Abstract
Food resources contaminated with spoilage or pathogenic microorganisms pose severe problems to all higher organisms. Here, we describe a food-hygienic strategy of the emerald cockroach wasp Ampulex compressa. The wasp larvae develop on and inside the American cockroach Periplaneta americana, a host that can harbor various putrefactive microbes, as well as human and insect pathogens. From P. americana, we isolated the Gram-negative bacterium Serratia marcescens, which is a potent entomopathogen that can rapidly kill insect larvae. It is also known as a food contaminant and as an opportunistic human pathogen. Using behavioral observations and chemical analyses, we demonstrated that A. compressa larvae impregnate their cockroach hosts from inside with large amounts of an oral secretion containing a blend of γ-lactones and isocoumarins with (R)-(-)-mellein [(R)-(-)-3,4-diydro-8-hydroxy-3-methylisocoumarin] and micromolide [(4R,9Z)-octadec-9-en-4-olide] as dominant components. We fractionated hexane extracts of the secretion and investigated the antimicrobial properties of the fraction containing the lactones and isocoumarins, as well as of synthetic (R)-(-)-mellein and micromolide, against S. marcescens and a Gram-positive bacterium, Staphylococcus hyicus, in broth microdilution assays. The test fraction inhibited growth of both tested bacteria. The activity of the fraction against S. marcescens was explained by (R)-(-)-mellein alone, and the activity against S. hyicus was explained by the combined action of (R)-(-)-mellein and micromolide. Our data suggest that the specific combination of antimicrobials in the larval secretion provides an effective frontline defense against the unpredictable spectrum of microbes that A. compressa larvae may encounter during their development inside their cockroach hosts.
Keywords: antibiotic, food hygiene, jewel wasp
Microbe-contaminated food poses severe threats to all animals from invertebrates to vertebrates, including humans. Microbial decomposers not only may cause the food to rot but also are often responsible for serious, sometimes even lethal, diseases when ingested with the food (1–4). Food hygiene is thus of vital importance to avoid potentially severe hazards associated with microbe-laden food resources.
Insects are increasingly recognized as sources of microbial food contaminations (5). In particular, cockroaches, such as the peridomestic American cockroach Periplaneta americana (Blattaria, Blattidae), are suspected vectors of food spoilage and pathogenic microbes (6, 7). Owing to their unsanitary lifestyle, they can pick up, carry, and transfer a plethora of bacteria and fungi (6–14) and hence represent a potential health problem to humans, as well as wild and domestic animals.
Animals that feed on cockroaches are especially at risk for acquiring infections from their contaminated food. One of these species is the emerald cockroach wasp Ampulex compressa F. (Hymenoptera, Ampuliciae), which relies on cockroaches such as P. americana for food for its offspring (15). The A. compressa female attacks a cockroach, stings it, and drags the then-docile host to a hiding place, where it glues an egg to the coxa of one of the mesothoracic legs of the cockroach. When the wasp’s egg hatches, the larva first imbibes hemolymph through a hole in the thoracic cuticle of the still living cockroach. About 7 d after oviposition (at 27 °C), the larva moves inside the cockroach and feeds on the interior organs, causing the death of the host. During this time, the host cockroach represents both the food and the microenvironment of the larva. After eroding the cockroach almost completely, the larva spins itself into a cocoon inside the cockroach carcass, pupates, and ecloses as an adult about 6 wk after oviposition.
During the relatively long period of development and metamorphosis inside the cockroach, the larvae and pupae of A. compressa have to cope with competitive, putrefactive microorganisms that can degrade their larval food, as well as with harmful entomopathogens. To preserve their food source and secure their survival, A. compressa larvae can thus be expected to have evolved effective antimicrobial strategies, such as the deployment of antimicrobial compounds that inhibit growth of antagonistic bacteria and fungi.
In the present study, we screened P. americana hosts for the presence of possible pathogens using microbiological and molecular techniques. We extracted P. americana cockroaches parasitized by A. compressa and performed chemical analyses for structure elucidation and quantification of the compounds present in these extracts. We unraveled the source of the identified chemicals and, as a final step, examined their antimicrobial activity against an isolated pathogen and another bacterium in vitro.
Results
To detect microorganisms that may threaten A. compressa larvae during their development, we probed the outer surface and inner tissue of four P. americana cockroaches with microbiological techniques. From four of the surface samples and three of the tissue samples, we obtained bacterial colonies on agar plates. Nearly all of the colonies showed conspicuous pink pigmentation, and we focused on these pink colonies for further analyses. After purification of the bacterial strains, their DNA was extracted and partial 16S rRNA sequences were amplified by PCR and sequenced. The obtained partial 16S rRNA sequences of the four surface isolates (563, 586, 646, and 1,053 bp) and of the three tissue isolates (479, 492, and 510 bp) were all identical (GenBank accession no. of an exemplary 1,053 bp sequence: JX448402) and showed 99% homology with a published 16S rRNA gene sequence of Serratia marcescens (GenBank accession no. EF035134.1).
