Abstract
To maintain homeostasis, hypothalamic neurons in the arcuate nucleus must dynamically sense and integrate a multitude of peripheral signals. Blood-borne molecules must therefore be able to circumvent the tightly sealed vasculature of the blood–brain barrier to rapidly access their target neurons. However, how information encoded by circulating appetite-modifying hormones is conveyed to central hypothalamic neurons remains largely unexplored. Using in vivo multiphoton microscopy together with fluorescently labeled ligands, we demonstrate that circulating ghrelin, a versatile regulator of energy expenditure and feeding behavior, rapidly binds neurons in the vicinity of fenestrated capillaries, and that the number of labeled cell bodies varies with feeding status. Thus, by virtue of its vascular connections, the hypothalamus is able to directly sense peripheral signals, modifying energy status accordingly.
Keywords: hormone diffusion, in vivo imaging, median eminence, metabolism
Continuous integration of peripheral signals by neurons belonging to the arcuate nucleus of the hypothalamus (ARH) is critical for central regulation of energy balance and neuroendocrine function (1). To dynamically report alterations to homeostasis and ensure an appropriate neuronal response, blood-borne factors such as hormones must rapidly access the central nervous system (CNS). This is particularly evident in the case of food intake, which is regulated by a plethora of circulating satiety signals (2) whose levels fluctuate in an ultradian manner. Despite this, it remains unclear how key energy status-signaling hormones such as ghrelin can be rapidly sensed by target neurons to alter feeding responses (3). Elucidation of the mechanisms underlying molecule entry into the brain is important for understanding not only normal maintenance of homeostasis but also how this is perturbed during common pathologies such as obesity and diabetes (4, 5).
Although molecule transport mechanisms within the ARH are poorly characterized, they likely assume one of two forms. First, chronic feedback may be accomplished by uptake of circulating molecules into the ARH via saturable receptor-mediated transport at the level of the choroid plexus and/or blood–brain barrier (BBB) (6–9). Second, the ARH is morphologically located in close apposition to the median eminence (ME), a circumventricular organ composed of fenestrated capillaries. Because these vessels project toward the ventromedial ARH (vmARH), they could represent a direct vascular input for passive diffusion of peripheral molecules into the hypothalamus (10–13). So far, study of the functional importance of fenestrated capillaries in molecule entry into the metabolic brain has been impeded by lack of appropriate tools.
To evaluate the role of fenestrated ME/ARH capillaries in rapid detection of peripheral signals by the hypothalamus, we used a recently developed in vivo imaging approach to visualize in real time the extravasation of fluorescent molecules (14). Ghrelin was chosen as a candidate hormone because its acute effects upon feeding behavior (15), together with a short circulating half-life, demand the presence of rapid and precise sensing mechanisms. Although ghrelin’s orexigenic effects on hypothalamic feeding centers are well documented (16, 17), it remains unclear how peripherally secreted hormone accesses this BBB-protected site, as a specialized transport system from the circulation to the brain is yet to be identified (18). Here, we show that circulating fluorescently labeled ghrelin diffuses through fenestrated capillaries of the ME, which project to the vmARH before rapidly binding nearby neuropeptide Y (NPY)- and proopiomelanocortin (POMC)-expressing neurons, the two functionally opposing neuron populations implicated in regulation of food intake in the ARH. Thus, our data support a role for ARH-residing neurons in eliciting ghrelin’s effects on feeding behavior through direct and rapid sensing of circulating ghrelin. Furthermore, we demonstrate that this process is inherently plastic as it can be manipulated in a nutrient-dependent manner by simply using a controlled fasting–refeeding paradigm. As such, hypothalamic neurons are able to monitor peripheral energy balance directly through their vascular inputs, allowing rapid organismal adaptation to prevailing metabolic state.
Results
In Vivo Permeability of Fenestrated Vessels in the Median Eminence.
