Abstract
Purpose
Intensity-modulated radiotherapy (IMRT) is increasingly incorporated into therapy for pancreatic cancer. A concern regarding this technique is the potential for geographic miss and decreased local control. We analyzed patterns of first failure among patients treated with IMRT for resected pancreatic cancer.
Methods and Materials
Seventy-one patients who underwent resection and adjuvant chemoradiation for pancreas cancer are included in this report. IMRT was used for all to a median dose of 50.4 Gy. Concurrent chemotherapy was 5-FU–based in 72% of patients and gemcitabine-based in 28%.
Results
At median follow-up of 24 months, 49/71 patients (69%) had failed. The predominant failure pattern was distant metastases in 35/71 patients (49%). The most common site of metastases was the liver. Fourteen patients (19%) developed locoregional failure in the tumor bed alone in 5 patients, regional nodes in 4 patients, and concurrently with metastases in 5 patients. Median overall survival (OS) was 25 months. On univariate analysis, nodal status, margin status, postoperative CA 19-9 level, and weight loss during treatment were predictive for OS. On multivariate analysis, higher postoperative CA19-9 levels predicted for worse OS on a continuous basis (p < 0.01). A trend to worse OS was seen among patients with more weight loss during therapy (p = 0.06). Patients with positive nodes and positive margins also had significantly worse OS (HR for death 2.8, 95% CI 1.1–7.5; HR for death 2.6, 95% CI 1.1–6.2, respectively). Grade 3–4 nausea and vomiting was seen in 8% of patients. Late complication of small bowel obstruction occurred in 4 (6%) patients.
Conclusions
This is the first comprehensive report of patterns of failure among patients treated with adjuvant IMRT for pancreas cancer. IMRT was not associated with an increase in local recurrences in our cohort. These data support the use of IMRT in the recently activated EORTC/US Intergroup/RTOG 0848 adjuvant pancreas trial.
Keywords: pancreatic cancer, chemoradiation, IMRT, patterns of failure
Introduction
Although the use of adjuvant chemoradiation (CRT) in the treatment of resected pancreatic cancer has been one of the most hotly debated topics in gastrointestinal oncology in recent years, the use of this therapy is a widely accepted standard of care in the United States. In addition to debate over whether adjuvant CRT is associated with improved survival in patients with resected pancreatic cancer, questions have been raised about the potential toxicity of adjuvant CRT. Intensity-modulated radiation therapy (IMRT) has been shown to decrease gastrointestinal toxicity by reducing the radiation dose to critical structures in the upper abdomen, including the kidneys, stomach, and small bowel. One concern regarding the utilization of IMRT in this patient population is the possibility for geographic miss secondary to the increased conformality of the IMRT-based treatment plans and consequent higher risk of local failure.
This analysis is an attempt to elucidate more information regarding the natural history of resected pancreatic cancer treated adjuvantly with IMRT-planned concurrent CRT. Specifically, our goal was to determine the patterns of first failure as well as predictors of overall survival in this cohort of patients. Data from two high-volume academic centers (The Johns Hopkins Medical Institutions and the University of Maryland Medical Center) were combined in an attempt to increase the power of the analysis.
Methods and Materials
Patient charts were retrospectively reviewed under a protocol approved by both departments’ internal institutional review boards. All patients provided informed consent for treatment. Patients were evaluated by the treating radiation oncologist at 1 month after completing treatments and at 3-month intervals thereafter. Restaging computed tomography (CT) scans of the chest, abdomen, and pelvis were routinely obtained at 3- to 4-month intervals. The site of first failure was determined by a careful review of these serial scans as well as of the pertinent clinical documentation by the patients’ treating oncologists. Patients who failed simultaneously at more than one site were documented as such. Kaplan-Meier and Cox regression analyses were performed to analyze possible predictors of overall survival. Survival times were calculated from the date a tissue diagnosis of pancreatic adenocarcinoma was established.
A total of 71 consecutive patients, treated between May 2005 and January 2009, are included in this report. Median patient age was 62 years (range, 35–83 years). Surgical resection of a pancreatic tumor was performed before CRT in all patients. The most commonly performed resection was pancreaticoduodenectomy (72%), with negative surgical margins achieved in 68% of patients. Limited vascular resection was performed in selected patients. The majority of patients (68%) had one or more metastatic lymph nodes. Table 1 shows details of patient demographics, surgical findings, and staging. Seven patients (10% of the cohort) had periampullary tumors. All of these patients had involved regional lymph nodes, and in terms of treatment paradigm and target volumes, were managed similarly to patients with pancreatic head primaries.
