Abstract
Advances in human genetics are leading to the discovery of new disease-causing mutations at a remarkable rate. Many such mutations, however, occur in genes that encode for proteins of unknown function, which limits our molecular understanding of, and ability to devise treatments for, human disease. Here, we use untargeted metabolomics combined with a genetic mouse model to determine that the poorly characterized serine hydrolase α/β-hydrolase domain-containing (ABHD)12, mutations in which cause the human neurodegenerative disorder PHARC (polyneuropathy, hearing loss, ataxia, retinosis pigmentosa, and cataract), is a principal lysophosphatidylserine (LPS) lipase in the mammalian brain. ABHD12−/− mice display massive increases in a rare set of very long chain LPS lipids that have been previously reported as Toll-like receptor 2 activators. We confirm that recombinant ABHD12 protein exhibits robust LPS lipase activity, which is also substantially reduced in ABHD12−/− brain tissue. Notably, elevations in brain LPS lipids in ABHD12−/− mice occur early in life (2–6 mo) and are followed by age-dependent increases in microglial activation and auditory and motor defects that resemble the behavioral phenotypes of human PHARC patients. Taken together, our data provide a molecular model for PHARC, where disruption of ABHD12 causes deregulated LPS metabolism and the accumulation of proinflammatory lipids that promote microglial and neurobehavioral abnormalities.
Keywords: lipidomics, neuroinflammation
Genome mapping and sequencing have facilitated determination of the genetic basis for over 3,500 inherited diseases in humans (1), with additional pathogenic mutations still being discovered. For many Mendelian disorders, however, the molecular and cellular mechanisms that link genotype to disease phenotype remain poorly understood. This problem is perhaps most apparent for diseases where the causative mutations occur in genes that encode for proteins with unannotated or poorly characterized functions. Here, we focus on one such disease - the neurodegenerative disorder PHARC (polyneuropathy, hearing loss, ataxia, retinosis pigmentosa, and cataract).
PHARC [Mendelian Inheritance in Man (MIM) no. 612674; Online Mendelian Inheritance in Man database, http://omim.org] is a rare, autosomal recessive disorder that causes polymodal sensory and motor defects associated with demyelination of sensomotor neurons, retinal dystrophy, and cerebellar atrophy (2). PHARC symptoms are slowly progressive and begin in the childhood or teenage years; heterozygous carriers are unaffected (3). In 2010, PHARC was reported to be caused by homozygous mutations in ABHD12, which codes for the poorly characterized serine hydrolase enzyme α/β-hydrolase domain-containing (ABHD)12 (3). To date, five distinct ABHD12 mutations have been identified in patients with PHARC, all of which are expected to lead to complete loss of ABHD12 expression (3, 4). PHARC, therefore, likely represents a human ABHD12 null model.
We demonstrated previously that ABHD12 is a membrane-bound enzyme that exhibits monoacylglycerol (MAG) lipase activity in vitro (5). These initial data suggested that ABHD12 could contribute to the metabolism of the endogenous cannabinoid 2-arachidonoylglycerol (2-AG) in the nervous system. Nonetheless, the physiological metabolites regulated by ABHD12 in vivo, and the molecular and cellular mechanisms by which this enzyme contributes to PHARC, are unknown. Here, we have addressed these important questions by generating ABHD12−/− mice and analyzing these animals for metabolomic and behavioral phenotypes. Young ABHD12−/− mice (<6 mo old) were mostly normal in their behavior; however, as these animals age, they develop an array of PHARC-related phenotypes, including defective auditory and motor behavior, with concomitant cellular pathology indicative of a neuroinflammatory response. Metabolomic characterization of brain tissue from ABHD12−/− mice revealed striking elevations in a series of lysophosphatidylserine (LPS) lipids that occur before the onset of neuroinflammatory and behavioral defects. We confirmed that LPS lipids are direct substrates for ABHD12 and that this enzyme is a major contributor to bulk brain LPS lipase activity. These data, taken together, demonstrate that ABHD12−/− mice are a suitable animal model for investigating the mechanistic basis of PHARC and point to a functional role for an ABHD12-regulated LPS pathway in this disease.
Results
Targeted Disruption of the Abhd12 Gene.
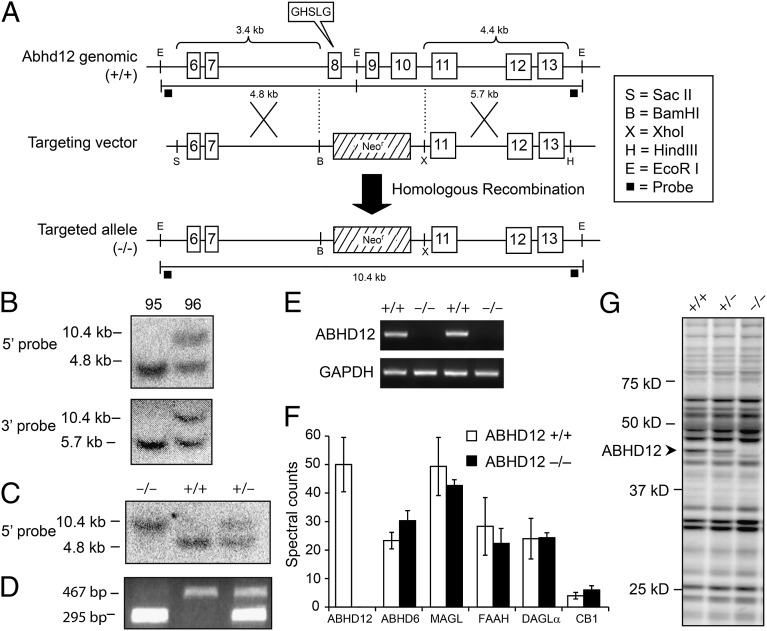
To generate mice lacking ABHD12, a targeting construct was designed to delete exons 8–10 of the Abhd12 gene, including the predicted catalytic serine S246 contained within a canonical GXSXG motif, upon homologous recombination in C57BL/6-derived embryonic stem (ES) cells (Fig. 1A). One targeted ES clone, 96, was identified by Southern blotting (Fig. 1B) and used to generate chimeric mice. These chimeras gave germ-line transmission of the targeted mutation, as determined by Southern blotting (Fig. 1C) and PCR genotyping (Fig. 1D). Mice homozygous for the targeted allele (ABHD12−/− mice) were viable and born at the expected Mendelian frequency. Loss of ABHD12 mRNA (Fig. 1E), protein (Fig. 1F), and activity (Fig. 1G) were confirmed in the brains of ABHD12−/− mice by RT-PCR, liquid chromatography–mass spectrometry (LC-MS) proteomics, and activity-based protein profiling (ABPP) (6), respectively. Levels of other protein constituents of the endocannabinoid system were unaffected by ABHD12 disruption (Fig. 1F).