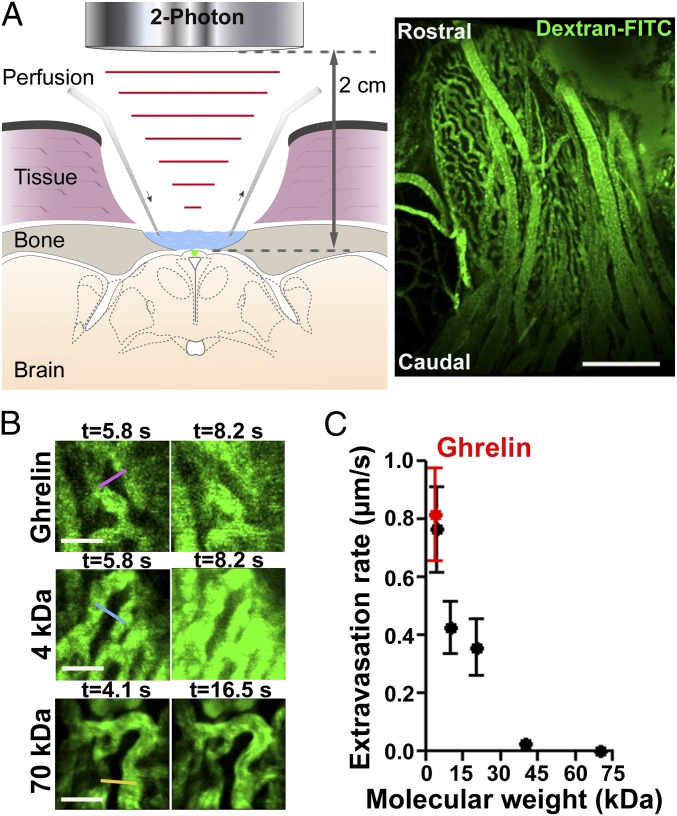
To access deep structures on the ventral surface of the brain and directly visualize vessels of the ME in vivo, surgical approaches developed for functional imaging of the pituitary (14) were combined with a multiphoton microscope adapted with long-working distance (2 cm) objectives (Fig. 1A). A coronal view of the ME and the ARH is schematized in Fig. 1A (Left), and a representative image of the median eminence vasculature (ventral view) is shown in Fig. 1A (Right). Fluorescence intensity variations in the ME parenchyma were recorded in vivo following i.v. injection of fluorescent dextrans (Fig. 1B and Movie S1), and a transient increase in fluorescence intensity could be detected in the parenchyma when molecules were able to diffuse through the capillaries (Fig. 1B). Fluorescence intensity variations over time postinjection in selected regions of interest were measured (Fig. 1B, color bars), allowing calculation of extravasation rates across fenestrated capillaries for each fluorescent molecule (Fig. 1C). Molecule size cutoff was below 70 kDa, with a step-like decrease in permeability rate between 20 and 40 kDa (Fig. 1C) (n = 4–16 movies from three to eight animals per molecule).
Fig. 1.
In vivo extravasation of molecules through fenestrated vessels in the median eminence (ME). (A) Schematic representation of the imaging setup (Left), and representative image of the ME vasculature acquired in vivo (Right). Z-projection of a 100-µm stack. Green, The 150-kDa dextran-FITC. (Scale bar: 130 µm.) (B) Fluorescence variation in the ME parenchyma at two time points after i.v. injection of fluorescent ghrelin (Top), 4-kDa dextran (Middle), and 70-kDa dextran (Bottom). Fluorescence variations were measured in regions of interest (color bars). (Scale bar: 20 µm.) Green, FITC. (C) Molecule extravasation rate in vivo as a function of molecular weight (mean ± SEM, n = 4–16 movies from 3 to 8 animals per molecule).
To investigate the transfer rate of ghrelin (3.3 kDa), a recently developed bioactive fluorescently labeled ghrelin derivative (3.8 kDa), capable of specifically binding and activating growth hormone secretagogue receptor-1a (GHS-R-1a) (19, 20), was used. The extravasation rate of fluorescent ghrelin across the ME capillary barrier was comparable to that of 4-kDa FITC-conjugated dextran (∼0.8 µm/s) (Fig. 1C and Movie S2) (n = 8 movies from three animals), suggesting that ghrelin extravasates passively but rapidly (second range) through fenestrated capillary branches of the ME, which are known to project to the vmARH (Fig. 2A, asterisk) (12, 13).
