Abstract
Penicillium chrysogenum is a filamentous fungus of major medical and historical importance, being the original and present-day industrial source of the antibiotic penicillin. The species has been considered asexual for more than 100 y, and despite concerted efforts, it has not been possible to induce sexual reproduction, which has prevented sexual crosses being used for strain improvement. However, using knowledge of mating-type (MAT) gene organization, we now describe conditions under which a sexual cycle can be induced leading to production of meiotic ascospores. Evidence of recombination was obtained using both molecular and phenotypic markers. The identified heterothallic sexual cycle was used for strain development purposes, generating offspring with novel combinations of traits relevant to penicillin production. Furthermore, the MAT1-1–1 mating-type gene, known primarily for a role in governing sexual identity, was also found to control transcription of a wide range of genes with biotechnological relevance including those regulating penicillin production, hyphal morphology, and conidial formation. These discoveries of a sexual cycle and MAT gene function are likely to be of broad relevance for manipulation of other asexual fungi of economic importance.
Keywords: sexual recombination, secondary metabolism, ascomycete
Filamentous fungi are of great value to the pharmaceutical industry because of their extensive secondary metabolism (1). Examples of fungal products include statins from Aspergillus terreus and Penicillium citrinum, immunosuppressants from Tolypocladium inflatum and Penicillium brevicompactum, and antibiotics from Acremonium chrysogenum and Penicillium chrysogenum. Strain improvement programs generally use random mutagenesis and, more recently, recombinant technologies to generate improved derivatives (2). A common feature of most industrial filamentous fungi is that they lack a sexual cycle, which has prevented the generation of novel strains by sexual crossing. This method offers particular advantages because crosses can be set up between isolates with different desirable traits, and meiotic recombination occurs throughout the whole genome, potentially generating considerable genetic variation for screening purposes (2).
P. chrysogenum is the major industrial source of the beta-lactam antibiotic penicillin, which has annual worldwide sales of about US$ 8 billion (3). Sir Alexander Fleming made the fortuitous discovery of penicillin as a result of a contaminant, P. chrysogenum, inhibiting growth of a bacterial culture. Fifteen years later, a higher-yielding strain, NRRL1951, was isolated at the US Department of Agriculture Northern Regional Research Laboratory (NRRL) in Peoria, Illinois, from a moldy cantaloupe, which generated sufficient amounts for the commercial production of penicillin (4). Since then, conventional mutagenesis programs have been used to develop strains with elevated penicillin titers. All P. chrysogenum production strains currently used worldwide are derivatives of NRRL1951 and show amplification of the genomic region encoding penicillin biosynthesis genes (5). Recent phylogenetic analyses have revealed that P. chrysogenum sensu lato is composed of at least two distinct species, Penicillium rubens and P. chrysogenum sensu stricto, with Fleming’s strain and NRRL1951 reidentified as P. rubens (6, 7). However, for the purposes of this study, we refer to all isolates as P. chrysogenum given that this is a nomen conservandum (8).
P. chrysogenum is only known to reproduce by asexual means. However, accumulating evidence suggests that it might have the potential for sexual reproduction with an unidentified or “cryptic” sexual stage present (9). We recently discovered mating-type (MAT) and pheromone signaling genes in P. chrysogenum (10), which are involved with mating in other sexual fungi (11). For sex to occur in heterothallic (obligate outcrossing) ascomycete fungi, complementary MAT1-1 and MAT1-2 isolates must be present (11). Significantly, a MAT1-1 locus, with a MAT1-1–1 gene encoding a putative alpha-box transcription factor, is present in NRRL1951 and all its derivatives, whereas the original Fleming strain contains the opposite MAT1-2 locus (10). In addition, recombination has been reported within natural populations of P. chrysogenum together with a near 1:1 distribution of MAT1-1 and MAT1-2 isolates (6), and there is evidence of repeat induced point mutation in the genome, a process associated with meiosis (12).
Recent findings suggest that sexual reproduction can be triggered in supposedly asexual fungi (13–16) if the correct growth conditions are identified (17, 18). The principle aim of the current study was therefore to determine whether a functional sexual cycle could be induced in P. chrysogenum, using knowledge of MAT gene organization in the species to set up directed crosses between known MAT1-1 and MAT1-2 isolates, and if the sexual cycle could be used for strain development purposes. We also investigated whether MAT genes, which are defined primarily by their role in governing sexual identity (11, 19), might have additional roles in regulating other developmental processes of biotechnological relevance.