Fig. 1.
Generation and initial characterization of ABHD12−/− mice. (A) ABHD12-targeting vector designed to replace Abhd12 exons 8–10 with a neomycin (Neo) selection cassette upon homologous recombination. (B) Targeted clone no. 96 was identified by Southern analysis of EcoRI-digested ES cell genomic DNA with external 5′ and 3′ probes. Germ-line transmission was confirmed by Southern (C) and PCR (D) genotyping of genomic tail DNA. (E) RT-PCR confirmed loss of ABHD12 mRNA in ABHD12−/− mice. (F) Untargeted MS-based proteomics confirmed absence of ABHD12 protein in ABHD12−/− brain tissue. Protein levels of other measured constituents of the endocannabinoid system were equivalent between genotypes. Values represent the mean ± SEM (n = 3). (G) Lack of ABHD12 activity in ABHD12−/− mice was confirmed by ABPP of brain membrane proteomes with the serine hydrolase probe fluorophosphonate (FP)-rhodamine. Other serine hydrolase activities were unaltered in ABHD12−/− brain proteome. Fluorescent gel shown in grayscale.
ABHD12−/− Mice Develop an Age-Dependent, PHARC-Related Phenotype.
ABHD12−/− mice were fertile and largely indistinguishable from their WT (ABHD12+/+) and heterozygous (ABHD12+/−) littermates at early stages of life. ABHD12+/+, ABHD12+/−, and ABHD12−/− mice were assessed in a battery of behavioral tests potentially relevant to the symptoms of PHARC disease. Initial behavioral studies were performed with relatively young mice (5–6 mo old), but considering that neurodegenerative phenotypes often only become apparent in aged mice, even for disease models with childhood onset in humans (7–10), this panel of tests was repeated when mice were 11–12 and 17–18 mo old.
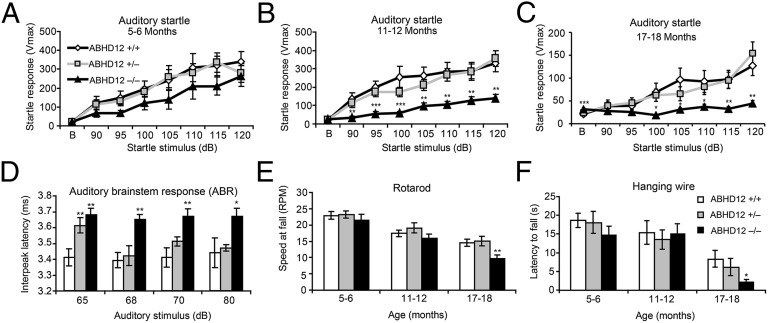
Young ABHD12−/− mice behaved similarly to control littermates except for modest hypermotility, which was transitory and not observed during the later testing sessions (Fig. S1). A slight reduction in the auditory startle reflex was also observed in 5- to 6-mo-old ABHD12−/− mice, although this effect failed to reach statistical significance (Fig. 2A). However, when retested at 11–12 mo, ABHD12−/− mice exhibited a significantly blunted startle response (Fig. 2B). At 17–18 mo old, ABHD12−/− mice failed to startle above baseline, whereas ABHD12+/+ and ABHD12+/− mice maintained a measurable startle response (Fig. 2C). Decreased auditory startle response in ABHD12−/− mice could be caused by hearing loss [a known PHARC symptom (3)] in these animals. To investigate the ability of ABHD12−/− mice to process auditory information, auditory brainstem responses (ABRs) were measured in a cohort of 9- to 10-mo-old mice after confirming that ABHD12−/− mice exhibit a reduced auditory startle response at this age (Fig. S1). The sinusoidal waveform of the brainstem auditory evoked potential (BAEP) was intact in ABHD12−/− mice (Fig. S1), and no genotypic differences were detected in peak amplitude (Table S1). However, absolute latencies of waves IV, V, and VII (Table S1) and interpeak latencies of waves I–IV were delayed in ABHD12-disrupted mice (Fig. 2D), confirming impaired auditory signaling in these animals and suggesting that the hearing loss induced by ABHD12 disruption may be caused by reduced auditory conductance.
Fig. 2.