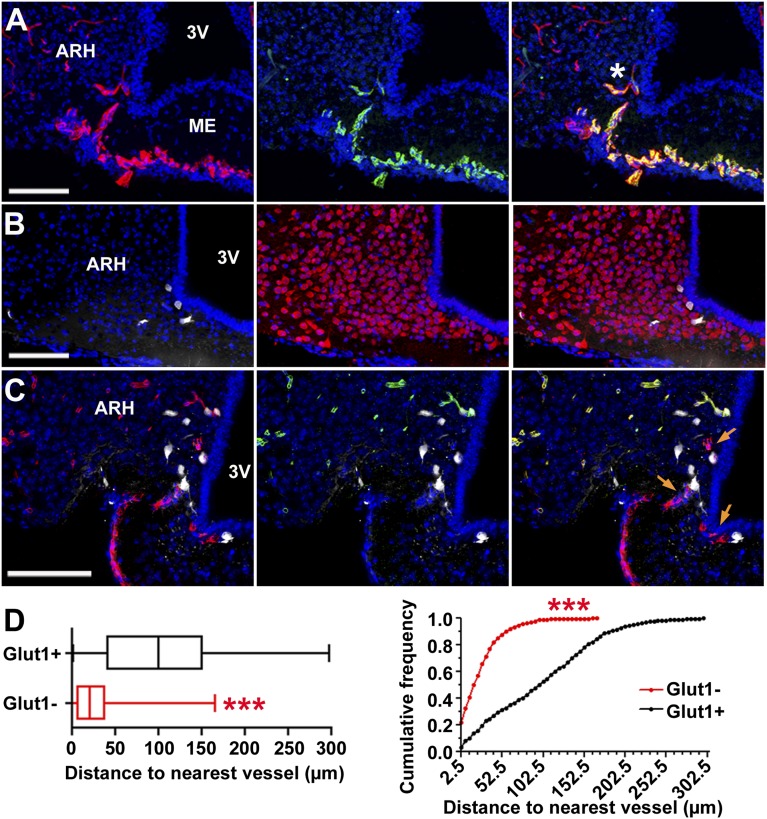
Fig. 2.
Fluorescent ghrelin binds to neurons in the ME/ARH region in proximity to BBB-free vessels. (A–C) Confocal images of brain frozen sections 5–10 min after i.v. injection of fluorescent ghrelin (B and C). (Scale bar: 100 µm.) (A–C) Blue, Nuclei (Hoechst). (A) Capillaries (rhodamine-lectin, red) in the ME/ARH express the fenestration marker MECA-32 (green) and project to the ARH (asterisk). (B) Fluorescent ghrelin (white) labels hypothalamic neurons (HuC/D, red). (C) Ghrelin-labeled neurons (white) are primarily located in the vicinity of vessels (CD31, red) (orange arrows) not expressing the blood–brain barrier marker Glut1 (green). (D) Distances between ghrelin-labeled neurons and nearest Glut1-negative or Glut1-positive vessel (>600 neurons, n = 4 animals). Wilcoxon rank-sum test of medians (Left) and Kolmogorov–Smirnov analysis of cumulative frequency distributions (bin size, 2.5 µm) (Right) indicate ghrelin-labeled neurons are in closer vicinity to Glut1-negative vessels (***P < 0.001).
Ghrelin Rapidly and Specifically Binds to Neurons in the Vicinity of Fenestrated Capillaries in the ARH.
We assessed rapid peptide binding in the vicinity of fenestrated capillaries projecting deeper within the ARH (Fig. 2A, asterisk), by injecting a bolus of bioactive fluorescent ghrelin i.v. before euthanizing animals 5–10 min later. Fluorescent ghrelin binding was localized to cell bodies in both the ME and vmARH, corresponding to hypothalamic HuC/D-positive neurons (100%; n = 18 slices from three animals) (Fig. 2B). Intracellular labeling was consistent with rapid internalization of GHS-R-1a bound to fluorescent ghrelin (21). Supporting a functional role of fenestrated capillaries in fast hormone entry into the ARH was the observation that ghrelin-labeled neurons were located significantly closer to capillary branches, which did not express the BBB marker Glut1 compared with BBB-protected Glut1-positive vessels (26.6 ± 1.6 vs. 100.8 ± 2.9 µm, mean ± SEM, 123–250 neurons per animal, n = 4 animals) (Fig. 2 C and D). No ghrelin-labeled cells could be detected under the same conditions in other BBB-protected areas of the brain, such as the CA3 and CA1 regions (n = 3 animals), which densely express GHS-R-1a (22).