Results
Induction of a Sexual Life Cycle in P. chrysogenum.
Applying knowledge of MAT gene presence, we set up 24 crosses between P. chrysogenum strains of known MAT1-1 and MAT1-2 genotype. These strains were either wild type (from different geographic origins) or production strains with high penicillin titers (Table S1). Various combinations of growth media and pairings were tested, including the use of conditions recently shown to induce sexual reproduction in Aspergillus fumigatus and related aspergilli (18, 20). When crosses were performed on different oatmeal agars in sealed Petri dishes in the dark at 15–27 °C, cleistothecia [sexual reproductive structures characteristic of Penicillium species and some other ascomycete fungi (18, 21)] were formed in most but not all crosses in the contact zone of the two mating partners after incubation from 5 wk to 3 mo, but these were sterile with no ascospores produced.
However, in one cross, Q176 (MAT1-1; a derivative of NRRL1951) × IB 08/921 (MAT1-2; wild type), when the oatmeal agar was supplemented with biotin, cleistothecia were produced within 5 wk that contained viable ascospores (Fig. 1A and Table S1). Thus, the addition of biotin was necessary for completion of the sexual cycle. Biotin is also essential for sexual development in certain Sordaria and Chaetomium species (22).
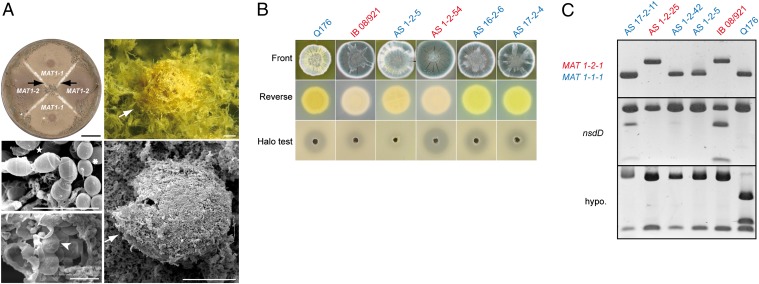
Fig. 1.
The sexual cycle of P. chrysogenum. (A) Paired culture of Q176 (MAT1-1) and IB 08/921 (MAT1-2) incubated for 5 wk at 20 °C in the dark on oatmeal agar supplemented with biotin. Cleistothecia were found at the junction zones (arrows) of mycelia from both mating types. Scale bar: 2 cm (Top Left). Light (Upper Right) and scanning electron (Middle and Bottom Left and Lower Right) micrographs illustrate cleistothecia (arrows), asci (arrowhead), and ascospores (star), with a chain of smaller conidia for comparison (asterisk). Scale bars: 10 µm (Middle and Bottom Left) and 100 μm (Lower Right). (B) Phenotypic evidence of recombination in selected ascospore progeny (AS), as indicated, compared with parental isolates Q176 and IB 08/921. Front and reverse views show conidial density and chrysogenin formation, respectively. The Bottom row shows results of a penicillin bioassay with halo formation on a bacterial lawn. (C) Molecular evidence of recombination by PCR analysis (Top) and restriction fragment length polymorphism (RFLP) analysis (Middle and Bottom). DNA from four ascospore lineages (designated AS) and two parental strains (Q176 and IB 08/921) was used for analysis with mating-type locus-specific primers. Mating types are distinguished by the size of PCR amplicons. For RFLP analysis, gene-specific primers were used for amplification of DNA, followed by restriction enzyme digestion with PdmI (nsdD) and HinfI (hypo.; Pc24g01940). Strain designation color indicates either MAT1-1 (blue) or MAT1-2 (red) genotype.
Molecular and Phenotypic Evidence for Recombination in the Ascospore Offspring.