ABHD12−/− mice develop age-dependent PHARC-related behaviors. (A–C) Eleven- to 12-month-old (B) and 17- to 18-mo-old (C), but not 5- to 6-mo-old (A), ABHD12−/− mice (black triangles) showed significantly reduced auditory startle reflex compared with ABHD12+/− (gray squares) and ABHD12+/+ (white diamonds) littermate controls (n = 8–19 per group). (D) The auditory brainstem response (ABR) was impaired in 9- to 10-mo-old ABHD12−/− mice (black bars), which showed delayed interpeak latency of waves I–IV. ABHD12+/− mice (gray bars) also showed a modest defect in ABR compared with ABHD12+/+ mice (white bars) that was only observed at the lowest auditory stimulus (n = 5 per genotype). (E and F) Seventeen- to 18-mo-old ABHD12−/− mice displayed mild ataxia (E) and a reduced latency to fall (F) in the rotarod and hanging wire test, respectively (n = 16–19 per genotype). Data represent the averages ± SEM. Variance among genotypes was assessed by two-way ANOVA and Fisher’s protected least-significant difference (PLSD) test. The Student’s t test was used to determine significance for each individual dB pulse tested in A–C. *P < 0.05; **P < 0.01; ***P < 0.001 vs. ABHD12+/+ mice.
Seventeen- to 18-month-old ABHD12−/− mice exhibited additional deficits in the accelerating rotarod (Fig. 2E) and hanging wire (Fig. 2F) tests that were not observed at younger ages. The propensity of ABHD12−/− mice to fall off of the rotarod at slower speeds than control mice is indicative of mild motor incoordination, mirroring the ataxia observed in human PHARC patients (3). Similarly, decreased latency to fall in the hanging wire test suggests muscle weakness in aged ABHD12−/− mice, consistent with the peripheral neuropathy experienced by PHARC patients (3). On the other hand, ABHD12−/− mice appeared to possess normal ocular structure and function, because we did not detect significant alterations in visual performance in the optomotor tracking test (Fig. S1) or lens opacity in these animals. Taken together, these results showed that ABHD12−/− mice develop several, but not all, of the main features of PHARC disease, including hearing loss, ataxia, and symptoms of polyneuropathy, indicating that these animals are a suitable model to study the endogenous function of ABHD12 and the molecular basis for PHARC disease.
Enhanced Microglial Activation in Brain Tissue from ABHD12−/− Mice.
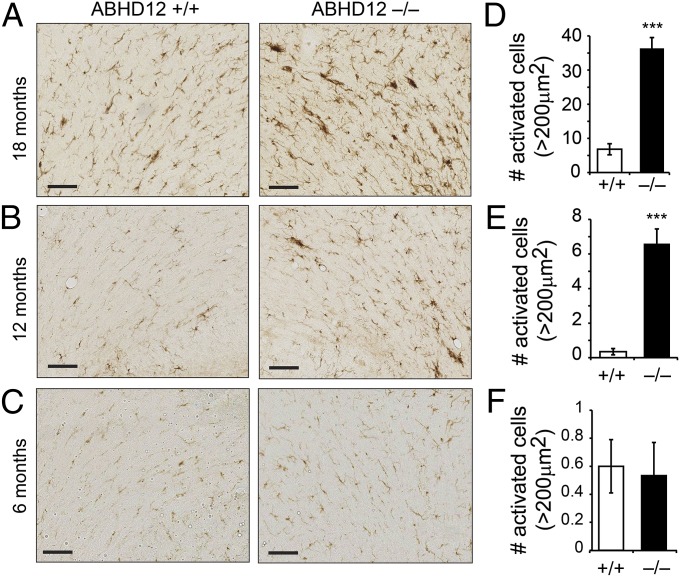
We next studied brains from old (18–21 mo) ABHD12+/+ and ABHD12−/− mice by histological analysis to investigate the cellular basis for the late-onset neurobehavioral abnormalities observed in ABHD12−/− mice. Hematoxylin and eosin (H&E) staining, Luxol Fast Blue (LFB) staining, Nissl staining, calbindin D28k immunohistochemistry, and glial fibrillary acid protein (GFAP) immunohistochemistry revealed no obvious differences in gross brain organization, myelin, neurons, Purkinje neurons, and astrocytes, respectively, between ABHD12+/+ and ABHD12−/− brains (Fig. S2). On the other hand, immunostaining for ionized calcium-binding adaptor molecule (Iba)1, which is specifically expressed in microglia and macrophages, revealed a dramatic genotypic difference in the morphology of microglia in the cerebellum (Fig. 3 A–C). Quantitation of Iba1 immunoreactivity identified significantly more activated microglia (i.e., Iba1-reactive cells with enlarged soma size and retracted processes) in sections from 18-mo-old ABHD12−/− mice (Fig. 3D). A significant increase in activated microglia was also observed in cerebellar sections from 12-mo-old ABHD12−/− mice (Fig. 3 B and E), a time point at which these animals displayed normal rotarod behavior (Fig. 2E). In contrast, WT microglial morphology was found in cerebellar sections from 6-mo-old ABHD12−/− mice (Fig. 3 C and F). These data indicate that disruption of ABHD12 promotes an age-dependent neuroinflammatory response in the brain that temporally precedes the onset of cerebellar-mediated behavioral abnormalities.
Fig. 3.
ABHD12 disruption causes age-dependent increases in microglial activation in the mouse brain. (A–C) Representative images of Iba1 immunostaining for microglia in the cerebellum of 18-mo-old (A), 12-mo-old (B), and 6-mo-old (C) ABHD12+/+ and ABHD12−/− mice. (D–F) Quantifying the number of enlarged cells (>200 μm2) revealed significantly more activated microglia in cerebellar sections from 18-mo-old (D) and 12-mo-old (E) ABHD12−/− mice compared with ABHD12+/+ mice. No genotype differences were observed at 6 mo of age (F). Data represent the averages ± SEM. ***P < 0.001 (n = 3–4 per genotype per age group). (Scale bar, 100 μm.)
Identification of Brain Metabolites Deregulated by ABHD12 Disruption.