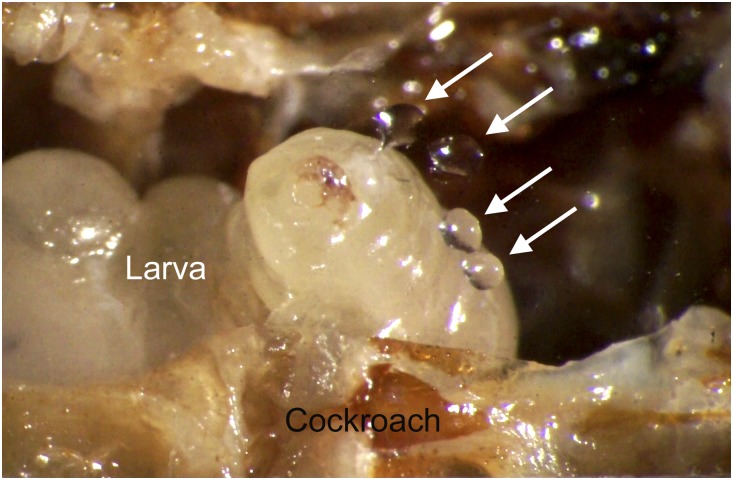
To elucidate the antimicrobial strategy that A. compressa larvae use, we monitored 8-d-old larvae inside their hosts through small holes in the abdominal cuticle covered by coverslips. The larvae could be observed to repeatedly deposit clear droplets of an oral secretion into the nearly empty body cavity of the cockroach, as well as onto the coverslips (Fig. 1 and Movie S1). The larvae then dispersed these droplets inside their hosts’ bodies. Droplets of the secretion were directly probed from the coverslips by solid-phase microextraction (SPME) and analyzed by coupled gas chromatography–mass spectrometry (GC/MS). Apart from some typical insect hydrocarbons, the secretion contained nine more polar compounds that were neither previously known from A. compressa nor from P. americana. We therefore considered these compounds as candidate antimicrobial substances. The two major compounds of this mixture (1 and 8 in Table 1 and Fig. 2) were of particular interest.
Fig. 1.
A larva of A. compressa inside its P. americana host applying droplets of secretion (arrows) to a coverslip that covers an artificial opening in the cockroach cuticle (Movie S1).
Table 1.
Chemical composition of the secretion of A. compressa larvae and mean amounts and respective proportions of larval compounds found on single P. americana hosts parasitized by A. compressa (n = 10)
| No. | LRI | Compound | Larval secretion | Amount per cockroach (µg) | Proportion (%) |
| 1 | 1,550 | (R)-(-)-mellein* | + | 1,900 ± 1,442 | 87 ± 5 |
| 2 | 1,660 | 7-Hydroxymellein† | + | 54 ± 110 | 1.3 ± 3.0 |
| 3 | 1,713 | 4-Hydroxymellein† | + | 24 ± 21 | 1 ± 0.3 |
| 4 | 1,878 | 5-Hydroxymellein† | + | 1.7 ± 5 | 0.04 ± 0.14 |
| 5 | 2,107 | (R)-Hexadecan-4-olide‡ | + | 6.5 ± 7 | 0.22 ± 0.17 |
| 6 | 2,207 | Heptadecan-4-olide§ | + | 0.6 ± 1.2 | 0.01 ± 0.03 |
| 7 | 2,273 | Octadeca-9,12-dien-4-olide§ | + | 10 ± 9.2 | 0.46 ± 0.23 |
| 8 | 2,284 | (4R,9Z)-octadec-9-en-4-olide/micromolide‡ | + | 216 ± 205 | 8.6 ± 3.8 |
| 9 | 2,313 | (R)-octadecan-4-olide‡ | + | 41 ± 43 | 1.5 ± 0.8 |
The two major compounds are in boldface type. Numbers correspond to the numbers in Fig. 2. LRI, linear retention index (calculated in relation to n-alkanes); +, compound is present in larval secretion. Values are means ± SD.
*Identified by comparing the mass spectrum, retention time on a nonpolar GC column, and optical rotation value with a synthetic reference compound.
†Tentatively identified by comparing the mass spectrum with published mass spectra (SI Materials and Methods).
‡Identified by comparing the mass spectrum and retention times on a nonpolar and an enantioselective GC-column with a synthetic reference compound.
§Tentatively identified by interpretation of the mass spectrum (SI Materials and Methods).
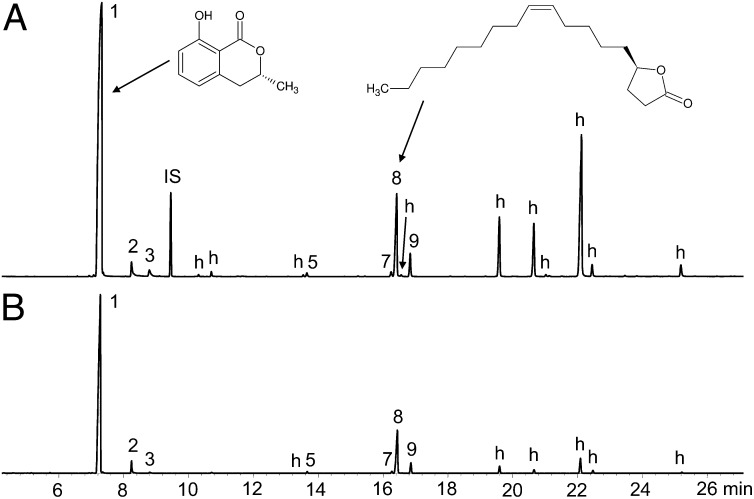
Fig. 2.
Total ion current chromatograms of (A) a solvent extract of a P. americana parasitized by A. compressa and (B) a SPME sample of the secretion of an A. compressa larva. Although the relative amounts of compounds showed a broad variation, the chromatogram can be considered typical. The peaks of minor components are not always visible due to the magnification used. Peak numbers correspond to the numbers in Table 1. h, hydrocarbon; IS, internal standard; 1, (R)-(-)-mellein; 8, micromolide (Table S1 and Fig. S1).
To obtain larger amounts of the target compounds for structure elucidation and in vitro tests of antimicrobial activity, we extracted them with hexane from P. americana cockroaches parasitized by A. compressa. These extracts contained the target compounds in similar proportions as detected in the larval secretion (Fig. 2 and Table 1). Additionally, some typical cuticular hydrocarbons from P. americana and A. compressa were detected (Table S1). Control extracts of unparasitized P. americana contained only hydrocarbons (Fig. S1 and Table S1).