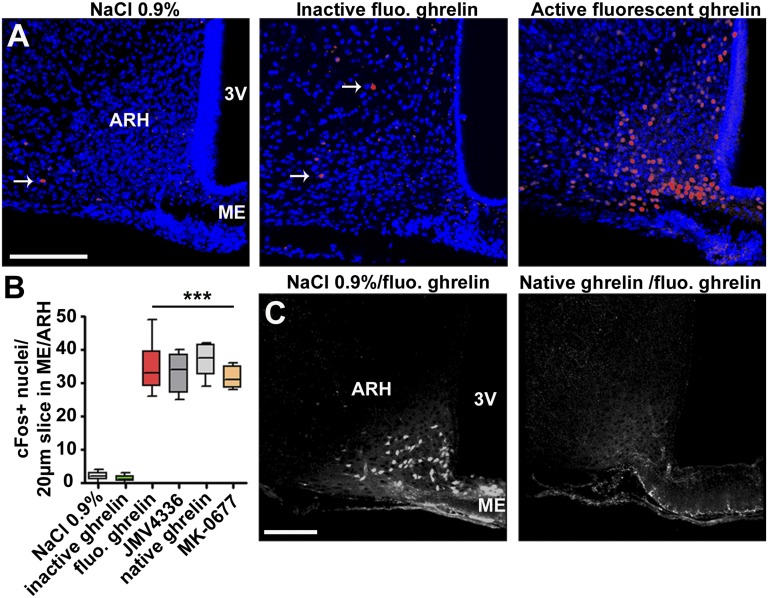
Because expression levels of the immediate-early genes, c-fos, are up-regulated in the ARH following systemic ghrelin administration (17), the induction of Fos protein was measured in hypothalamic neurons following in vivo treatment with tagged hormone (Fig. 3A). Two hours after fluorescent ghrelin injection, an increase in c-Fos expression was observed in the ARH, comparable with that obtained following i.v. injection of native rat/mouse ghrelin and GHS-R-1a agonists JMV4336 and MK-0677 (23, 24) (Fig. 3 A and B; n = 3 animals per condition). Specificity of fluorescent ghrelin binding was assessed by competition experiments using pretreatment with excess native hormone or GHS-R-1a agonist; complete displacement of fluorescent ghrelin from neurons could be achieved (Fig. 3C; n = 3 animals per condition). In addition, i.v. injection of 25 nmol of either fixable 3-kDa rhodamine-labeled dextran or inactive FITC-labeled ghrelin before killing failed to label any cells in the ME/ARH region (n = 3 animals per condition).
Fig. 3.
Fluorescent ghrelin activity and binding specificity. (A and C) Representative confocal images of coronal sections of the ME/ARH region. Twenty-micrometer z-projections are shown. (Scale bar: 150 µm.) (A) Fluorescent bioactive ghrelin induces c-Fos expression in the ARH. Images of brain slices from mice killed 2 h after i.v. injection of 0.9% NaCl (Left), 25 nmol of inactive fluorescent ghrelin (Center), or 25 nmol of active fluorescent ghrelin (Right). Blue, Hoechst, red, c-Fos. The white arrows indicate c-Fos–positive nuclei. (B) Quantification of the number of c-Fos–positive nuclei in the ARH per 20-µm-thick slice following treatment with different GHS-R-1a agonists. All active agonists induced c-Fos in a significant manner compared with saline or inactive ghrelin (one-way ANOVA, ***P < 0.001, n = 12–30 slices from three animals per condition). (C) Competition experiment. Injection (i.v.) of commercial ghrelin (rat, mouse) 15 min before i.v. injection of active fluorescent ghrelin prevented labeling of cell bodies in the ARH (right panel vs. control left panel) (n = 3 animals per condition). White, active fluorescent ghrelin.
Ghrelin Labels Primarily Appetite-Modifying Neurons in a Metabolic State-Dependent Manner.
To determine specificity of fluorescent ghrelin binding in known ghrelin-responsive neuron populations, we used NPY-eGFP transgenic mice (21) and performed immunostaining for β-endorphin, a marker of POMC-neurons (25) (Fig. S1A). Of the 340 ± 19 (mean ± SEM) ghrelin-labeled neurons detected in the ARH (1,200 µm in length; divided into 24 slices, 50 µm thick) (n = 11 animals, fed on standard chow), 35 ± 4% and 41 ± 3% corresponded to NPY- and β-endorphin–expressing neurons, respectively (n = 4 animals per group) (Fig. S1B). Of note, only a small proportion of the total NPY- and β-endorphin–expressing neuron population was labeled (up to 3%, n = 4 animals/group), suggesting that only a subset of neurons was targeted by ghrelin.