Characterization of ABHD12−/− mice confirmed a minor defect in brain 2-AG metabolism. Protein homogenates prepared from ABHD12+/+ and ABHD12−/− brains hydrolyzed 2-AG to similar extents, but ABHD12−/− homogenates showed a significantly greater reduction in 2-AG hydrolysis following inhibition of the principal brain 2-AG hydrolase, MAG lipase (MAGL) (Fig. S3). Bulk levels of 2-AG and other MAGs, however, were unchanged in ABHD12−/− brains (Fig. S3). Additionally, ABHD12−/− mice did not exhibit any of the classical signs of enhanced signaling through the central cannabinoid receptor CB1: analgesia, hypomotility, or hypothermia (Fig. S3). These results, taken together, suggested that the behavioral and cellular pathology elicited by ABHD12 disruption may be attributable to deregulation of nonendocannabinoid metabolites. To more fully explore this possibility, we performed a metabolomic analysis of ABHD12+/+ and ABHD12−/− brains by untargeted LC-MS (11, 12).
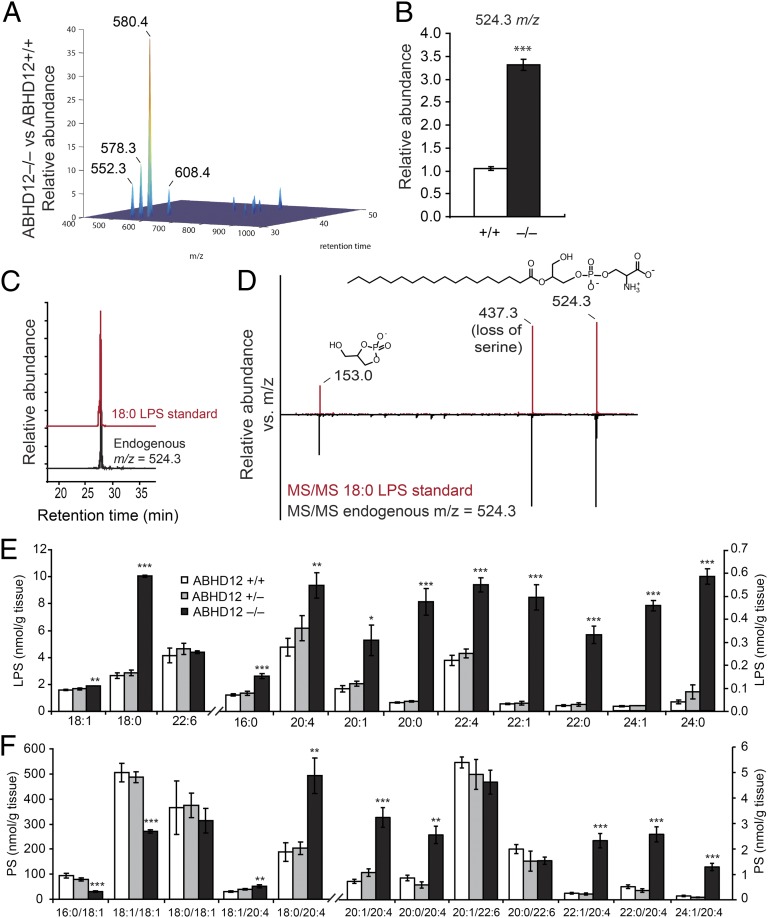
LC-MS profiling of organic-soluble brain fractions from 2- to 6-mo-old ABHD12+/+ and ABHD12−/− mice combined with data analysis using the XCMS program (13) identified several metabolites that were significantly altered in ABHD12−/− brains (Fig. 4A and Table S2). The largest fold-change differences were observed for metabolites with m/z values of 552.3, 578.3, 580.4, and 608.4, all of which were detected in the negative-ion mode and were dramatically (∼6- to 40-fold) elevated in ABHD12−/− brains (Fig. 4A and Table S2). Based on the differences in m/z values and LC retention times of these metabolites, we hypothesized that they might represent related members of a common lipid class differing in acyl chain length and degree of unsaturation. Targeting these metabolites by Fourier transform ion cyclotron resonance (FT-ICR)-MS allowed mass determination within 2 ppm accuracy and calculation of their molecular formulas, which matched those of very long chain lysophosphatidylserine (VLC-LPS) lipids (Table S3).
Fig. 4.
Deregulated (lyso)phosphatidylserine [(L)PS] metabolites in brain tissue from ABHD12−/− mice. (A) Untargeted metabolomics identified several metabolites with altered brain levels in 2- to 6-mo-old ABHD12−/− mice compared with ABHD12+/+ mice. The m/z values with the largest fold-change differences between genotypes are marked. (B) Relative peak intensity of a metabolite with an m/z value of 524.3, corresponding to 18:0 lysophosphatidylserine (LPS), in ABHD12−/− vs. ABHD12+/+ brain tissue. (C and D) The m/z 524.3 metabolite (black trace) exhibited the same LC elution time (C) and MS/MS fragmentation pattern (D) as a synthetic 18:0 LPS standard (red trace). Key daughter ions in the MS/MS analysis include 153.0 (dehydro-glycerophosphate) and 437.3 [18:0 lysophosphatidic acid (LPA)]. (E and F) Targeted MRM measurements of LPS (E) and PS (F) species from brain tissue of ABHD12+/+ (white bars), ABHD12+/− (gray bars), or ABHD12−/− (black bars) mice. Data are presented as average values ± SEM (n = 3–5 per group). *P < 0.05; **P < 0.01; ***P < 0.001 vs. ABHD12+/+ (Student’s t test).
Manual inspection of the LC-MS data revealed that an additional metabolite with an m/z value of 524.3, corresponding to 18:0 LPS, was also elevated in ABHD12−/− brain metabolomes (Fig. 4B). Supporting this molecular assignment, the endogenous m/z 524.3 metabolite and a synthetic 18:0 LPS standard displayed the same LC elution time (Fig. 4C) and fragmentation spectra (Fig. 4D). Fragmentation spectra for the m/z 578.3, 580.4, and 608.4 metabolites, which, like the spectrum for 18:0 LPS (Fig. 4D), all possessed a common peak at m/z = 153, corresponding to the glycerophosphate–H2O moiety, and a peak at the parent m/z minus 87, corresponding to the neutral loss of serine (Fig. S4), permitted their structural assignment as 22:1, 22:0, and 24:0 LPS species, respectively.
