence level. Error bars indicate sd (n = 4).
(C) IAA synthesis rates in wild-type Ws and the hexose-accumulating line dpe2-3. The seedlings were incubated in the end of the light period. Error bars indicate sd (n = 4).
Figure 6.
IAA Biosynthesis Is Altered in Hexose-Accumulating and PIF Mutant and Overexpressing Lines.
IAA biosynthesis rates in the wild-type lines Col-0 and Ler and in the gin2-1 mutant line (in Ler background) (A) and in the lines PIF5-OX and pifq (Col-0 background) (B) after 20-h incubation in liquid medium. White bars indicate IAA biosynthesis rates after incubation without sugar and dark bars after incubation with 5% Glc. Error bars denote se (n = 4).
PIF Proteins Are Negative Regulators of Glc-Induced IAA Biosynthesis
A member of the PIF gene family has recently been shown to interact with elements in the promoter of multiple genes involved in auxin biosynthesis and signaling (Franklin et al., 2011; Hornitschek et al., 2012). Given that, like sugars and auxin, the PIF proteins have been suggested to be central regulators of multiple growth, development, and environmental response processes, we investigated whether the link between Glc levels and IAA biosynthesis is impaired in pif mutant lines. Indeed, the ability of Glc to induce IAA biosynthesis was upregulated in the pif1 pif3 pif4 pif5 quadruple mutant line compared with the wild type (Figure 6B). This suggests that PIF proteins play a role but that they act as negative regulators of the process. Commensurate with these results, the response to Glc was significantly reduced in a line overexpressing PIF5 (Figure 6B). Taken together, the data indicate that carbohydrate levels affect multiple IAA biosynthesis pathways and that the response is at least partially coordinated through PIF-mediated pathways.
DISCUSSION
It is well established that auxin has a defined function in controlling cell specificity, expansion, and proliferation in plants. Considerable evidence has also accumulated showing auxin as a likely component of a system that translates variables such as light capture into growth responses (Halliday et al., 2009). Sugars similarly are known to play a central role in the regulation of growth and development. Like auxin, sugar responses are dose dependent and sugar also has the capacity to function as a signaling molecule (Rolland et al., 2006). Plants sense their carbohydrate status and respond to favorable conditions by allocating resources appropriate to the opportunities and limitations in their environment. Without an appropriate growth response, increasing carbohydrate levels can lead to feedback inhibition of photosynthesis and, therefore, a failure to take full advantage of the light resource. Similarly, the PIF proteins are integral to multiple aspects of growth and development, including a capacity for the growth response to shading. Given the known importance of these three elements in growth control, we hypothesized that they are interrelated.
Exogenous Sugar Induces IAA Biosynthesis in Arabidopsis Seedlings
We demonstrated that biosynthesis of IAA, the main auxin in plants, is strongly upregulated by exogenous Glc and by low and physiologically relevant levels of the sugar (Figure 1B; see Supplemental Figure 1B online). We also showed that Glc increases levels of two of the main IAA precursors, ANT and TRP, and the main IAA catabolite, oxIAA (Figure 1B). In the control where no Glc was added, there was a dramatic increase in the level of the IAA precursor TRP (Figure 1A). The majority of the IAA biosynthesis pathways described to date use TRP as a precursor (Normanly, 2010; Mano and Nemoto, 2012). Importantly, despite the dramatic increase in TRP in the control, there was no increase in IAA levels in these plants. There is a major difference in the pool size of TRP compared with IAA, with TRP levels around 500 to 1000 times higher than IAA (Figures 1B and 4B). Since TRP is also a precursor for the biosynthesis of defense compounds, such as camalexin and indoleglucosinolates (Figure 4A), and is incorporated into proteins, it is not unexpected that the flow of TRP into the IAA biosynthetic pathways would be largely independent of the level of the amino acid. However, our data clearly indicate that Glc is a trigger for one or more elements that regulate the flow of TRP metabolites through the IAA biosynthetic pathways, since IAA and several IAA precursors downstream of TRP were upregulated by Glc (Figure 4B). In addition to affecting IAA biosynthesis, Glc treatment increased the concentration of the major IAA catabolite, oxIAA, and two major IAA conjugates (IAAsp and IAGlu), most likely as a consequence of the increased IAA level (Figures 1B and 4B). These IAA metabolites have previously been shown to be strongly upregulated in IAA-overproducing lines (Novák et al., 2012) and after treatment with exogenous IAA (Östin et al., 1998). Taken together, the induced biosynthesis and metabolism data indicate that auxin homeostasis operates to serve immediate, dynamic processes, such as demonstrated here in the response to Glc.