For structure elucidation, hexane extracts of parasitized cockroaches were purified by adsorption chromatography using a silica gel column and dichloromethane as the eluent, fractionated by size-exclusion high-performance liquid chromatography (SE-HPLC), and subsequently analyzed by GC/MS. The mass spectrum of major compound 1 (Fig. S2) exhibited a molecular ion at m/z 178 and matched mass spectral data published for the isocoumarin derivative mellein (16, 17). The optical rotation value of the purified compound 1 [(αD) = −102° (CH2Cl2, c = 0.05)] was in agreement with that reported for (R)-(-)-mellein [(R)-(-)-3,4-diydro-8-hydroxy-3-methylisocoumarin] (18). To confirm the identity of compound 1, we synthesized (R)-(-)-mellein by published methods (19). Mass spectral data, retention time, and optical rotation of the synthesized (R)-(-)-mellein matched the data of the natural product. Thus, compound 1 was unambiguously identified as (R)-(-)-mellein. Apart from (R)-(-)-mellein, the oral secretion of A. compressa larvae contained some structurally related minor compounds, which were tentatively identified as hydroxylated mellein derivatives (Fig. 2 and Table 1).
The mass spectrum of compound 8 (Fig. S3) had a molecular ion at m/z 280 and matched mass spectral data reported for a monounsaturated C18-γ-lactone (20–22). Derivatization of compound 8 with dimethyl disulfide (23) resulted in a product showing a molecular ion at m/z 374 and diagnostic ions at m/z 173 and 201 (Fig. S3), which is indicative of a double bond in position 9 (20). To elucidate the absolute configuration at carbon atom 4, we analyzed compound 8 and a racemic mixture of synthetic (9Z)-octadec-9-en-4-olide, as well as the pure (S)-enantiomer on an enantioselective β-DEX-225 GC column. Compound 8 coeluted with the earlier eluting (R)-enantiomer. To confirm the identity and absolute configuration of compound 8, we synthesized (4R,9Z)-octadec-9-en-4-olide according to refs. 24 and 25. The synthetic compound and the natural compound had identical mass spectra and retention times on both conventional and enantioselective GC columns. The synthesis of the (Z)-configured lactone yielded the (E)-configured isomer as a by-product that eluted slightly after the (Z)-configured isomer on the nonpolar GC column (20). Comparison with the natural product revealed that it contained only the (Z)-configured isomer. Therefore, the identity of compound 8 could be confirmed as (4R,9Z)-octadec-9-en-4-olide, also known under the common name micromolide (22), which will be used in the following text. Apart from micromolide, the oral secretion of A. compressa contained minor amounts of some additional long-chain γ-lactones (Fig. 2 and Table 1).
Quantitative analysis of single parasitized cockroaches (Table 1) revealed that (R)-(-)-mellein was by far the most prevalent compound, followed by micromolide. The total amount of the nine larval compounds per nest varied between 517 and 5,394 µg with a mean of 2,254 ± 1,869 µg.
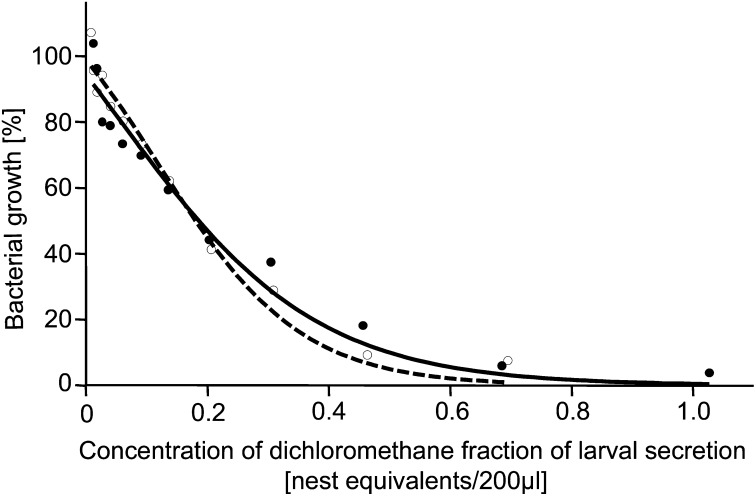
As a next step, we tested our hypothesis that the oral secretion applied by A. compressa larvae to their cockroach hosts provides protection against antagonistic microbes. To this end, we tested the antibacterial activity of the dichloromethane fraction containing (R)-(-)-mellein and micromolide in broth microdilution assays against the isolated Gram-negative entomopathogen S. marcescens and against Staphylococcus hyicus as a selected Gram-positive bacterium. We determined the IC50, i.e., the concentration of the antimicrobial compounds that is required to reduce bacterial growth by 50%, and we defined a “nest equivalent” as the average amount of antimicrobials found in one parasitized cockroach containing an A. compressa cocoon. The dichloromethane fraction effectively inhibited growth of S. marcescens (Fig. 3). There was a pronounced dose–response relationship with an IC50 of 0.18 nest equivalents/200 µL broth (Materials and Methods and SI Materials and Methods). A similar effect was found against S. hyicus, with an IC50 of 0.19 nest equivalents/200 µL. Thus, the fraction of the larval secretion containing (R)-(-)-mellein and micromolide clearly showed activity against both tested bacteria.
Fig. 3.
Percentage of growth relative to controls of bacteria grown in the presence of varying concentrations of the dichloromethane fraction of the larval secretion. Growth was measured as optical density in broth microdilution assays. Logistic regressions with Levenberg–Marquardt correction were fitted to the data. Open circles and dashed line: S. marcescens; filled circles and solid line: S. hyicus.