In addition to the striking accumulation of VLC-LPS species identified in ABHD12−/− brains, more modest alterations were also found in other brain metabolites by lipidomic analysis (Fig. 4A and Table S2), including select phosphatidylserine (PS) (Fig. S4), phosphatidylglycerol (PG) (Fig. S4), and lysophosphatidylinositol (LPI) lipids (Table S2). In contrast, all other (lyso)phospholipid and free fatty acid levels examined were equivalent between genotypes (Table S2). Finally, we detected two brain metabolites elevated in ABHD12−/− mice that remain structurally unidentified (m/z values of 718.6 and 814.6; Table S2).
We used targeted multiple reaction monitoring (MRM) MS methods to confirm our untargeted lipidomic findings, including the dramatic elevations in VLC-LPS lipids caused by ABHD12 disruption (Fig. 4E and Tables S4 and S5). Targeted experiments, on the other hand, failed to detect VLC-LPI or VLC-LPG species in ABHD12+/+ or ABHD12−/− brains. Our targeted analyses also confirmed changes in other lipid metabolites in ABHD12−/− brains (Table S4), including PS lipids, which displayed a notably remodeled acyl chain distribution with selective enrichment of arachidonate-containing species (Fig. 4F). An altered LPS and PS lipid profile was also found in brain regions (cerebellum, cortex, and hippocampus) from ABHD12−/− mice, with the greatest absolute levels and accumulation of VLC-LPS detected in the cerebellum of these animals (Table S6). In contrast, LPS and PS levels were largely unchanged in several ABHD12−/− peripheral tissues, with less than twofold differences observed among genotypes (Table S6).
In summary, targeted and untargeted lipidomic profiling demonstrated that deregulation of brain (L)PS pathways is a major metabolic consequence of genetic ablation of ABHD12.
ABHD12 Is a Principal LPS Hydrolase in the Mouse Brain.
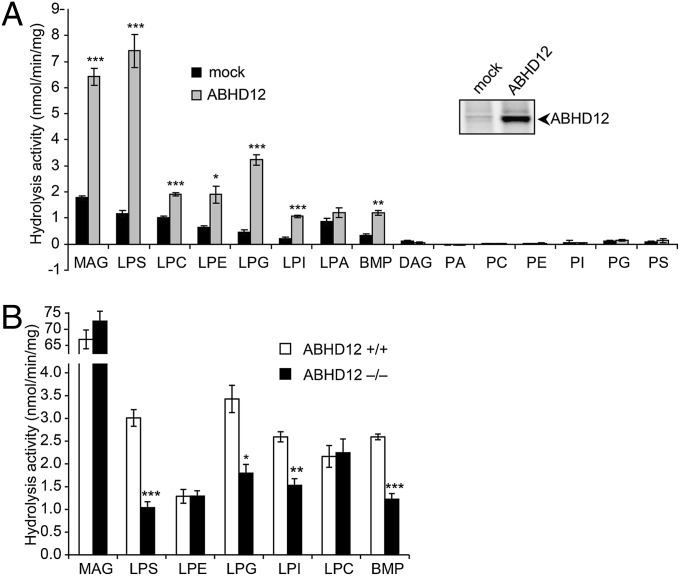
To determine whether any of the altered lipids in ABHD12−/− brains are direct substrates or products of ABHD12, we assayed this enzyme against a panel of neutral lipids and (lyso)phospholipids (Table S7). Consistent with previous work, ABHD12 showed MAG hydrolase activity (Fig. 5A). ABHD12 also hydrolyzed several lysophospholipid species, with the highest activity being observed for LPS (Fig. 5A). ABHD12 hydrolyzed LPS lipids bearing both saturated (C16:0) and unsaturated (C20:4) acyl chains (Fig. S5). In contrast, ABHD12 did not display significant activity against lipid species bearing a doubly acylated glycerol backbone (Fig. 5A).
Fig. 5.
ABHD12 is a major brain LPS lipase. (A) Membrane homogenates from HEK293T cells transfected with a murine ABHD12 cDNA (gray bars) displayed increased hydrolytic activity compared with mock-transfected cell membranes (black bars) toward several lysophospholipids and other singly acylated glycerolipids (e.g., MAG, BMP). In contrast, doubly acylated glycerolipids (e.g., DAG, PA, PC, PE, PI, PG, PS) were not hydrolyzed by ABHD12. See Table S7 for more information on lipid species tested as ABHD12 substrates. (Inset) Active ABHD12 expression in transfected cells was confirmed by ABPP with the FP-rhodamine probe. (B) ABHD12−/− brain membrane homogenates (black bars) displayed significantly reduced hydrolytic activity toward 18:1 LPS, LPG, LPI, and BMP compared with ABHD12+/+ brain homogenates (white bars). Data are presented as average values ± SEM (n = 3 per group). *P < 0.05; **P < 0.01; ***P < 0.001 vs. mock or ABHD12+/+ homogenates (Student’s t test).