Treatment with 3% Suc elicited a pronounced increase in the rate of IAA biosynthesis (see Supplemental Figure 3A online). This increased efficacy may be explained by Suc entering the tissue more efficiently than Glc, through active uptake into cells, or that Suc is degraded after uptake into Glc and Fru monomers. Although mannitol, a slowly metabolizable sugar, was able to induce an increase in the transcripts for some IAA biosynthetic pathway elements (Figure 3), IAA levels were not altered by treatment with this Glc analog (see Supplemental Figure 1A online). One auxin biosynthetic gene, YUCCA9, did not respond to mannitol and was rapidly upregulated by Glc in a concentration-dependent manner. It may be that under normal circumstances, of the four auxin biosynthetic genes identified as being upregulated by Glc, YUCCA9 is the key mediator of Glc-induced auxin biosynthesis. Whereas single or double gene knockout lines for YUCCA9 and other IAA biosynthesis pathway elements did not affect the capacity of Glc to induce IAA, redundancies within and between pathways may have masked the effects of the mutations (Normanly, 2010; Mano and Nemoto, 2012). Although knocking out a single auxin biosynthetic gene, TAA1, eliminates the capacity of plants to respond appropriately to shade avoidance inducing conditions (Tao et al., 2008), it may be that in the case of Glc induction, a severe downregulation of individual or multiple pathways is required before an overall effect of sugars on auxin biosynthesis would be observed. We are currently creating multiple gene knockout mutant lines to determine the scope of genetic recruitment into the response mechanism.
The lack of IAA induction with mannitol indicates both that the response depends on a capacity of the sugars to be metabolized and that it is not due to changes in the osmotic potential of the treatment media. Glc has been shown to have some negative effects on Arabidopsis development (León and Sheen, 2003). To obviate this risk, experiments were conducted for comparatively brief periods of time. Two other hormones known to be involved in sugar responses, ABA and ethylene (Rolland et al., 2006), did not induce IAA biosynthesis, further indicating the specificity of the response. Although the secondary effects of stress or osmotic changes cannot be entirely ruled out, together the evidence strongly suggests that endogenous, physiological levels of soluble sugars are key elicitors of IAA biosynthesis.
Genetic and Diurnal Variations in Carbohydrate Levels Correlate with IAA Biosynthesis Rates
To identify contexts within which the sugar induction of IAA biosynthesis might operate, we monitored the rates of IAA biosynthesis at different times during the diurnal cycle, when endogenous sugars are known to rise and fall. We demonstrated that the rates of IAA biosynthesis are significantly reduced at the end of the dark period when soluble sugars are typically at their lowest in the diurnal cycle. Conversely, IAA levels were high at the end of the light period, when endogenous sugars normally peak (Figures 5A and 5B; Smith and Stitt, 2007) and when there is a high rate of elongation growth (Stewart et al., 2011). Maintaining lighting during the normal dark period led to an increase in the rate of IAA biosynthesis, indicating that IAA biosynthesis rates correlate with light and the diurnal cycle, rather than being regulated by circadian oscillations (Figure 5B). We explored this further using the mutant line dpe2-3, which shows increased endogenous hexose levels as a result of a mutation in a gene encoding a cytosolic disproportionating enzyme (Chia et al., 2004). Consistent with the hypothesis that light induction of IAA biosynthesis is due to the associated levels of free sugars, the dpe2-3 mutant had elevated IAA biosynthesis compared with the wild-type control (Figure 5C). From our data, it is tempting to hypothesize that free sugar levels and IAA biosynthesis provide a mechanism for dynamically regulating growth rates commensurate with the physiological status of the plant.