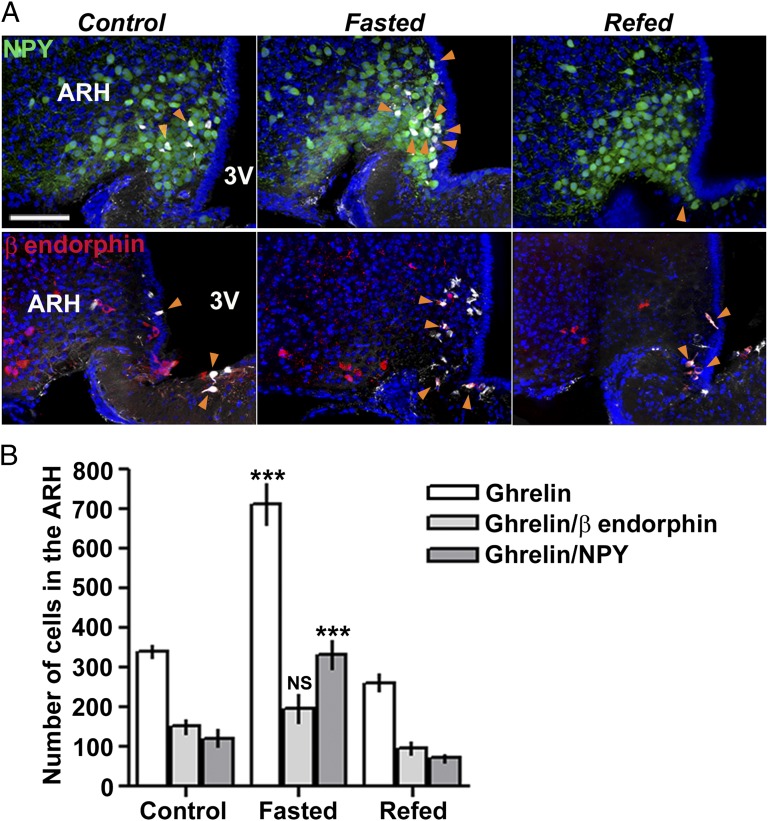
Finally, we investigated whether feeding status of the animals could alter fluorescent ghrelin binding, by subjecting animals to a controlled fasting–refeeding schedule. A significant increase in numbers of both total and NPY fluorescent ghrelin-positive neurons was detected in 24-h–starved animals, and this could be reversed by refeeding the mice over a 24-h period (Fig. 4) (n = 11 and 4 animals, respectively). By contrast, the number of ghrelin-labeled POMC neurons did not significantly vary (Fig. 4) (n = 4 animals).
Fig. 4.
Fluorescent ghrelin labels NPY but not β-endorphin–expressing neurons in a metabolic state-dependent manner. (A) Confocal images of ME/ARH region following i.v. injection of fluorescent ghrelin; NPY-eGFP transgenic mouse (Top) (green, GFP) and β-endorphin immunostaining (Bottom) (red). The orange arrowheads indicate ghrelin-labeled (white) NPY (top) or β-endorphin (bottom) neurons under control conditions (Left), following 24-h fasting (Center), and 24-h fasting plus 24-h refeeding (Right). (Scale bar: 100 µm.) (B) Quantification of ghrelin-labeled neurons in the whole ME/ARH region under control conditions, following 24-h fasting, and 24-h fasting plus 24-h refeeding (15–24 slices per animal, n = 4–11 animals per condition). Numbers were normalized to correspond to total ME length (1,200 µm; 24 slices, 50 µm thick). Fasting induced a significant increase in both total number and number of NPY ghrelin-labeled neurons, which was reversed by refeeding (one-way ANOVA, ***P < 0.001), whereas fasting had no significant effect on the total number of ghrelin-labeled β-endorphin–expressing neurons (one-way ANOVA, P > 0.05).
Discussion
The regulation of a variety of homeostatic functions depends upon central integration of peripheral feedback signals. Brain–periphery cross talk therefore requires tight control of molecule entry and/or dynamic sensing to adapt responses to physiological state. With obesity and diabetes reaching epidemic proportions, research efforts have focused on understanding how hormones such as ghrelin, insulin, or leptin functionally target appetite-regulating neurons of the hypothalamus. However, the fundamental mechanisms by which peripherally secreted hormones access hypothalamic neurons remain poorly characterized. By using tractable bioactive ghrelin together with in vivo multiphoton imaging techniques, we have uncovered a functional role for fenestrated vessels of the ME/ARH in rapid blood-borne molecule sensing by the metabolic brain. It is anticipated that these findings will not only be valuable for understanding the basic mechanisms underlying appetite regulation but can also be extended to a range of other homeostatic functions that rely on integration of peripheral signals by the CNS.
Fenestrated capillaries of the ME possess pores of 50–80 nm in diameter, encompassed by a permeable diaphragm composed of radial fibrils (26, 27). Passive molecule diffusion through fenestrae can be both size and charge limited (28), and it is reasonable to assume that the ME displays similar selectivity. To study passive diffusion of molecules and exclude other potential rate-limited transport mechanisms, it is necessary to follow the time course of events at the blood–brain interface in real time. Using our approaches, we were able to show that relatively small circulating molecules (≤40 kDa in size) could freely and rapidly diffuse through fenestrated capillaries of the ME, a finding with implications for the study of non–receptor-dependent mechanisms of hormone and drug uptake into the CNS. Furthermore, as saturable transporters are modulated by physiological status and pathological conditions (29, 30), modifications in vessel permeability could constitute yet another level of regulation for molecule diffusion into the metabolic brain.