We next assayed brain membrane homogenates prepared from ABHD12+/+ and ABHD12−/− mice for hydrolytic activity against the lipid species cleaved by recombinant ABHD12. These activity measurements revealed that ABHD12 is a principal LPS hydrolase in the mouse brain, accounting for ∼70% of total membrane lysate activity (Fig. 5B). This reduction in hydrolytic activity in ABHD12−/− brains was observed for both saturated (16:0) and unsaturated (20:4) LPS lipids (Fig. S5). ABHD12−/− brain homogenates also displayed a significant reduction in LPI, LPG, and BMP (bismonoacylglycerolphosphate) hydrolase activities (Fig. 5B), although we observed only modest alterations (<twofold) in a subset of LPI and LPG species and no changes in BMP levels in ABHD12−/− brain metabolomes (Fig. S4). In contrast, the hydrolysis of MAG, LPC (lysophosphatidylcholine), and LPE (lysophosphatidylethanolamine) was unaltered in membrane lysates from ABHD12−/− brains (Fig. 5B).
These results, when combined with the findings from our metabolomic analysis of ABHD12−/− brains, indicate that ABHD12 functions as a major LPS lipase in the mammalian nervous system.
Discussion
Human genetic studies have recently discovered mutations in the ABHD12 gene as the causative basis for the neurodegenerative disease PHARC (3). Understanding the role that ABHD12 plays in nervous system, however, requires elucidation of the enzyme’s endogenous metabolic function. With this goal in mind, we have generated and characterized ABHD12−/− mice. We show that these animals develop age-dependent, PHARC-like behavioral abnormalities, including hearing disruptions, ataxia, and muscle weakness, that are accompanied by microglial activation in the brain. That activated microglia were detected in the brain at time points preceding behavioral deficits points to a potential neuroinflammatory basis for the development and progression of ABHD12-dependent PHARC pathologies.
Using a combination of untargeted and targeted metabolomics, we discovered that ABHD12−/− brains contain significant elevations in LPS lipids, including massive (>10-fold) increases in several VLC-LPS lipids. We also detected substantial changes in PS lipids that appear to reflect remodeled acyl chain distribution. We suspect that this latter metabolic phenotype may be a secondary consequence of ABHD12-mediated changes in LPS lipids, given that ABHD12 shows robust LPS lipase, but not PS lipase activity. Importantly, the alterations in (L)PS lipid levels occurred in young ABHD12−/− mice (2–6 mo old) before the onset of microglial activation or neurobehavioral abnormalities. These data thus support a model where deregulated (L)PS metabolism is a cause, rather than a consequence, of PHARC-like syndrome in ABHD12−/− mice (see below).
How might accumulation of LPS in the brains of ABHD12−/− mice contribute to neuroinflammation and PHARC-like behavioral defects? LPS has been shown to be a bioactive lipid that produces several cellular effects (14), including mast cell degranulation (15–17), macrophage clearance of apoptotic cells (18, 19), leukemic cell stimulation (20), and chemotactic migration of human glioma cells (21) and murine fibroblasts (22). Although the bioactivity of LPS in the nervous system has not been well studied, our findings support a model where LPS exhibits similar immunomodulatory activities to stimulate microglial function in the brain.
Reports on VLC-LPS are scarce in the literature. Interestingly, however, parasitic worms that cause schistosomiasis, a chronic blood-vascular disease common in the developing world, produce many of the same VLC-LPS species that are highly elevated in ABHD12−/− brains (23). Parasite-derived VLC-LPS, but not synthetic 16:0 LPS, was found to activate toll-like receptor (TLR)2 on dendritic cells and modify their T-cell–stimulating activity. TLR2 recognizes many pathogen-derived molecules and is expressed in the CNS by glial cells (microglia, astrocytes, oligodendrocytes) and neurons (24). TLR2 agonists initiate a proinflammatory response by microglia (25) and can induce microglial-mediated neuronal death in primary neural cultures (26, 27) and in vivo (28). We hypothesize the VLC-LPS species that accumulate following ABHD12 disruption could serve as endogenous TLR2 ligands, leading to microglial activation and neuroinflammation in the brain.
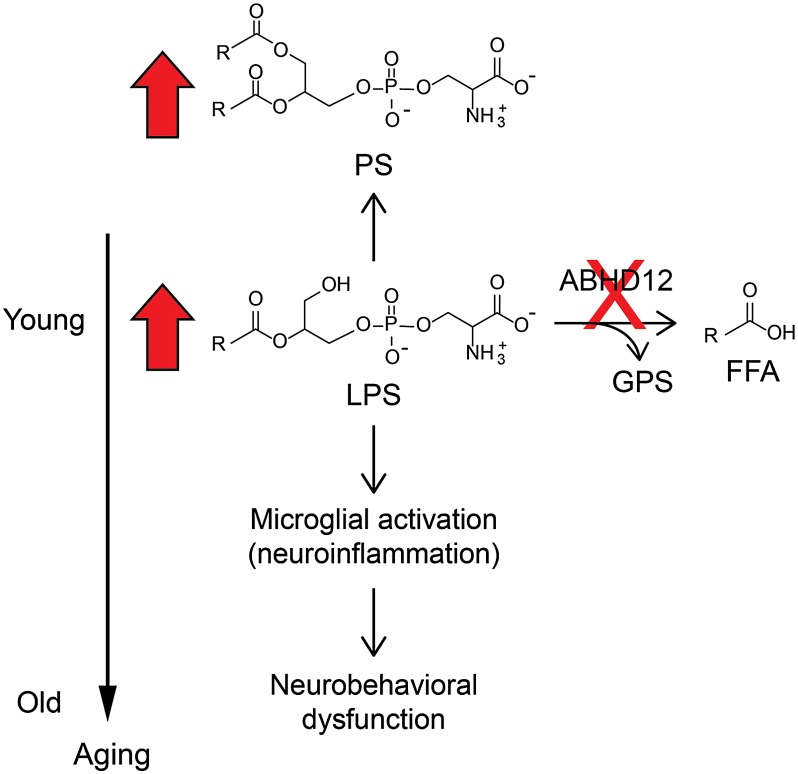