To verify that sexual outcrossing had occurred, it was necessary to provide evidence of recombination. Therefore, more than 150 ascospore progeny from the Q176 × IB 08/921 cross were isolated. These parental isolates are phenotypically distinct: Q176 produces pale green conidia, the yellow pigment chrysogenin, and shows an elevated penicillin titer relative to most wild-type strains, whereas IB 08/921 produces dark green conidia, no detectable chrysogenin, and only has weak antibacterial activity. As shown in Fig. 1B, recombinant strains were found with novel characteristics derived from both parents. For example, offspring AS 16-2-6 had dark green conidia, chrysogenin production, and a distinct halo indicating significant penicillin biosynthesis. A very notable phenotype was seen in offspring AS 1-2-54, which lacked chrysogenin production (similar to parent IB 08/921), but which formed a marked clearing zone (similar to parent Q176). Chrysogenin contaminates crystalline penicillin powders, and during the 20th century commercial producers had to extract this pigment, resulting in a reduced penicillin yield (4). Thus, we demonstrate here that sexual crossing offers a way to bring together previously separate traits of interest to develop improved strains that have high penicillin titer and lack chrysogenin. This sexual crossing and offspring screening approach could also be applied to different fungi for the removal of other unwanted secondary metabolites such as mycotoxins (23).
Ascospore offspring were also screened for recombination at the molecular level. After comparing sequences from a total of 56 genes, 11 genes were identified across seven contigs of the P. chrysogenum genome that exhibited restriction site polymorphisms between the parental isolates, meaning that different alleles could be distinguished by a single restriction enzyme digest (Fig. 1C and Fig. S1 A and B). In addition, mating type was determined by Southern hybridization of genomic DNA with MAT-specific probes or PCR analysis with MAT-specific primer pairs (Fig. 1C). Consistent with the phenotypic data, screening of 36 recombinant ascospore lines revealed at least five different recombinant molecular phenotypes indicating independent chromosomal assortment (groups 1–5; Table 1). A strong bias toward the group 1 phenotype was observed, possibly explained by genome heterogeneity of the two unrelated mating partners preventing recombination of certain linkage groups. Two of the marker genes, nsdD and stuA, were present on the same single contig. Three of the progeny groups (groups 2, 4, and 5) contained nsdD and stuA alleles derived from the different parental strains, demonstrating that intrachromosomal recombination had occurred. Evidence of recombination was also obtained from a second cross of two F1 progeny from the original Q176 × IB 08/921 cross (Fig. S1C). Overall, these data confirmed that P. chrysogenum possesses a heterothallic sexual breeding system.
Table 1.
Molecular characterization of ascospore progeny from cross Q176 × IB 08/921
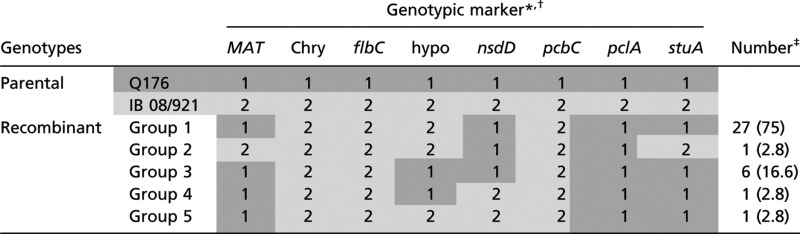 |
Progeny were classified into five recombinant groups based on 12 genotypic markers (eight shown) obtained by Southern hybridization or RFLP analysis (see Fig. 1C and Fig. S1). Numerical values (1 and 2) indicate whether the gene was derived from the MAT1-1 (dark gray shading) or the MAT1-2 (light gray shading) parent, respectively. Gene abbreviations and products: Chry, putative chrysogenin synthase; flbC, C2H2 conidiation transcription factor; hypo, hypothetical gene (Pc24g01940); nsdD, GATA-type sexual development transcription factor; pcbC, isopenicillin N synthase; pclA, phenylacetyl-CoA ligase; stuA, helix–loop–helix transcription factor.
*The following genes failed to identify additional recombinant phenotypes, most probably due to linkage with other marker genes (see Fig. S1B): flbB, bZIP transcription factor; fluG, developmental activator; penDE, acyl-CoA:isopenicillin N acyltransferase; UDP, UDP-glucose 4-epimerase like.
‡Number of progeny per group; number in parentheses indicates the percentage.
Construction of MAT1-1–1 Deletion and Overexpression Strains.