Table 1.
Patient demographics and staging
| Sex | |
| Male | 37 (52%) |
| Female | 34 (48%) |
| T stage | |
| T1 | 6 (8%) |
| T2 | 13 (18%) |
| T3 | 49 (68%) |
| T4 | 3 (4%) |
| N stage | |
| Node negative | 22 (31%) |
| Node positive | 48 (67%) |
| None sampled | 1 (1%) |
| Tumor site | |
| Head/neck | 48 (68%) |
| Body/tail | 15 (21%) |
| Ampulla | 7 (10%) |
| Treating Institution | |
| JHMI | 43 (61%) |
| UMD | 28 (39%) |
| Surgery | |
| Whipple | 51 (72%) |
| Distal pancreatectomy | 16 (22%) |
| Total pancreatectomy | 4 (6%) |
| Margin status | |
| Negative | 48 (68%) |
| Positive | 23 (32%) |
Abbreviations: JHMI = The Johns Hopkins Medical Institutions; UMD = University of Maryland.
Radiation treatment was given concurrently with either capecitabine (750–825 mg/m2, divided in twice-daily doses and given Monday-Friday with radiation treatment) or gemcitabine (600 mg/m2). Median radiation dose was 50.4 Gy given in 28 1.8-Gy fractions. All patients underwent CT-guided simulation with oral contrast and with IV contrast when diagnostic intravenous (IV) contrast-enhanced scans were not available for fusion. Four-dimensional CT planning techniques were not routinely used for the cohort of patients in this report. Preoperative CT scans were used to aid in the delineation of the tumor bed. Inverse-planned IMRT was used to generate optimized treatment plans for each patient. The IMRT technique used has been described in detail in a prior publication (1). Briefly, treatment volumes were constructed as per the same guidelines used to construct the three-dimensional (3D) fields used in Radiation Therapy Oncology Group (RTOG) 97-04. The initial treatment field was prescribed 45 Gy and was based on a CTV including the resection bed, celiac, peripancreatic, pancreaticoduodenal, porta hepatic, and paraaortic lymph node basins extending from approximately T10 through L3. The major dose-limiting normal tissue structures were the spinal cord, kidneys, liver, and bowel (with specific constraints shown in Table 2). A sequential small field boost encompassed the resection bed (plus a 1–1.5 cm margin) including any areas of positive margin as determined by review with the treating surgeon. The small field boost was prescribed 5.4 Gy in patients with negative margins and 9–14.4 Gy in patients with positive margins.
Table 2.
Normal tissue constraints for intensity-modulated radiotherapy treatment plans
| Organ | Volume (%) | Dose (Gy) |
|---|---|---|
| Ipsilateral kidney | 33 | <18 |
| Contralateral kidney | 66 | <18 |
| Bowel | 5 | <54 |
| 10 | 50 | |
| 15 | <45 | |
| 50 | <20 | |
| Spinal cord | Maximum dose | 45 |
| Liver | 60 | <30 |
Results
At a median follow-up of 24 months, 52 patients (73%) had experienced treatment failure. Three patients (6%) were lost to follow-up. The predominant failure pattern was the development of distant metastases without accompanying local failure. Thirty-seven patients (71% of failures) developed distant metastases, with the majority of metastatic disease (24/37 patients, 65%) developing in the liver. Fourteen patients developed local (tumor bed) or regional (draining lymph nodes) failures. Of these patients, 9 had isolated locoregional recurrences (confined to tumor bed or draining lymph nodes, and 5 had simultaneous locoregional failure and distant metastatic disease. Table 3 provides further details regarding failure patterns.
Table 3.
Patterns of failure
| Failure Site | Number (% of Total Patients) |
|---|---|
| Locoregional | 14 (21%) |
| Local only | 5 (7%) |
| Regional only | 3 (4%) |
| Local and regional | 1 (1%) |
| Locoregional and distant | 5 (7%) |
| Distant metastases | 37 (51%) |
| Liver | 24 (33%) |
| Lung | 9 (12%) |
| Mesentery | 6 (8%) |
| Other | 3 (4%) |
| NED | 17 (24%) |
| Lost to follow-up | 3 (4%) |
Abbreviation: NED = no evidence of disease.