To elucidate the role of the two major compounds of the larval secretion separately, we tested synthetic (R)-(-)-mellein and micromolide in broth microdilution assays. (R)-(-)-mellein alone exhibited antibacterial activity against S. marcescens with an IC50 of 0.11 nest equivalents/200 µL. The highest concentration showed complete growth inhibition. Against S. hyicus, (R)-(-)-mellein was only mildly active with a growth inhibition of 66% at the highest concentration of about 0.7 nest equivalents/200 µL. Micromolide was hardly active against S. marcescens when tested alone; only the two highest concentrations affected its growth (inhibition of 16% and 26%, respectively). Against S. hyicus, micromolide was active with an IC50 value of 1.4 nest equivalents/200 µL. Concentrations of ≥2.7 nest equivalents/200 µL completely inhibited growth of S. hyicus. Both (R)-(-)-mellein and micromolide thus inhibited bacterial growth in vitro but showed differential activity against the Gram-negative and Gram-positive target strains.
The antibacterial activity of (R)-(-)-mellein alone could explain the inhibitory effect of the fractionated secretion against the Gram-negative S. marcescens in our tests. However, neither (R)-(-)-mellein nor micromolide alone could explain the effect against the Gram-positive S. hyicus. We therefore performed an additional broth microdilution experiment to test whether the antimicrobial activity of the bioactive fraction was due to the combined activity of (R)-(-)-mellein and micromolide. A blend of the two synthetic compounds in the same ratio as found in the bioactive fraction showed the same activity as this fraction (median growth inhibition ± median absolute deviation: 91 ± 1.17% vs. 92 ± 1.17%; n = 6, U = 17, exact P = 0.9).
Discussion
As cockroaches are the exclusive hosts of A. compressa larvae, representing both their food source and microenvironment, the wasp larvae need effective defense mechanisms against competitive, putrefactive, and pathogenic microbes (8–13). In this study, we found clear evidence that A. compressa larvae are capable of coping with antagonistic microbes inside their P. americana hosts by using a mixture of antimicrobials present in their oral secretion.
We isolated the bacterium S. marcescens as the by far most abundant microbe from P. americana host cockroaches. S. marcescens (Enterobacteriaceae) is a ubiquitous, Gram-negative bacterium and a common colonizer of cockroaches (10–13). It is responsible for food contamination (26) and causes disease in plants (27), invertebrates (4, 26, 28), and vertebrates, including humans (29). Most important for this study, S. marcescens has been shown to cause severe septicemia in insect larvae, leading to their rapid death (4, 28, 30). S. marcescens thus may pose severe threats to the developing A. compressa offspring, which have to defend themselves against this virulent microbe.
Our behavioral observations and chemical analyses revealed that the larvae excreted and dispersed an oral secretion inside their cockroach hosts. This larval secretion contained the two major compounds (R)-(-)-mellein and micromolide, as well as some minor compounds. The same composition of compounds could be extracted from P. americana cockroaches parasitized by A. compressa in large quantities but not from unparasitized cockroaches. We thus conclude that the wasp larvae impregnate their cockroach hosts with these compounds from inside.
The dichloromethane fraction of the secretion containing (R)-(-)-mellein and micromolide showed antibacterial activity against S. marcescens and S. hyicus. Even though it is not known whether our chosen test strain, S. hyicus, is a natural target of the larval secretion, our assays demonstrate the potential of the larval secretion to inhibit growth of both Gram-negative as well as Gram-positive bacteria. These findings suggest that the wasp larvae sanitize their hosts with an antimicrobial secretion. The results of our assays revealed that the antimicrobial activity of the larval secretion is mediated by the two major compounds identified in this study. We could demonstrate that (R)-(-)-mellein effectively inhibits the growth of the Gram-negative S. marcescens and that it is mildly active against the Gram-positive S. hyicus. (R)-(-)-mellein has previously been isolated from diverse fungi (e.g., refs. 31 and 32), plants (33), and insects (34, 35). It has been shown to have antibacterial activity against standard and methicillin-resistant strains of Staphylococcus aureus (36). Moreover, it has antifungal activity (37, 38) and is a potent inhibitor of hepatitis C virus protease NS3 (39). However, to our knowledge, (R)-(-)-mellein has never been reported before as an antimicrobial agent used by an insect.
The second major compound of the larval secretion, micromolide, displayed clear growth-inhibiting properties against S. hyicus, but not against S. marcescens. Micromolide, which has previously been isolated as the sex pheromone of the currant stem girdler Janus integer [Hymenoptera, Cephidae (20, 21)] and from the bark of Micromelum hirsutum [Sapindales, Rutaceae (22)], is one of the most active compounds against the causative agent of tuberculosis, Mycobacterium tuberculosis and is thought to be a promising lead compound for the development of new antituberculosis drugs (22).
The combination of (R)-(-)-mellein and micromolide, as found in the secretion of A. compressa larvae, has so far not been reported from any natural source. As this blend of compounds can be seen as the result of an evolutionary arms race between the wasp and pathogenic microorganisms over long periods of time, one can expect that the use of this particular mixture lends a decisive advantage to the wasp larvae with regard to survival and/or well-being. Our antimicrobial assays revealed that the effect of the bioactive fraction against S. marcescens could be explained by the activity of (R)-(-)-mellein alone. Against S. hyicus, the synthetic blend of micromolide and (R)-(-)-mellein was similarly active in our assays compared with the bioactive fraction. This suggests that the inhibitory effect of the fraction against S. hyicus is mediated by the combined activity of micromolide and (R)-(-)-mellein. Hence, our own results and previous reports (22, 36–39) suggest that the specific combination of antimicrobials present in the larval secretion of A. compressa exert activity against a wide range of different microbes.
Such a broad-spectrum defense may be indispensable to the life of A. compressa larvae. The range of microbes that A. compressa larvae may encounter during their development in their hosts is unpredictable and may encompass all different kinds of microbes, such as various Gram-positive and Gram-negative bacteria, mycobacteria, viruses, yeasts, and filamentous fungi (8, 9, 11, 12, 14, 40). An effective strategy to gain a reliable and enduring protection against a broad spectrum of microbes may therefore be the application of large amounts of a mixture of several relatively unspecific antimicrobial compounds (41, 42).