These findings indicated that the MAT genes were functional in P. chrysogenum in relation to sexual reproduction. This encouraged us to study the functionality of MAT genes in regard to other developmental pathways relevant to penicillin biosynthesis in P. chrysogenum. Strain P2niaD18, which has a high penicillin V titer under laboratory conditions and is a MAT1-1 derivative of NRRL1951, was chosen for study (10). An additional strain, ∆Pcku70, with a deleted ku70 gene was constructed from P2niaD18 to promote efficient gene replacement (24). ∆Pcku70 was then transformed with a construct in which the MAT1-1–1 ORF was replaced with a phleomycin resistance cassette (Fig. S2A), leading to the production of two mutants (∆MAT1-1–1 EK5 and EK6) lacking the MAT1-1–1 gene. A complemented control strain (∆MAT1-1–1::MAT1-1–1) was then constructed by reinserting the MAT1-1–1 gene together with a terbinafine resistance marker (25). Finally, two MAT1-1–1 overexpression strains (P2::MAT1-1–1 T2 and T5) were constructed by transforming P2niaD18 with an insert with the MAT1-1–1 gene under control of the constitutive gpd promoter of Aspergillus nidulans. All recombinant strains were genetically characterized (Fig. S2 A–C) and are subsequently referred to as “recombinant MAT1 strains.” As predicted from previous studies with MAT gene deletants of A. nidulans, Gibberella zeae, and Sordaria macrospora (26–28), the ∆MAT1 deletion strains were sterile when crossed to the fertile MAT1-2 isolate IB 08/921, being unable to form ascospores, although cleistothecia were formed (Table S1).
Penicillin Production Is Regulated by the MAT1-1–1 Gene.
We first assessed functionality of MAT1-1–1 in penicillin production using a bioassay measuring clearing zone formation in bacterial lawns (Fig. 2A). Overexpression and complemented strains did not deviate significantly from the parental strains. However, both ∆MAT1 mutants showed a significant reduction in penicillin biosynthesis throughout the time course compared with control strains (e.g., a 60% reduction at 72 h), although all strains exhibited similar mycelial dry weight production. The reduced penicillin titer was confirmed by HPLC analysis (Fig. S3A). That MAT genes can influence fungal secondary metabolite production is a previously undescribed finding of high industrial relevance because many other filamentous fungi are used as the sources of key natural products (1, 17).
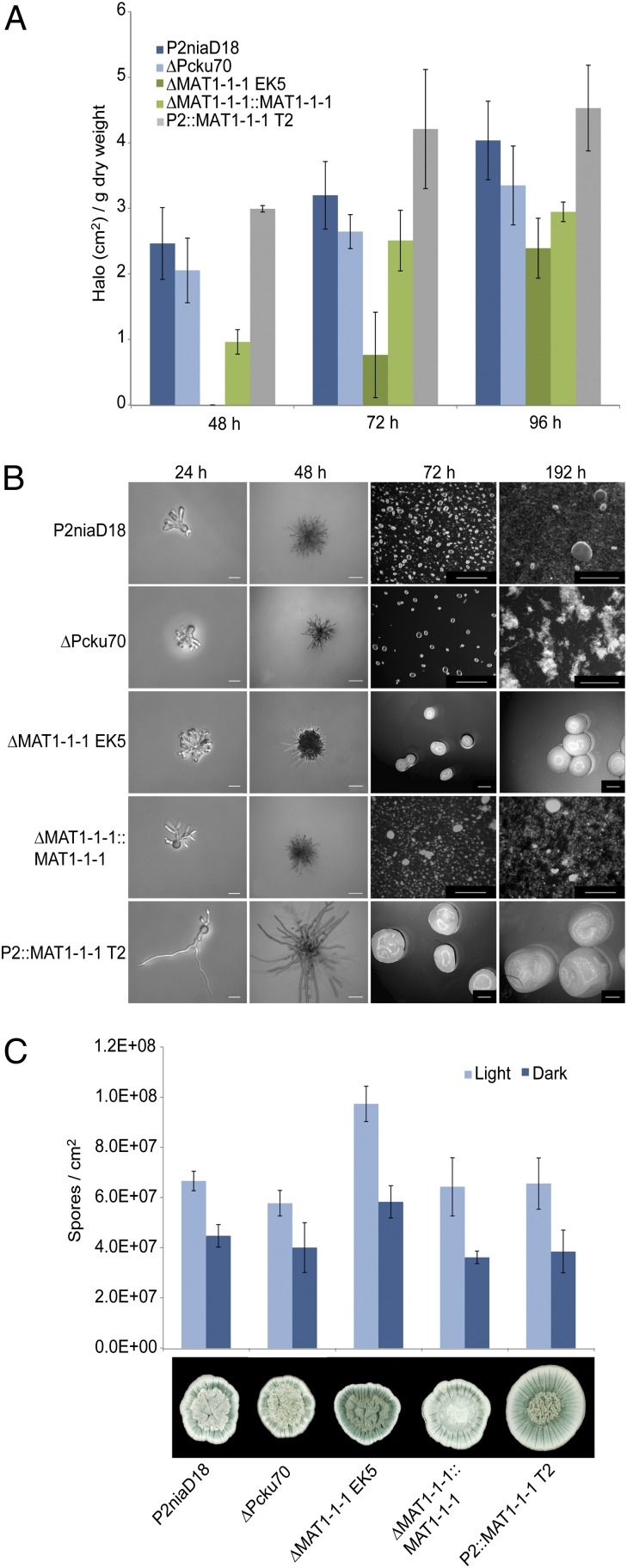
Fig. 2.