Because, unlike Glc and Suc, mannitol, a slowly metabolizable sugar, failed to induce IAA biosynthesis, it appears that the induction depends on a mechanism originating from products of the metabolism of the sugars. The Arabidopsis hexokinase HXK1 mutant gin2-1 has a reduced capacity for hexose phosphorylation and as a result is impaired in Glc-driven signal transduction (Moore et al., 2003). In our experiments, the gin2-1 mutant had a lower basal level of IAA and a reduced capacity for induction of IAA biosynthesis by Glc compared with the corresponding wild-type (Ler) (Figure 6A). This suggests that the phosphorylation status of the sugars is important for their capacity to induce IAA biosynthesis. Whether the induction of auxin biosynthesis by sugars involves a signaling cascade initiated from Suc, as suggested by Gonzali et al. (2005), hexoses, or glycolysis activity or intermediates remains to be investigated. The fact that the gin2-1 mutant retains 50% of the wild-type Glc kinase activity (Moore et al., 2003) suggests also that HXK1-independent pathways can operate in plants, and the observation that auxin biosynthesis can be upregulated by Glc in gin2-1, although to a lesser extent than in the wild type (Figure 6A), indicates that HXK1-independent pathways also operate in this response. Our data suggest a role for both sugar metabolism and signaling in the regulation of auxin biosynthesis.
PIF Genes Negatively Regulate Glc-Induced IAA Biosynthesis
The seven PIF genes in Arabidopsis belong to a 15-member subfamily of basic helix-loop-helix transcriptional regulators that have been shown to have diverse functions and targets in plants (Leivar and Quail, 2011). From the study of pif mutant lines, it is clear that PIF proteins are involved in the regulation of IAA homeostasis (Franklin et al., 2011; Sun et al., 2012). It is equally clear that Suc can regulate PIF gene expression (Stewart et al., 2011). Given that like auxin and Suc the PIF proteins have been implicated in the regulation of growth and development (Leivar and Quail, 2011), we examined whether the pif1-1 pif3-3 pif4-2 pif5-3 (pifq) quadruple mutant line and a PIF5 overexpressor (PIF5-OX) line had an altered capacity for Glc induction of IAA. Under our conditions, the capacity of Glc to induce IAA was heightened in the pifq mutant compared with the wild type (Figure 6B). One possible explanation for this is that the pifq mutant is hypersensitive to sugars. However, it has been shown recently that the normal capacity of sugar to induce hypocotyl elongation in wild-type plants is lost in the pifq mutant line (Liu et al., 2011; Stewart et al., 2011), suggesting an insensitivity rather than a hypersensitivity. It is intriguing that our data indicate that the sugar in the experiments of Stewart et al. (2011) should have led to an increase in auxin, and the hormone is well known to promote hypocotyl elongation (Lilley et al., 2012; Takahashi et al., 2012). It is likely that the pifq mutant lacks a capacity to respond appropriately to the auxin that was presumably induced by Suc in the experiments of Stewart et al. (2011).
The enhanced Glc induction of IAA biosynthesis in the pifq line indicates that the PIF proteins act as negative regulators of sugar-induced IAA biosynthesis. In support of this explanation, the PIF5-OX line showed a severely reduced capacity for Glc induction of IAA biosynthesis (Figure 6B). However, if this is correct, the response mechanism is likely to be complex because PIF gene expression is upregulated by Suc (Stewart et al., 2011) and PIF4 has been shown to bind to promoter elements of the auxin biosynthetic genes TAA1 and CYP79B2 and to induce their expression under elevated temperatures (Franklin et al., 2011). Like auxin and Suc, PIF proteins are known to regulate environmental response processes (Leivar and Quail, 2011). Although it is possible that the PIF proteins act as master switches in the sugar induction of IAA biosynthesis, clearly, the connections between these elements are complex and require further definition.
In this work, we established a regulatory link between light, carbon, and IAA metabolism. Combined, our data indicate that products of photosynthesis regulate IAA biosynthesis and that this regulation is mediated by PIF proteins, adding an important additional dimension to our understanding of the roles played by this family of transcription regulators. It has been established that each of the three elements, sugars, auxin, and PIF proteins are capable of acting as regulators of growth, development, and environmental responses. We propose that together they form a control mechanism that dynamically coordinates growth in response to a changing environment (see Supplemental Figure 6 online).