The focus of the present study was to investigate the major compounds of the larval secretion. The methods used for extraction and fractionation were optimized to achieve this goal. Hence, the crude larval secretion might contain additional polar compounds that contribute to its antimicrobial property but that were not covered by our analytical approach. However, taking into account that the concentrations of (R)-(-)-mellein and micromolide in the antimicrobial assays are similar to those found in the cockroaches (SI Materials and Methods), the IC50 values determined here for S. marcescens and S. hyicus indicate that the average amount of antimicrobials released by a single larva is ample to effectively impair bacterial growth in its cockroach host. The large amounts of antimicrobials deployed suggest that A. compressa larvae invest a considerable portion of their resources into their antimicrobial defense.
Given that virtually all insect species are threatened by pathogenic and competing microorganisms, it can be assumed that all species have to use some kind of countermeasure. Recently, such antimicrobial defense mechanisms of insects have received growing attention, and their potential as valuable sources of natural products and therapies has been acknowledged (43–47).
Food hygiene may be of vital importance, especially to the vulnerable early developmental stages of insects to evade the threats of antagonistic microbes. Even though the antimicrobial strategies used to protect the immatures of insects from food-associated microbes remain rather unexplored, there are some examples that demonstrate the diversity and ingenuity of remedial measures that insect larvae or their parents use against food-associated microbes. Larvae of the parasitoid ichneumonoid wasp Pimpla turionellae, for example, discharge an antimicrobial anal secretion of unknown composition inside their lepidopteran host pupae to secure their survival (48). Females of the parasitoid wasp Philanthus triangulum embalm their prey bees with a lipid secretion that reduces water condensation and in this way inhibit the growth of fungal decomposers on the larval provisions (49). Adult burying beetles of the genus Nicrophorus use antimicrobial oral and anal exudates to protect the carrion that serves as larval food from microbes (46, 50).
We finally conclude that the impregnation of the host with large amounts of antimicrobial substances is a (food-) hygienic behavior of the A. compressa larva that grants protection against antagonistic microbes during development inside the cockroach host. The secretion of a blend of antimicrobials with broad-spectrum activity seems to represent an essential frontline defense strategy against diverse putrefactive and entomopathogenic microbes.
Materials and Methods
Insects.
P. americana parasitized by A. compressa were obtained from laboratory populations kept at the University of Regensburg (SI Materials and Methods).
Isolation and Identification of Microorganisms from Cockroaches.
The outer surfaces of four P. americana were probed with nylon-flocked swabs (microRheologics), which were then streaked onto lysogeny broth (LB) agar plates. Four additional P. americana cockroaches were surface-sterilized with 70% (vol/vol) ethanol and dissected with sterile dissecting scissors and forceps to remove thoracic muscle tissue, as this is the tissue that A. compressa larvae feed on. The muscle tissue was then streaked onto LB agar plates. The plates were incubated at 30 °C until visible growth of microbes. Morphological characteristics of the cultivated strains were recorded. Single bacterial colonies were transferred twice to new LB agar plates to obtain pure strains. Genetic analyses were performed as described in SI Materials and Methods.
Behavioral Observations.
To study the behavior of A. compressa larvae inside their hosts, parasitized P. americana (n = 7) were dissected 8 d after oviposition by the A. compressa female. The abdominal cavity of the cockroach was opened by carefully removing the right half of each sternite. The hole in the cockroach carcass was covered by a glass coverslip, which allowed the observation of the larva. For documentation of the behavior, high-definition movies and photos were taken with a Sony NEX-5 digital camera mounted on a Zeiss OPMI stereomicroscope.
A. compressa larvae could be observed excreting small droplets of liquid onto the coverslip (Results). These droplets of larval secretion were taken up with a SPME fiber (100 µm polydimethylsiloxane; Supelco) by wiping the fiber in the droplets. The fiber was then allowed to air dry for 1 min and subsequently analyzed by coupled GC/MS (SI Materials and Methods).
Isolation and Fractionation of Chemical Compounds.
Preliminary GC/MS analyses of extracts made with hexane, dichloromethane, and methanol revealed no marked differences. Given that compounds 1 and 8 were by far the most prevalent compounds in each of the extracts, we focused on the isolation and identification of these two major compounds (and the structurally related minor compounds) to test our hypothesis of their antibacterial activity.
For identification of putative antimicrobials, five parasitized cockroaches containing A. compressa cocoons were extracted in 10 mL hexane for 10 min. The crude hexane extract was then fractionated by column chromatography on a silica gel column (Chromabond 500 mg; Macherey and Nagel) with hexane and dichloromethane (8 mL each) as the mobile phase to separate nonpolar hydrocarbons from the more polar target compounds. GC/MS analyses revealed that the hexane fraction contained only hydrocarbons, whereas the dichloromethane fraction contained the more polar target compounds. Using dichloromethane as the mobile phase, the dichloromethane fraction of the extract was further fractionated by SE-HPLC (SI Materials and Methods) to separate (R)-(-)-mellein and the minor isocoumarine derivatives from micromolide and the minor γ-lactones. Two fractions containing the γ-lactones (fraction 1, retention time 6.35–7.20 min) and the isocoumarins (fraction 2, 7.34–8.04 min), respectively, were collected manually.
Identification of Chemical Compounds.
Identification of the putatively antimicrobial compounds was accomplished by use of GC/MS, enantioselective gas chromatography, and determination of optical rotation (SI Materials and Methods). Iodine-catalyzed methylthiolation using dimethyl disulfide (23) was performed to determine the positions of the double bonds of unsaturated compounds.