In summary, our findings provide a working model to explain the molecular basis for PHARC, wherein ABHD12 disruption produces, in a temporally ordered manner, deregulated (L)PS metabolism that includes elevations in proinflammatory VLC-LPS lipids, followed by microglial activation and the onset of neuroinflammation and, finally, an array of neurobehavioral abnormalities (Fig. 6). We should, however, clarify that ABHD12−/− mice do not appear to exhibit all features of human PHARC. We did not, for instance, observe significant optomotor defects or cerebellar atrophy in ABHD12−/− mice. Of course, difficulties in fully recapitulating human neurodegenerative diseases with genetic mouse models is a well-recognized challenge (29, 30), and we believe, overall, that ABHD12−/− mice display many of the key features of PHARC. Perhaps most importantly, these animals provide a valuable tool to decipher the molecular and cellular basis for PHARC. In this regard, although the major metabolic changes caused by ABHD12 ablation corresponded to (L)PS lipids, we cannot rule out a potential role for (L)PGs, (L)PIs, or other ABHD12-regulated metabolites in the development of PHARC. Additionally, although our results do not support a role for ABHD12 as a regulator of bulk endocannabinoid signaling in the mammalian brain, it remains plausible that this enzyme could control local 2-AG accumulation in specific proinflammatory brain cells, such as microglia. Cannabinoid type 2 (CB2) receptors, in particular, are up-regulated in activated microglia associated with Alzheimer's and multiple sclerosis plaques (31, 32) and are thought to regulate microglial motility (33). Microglial/glial CB2 activation, however, is generally considered neuroprotective, and it has been associated with a reduction in neurotoxic factor release (34). Determining whether ABHD12 regulates 2-AG in primary microglia and how the loss of ABHD12 affects CB2-mediated microglial activity are fertile topics for future research. Finally, we speculate that, if elevated LPS lipids prove to be a causative agent in PHARC, then identifying the enzymatic source for these proinflammatory lipids in the nervous system (i.e., the PS lipase that produces VLC-LPS lipids) could provide an attractive molecular target for therapeutic intervention.
Fig. 6.
Working model to explain the molecular and cellular basis for ABHD12-dependent neuropathology. Our results point to a model where loss of ABHD12 leads to accumulation of the enzyme’s endogenous LPS substrates, as well as corresponding (indirect) alterations in PS lipids. These metabolic changes occur in young mice (<6 mo), before the appearance of cellular or behavioral signs of neuropathology. By 12 mo of age, neuroinflammatory markers, such as cerebellar microglial activation, are observed in ABHD12−/− mice, which are then followed by PHARC-related behavioral deficits that intensify with age. FFA, free fatty acid; GPS, glycerophosphate.
Materials and Methods
An extended section is provided in SI Materials and Methods.
Generation of ABHD12−/− Mice.
Constitutive ablation of the Abhd12 gene was achieved on the C57BL/6 genetic background using standard gene targeting techniques. All mice used in this study were generated from breeding ABHD12+/− mice.
Biochemical Studies.
Activity-based protein profiling (ABPP) and substrate activity assays of mouse brain and transfected HEK293T cell homogenates were performed as described previously (5, 35). Untargeted LC-MS proteomic analysis of brain homogenates was performed as previously described (35).
Behavioral Studies.
Tests for locomotor activity, startle response, auditory brainstem response, rotarod performance, hanging wire performance, and optomotor activity were performed as described in the SI Materials and Methods.
Histological and Immunohistochemical Analyses.
Slide-mounted sections from 18- to 21-mo-old ABHD12+/+ and ABHD12−/− mice (n = 4) were stained with H&E to visualize gross brain organization, LFB to visualize myelin, and Nissl (cresyl violet) to visualize neurons. Immunohistochemistry was performed on frozen free-floating sections as described previously (36). Sections from 18- to 21-mo-old mice (n = 4) were immunostained with a GFAP antibody to visualize astrocytes or a calbindin antibody to visualize Purkinje neurons. Microglia were visualized in sections from 6-, 12-, and 18-mo-old ABHD12+/+ and ABHD12−/− mice (n = 3–4 per genotype per age) using an antibody against Iba1 and the number of enlarged microglia (>200 μm) in matching cerebellar sections were counted using ImageJ software (National Institutes of Health).
LC-MS Metabolite Profiling.
Untargeted metabolomics analysis was performed on organic-soluble brain extracts from 2- to 6-mo-old ABHD12+/+ and ABHD12−/− mice (n = 3–5) as described previously (11). The molecular assignment of ABHD12-regulated metabolites was achieved by high mass-accuracy measurements, MS/MS fragmentation analysis, and coelution studies with synthetic lipid standards (37). Tissue lipid levels in ABHD12−/−, ABHD12+/−, and ABHD12+/+ littermates (6 mo old; n = 5) were quantified by targeted MRM methods (35).
Supplementary Material
Acknowledgments
We thank Sergey Kupriyanov and Greg Martin [The Scripps Research Institute (TSRI) Mouse Genetics Core], Amanda Roberts and Salvador Huitron-Resendiz (TSRI Mouse Behavioral Assessment Core), Margie Chadwell and Anita San-Soucie (TSRI Histology Core), and Holly Pugh for technical assistance. This work was supported by US National Institutes of Health Grants DA017259 and DA009789.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217121110/-/DCSupplemental.
References
- 1.Online Mendelian Inheritance in Man 2012. McKusick-Nathans Institute of Genetic Medicine (John Hopkins University, Baltimore). Available at http://omim.org. Accessed September 15, 2012.