METHODS
Plant Material and Growth Conditions
Wild-type Arabidopsis thaliana ecotypes used in this study were Col-0, Ler, and Ws. The mutant lines yuc8 and yuc9 (Tao et al., 2008), yuc8 yuc9 (Hentrich, 2010), sav3-2 (Tao et al., 2008), tar1-1, tar2-1, and tar1-1 tar2-1 (Stepanova et al., 2008), pif1-1 pif3-3 pif4-2 pif5-3 (Leivar et al., 2008), and PIF5-OX (Lorrain et al., 2008) were in the Col-0 background. The gin2-1 line (Moore et al., 2003) was in the Ler background, and the dpe2-3 line (Chia et al., 2004) was in the Ws background. T-DNA insertions into the CYP79B2 and CYP79B3 genes in Col-0 background were obtained through the Nottingham Arabidopsis Stock Centre and crossed. The single cyp79B2 and cyp79B3 mutants and the double mutant line cyp79B2 cyp79B3 were genotyped by gene-specific primers that are described in Supplemental Table 1 online. The seeds were sterilized using 75% ethanol with 0.1% (v/v) Tween 20 for 5 min, followed by treatment with 95% ethanol for 30 s. Most of the liquid was removed with a pipette, and the remaining liquid was evaporated in a fume hood. The seeds were sown on Murashige and Skoog plates (1× Murashige and Skoog medium, 50 mM MES, and 1% agar, pH 5.7) and stratified at 4°C for 3 d before placing in long-day photoperiod growth rooms (18 h light/6 h dark). The growth light was provided from cool white fluorescent lamps with an average intensity of 150 μE. Daily temperatures varied between 22 and 18°C for light and dark periods, respectively.
Treatments and Sample Homogenization
For the sugar treatments, 10 DAG, the seedlings were immersed into liquid medium (1× Murashige and Skoog medium and 50 mM MES, pH 5.7) in plastic Petri dishes 2 h after the onset of light. They were first incubated in darkness for 4 to 5 h (pretreatment) with gentle shaking and then transferred into incubation media (treatment) with or without sugars (Glc, Suc, and mannitol), 40 μM 1-aminocyclopropane-1-carboxylic acid, or 40 μM ABA, and further incubated for 6 to 20 h in darkness. To inhibit protein synthesis, the treatment medium was supplemented with 20 mg/mL CHX, and to measure the rate of IAA biosynthesis, the treatment medium was supplemented with 30 or 60% deuterium oxide (Ljung et al., 2005). Harvested samples were dried gently with tissue paper to remove excess liquid and then snap frozen in liquid nitrogen after weighing using a precision scale (Mettler Toledo AT20). The 10 DAG plants were also used for the Glc treatment on plates. The treatment commenced 6 h after the onset of light. At the conclusion of the treatments, the samples were homogenized for quantification of IAA and its metabolites, and RNA extraction and metabolomics analyses, either in small scintillation vials or in Eppendorf tubes for 2 min using a MixerMill MM 301 bead mill and tungsten carbide beads. Ground samples were immediately used for analyses or frozen in liquid nitrogen and stored at −80°C.
RNA Extraction and Gene Expression Analysis
For RNA extraction, 60 mg of homogenized plant material was weighed using a frozen spatula and further homogenized for 1 min in Total RNA Lysis solution (Bio-Rad) with β-mercaptoethanol. RNA was extracted using the Aurum Total RNA kit (Bio-Rad). Two micrograms of total RNA was used for gene expression analysis with 24k ATH1 gene chip (International Affymetrix Service). The raw gene chip data was GCRMA normalized, and Venn diagrams were drawn based on contrasts between different treatments using affylmGUI package in R. Fold changes in gene expression were calculated for the average expression values against the average of mock treatment. For quantitative PCR validation of gene expression, Glc treatment was repeated; samples were harvested and homogenized for 2 min in total RNA lysis solution (Bio-Rad) with β-mercaptoethanol. One microgram of total RNA was used as a template for cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad) according to manufacturer’s instructions. Transcript levels were measured with CFX96 real-time PCR detection system (Bio-Rad) using iQ SYBR Green Supermix reagents (Bio-Rad). Reference genes TIP41-like, UBQ10, APT1, and eF1a were analyzed, and the stability of the genes was calculated using the SLqPCR package in R (Vandesompele et al., 2002). The geometric average of all four genes was used for normalization. Oligonucleotide primers used for quantitative PCR analysis are described in Supplemental Table 2 online.