(R)-(-)-mellein was synthesized according to ref. 19, and its purity was confirmed by comparing the NMR spectrum, melting point, and optical rotation value with data previously reported (18). Micromolide was synthesized according to refs. 24 and 25, and its purity was confirmed by comparing the NMR spectrum, melting point, and optical rotation value with data previously reported (51). A racemic mixture of (9Z)-octadec-9-en-4-olide and enantiomerically pure (4S,9Z)-octadec-9-en-4-olide were kindly provided by Allard Cossé, Agricultural Research Service, Department of Agriculture, Preoria, IL. Racemic mixtures of hexadecan-4-olide and octadecan-4-olide were kindly provided by Lukas J. Goossen and Dominik M. Ohlmann, Fachbereich Chemie, Technische Universität Kaiserslautern, Germany (52).
(R)-(-)-mellein was identified in the natural sample by comparing the mass spectrum, retention time on a nonpolar GC column, and optical rotation value with data obtained by analysis of the synthetic reference compound. The optical rotation values were measured with a Perkin-Elmer 241 polarimeter (c = 0.05 in dichloromethane). Micromolide and the two minor lactones, hexadecan-4-olide and octadecan-4-olide, were identified by comparing their mass spectra and retention times on both nonpolar and enantioselective GC columns with data obtained by the analyses of the synthetic reference compounds. For information on the minor compounds, see SI Materials and Methods. To confirm the absolute configuration of the γ-lactones 8 (micromolide), 5, and 9, we performed enantioselective gas chromatography on a β-DEX 225 stationary phase (SI Materials and Methods). Hydrocarbons in the nonpolar fraction were identified as previously described (53).
Quantification of Chemical Compounds.
For quantification of the antimicrobials found on single parasitized cockroaches, 10 cockroaches containing A. compressa cocoons were individually extracted in 4 mL of dichloromethane containing 0.05 mg⋅mL−1 octadecane as an internal standard under gentle agitation for 2 h. As controls, eight unparasitized cockroaches were extracted in the same way. An aliquot of 1 µL of each sample was used for GC/MS analysis. Quantification of (R)-(-)-mellein and micromolide in individual extracts was done by the internal standard method. For this purpose, calibration curves were created by analyses of a dilution series of synthetic (R)-(-)-mellein and micromolide dissolved in dichloromethane containing the internal standard. Values given are means ± 1 SD.
In Vitro Tests of Antimicrobial Activity: Broth Microdilution Assays.
The antibacterial activity of the dichloromethane fraction of the larval secretion containing (R)-(-)-mellein and micromolide was investigated with broth microdilution assays (SI Materials and Methods) against the target strains S. marcescens (isolated in the present study) and S. hyicus (obtained from the Institute of Microbiology at the University of Regensburg). S. marcescens was used because it was isolated from P. americana in this study and represents a potential threat to A. compressa immatures. S. hyicus was chosen to test the activity of the dichloromethane fraction of the larval secretion against a representative of the Gram-positive bacteria. Synthetic samples of (R)-(-)-mellein and micromolide were assayed individually for their antibacterial activity.
The data were visualized as concentration vs. growth response curves. Nonlinear regression was performed by fitting logistic regression lines with a Levenberg–Marquardt correction to the data set using the statistics software package PAST, Version 2.5 (54). The IC50 was calculated by using the regression equations.
To verify that the antibacterial activity of the dichloromethane fraction was mediated by (R)-(-)-mellein and micromolide and not by an unknown nonvolatile component that was not detectable in our GC/MS analyses, we tested the antimicrobial activity of a synthetic blend of (R)-(-)-mellein and micromolide in the same ratio as found in the bioactive dichloromethane fraction against this fraction in broth microdilution experiments against S. hyicus (SI Materials and Methods). The median growth inhibition of the fraction and the synthetic blend was compared with an exact Mann–Whitney U test.
Supplementary Material
Acknowledgments
G.H. thanks Erhard Strohm for his unwavering and universal support. Reinhard Wirth is acknowledged for providing valuable support in all microbiological issues and Agnes Paech for technical assistance and tending the cockroaches and wasps. Sylvia Cremer and Jürgen Heinze are acknowledged for providing access to the microplate reader and Simon Tragust for providing technical assistance. Oliver Reiser is gratefully acknowledged for providing financial support. We thank three anonymous reviewers for their helpful comments on a previous version of the manuscript and Jennifer McCaw for proofreading the manuscript. The study was supported by a grant from the Forschungsrat of the University of Regensburg (to G.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. JX448402).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213384110/-/DCSupplemental.
References
- 1.Janzen D. Why fruits rot, seeds mold, and meat spoils. Am Nat. 1977;111(980):691–713. [Google Scholar]
- 2.Scallan E, et al. Foodborne illness acquired in the United States: Major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Food Safety Authority 2011. Tracing seeds, in particular fenugreek (Trigonella foenum-graecum) seeds, in relation to the Shiga toxin-producing E. coli (STEC) O104:H4 2011 Outbreaks in Germany and France. www.efsa.europa.eu/de/supporting/doc/176e.pdf.