After establishing the pattern of failure, treatment plans of all the patients with local failure were reviewed to determine whether the recurrences occurred within the original target volume. Two patients had nodal recurrences that occurred outside the original planning target volume (PTV). One of these patients had a pancreatic head tumor with an isolated nodal recurrence in the lower para-aortic region. The other patient had a pancreatic tail mass and developed a nodal recurrence in the mid-medial porta hepatis, which had not been included in the original target volume. Two other patients had local failures at the margins of the original PTV. Of the 10 remaining patients with local or regional failures, all occurred within the 45-Gy line of the original treatment plan.
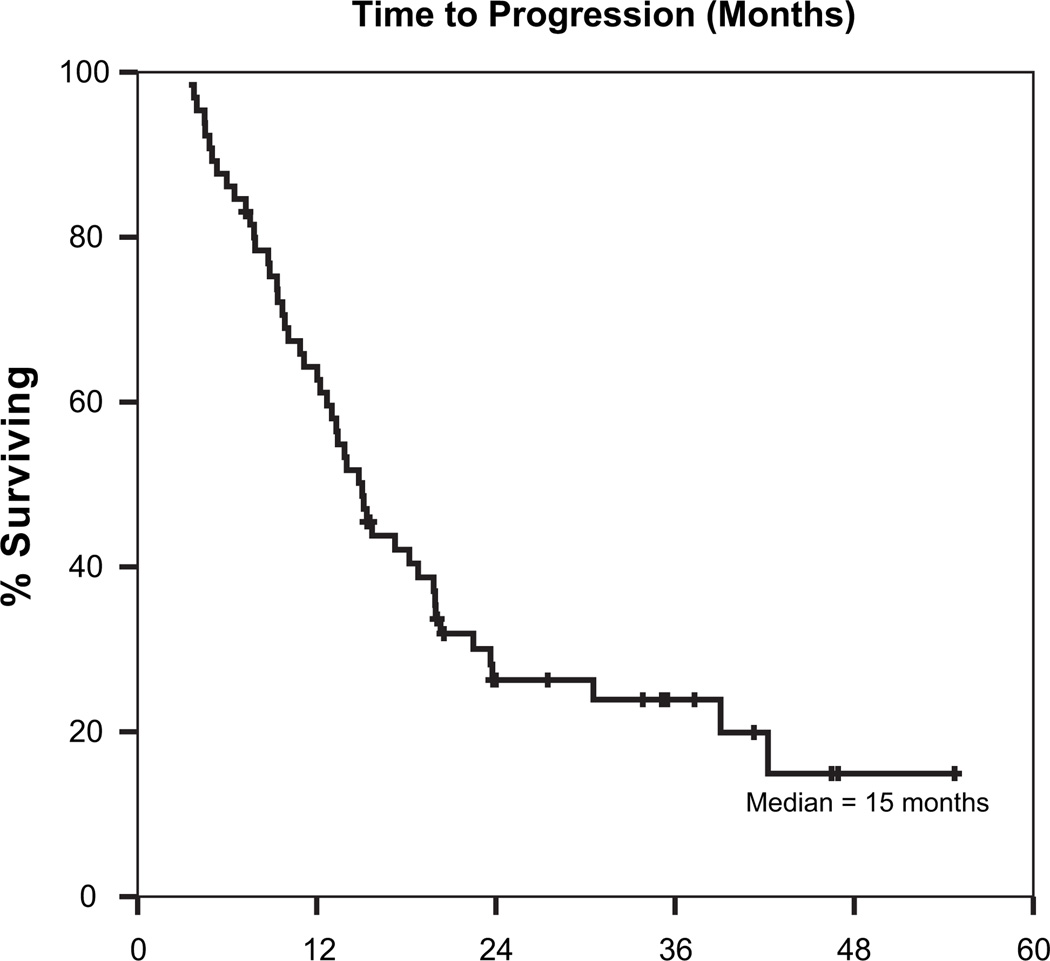
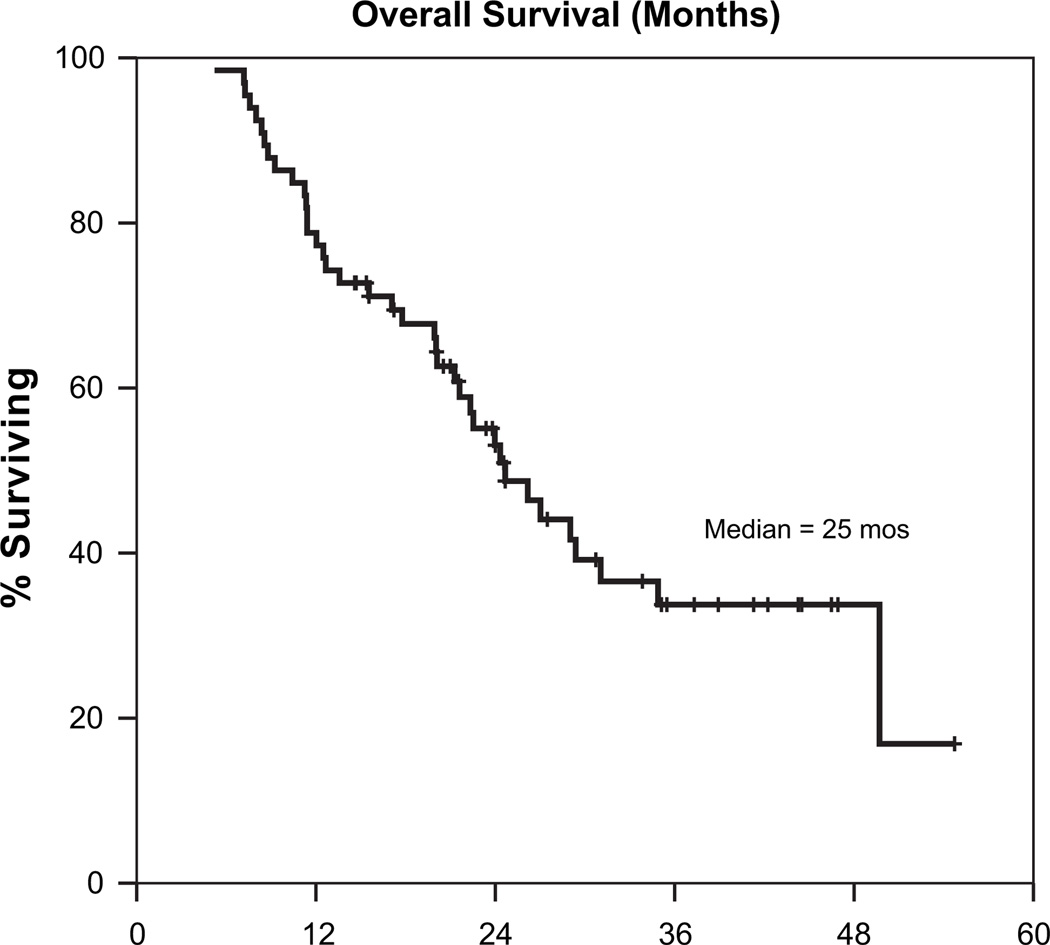
The median time to tumor progression was 15 months, and the median overall survival was 25 months. Figures 1 and 2 are Kaplan-Meier curves depicting progression-free and overall survival (respectively) for the entire cohort of patients. On univariate analyses, margin status, nodal stage, and postoperative CA19-9 levels were all predictive of overall survival. Table 4 shows details of overall survival by margin status, nodal stage, and post-operative CA19-9. On multivariate analysis using a Cox regression model, postoperative CA19-9 levels were strongly predictive for overall survival on a continuous basis (p = 0.005). Additionally, patients with positive margins had a higher risk of death (HR 2.6, 95% CI 1.1–6.2), as did those with nodal involvement (HR 2.8, 95% CI 1.1–7.5). A trend toward worse survival was also noted among patients with a greater degree of weight loss during adjuvant chemoradiation (on a continuous basis, p = 0.06).
Fig. 1.
Time to progression (months), all patients.
Fig. 2.
Overall survival (months) all patients.
Table 4.