Functional analysis of the MAT1-1–1 gene. (A) Results of a bioassay used to assess penicillin production in parental and representative recombinant MAT1 strains from 48 to 96 h growth. Error bars represent mean ± SD (n = 3) from three independent experiments measuring halo formation. (B) Hyphal morphology of germinating conidia on solid media (24 h, 48 h) and subsequent pellet formation in liquid shaking cultures (72 h, 192 h) formed by parental and representative recombinant MAT1 strains as indicated. Micrographs are representative of three independent experiments. Scale bars: 20 μm (24 h), 100 μm (48 h), and 2,000 μm (72 and 192 h). (C) Quantification of conidia production by parental and representative MAT1 recombinant strains after 168 h growth on complete culture medium in the light or dark. Bottom panel shows typical morphology of respective strains.
MAT1-1–1 Gene Controls Hyphal Morphology, Conidiation, and Pellet Formation.
We next assessed the effect of MAT1-1–1 expression on hyphal morphology. This is an important industrial trait because fungi exhibit distinct morphologies in submerged culture depending on the extent of branching and/or elongation of hyphae. Freely dispersed hyphal suspensions can be formed that are highly viscous or hyphae may aggregate to form “pellets” with lower viscosity. Inspection of strains revealed important morphological differences when grown for 24–48 h on solid or in shaken liquid media (Fig. 2B and Fig. S3B). Conidia of the parental and complemented strains germinated mostly to yield one or two hyphae exhibiting dichotomous branching. By contrast, conidia of the overexpression strains exhibited long germinating hyphae without any terminal branching. Conidia of the ∆MAT1 strains produced short hyphae with intensively branching tips, often with more than two emergent hyphae. These phenotypic differences were confirmed quantitatively (Fig. S3 C and D). Cultures were then grown from 72 to 192 h in shaken liquid culture, comparable to applied fermentation conditions. Phenotypic differences were even more pronounced, with gene deletion and overexpression strains producing significantly larger pellets than control strains (Fig. 2B and Fig. S3E). Thus, these previously undescribed results demonstrated that MAT genes can influence the morphology and polarity of germinating hyphae.
The influence of MAT1-1–1 expression on conidial formation was also investigated. There were clear differences in sporulation between parental and deletion strains when plated on solid media. An approximate 25% increase in sporulation was seen in both ∆MAT1 strains relative to other strains when grown in the light (Fig. 2C). Again, this is a unique report of MAT genes influencing asexual sporulation in fungi.
Microarray Time-Course Analysis of MAT1-1–1 Regulated Gene Expression.