- 4.Flyg C, Kenne K, Boman HG. Insect pathogenic properties of Serratia marcescens: Phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. J Gen Microbiol. 1980;120(1):173–181. doi: 10.1099/00221287-120-1-173. [DOI] [PubMed] [Google Scholar]
- 5.Blazar JM, Lienau EK, Allard MW. Insects as vectors of foodborne pathogenic bacteria. Terr Arthropod Rev. 2011;4(1):5–16. [Google Scholar]
- 6.Rueger ME, Olson TA. Cockroaches (Blattaria) as vectors of food poisoning and food infection organisms. J Med Entomol. 1969;6(2):185–189. doi: 10.1093/jmedent/6.2.185. [DOI] [PubMed] [Google Scholar]
- 7.Rust MK. In: Public Health Significance of Urban Pests. 2. Cockroaches. Bonnefoy X, Kampen H, Sweeney K, editors. Copenhagen: World Health Organization Europe; 2008. pp. 53–84. [Google Scholar]
- 8.Fakoorziba MR, Eghbal F, Hassanzadeh J, Moemenbellah-Fard MD. Cockroaches (Periplaneta americana and Blattella germanica) as potential vectors of the pathogenic bacteria found in nosocomial infections. Ann Trop Med Parasitol. 2010;104(6):521–528. doi: 10.1179/136485910X12786389891326. [DOI] [PubMed] [Google Scholar]
- 9.Lemos AA, et al. Cockroaches as carriers of fungi of medical importance. Mycoses. 2006;49(1):23–25. doi: 10.1111/j.1439-0507.2005.01179.x. [DOI] [PubMed] [Google Scholar]
- 10.Prado MA, Gir E, Pereira MS, Reis C, Pimenta FC. Profile of antimicrobial resistance of bacteria isolated from cockroaches (Periplaneta americana) in a Brazilian health care institution. Braz J Infect Dis. 2006;10(1):26–32. doi: 10.1590/s1413-86702006000100006. [DOI] [PubMed] [Google Scholar]
- 11.Pai H-H, Chen W-C, Peng C-F. Cockroaches as potential vectors of nosocomial infections. Infect Control Hosp Epidemiol. 2004;25(11):979–984. doi: 10.1086/502330. [DOI] [PubMed] [Google Scholar]
- 12.Pai H-H, Chen W-C, Peng C-F. Isolation of bacteria with antibiotic resistance from household cockroaches (Periplaneta americana and Blattella germanica) Acta Trop. 2005;93(3):259–265. doi: 10.1016/j.actatropica.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Chaichanawongsaroj N, Vanichayatanarak K, Pipatkullachat T, Polrojpanya M, Somkiatcharoen S. Isolation of gram-negative bacteria from cockroaches trapped from urban environment. Southeast Asian J Trop Med Public Health. 2004;35(3):681–684. [PubMed] [Google Scholar]
- 14.Baumholtz MA, Parish LC, Witkowski JA, Nutting WB. The medical importance of cockroaches. Int J Dermatol. 1997;36(2):90–96. doi: 10.1046/j.1365-4362.1997.00077.x. [DOI] [PubMed] [Google Scholar]
- 15.Haspel G, Gefen E, Ar A, Glusman J-G, Libersat F. Parasitoid wasp affects metabolism of cockroach host to favor food preservation for its offspring. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191(6):529–534. doi: 10.1007/s00359-005-0620-1. [DOI] [PubMed] [Google Scholar]
- 16.Harris JP, Mantle PG. Biosynthesis of ochratoxins by Aspergillus ochraceus. Phytochemistry. 2001;58(5):709–716. doi: 10.1016/s0031-9422(01)00316-8. [DOI] [PubMed] [Google Scholar]
- 17.Jeleń HH, Grabarkiewicz-Szczesna J. Volatile compounds of Aspergillus strains with different abilities to produce ochratoxin A. J Agric Food Chem. 2005;53(5):1678–1683. doi: 10.1021/jf0487396. [DOI] [PubMed] [Google Scholar]
- 18.Sadequl Islam M, Ishigami K, Watanabe H. Synthesis of (-)-mellein, (+)-ramulosin, and related natural products. Tetrahedron. 2007;63:1074–1079. [Google Scholar]
- 19.Saito F, et al. Synthesis and absolute configuration of (+)-pseudodeflectusin: Structural revision of aspergione B. Eur J Org Chem. 2006;2006(21):4796–4799. [Google Scholar]
- 20.Cossé AA, Bartelt RJ, James DG, Petroski RJ. Identification of a female-specific, antennally active volatile compound of the currant stem girdler. J Chem Ecol. 2001;27(9):1841–1853. doi: 10.1023/a:1010412826373. [DOI] [PubMed] [Google Scholar]
- 21.James DG, Petroski RJ, Cossé AA, Zilkowski BW, Bartelt RJ. Bioactivity, synthesis, and chirality of the sex pheromone of currant stem girdler, Janus integer. J Chem Ecol. 2003;29(10):2189–2199. doi: 10.1023/a:1026210111334. [DOI] [PubMed] [Google Scholar]
- 22.Ma C, et al. Anti-tuberculosis constituents from the stem bark of Micromelum hirsutum. Planta Med. 2005;71(3):261–267. doi: 10.1055/s-2005-837826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard RW. In: Insect Lipids: Chemistry, Biochemistry and Biology, Cuticular Hydrocarbons and Chemical Communication. Stanley-Samuelson DW, Nelson DR, editors. Lincoln, NE: Univ of Nebraska Press; 1993. pp. 179–226. [Google Scholar]
- 24.Habel A, Boland W. Efficient and flexible synthesis of chiral gamma- and delta-lactones. Org Biomol Chem. 2008;6(9):1601–1604. doi: 10.1039/b801514g. [DOI] [PubMed] [Google Scholar]
- 25.Haynes SW, Sydor PK, Corre C, Song L, Challis GL. Stereochemical elucidation of streptorubin B. J Am Chem Soc. 2011;133(6):1793–1798. doi: 10.1021/ja109164t. [DOI] [PubMed] [Google Scholar]
- 26.Li B, et al. Characterization and comparison of Serratia marcescens isolated from edible cactus and from silkworm for virulence potential and chitosan susceptibility. Braz J Microbiol. 2011;42(1):96–104. doi: 10.1590/S1517-83822011000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rascoe J, et al. Identification, phylogenetic analysis, and biological characterization of Serratia marcescens strains causing cucurbit yellow vine disease. Phytopathology. 2003;93(10):1233–1239. doi: 10.1094/PHYTO.2003.93.10.1233. [DOI] [PubMed] [Google Scholar]