Because diffusion dynamics for inert sugars may differ from those for bioactive hormones, we investigated the diffusion of functional fluorescently labeled ghrelin, a pleiotropic hormone for which mechanism of entry into the ARH remains elusive (31). Our results demonstrate that ghrelin also crosses fenestrated capillaries in the ME through passive diffusion. Although we cannot exclude a role for additional receptor-mediated transport processes in the ARH, no specific ghrelin uptake mechanisms could be identified in the murine BBB (18), and the rate of transport of ghrelin across the BBB was much lower when studied by brain perfusion vs. i.v. injection (32). Although systemic administration of ghrelin has previously been shown to induce hypothalamic c-fos expression (17), the current studies demonstrate sensing of hormone by ARH neurons over timescales (5 vs. 30 min) that are more compatible with the acute orexigenic effects of ghrelin on food intake (15). In addition, the close proximity of ghrelin-labeled neurons to fenestrated capillaries projecting from the ME into the ARH supports an important functional role for this vascular route in rapid molecule entry into the metabolic brain. In accordance with previous observations (22), the absence of labeling in BBB-protected areas distant from circumventricular organs suggests that direct ghrelin effects in other brain regions involved in nonhomeostatic feeding behaviors (33–35) may be mediated through either indirect actions (e.g., via neuronal relays) or slower transport mechanisms. Given that ME/ARH fenestrated capillaries are permissive for molecules as large as 20–40 kDa, it is not unfeasible that other key hormones involved in energy homeostasis such as leptin (16 kDa), insulin (5.8 kDa), or GLP-1 (4.1 kDa) might also use this vascular route to access metabolic-sensing neurons.
Reflecting its role in food intake regulation, fluorescent ghrelin primarily bound appetite-modifying NPY and POMC neurons, and cytoplasmic labeling was consistent with the rapid internalization of a receptor–hormone complex (21). Although nearly all NPY neurons express GHS-R-1a receptors (36), and one-third up-regulate c-fos mRNA expression following GHS-R-1a agonist treatment (37), we found that numbers of ghrelin-labeled NPY neurons were relatively small (∼3% of the population). The different treatment timescales, marked species divergence in GHS-R-1a expression (rat vs. mouse) (38), and detection methods used (in situ hybridization vs. fluorescence) could explain the lower proportion of detected NPY neurons. These numbers were nonetheless within the range of that recently shown to be sufficient to stimulate food intake (∼300 neurons and above) (39). By contrast, labeling of POMC neurons was more surprising given that ghrelin is generally acknowledged to indirectly target this population via GHS-R-1a–expressing presynaptic NPY neurons (25, 40). Together with ex vivo studies demonstrating direct effects of ghrelin on POMC neuron electrical activity (25), our results clearly suggest that POMC neurons may be a direct target for ghrelin in vivo.
Feeding status dynamically regulated the number of ghrelin-labeled neurons. Although the number of POMC ghrelin-labeled neurons remained unchanged, as would be expected from a neuronal population that displays minimal activity in the fasted state (41), we observed a reversible increase in the number of NPY ghrelin-labeled neurons following 24-h fasting. These results appear to be consistent with the physiological necessity to secure robust feeding responses following a fasting period, the direct correlation between quantity of ingested food and number of stimulated NPY neurons (39), the increase in GHS-R mRNA expression in the ARH following short-term fasting (42, 43), and the regulation by nutritional state of the effects of ghrelin on Fos protein expression in the ARH (17, 44). The unique vmARH milieu irrigated by fenestrated capillaries may therefore not only provide a niche for neurogenic tanycytes (45) but may also support a population of “scout neurons” that are able to rapidly sense peripheral signals and coordinate more global responses.
In summary, we have demonstrated that (i) circulating hormones such as ghrelin can freely and rapidly diffuse through fenestrated capillaries of the ME and into their site of action within the ventromedial ARH; (ii) hypothalamic neurons may play an important role in eliciting ghrelin’s effects on feeding behavior through direct and rapid sensing of circulating ghrelin levels; (iii) the number of labeled NPY neurons is compatible with that recently shown to underlie acute ghrelin effects on food intake (39); and (iv) feeding status modifies the capacity of NPY neurons to bind ghrelin. The use of in vivo imaging in combination with the development of fluorescently labeled ligands that retain biological activity could therefore help unveil access routes and in vivo neuronal targets not only for endogenous molecules but also for novel appetite-modifying drugs intended for weight management.
Materials and Methods
A brief outline is provided; for full details, see SI Materials and Methods.