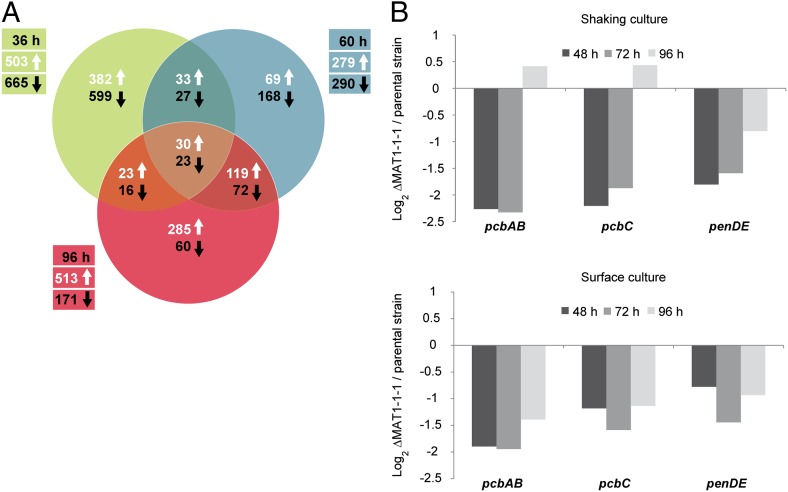
To understand the molecular basis for the observed phenotypes, a microarray time-course experiment was performed to investigate MAT1-1–1 dependent transcriptional regulation further, comparing expression of the ∆MAT1 mutant relative to the ∆Pcku70 parent up to 96 h growth. A total of 2,421 genes showed differential regulation over this period, as defined by a threshold of at least twofold change in expression levels (Fig. 3A). Between 23 and 30 genes of mostly unknown function were down- or up-regulated, respectively, at all time points (Table S2). Consistent with the data above, genes related to conidiation and morphology, (e.g., PcbrlA, PcdewA, PcdewB) were down-regulated in the ∆MAT1 strain (Table S2). The three penicillin biosynthesis genes (pcbAB, pcbC, penDE) were also down-regulated at 60 and 96 h; this result was confirmed by quantitative real-time PCR (qRT-PCR) analysis (Fig. 3B). Other microarray studies have also demonstrated that MAT genes have a wide-ranging effect on fungal gene expression (26, 29–31).
Fig. 3.
MAT1-1–1 dependent transcriptional regulation. (A) Venn diagram of differentially regulated genes in the ∆MAT1-1–1 EK5 strain. For array analysis, mRNA was used from cultures grown for 36, 60, and 96 h. Arrows indicate transcriptionally up- or down-regulated genes. (B) qRT-PCR analysis to quantify transcriptional expression of the penicillin biosynthesis genes in ΔMAT1-1–1 strains EK5 and EK6 when grown as liquid shaking or surface cultures. Values are mean log2-transformed average expression ratios of at least three biological replicates from two independently derived deletion strains (mean ΔMAT1-1–1 EK5/EK6 n ≥ 3) relative to the ΔPcku70 parental strain.
Functionality of the P. chrysogenum Pheromone and Pheromone Receptor Genes.
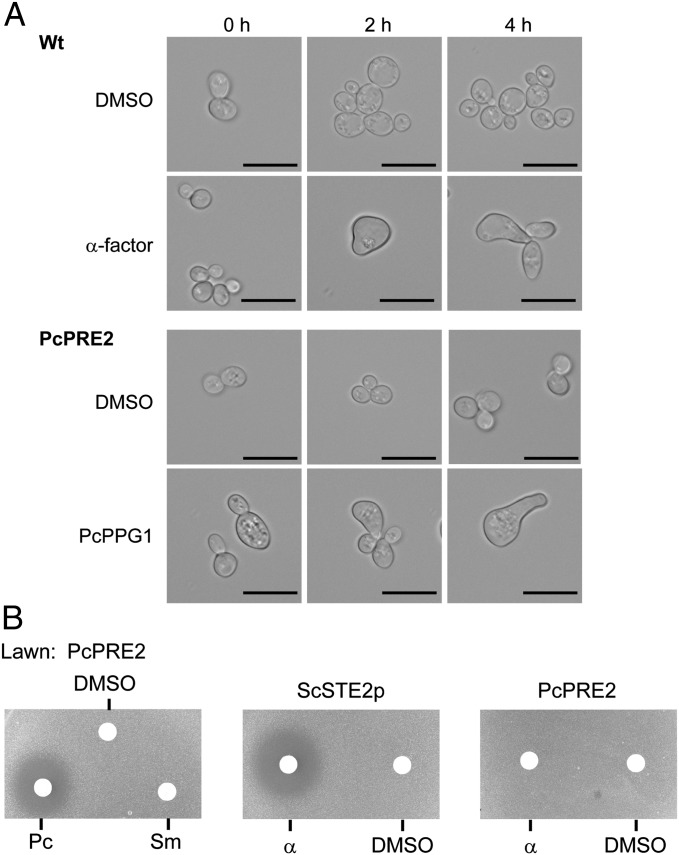
The microarray analysis also revealed that three elements of a putative pheromone signaling pathway were expressed, comprising a previously identified pheromone precursor (Pcppg1) and two pheromone receptor (Pcpre1, Pcpre2) genes (10) (Fig. S4). We therefore examined their functionality using yeast bioassays. Successful pheromone binding and signaling would be expected to result in cell cycle arrest and change in cell morphology and lead to formation of a halo in lawns of Saccharomyces cerevisiae (32–34). On the basis of similarity to the S. cerevisiae MFα proteins, the P. chrysogenum Pcppg1 gene was predicted to produce a decapeptide pheromone of sequence KWCGHIGQGC, expected to bind to the cognate PcPRE2 receptor protein. And indeed, S. cerevisiae wild-type cells (ScSTE2p) or yeasts heterologously expressing PcPRE2 exhibited polarized growth, leading to pear-shaped forms (shmoos) of unconjugated haploid cells, in response to either the native S. cerevisiae α-factors or the synthetic decapeptide pheromone PcPPG1, respectively (35) (Fig. 4A). This finding was further confirmed by bioassays in which addition of the synthetic PcPPG1 pheromone to lawns of S. cerevisiae that were heterologously expressing PcPRE2 resulted in halo formation (Fig. 4B). Thus, PcPRE2 and PcPPG1 represent a functional pheromone-receptor pair likely involved in the observed mating of P. chrysogenum.