Univariate predictors of overall survival
| Clinical Factor | Median Overall Survival (Months) |
p |
|---|---|---|
| Margin status | ||
| Positive | 14 | 0.001 |
| Negative | 29 | |
| Postoperative CA19-9 | ||
| >90 | 11 | <0.001 |
| <90 | 29 | |
| Nodal status | ||
| Node positive | 22 | 0.007 |
| Node negative | 49 |
In terms of toxicity, treatment was overall well-tolerated. Median weight loss during therapy was 3.5%. Nausea/vomiting was limited to grade 1 or 2 in 70% of patients; Grade 3 nausea/vomiting occurred in 8% of patients. Grade 2 diarrhea was noted in 21𰀥 of patients; no patient had worse than grade 2 diarrhea. Five patients (7%) developed late toxicity of either small bowel obstruction or fistula.
Discussion
This series is the first to provide a comprehensive assessment of the patterns of first failure in patients with resected pancreatic cancer undergoing IMRT-planned concurrent chemoradiation. Our previous work in IMRT for pancreatic cancer focused on evaluating the toxicity profile of this therapy and demonstrated that IMRT-planned treatments were associated with improved acute toxicity profiles. As institutional experience grew and follow-up times lengthened, it seemed logical to evaluate whether or not IMRT-planned adjuvant radiation was associated with any change in the natural history of the disease. In this report, no association between the use of IMRT for radiation therapy planning and an increased local failure rate was observed.
Distant metastases dominate the previously documented failure patterns in most reports of prospective adjuvant therapy in pancreatic cancer. Our results are concordant with these prior publications that demonstrate distant metastatic disease as the primary pattern of failure in this patient population. In the most modern series investigating adjuvant CRT (RTOG 9704), locoregional disease was a component of treatment failure in 33% of patients; >70𰀥 of patients in both the 5-FU (control) and gemcitabine (experimental) groups developed distant metastases as a component of first failure (2). The control group of the CONKO-001 trial, which was assigned to observation after resection, experienced distant metastasis rates of 49% and local failure rates of 41% as compared with 56% and 34% in the treatment (chemotherapy with gemcitabine but not radiation) group (3).
Nonrandomized, institutional data examining the role of adjuvant chemoradiation in the treatment of pancreatic cancer is also available. Hattangadi et al. reported on a series of 86 patients treated at Massachusetts General Hospital with external beam radiation and concurrent continuous-infusion 5-FU (4). Slightly less than half the patients (43%) received gemcitabine after completing concurrent CRT. The median overall survival in this group was 22 months, with a 3-year distant metastasis rate of 87%. The five-year rate of locoregional failure was 36%. Combined data from the Mayo Clinic and Johns Hopkins Hospital showed a 5-year overall survival of 22.3% and median overall survival of 21.1 months in a group of 583 patients with resected pancreatic cancer who received adjuvant CRT (5). Patterns of failure were not reported in this series. Data from the individual institutions are also available, as summarized in Table 5 along with the results of the recent large randomized clinical trials described previously).
Table 5.
Summary of recent adjuvant clinical trial results in pancreatic cancer
| Institution/Author | n | Median/5-year OS | Local Failure | Metastatic Disease |
|---|---|---|---|---|
| RTOG 9704 Regine et al. | 451 | 20.5 months/22% (gemcitabine arm) 17.2 months/18% (5-FU arm) | 28% (all patients) | 73% (all patients) |
| CONKO-001 Oettle et al. | 368 | 22.1 months/23% (gemcitabine arm) 20.2 months/11.5% (observation arm) | 34% (gemcitabine) 41% (observation) | 56% (gemcitabine) 49% (observation) |
| Johns Hopkins Herman et al. (9) | 271 | 21.2 months/20.1% | Not reported | |
| Mayo Clinic Miller et al. (10) | 269 | 25.2 months/28% | Not reported | |
Abbreviations: OS = overall survival; RTOG = Radiation Therapy Oncology Group.
Two reports evaluating patterns of failure in small cohorts of patients receiving adjuvant IMRT-based CRT have been published. These series are somewhat limited because of small numbers of patients and short follow-up. One series (Ben-Josef et al.) reported a single local failure among 7 patients (14%) with a median follow up of 8.5 months (6). A second series (Milano et al.) reported no local failures among eight patients at a median follow-up of 10 months (7). With much longer median follow-up times, the results in this report show local control rates that compare quite favorably to previously published prospective trials. Table 4 summarizes the survival outcomes and patterns of failure (if available) from several of the most recently updated series. The current RTOG 0848/US Intergroup trial will provide an opportunity to validate this finding in a prospective setting.