Mass Spectrometry Analysis of Indolic Compounds, IAA Synthesis Rates, and Metabolites
Concentrations of IAA and different IAA metabolites (precursors, conjugates, and catabolites) were measured using liquid chromatography–tandem mass spectrometry (Agilent Instruments) as described by Novák et al. (2012). IAA synthesis rates (calculated as the ratio of labeled to unlabeled IAA) were analyzed as described by Ljung et al. (2005). For metabolic profiling, 20 mg of homogenized samples was prepared using an optimized extraction protocol and analyzed using a Pegasus III gas chromatograph/time-of-flight mass spectrometer (Leco), essentially following Gullberg et al. (2004). The chromatograms were processed with in-house developed scripts following Jonsson et al. (2005).
Statistical Analyses
Univariate statistics were performed in R (www.r-project.org) using analysis of variance (for more than three groups, bars not connected with letters show statistical significance, and asterisks show statistical difference to 0% control) or t tests (for control versus treatment type data, asterisks show statistical difference), always at 95% confidence level. Multivariate analysis was done using SIMCA-P+ 12 (Umetrics).
Accession Numbers
Sequence data from this article (including the origins of knockout and overexpressing lines analyzed) can be found in the GenBank/EMBL data libraries or in the Arabidopsis Genome Initiative database under the following accession numbers:YUC8 and yuc8, At4g28720; YUC9 and yuc9, At1g04180; CYP79B2 and cyp79B2, At4g39950; CS27941, SALK_113348C; CYP79B3 and cyp79B3, At2g22330; CS413556, GK-142B08-012878; TAA1 and sav3-2, At1g70560; TAR1 and tar1-1, At1g23320; TAR2 and tar2-1, At4g24670; PIF1 and pif1-1, At2g20180; PIF3 and pif3-3, At1g09530; PIF4 and pif4-2, At2g43010; PIF5 and pif5-3, PIF5-OX, At3g59060; HXK1 and gin2-1, At4g29130, N6383; DPE2 and dpe2-3, At2g40840, N6486.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Changes in Osmoticum and Immersion into Liquid Medium Do Not Induce IAA Biosynthesis.
Supplemental Figure 2. Exogenous Glc Rescues the Carbohydrate Balance in Seedlings Incubated in Liquid Medium.
Supplemental Figure 3. Metabolizable Sugars Induce IAA Biosynthesis in Both Root and Shoot Tissues.
Supplemental Figure 4. Mild and Strong Treatments with Glc Create a Similar Response in Gene Expression.
Supplemental Figure 5. IAA Biosynthesis Mutant Lines Do Not Show Uncoupling of IAA Induction from Glc Treatment.
Supplemental Figure 6. Environmental Control of IAA Homeostasis and Growth through the PIF Genes.
Supplemental Table 1. Oligonucleotide Primers Used for Genotyping.
Supplemental Table 2. Oligonucleotide Primers Used for Gene-Specific qPCR.
Acknowledgments
We thank Jose Alonso, Stephan Pollmann, Peter Quail, Yunde Zhao, Christian Fankhauser, Joanne Chory, and Alison Smith for generously sharing seed stocks. We also thank Roger Granbom and Gun Lövdahl for excellent technical assistance. This work was supported by grants from Kempestiftelserna (SMK-2937) and the Swedish Research Council (VR) (621-2010-5720).
AUTHOR CONTRIBUTIONS
I.S., G.S., B.J., and K.L. designed the research. I.S., O.N., and A.P. performed research. O.N. contributed a new analytical tool. Y.I. contributed new genetic material. I.S., O.N., and A.P. analyzed data. I.S., G.S., B.J., and K.L. wrote the article.