Fig. 4.
Bioassay of functionality of the P. chrysogenum pheromone and pheromone receptor genes. (A) Shmooing of S. cerevisiae in response to the synthetic pheromone PcPPG1 and to S. cerevisiae α-factor. MATa cells (YDB103, ste2Δ, sst2Δ) expressing the Pcpre2 (PcPRE2) gene were treated for 0, 2, or 4 h with either the synthetic pheromone PcPPG1 at 5 µM or DMSO. As a control, the wild type (Wt; MATa strain Y06055 sst2Δ) expressing the endogenous S. cerevisiae STE2 gene was treated with synthetic α-factor or DMSO. On average after 4 h, 50% of cells responded only to the specific pheromone by shmoo formation without any detected cross-reactivity of the pheromones. Scale bar, 10 µm. (B) Pheromone induced growth arrest (halo formation) of S. cerevisiae transformants expressing the P. chrysogenum Pcpre2 gene (PcPRE2; halo diameter: 2.3 ± 0.15 cm), or as a control the S. cerevisiae strain Y06055 (sst2Δ) expressing the endogenous STE2 pheromone receptor gene (ScSTE2p; halo diameter: 2.4 ± 0.13 cm). Averages of halo diameters from eight independent experiments (n = 8) were measured. No cross-reactivity of the pheromones could be detected. DMSO served as mock solution. α, S. cerevisiae α-factor pheromone; Pc, P. chrysogenum decapeptide pheromone (KWCGHIGQGC); Sm, S. macrospora undecapeptide (QWCRIHGQSCW).
Discussion
We have provided unequivocal evidence for a heterothallic sexual cycle involving production of recombinant ascospore progeny in P. chrysogenum and demonstrated that sexual crosses can be used to develop new strains with improved industrial characteristics. Sex in P. chrysogenum could be induced on oatmeal agar as reported recently for other supposedly “asexual” Aspergillus and Penicillium species (18, 20, 36, 37). However, it was necessary to supplement the agar with biotin to achieve complete sexual development, and similar supplementation might be required for other sexually recalcitrant species (17, 18). Even with biotin supplementation, not all of the crosses tested generated cleistothecia with ascospores, suggesting that P. chrysogenum is composed of isolates on a continuum of sexual fertility, rather than being purely sexual or asexual, as suggested by the “slow decline” hypothesis (38). Comparable results were obtained when highly derived industrial strains from Trichoderma, showing female sterility, were used in crosses with natural isolates of the teleomorph Hypocrea jecorina (14). In this context, a fitness trade-off between retention of mating ability and growth-rate advantage has been demonstrated in S. cerevisiae (39). Further work is now required to determine the extent of sexual fertility within natural populations of P. chrysogenum. The previously undescribed sexual state of P. chrysogenum was morphologically similar to that of known sexual Eupenicillium species, but no new teleomorph name is proposed in agreement with recent taxonomic revisions (18, 40).