- 28.Li B, et al. Effect of chitosan solution on the bacterial septicemia disease of Bombyx mori (Lepidoptera: Bombycidae) caused by Serratia marcescens. Appl Entomol Zool (Jpn) 2010;45:145–152. [Google Scholar]
- 29.Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 30.Sikorowski P, Lawrence A, Inglis G. Effects of Serratia marcescens on rearing of the tobacco budworm. Am Entomol. 2001;47(1):51–60. [Google Scholar]
- 31.Parisi A, Piattelli M, Tringali C, Magnano di san Lio G. Identification of the phytotoxin Mellein in culture fluids of Phoma tracheiphila. Phytochemistry. 1993;32(4):865–867. [Google Scholar]
- 32.Djoukeng JD, Polli S, Larignon P, Abou-Mansour E. Identification of phytotoxins from Botryosphaeria obtusa, a pathogen of black dead arm disease of grapevine. Eur J Plant Pathol. 2009;124:303–308. [Google Scholar]
- 33.Manigaunha A, Ganesh N, Kharya M. Morning glory: A new thirst in-search of de-novo therapeutic approach. Phytomedicine. 2010;2(1):18–21. [Google Scholar]
- 34.Kalinová B, et al. Composition and electrophysiological activity of constituents identified in male wing gland secretion of the bumblebee parasite Aphomia sociella. J Nat Prod. 2009;72(1):8–13. doi: 10.1021/np800434x. [DOI] [PubMed] [Google Scholar]
- 35.Bestmann HJ, Kern F, Schäfer D, Witschel MC. 3, 4-Dihydroisocoumarins, a new class of ant trail pheromones. Angew Chem Int Ed Engl. 1992;31:795–796. [Google Scholar]
- 36.Rukachaisirikul V, Arunpanichlert J, Sukpondma Y, Phongpaichit S, Sakayaroj J. Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PSU-M114. Tetrahedron. 2009;65(51):10590–10595. [Google Scholar]
- 37.Krohn K, et al. Dihydroisocoumarins from fungi: Isolation, structure elucidation, circular dichroism and biological activity. Phytochemistry. 1997;45(2):313–320. doi: 10.1016/s0031-9422(96)00854-0. [DOI] [PubMed] [Google Scholar]
- 38.Höller U, König GM, Wright AD. Three new metabolites from marine-derived fungi of the genera Coniothyrium and Microsphaeropsis. J Nat Prod. 1999;62(1):114–118. doi: 10.1021/np980341e. [DOI] [PubMed] [Google Scholar]
- 39.Dai JR, et al. Circumdatin G, a new alkaloid from the fungus Aspergillus ochraceus. J Nat Prod. 2001;64(1):125–126. doi: 10.1021/np000381u. [DOI] [PubMed] [Google Scholar]
- 40.Oothuman P, Jeffery J, Aziz AHA, Abu Bakar E, Jegathesan M. Bacterial pathogens isolated from cockroaches trapped from paediatric wards in peninsular Malaysia. Trans R Soc Trop Med Hyg. 1989;83(1):133–135. doi: 10.1016/0035-9203(89)90739-6. [DOI] [PubMed] [Google Scholar]
- 41.Kaltenpoth M, Göttler W, Herzner G, Strohm E. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr Biol. 2005;15(5):475–479. doi: 10.1016/j.cub.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 42.Kroiss J, et al. Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat Chem Biol. 2010;6(4):261–263. doi: 10.1038/nchembio.331. [DOI] [PubMed] [Google Scholar]
- 43.Röhrich CR, et al. Harmonine, a defence compound from the harlequin ladybird, inhibits mycobacterial growth and demonstrates multi-stage antimalarial activity. Biol Lett. 2012;8(2):308–311. doi: 10.1098/rsbl.2011.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poulsen M, Oh D-C, Clardy J, Currie CR. Chemical analyses of wasp-associated streptomyces bacteria reveal a prolific potential for natural products discovery. PLoS ONE. 2011;6(2):e16763. doi: 10.1371/journal.pone.0016763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dossey AT. Insects and their chemical weaponry: New potential for drug discovery. Nat Prod Rep. 2010;27(12):1737–1757. doi: 10.1039/c005319h. [DOI] [PubMed] [Google Scholar]
- 46.Rozen DE, Engelmoer DJP, Smiseth PT. Antimicrobial strategies in burying beetles breeding on carrion. Proc Natl Acad Sci USA. 2008;105(46):17890–17895. doi: 10.1073/pnas.0805403105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vilcinskas A. In: Insect Biotechnology: From Traditional Maggot Therapy to Modern Biosurgery. Vilcinskas A, editor. Vol 2. Heidelberg, Germany: Springer; 2011. pp. 67–75. [Google Scholar]
- 48.Führer E, Willers D. The anal secretion of the endoparasitic larva Pimpla turionellae: Sites of production and effects. J Insect Physiol. 1986;32(4):361–367. [Google Scholar]
- 49.Herzner G, Strohm E. Fighting fungi with physics: Food wrapping by a solitary wasp prevents water condensation. Curr Biol. 2007;17(2):R46–R47. doi: 10.1016/j.cub.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 50.Degenkolb T, Düring RA, Vilcinskas A. Secondary metabolites released by the burying beetle Nicrophorus vespilloides: Chemical analyses and possible ecological functions. J Chem Ecol. 2011;37(7):724–735. doi: 10.1007/s10886-011-9978-4. [DOI] [PubMed] [Google Scholar]
- 51.Mori K. Concise synthesis of (4R, 9Z)-octadec-9-en-4-olide, the female sex pheromone of Janus integer. Eur J Org Chem. 2005;2005(10):2040–2044. [Google Scholar]
- 52.Goossen LJ, Ohlmann DM, Dierker M. Silver triflate-catalysed synthesis of γ-lactones from fatty acids. Green Chem. 2010;12:197–200. [Google Scholar]
- 53.Herzner G, et al. Structure, chemical composition and putative function of the postpharyngeal gland of the emerald cockroach wasp, Ampulex compressa (Hymenoptera, Ampulicidae) Zoology (Jena) 2011;114(1):36–45. doi: 10.1016/j.zool.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Paleontologia Electronica. 2001;4(1):1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.