Glossary
- IAA
indole-3-acetic acid
- DAG
days after germination
- ANT
anthranilate
- TRP
tryptophan
- CHX
cycloheximide
- ABA
abscisic acid
- IAOx
indole-3-acetaldoxime
- IAM
indole-3-acetamide
- IAN
indole-3-acetonitrile
- IAAld
indole-3-acetaldehyde
- IAAsp
IAA-aspartate
- IAGlu
IAA-glutamate
- Ws
Wassilewskija
- Ler
Landsberg erecta
- Col-0
Columbia 0
- oxIAA
2-oxoindole-3-acetic acid
References
- Bhalerao R.P., Bennett M.J. (2003). The case for morphogens in plants. Nat. Cell Biol. 5: 939–943 [DOI] [PubMed] [Google Scholar]
- Chia T., Thorneycroft D., Chapple A., Messerli G., Chen J., Zeeman S.C., Smith S.M., Smith A.M. (2004). A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J. 37: 853–863 [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Lee S.H., Patel D., Kumar S.V., Spartz A.K., Gu C., Ye S., Yu P., Breen G., Cohen J.D., Wigge P.A., Gray W.M. (2011). Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl. Acad. Sci. USA 108: 20231–20235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freixes S., Thibaud M.-C., Tardieu F., Muller B. (2002). Root elongation and branching is related to local hexose concentration in Arabidopsis thaliana seedlings. Plant Cell Environ. 25: 1357–1366 [Google Scholar]
- Gonzali S., Novi G., Loreti E., Paolicchi F., Poggi A., Alpi A., Perata P. (2005). A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J. 44: 633–645 [DOI] [PubMed] [Google Scholar]
- Gullberg J., Jonsson P., Nordström A., Sjöström M., Moritz T. (2004). Design of experiments: An efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal. Biochem. 331: 283–295 [DOI] [PubMed] [Google Scholar]
- Halliday K.J., Martínez-García J.F., Josse E.M. (2009). Integration of light and auxin signaling. Cold Spring Harb. Perspect. Biol. 1: a001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentrich M. (2010). Moleculare Mechanismen der Octadecanoid-regulierten Indol-3-essigsäyre-Biosynthese. PhD dissertation (Ruhr-University Bochum, Bochum: Germany).
- Hornitschek P., Kohnen M.V., Lorrain S., Rougemont J., Ljung K., López-Vidriero I., Franco-Zorrilla J.M., Solano R., Trevisan M., Pradervand S., Xenarios I., Fankhauser C. (2012). Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 71: 699–711 [DOI] [PubMed] [Google Scholar]
- Jones B., Ljung K. (2012). Subterranean space exploration: The development of root system architecture. Curr. Opin. Plant Biol. 15: 97–102 [DOI] [PubMed] [Google Scholar]
- Jonsson P., Johansson A.I., Gullberg J., Trygg J., A J., Grung B., Marklund S., Sjöström M., Antti H., Moritz T. (2005). High-throughput data analysis for detecting and identifying differences between samples in GC/MS-based metabolomic analyses. Anal. Chem. 77: 5635–5642 [DOI] [PubMed] [Google Scholar]
- Kircher S., Schopfer P. (2012). Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 11217–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClere S., Schmelz E.A., Chourey P.S. (2010). Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol. 153: 306–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Oka Y., Liu T., Carle C., Castillon A., Huq E., Quail P.H. (2008). Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Quail P.H. (2011). PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P., Sheen J. (2003). Sugar and hormone connections. Trends Plant Sci. 8: 110–116 [DOI] [PubMed] [Google Scholar]
- Li P., Bohnert H.J., Grene R. (2007). All about FACE—Plants in a high-[CO2] world. Trends Plant Sci. 12: 87–89 [DOI] [PubMed] [Google Scholar]
- Lilley J.L., Gee C.W., Sairanen I., Ljung K., Nemhauser J.L. (October 16, 2012). An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. doi:10.1104/pp.112.205575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhang Y., Liu R., Hao H., Wang Z., Bi Y. (2011). Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J. Plant Physiol. 168: 1771–1779 [DOI] [PubMed] [Google Scholar]
- Ljung K., Hull A.K., Celenza J., Yamada M., Estelle M., Normanly J., Sandberg G. (2005). Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17: 1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S., Allen T., Duek P.D., Whitelam G.C., Fankhauser C. (2008). Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Mano Y., Nemoto K. (2012). The pathway of auxin biosynthesis in plants. J. Exp. Bot. 63: 2853–2872 [DOI] [PubMed] [Google Scholar]