One commonly voiced concern regarding the utilization of IMRT in pancreatic cancer, particularly in the postoperative setting, is the potential for geographic miss and worse local control. This arises from two sources: 1) targeting uncertainty and 2) increasingly conformal treatment plans, resulting in a suboptimal dose distribution to areas that might have received higher doses with the use of 3D conformal plans. In RTOG 9704, which used 3D conformal treatment planning, post hoc quality assurance analysis showed that 48% of treatment plans had one or more protocol deviations. More important, patients treated according to protocol had improved survival. These results clearly show that differences in planning techniques and targeting may significantly affect clinical outcomes, especially when highly conformal IMRT plans are used. For example, if a treating physician chose not to include draining nodal stations in the initial PTV, local control rates could be quite different than those reported here. We designed a uniform approach to treatment planning in an attempt to address this concern. Preoperative imaging was routinely integrated into the simulation process; additionally, treatment plans were developed in close consultation with experienced pancreatic surgeons who consistently marked tumor beds and areas of positive margins with radiopaque clips to assist in the treatment planning process. Furthermore, the targeted treatment area was designed to encompass the same nodal drainage fields as conventional 3D conformal radiation plans.
Organ motion within the abdomen may also have a significant impact on RT planning. In a recent analysis, Feng et al. described greater than expected motion, particularly in the craniocaudal plane, of unresected pancreatic tumors visualized on cine-MRI (8). This series reported a mean motion of 2 cm in both the superior and inferior directions. Based on this and other data, we have begun to routinely incorporate four-dimensional CT simulation into our IMRT planning techniques. In this series, we noted 2 patients with failures at the margins of the original PTV. We plan further analysis which will integrate data acquired from four-dimensional CT scans into an analysis of local failures relative to treated PTVs in the abdomen.
Despite the relatively low rates of local recurrence in this series, the development of distant metastases remained a significant clinical problem. Future directions in research include the integration of novel targeted agents such as erlotinib into treatment protocols, as well as the employment of radiation dose-escalation strategies such as stereotactic body radiation, in an attempt to improve the cure rate of this devastating disease.
Conclusion
The data summarized in this report do not substantiate the concern of worsening local control associated with IMRT-planned radiation therapy and support the use of IMRT in both the clinical trial setting and for routine adjuvant treatment of resected pancreatic cancer. Differences in planning techniques and target choice, as well as organ motion, may introduce significant variability in dose distribution within the abdomen and affect tumor control rates.
Summary.
IMRT is increasingly used for radiation treatment in pancreatic cancer but concerns exist regarding the potential for geographic miss. This study evaluated patterns of failure among patients treated with postoperative chemotherapy and IMRT-planned RT at two high-volume academic institutions. The loco-regional failure and metastatic rates were comparable to published 3D-CRT. The use of IMRT does not, therefore, appear to increase the risk of local failure in the post-operative scenario.
Footnotes
Conflict of interest: none.
References
- 1.Yovino S, Poppe M, Jabbour S, David V, Garofalo M, Pandya N, Alexander HR, Hanna N, Regine WF. Intensity-modulated radiation therapy significantly improves acute gastrointestinal toxicity in pancreatic and ampullary cancers. Int J Radiat Oncol Biol Phys. 2011 Jan 1;79(1):158–162. doi: 10.1016/j.ijrobp.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regine WF, Winter K, Abrams R, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 4.Hattangadi J, Hong T, Yeap B, et al. Results and patterns of failure in patients treated with adjuvant combined chemoradiation for resected pancreatic cancer. Cancer. 2009;115:3640–3650. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: The Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Milano M, Chmura S, Garofalo M, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: Toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445–453. doi: 10.1016/j.ijrobp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Feng M, Balter J, Normolle D. Characterization of pancreatic tumor motion using cine-MRI: Surrogates for tumor position should be used with caution. Int J Radiat Oncol Biol Phys. 2009;74:884–891. doi: 10.1016/j.ijrobp.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman JM, Swartz M, Hsu CC, et al. Analysis of fluorouracil-based chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: Results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller R, Iott M, Corsini M. Review of adjuvant radiochemotherapy for resected pancreatic cancer and results from Mayo Clinic for the 5th JUCTS symposium. Int J Radiat Oncol Biol Phys. 2009;75:364–368. doi: 10.1016/j.ijrobp.2008.11.069. [DOI] [PubMed] [Google Scholar]