- 2.Fiskerstrand T, et al. A novel Refsum-like disorder that maps to chromosome 20. Neurology. 2009;72(1):20–27. doi: 10.1212/01.wnl.0000333664.90605.23. [DOI] [PubMed] [Google Scholar]
- 3.Fiskerstrand T, et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: An inborn error of endocannabinoid metabolism. Am J Hum Genet. 2010;87(3):410–417. doi: 10.1016/j.ajhg.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenberger T, et al. Targeted next-generation sequencing identifies a homozygous nonsense mutation in ABHD12, the gene underlying PHARC, in a family clinically diagnosed with Usher syndrome type 3. Orphanet J Rare Dis. 2012;7(1):59. doi: 10.1186/1750-1172-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14(12):1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon GM, Cravatt BF. Activity-based proteomics of enzyme superfamilies: Serine hydrolases as a case study. J Biol Chem. 2010;285(15):11051–11055. doi: 10.1074/jbc.R109.097600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pujol A, et al. Late onset neurological phenotype of the X-ALD gene inactivation in mice: A mouse model for adrenomyeloneuropathy. Hum Mol Genet. 2002;11(5):499–505. doi: 10.1093/hmg/11.5.499. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler VC, et al. Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Hum Mol Genet. 2000;9(4):503–513. doi: 10.1093/hmg/9.4.503. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzetti D, et al. Repeat instability and motor incoordination in mice with a targeted expanded CAG repeat in the Sca1 locus. Hum Mol Genet. 2000;9(5):779–785. doi: 10.1093/hmg/9.5.779. [DOI] [PubMed] [Google Scholar]
- 10.Shinzawa K, et al. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: A model of human neurodegenerative disease. J Neurosci. 2008;28(9):2212–2220. doi: 10.1523/JNEUROSCI.4354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saghatelian A, et al. Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry. 2004;43(45):14332–14339. doi: 10.1021/bi0480335. [DOI] [PubMed] [Google Scholar]
- 12.Gross RW, Han X. Lipidomics at the interface of structure and function in systems biology. Chem Biol. 2011;18(3):284–291. doi: 10.1016/j.chembiol.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78(3):779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 14.Makide K, Kitamura H, Sato Y, Okutani M, Aoki J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 2009;89(3-4):135–139. doi: 10.1016/j.prostaglandins.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Bigon E, Bruni A, Mietto L, Toffano G. Lysophosphatidylserine-induced release of intra-cellular amines in mice. Br J Pharmacol. 1980;69(1):11–12. doi: 10.1111/j.1476-5381.1980.tb10876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin TW, Lagunoff D. Interactions of lysophospholipids and mast cells. Nature. 1979;279(5710):250–252. doi: 10.1038/279250a0. [DOI] [PubMed] [Google Scholar]
- 17.Smith GA, Hesketh TR, Plumb RW, Metcalfe JC. The exogenous lipid requirement for histamine release from rat peritoneal mast cells stimulated by concanavalin A. FEBS Lett. 1979;105(1):58–62. doi: 10.1016/0014-5793(79)80887-x. [DOI] [PubMed] [Google Scholar]
- 18.Frasch SC, et al. NADPH oxidase-dependent generation of lysophosphatidylserine enhances clearance of activated and dying neutrophils via G2A. J Biol Chem. 2008;283(48):33736–33749. doi: 10.1074/jbc.M807047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frasch SC, et al. Signaling via macrophage G2A enhances efferocytosis of dying neutrophils by augmentation of Rac activity. J Biol Chem. 2011;286(14):12108–12122. doi: 10.1074/jbc.M110.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KS, Lee HY, Kim M-K, Shin EH, Bae Y-S. Lysophosphatidylserine stimulates leukemic cells but not normal leukocytes. Biochem Biophys Res Commun. 2005;333(2):353–358. doi: 10.1016/j.bbrc.2005.05.109. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, et al. Lysophosphatidylserine stimulates chemotactic migration in U87 human glioma cells. Biochem Biophys Res Commun. 2008;374(1):147–151. doi: 10.1016/j.bbrc.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 22.Park KS, et al. Lysophosphatidylserine stimulates L2071 mouse fibroblast chemotactic migration via a process involving pertussis toxin-sensitive trimeric G-proteins. Mol Pharmacol. 2006;69(3):1066–1073. doi: 10.1124/mol.105.018960. [DOI] [PubMed] [Google Scholar]
- 23.van der Kleij D, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277(50):48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 24.Okun E, et al. Toll-like receptors in neurodegeneration. Brain Res Brain Res Rev. 2009;59(2):278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinsner A, et al. Highly purified lipoteichoic acid induced pro-inflammatory signalling in primary culture of rat microglia through Toll-like receptor 2: Selective potentiation of nitric oxide production by muramyl dipeptide. J Neurochem. 2006;99(2):596–607. doi: 10.1111/j.1471-4159.2006.04085.x. [DOI] [PubMed] [Google Scholar]
- 26.Neher JJ, et al. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186(8):4973–4983. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- 27.Lehnardt S, et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177(1):583–592. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann O, et al. TLR2 mediates neuroinflammation and neuronal damage. J Immunol. 2007;178(10):6476–6481. doi: 10.4049/jimmunol.178.10.6476. [DOI] [PubMed] [Google Scholar]
- 29.Sango K, et al. Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nat Genet. 1995;11(2):170–176. doi: 10.1038/ng1095-170. [DOI] [PubMed] [Google Scholar]
- 30.Lu J-F, et al. A mouse model for X-linked adrenoleukodystrophy. Proc Natl Acad Sci USA. 1997;94(17):9366–9371. doi: 10.1073/pnas.94.17.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benito C, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23(35):11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yiangou Y, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6(1):12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter L, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23(4):1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez AJ, García-Merino A. Neuroprotective agents: Cannabinoids. Clin Immunol. 2012;142(1):57–67. doi: 10.1016/j.clim.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Schlosburg JE, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13(9):1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomura DK, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long JZ, et al. Metabolomics annotates ABHD3 as a physiologic regulator of medium-chain phospholipids. Nat Chem Biol. 2011;7(11):763–765. doi: 10.1038/nchembio.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.