- Mishra B.S., Singh M., Aggrawal P., Laxmi A. (2009). Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 4: e4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Murano N., Akaike M., Nakamura K. (1997). Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for beta-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J. 11: 841–851 [DOI] [PubMed] [Google Scholar]
- Moore B., Zhou L., Rolland F., Hall Q., Cheng W.-H., Liu Y.-X., Hwang I., Jones T., Sheen J. (2003). Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Normanly J. (2010). Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harb. Perspect. Biol. 2: a001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák O., Hénykova E., Sairanen I., Kowalczyk M., Pospíšil T., Ljung K. (2012). Tissue-specific profiling of the Arabidopsis thaliana auxin metabolome. Plant J. 72: 523–536 [DOI] [PubMed] [Google Scholar]
- Nozue K., Harmer S.L., Maloof J.N. (2011). Genomic analysis of circadian clock-, light-, and growth-correlated genes reveals PHYTOCHROME-INTERACTING FACTOR5 as a modulator of auxin signaling in Arabidopsis. Plant Physiol. 156: 357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östin A., Kowalyczk M., Bhalerao R.P., Sandberg G. (1998). Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol. 118: 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson S.V., Johansson A.I., Kowalczyk M., Makoveychuk A., Wang J.Y., Moritz T., Grebe M., Benfey P.N., Sandberg G., Ljung K. (2009). An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 21: 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J., Friml J. (2009). Auxin transport routes in plant development. Development 136: 2675–2688 [DOI] [PubMed] [Google Scholar]
- Rolland F., Baena-Gonzalez E., Sheen J. (2006). Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Roycewicz P., Malamy J.E. (2012). Dissecting the effects of nitrate, sucrose and osmotic potential on Arabidopsis root and shoot system growth in laboratory assays. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367: 1489–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.M., Stitt M. (2007). Coordination of carbon supply and plant growth. Plant Cell Environ. 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Stavang J.A., Gallego-Bartolomé J., Gómez M.D., Yoshida S., Asami T., Olsen J.E., García-Martínez J.L., Alabadí D., Blázquez M.A. (2009). Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J. 60: 589–601 [DOI] [PubMed] [Google Scholar]
- Stepanova A.N., Robertson-Hoyt J., Yun J., Benavente L.M., Xie D.Y., Dolezal K., Schlereth A., Jürgens G., Alonso J.M. (2008). TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191 [DOI] [PubMed] [Google Scholar]
- Stewart J.L., Maloof J.N., Nemhauser J.L. (2011). PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS ONE 6: e19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Qi L., Li Y., Chu J., Li C. (2012). PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet. 8: e1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Hayashi K., Kinoshita T. (2012). Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 159: 632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H., Sugahara T., Kotake T., Nakagawa N., Sakurai N. (2010). Sugar treatment inhibits IAA-induced expression of endo-1,3:1,4-beta-glucanase EI transcripts in barley coleoptile segments. Physiol. Plant. 139: 413–420 [DOI] [PubMed] [Google Scholar]
- Tao Y., et al. (2008). Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H., Puech L., Fink S., Sundberg B. (1997). A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol. 115: 577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averiging of multiple internal control genes. Genome Biol. 3: RESEARCH0034.1–0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind J., Smeekens S., Hanson J. (2010). Sucrose: Metabolite and signaling molecule. Phytochemistry 71: 1610–1614 [DOI] [PubMed] [Google Scholar]
- Won C., Shen X., Mashiguchi K., Zheng Z., Dai X., Cheng Y., Kasahara H., Kamiya Y., Chory J., Zhao Y. (2011). Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 18518–18523 [DOI] [PMC free article] [PubMed] [Google Scholar]