Mice and in Vivo Surgery.
C57BL/6 mice were purchased from Janvier-SAS. NPY-eGFP mice (46) on a C57BL/6 background were sourced from The Jackson Laboratory. In all experiments, 7- to 15-wk-old male mice were used. All animal studies complied with the animal welfare guidelines of the European Community and were approved by the Direction of Veterinary Departments of Hérault and Nord, France (59-350134), and the Languedoc Roussillon Institutional Animal Care and Use Committees (CE-LR-0818). The ME was exposed using a surgery approach previously described for in vivo studies of the pituitary gland (14).
Long-Working Distance Multiphoton Imaging of Fluorescent Molecule Extravasation in Vivo.
Imaging of fluorescent molecule extravasation in the ME’s parenchyma in vivo was performed using a multiphoton microscope (Zeiss 7MP) adapted with a long-working distance objective M Plan Apo NIR 20×, 0.4 NA, 2.0-cm WD (Mitutoyo). Fluorescently labeled molecules were injected through an indwelling jugular catheter. Recordings in areas rich in tortuous vessels at the level of the ME and at a depth between 15 and 40 µm below the meninges, were started at the time of injection. Details of extravasation rates calculation are provided in SI Materials and Methods.
Fluorescent Hormones Synthesis and Binding in Vivo.
Both bioactive and inactive fluorescent ghrelin derivatives were developed by Cisbio Bioassays in collaboration with the Institut des Biomolécules Max Mousseron (Montpellier, France) (19). Active fluorescent ghrelin (25 nmol per animal) was injected i.v. into the tail vein or into the jugular vein under ketamine/xylazine anesthesia, and animals were killed 5–10 min postinjection, to assess in vivo binding. To test the influence of metabolic state on ghrelin binding, active fluorescent ghrelin (25 nmol) was injected in either wild-type or NPY-eGFP mice (46) fed on standard chow (RM3; Special Diet Services), after 24 h of fasting, or after 24 h of fasting followed by a 24-h refeeding period, and animals were killed 5–10 min postinjection.
Confocal Imaging.
Terminally anesthetized mice were perfused via the heart with 10 mL of PBS followed by 30 mL of 4% paraformaldehyde solution. In some experiments, vessels were labeled using rhodamine-labeled lectin (400 µg per mouse; Vector Laboratories) diluted in the perfusate (PBS). Brains were collected and prepared for confocal imaging. Details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. B. Engelhardt for antibodies to MECA-32; Dr. P. Le Tissier and Prof. D. G. Grattan for useful comments on the manuscript; and P. Fontanaud, M. Asari, G. Osterstock, M. Granier, P. Samper, and A. Guillou for technical assistance. We were supported by Agence Nationale de la Recherche (Gliodiabesity, PCV08_323168), Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Universities of Montpellier 1 and 2, University of Lille 2, National Biophotonics and Imaging Platform (Ireland), Infrastructures en Biologie, Santé et Agronomie, the Fondation pour la Recherche Medicale, Diabetes UK (R. D. Lawrence Fellowship), Institut Fédératif de Recherches 3 and 114, and Région Languedoc Roussillon (Imagerie du Petit Animal de Montpellier).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212137110/-/DCSupplemental.
References
- 1.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 2.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493(1):63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 5.Wilding JP. Neuropeptides and appetite control. Diabet Med. 2002;19(8):619–627. doi: 10.1046/j.1464-5491.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 6.Pi XJ, Grattan DR. Distribution of prolactin receptor immunoreactivity in the brain of estrogen-treated, ovariectomized rats. J Comp Neurol. 1998;394(4):462–474. doi: 10.1002/(sici)1096-9861(19980518)394:4<462::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, Lebel CR. Strategies for the delivery of leptin to the CNS. J Drug Target. 2002;10(4):297–308. doi: 10.1080/10611860290031895. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA. The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav. 2006;89(4):472–476. doi: 10.1016/j.physbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Skipor J, Thiery JC. The choroid plexus—cerebrospinal fluid system: Undervaluated pathway of neuroendocrine signaling into the brain. Acta Neurobiol Exp (Warsz) 2008;68(3):414–428. doi: 10.55782/ane-2008-1708. [DOI] [PubMed] [Google Scholar]
- 10.Cheunsuang O, Stewart AL, Morris R. Differential uptake of molecules from the circulation and CSF reveals regional and cellular specialisation in CNS detection of homeostatic signals. Cell Tissue Res. 2006;325(2):397–402. doi: 10.1007/s00441-006-0162-z. [DOI] [PubMed] [Google Scholar]
- 11.Palkovits M. Stress-induced activation of neurons in the ventromedial arcuate nucleus: A blood-brain-CSF interface of the hypothalamus. Ann N Y Acad Sci. 2008;1148:57–63. doi: 10.1196/annals.1410.062. [DOI] [PubMed] [Google Scholar]
- 12.Ciofi P, et al. Brain-endocrine interactions: A microvascular route in the mediobasal hypothalamus. Endocrinology. 2009;150(12):5509–5519. doi: 10.1210/en.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciofi P. The arcuate nucleus as a circumventricular organ in the mouse. Neurosci Lett. 2011;487(2):187–190. doi: 10.1016/j.neulet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Lafont C, et al. Cellular in vivo imaging reveals coordinated regulation of pituitary microcirculation and GH cell network function. Proc Natl Acad Sci USA. 2010;107(9):4465–4470. doi: 10.1073/pnas.0902599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grouselle D, et al. Pulsatile cerebrospinal fluid and plasma ghrelin in relation to growth hormone secretion and food intake in the sheep. J Neuroendocrinol. 2008;20(10):1138–1146. doi: 10.1111/j.1365-2826.2008.01770.x. [DOI] [PubMed] [Google Scholar]
- 16.Olszewski PK, Grace MK, Billington CJ, Levine AS. Hypothalamic paraventricular injections of ghrelin: Effect on feeding and c-Fos immunoreactivity. Peptides. 2003;24(6):919–923. doi: 10.1016/s0196-9781(03)00159-1. [DOI] [PubMed] [Google Scholar]
- 17.Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12(11):1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- 18.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302(2):822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 19.Leyris JP, et al. Homogeneous time-resolved fluorescence-based assay to screen for ligands targeting the growth hormone secretagogue receptor type 1a. Anal Biochem. 2011;408(2):253–262. doi: 10.1016/j.ab.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73(2):317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camiña JP, et al. Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue receptor 1a. Endocrinology. 2004;145(2):930–940. doi: 10.1210/en.2003-0974. [DOI] [PubMed] [Google Scholar]
- 22.Diano S, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9(3):381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 23.Moulin A, et al. Trisubstituted 1,2,4-triazoles as ligands for the ghrelin receptor: On the significance of the orientation and substitution at position 3. Bioorg Med Chem Lett. 2008;18(1):164–168. doi: 10.1016/j.bmcl.2007.10.113. [DOI] [PubMed] [Google Scholar]
- 24.Patchett AA, et al. Design and biological activities of L-163,191 (MK-0677): A potent, orally active growth hormone secretagogue. Proc Natl Acad Sci USA. 1995;92(15):7001–7005. doi: 10.1073/pnas.92.15.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 26.Bearer EL, Orci L. Endothelial fenestral diaphragms: A quick-freeze, deep-etch study. J Cell Biol. 1985;100(2):418–428. doi: 10.1083/jcb.100.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci USA. 1999;96(23):13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballermann BJ, Stan RV. Resolved: Capillary endothelium is a major contributor to the glomerular filtration barrier. J Am Soc Nephrol. 2007;18(9):2432–2438. doi: 10.1681/ASN.2007060687. [DOI] [PubMed] [Google Scholar]
- 29.Zlokovic BV, et al. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141(4):1434–1441. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]
- 30.Burguera B, et al. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes. 2000;49(7):1219–1223. doi: 10.2337/diabetes.49.7.1219. [DOI] [PubMed] [Google Scholar]
- 31.Fry M, Ferguson AV. 2010. Ghrelin: Central nervous system sites of action in regulation of energy balance. Int J Pept 2010:616757.
- 32.Banks WA, Burney BO, Robinson SM. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29(11):2061–2065. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11):2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Abizaid A, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116(12):3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Willesen MG, Kristensen P, Rømer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70(5):306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 37.Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138(2):771–777. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- 38.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494(3):528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41(5):711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- 41.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 42.Kim MS, et al. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport. 2003;14(10):1317–1320. doi: 10.1097/01.wnr.0000078703.79393.d2. [DOI] [PubMed] [Google Scholar]
- 43.Nogueiras R, et al. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes. 2004;53(10):2552–2558. doi: 10.2337/diabetes.53.10.2552. [DOI] [PubMed] [Google Scholar]
- 44.Tung YC, Hewson AK, Carter RN, Dickson SL. Central responsiveness to a ghrelin mimetic (GHRP-6) is rapidly altered by acute changes in nutritional status in rats. J Neuroendocrinol. 2005;17(6):387–393. doi: 10.1111/j.1365-2826.2005.01316.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee DA, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15(5):700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van den Pol AN, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29(14):4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.