Our results are of significance to the understanding of the biology and evolution of P. chrysogenum (a species of great importance in its own right) and are of industrial relevance because the sexual cycle now offers a valuable tool for strain improvement, such as increasing penicillin production. Sexual reproduction offers particular advantages over conventional mutagenesis and genetic recombinant technologies for a number of reasons (2). It involves recombination throughout the whole genome, thereby providing significant genetic variation for screening purposes. For many industrial processes, multiple genes might have to be manipulated to optimize strains, and gene-by-gene manipulations/mutations would be too slow to develop novel production strains in a reasonable time. Sexual reproduction offers an invaluable method to allow targeted crosses to be set up, providing a faster and economically cheaper procedure, without the need for prior knowledge of the genetic basis of traits of interest. Also, continued random mutagenesis and high-throughput screening can lead to undesirable deleterious mutations and genetic instability in producer strains (41). For example, production strains of P. chrysogenum contain multiple point mutations as well as major amplifications and deletions compared with the genome sequence of the progenitor Wisconsin strain (42). By contrast, sexual reproduction allows recombination of traits without introducing further mutations and offers a means to restore the fitness of industrial strains and eliminate ku70/ku80 mutations that can lead to genome instability (43).
Furthermore, we have demonstrated that the P. chrysogenum MAT1-1–1 gene regulates transcription of a wide range of genes including those controlling penicillin production, hyphal morphology, and conidial formation, all traits of biotechnological relevance. This is a major finding because MAT genes have previously been primarily known for their role in determining sexual identity, as well as for other aspects of sexual development (19). We had previously observed that hyphal morphology, and consequently pellet formation, in P. chrysogenum is dependent on a wide range of factors (44). Hyphal morphology in submerged fermentations is a highly important feature of production strains in large-scale fermenters because this can influence productivity as a result of oxygen depletion and nutritional gradients (45). The discovery that overexpression or deletion of the MAT1-1–1 gene significantly affected pellet formation indicates that manipulation of MAT genes might therefore provide a unique strategy for strain improvement. Overexpression of MAT genes might also increase the production of certain secondary metabolites of interest, given that the P. chrysogenum ∆MAT1 mutants showed a significant reduction in penicillin biosynthesis. The possible influence of the MAT1-2–1 gene family now merits future investigation.
Finally, these findings for P. chrysogenum are of broader significance because they indicate that sexual recombination might also be feasible for other filamentous fungi of economic importance that are assumed to be exclusively asexual, thus the findings are of general relevance to strain development and mating in fungi. The revelation of a sexual life cycle in P. chrysogenum illustrates an ongoing fungal “sexual revolution” (18) and the overall reproductive versatility of fungi, which exhibit a remarkable balance between sexual and asexual reproduction in response to different environmental conditions (46).
Materials and Methods
Strains and Growth Conditions.
Details of bacterial and fungal strains investigated in this study are summarized in SI Materials and Methods and Table S3. Maintenance and growth conditions were as described (47, 48). Growth conditions for P. chrysogenum are detailed in SI Materials and Methods. DNA-mediated transformation of P. chrysogenum to construct gene deletion and overexpression strains and further rescue of deletion strains (Fig. S2) were done in principal as described recently (49). Detailed information on strain constructions and functional analysis is provided in SI Materials and Methods and Tables S4 and S5.
Light and Scanning Electron Microscopy.
Details about specimen preparation are provided in SI Materials and Methods.
In vitro recombinant techniques, sequence analysis, and penicillin quantifications are detailed in SI Materials and Methods.
Mating and Analysis of Recombinant Ascospore Lines.
Strains of opposite mating type were inoculated onto either oatmeal agar medium (OA) (Pinhead Oatmeal; Odlums Group), OA (U.K.) (Traditional Rolled Oats; Quaker Oats), Köllnflocken (Kölln) medium, or Schmelzflocken (Schmelz) medium (Peter Kölln KGaA) (in each case 40 g/L), with or without addition of sildenafil citrate (Sil) (100 µM), vardenafil citrate (Var) (100 µM), or biotin (6.4 µg/L) after autoclaving. A 1 × 107 spore suspension of each isolate was prepared, and 20 µL of each suspension was inoculated as previously described (20). The plates were sealed with Parafilm and incubated at 15, 18, 20, or 27 °C in the dark. Further details for examination of crosses are provided in SI Materials and Methods.
Interaction Studies.
The interaction of pheromones and pheromone receptors from P. chrysogenum was studied using the heterologous yeast system that was described previously (32) and is detailed further in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to I. Godehardt, S. Mertens, K. Kalkreuter, and J. del Buono for their excellent technical assistance, G. Frenßen-Schenkel for the artwork, and V. Kock for help with the construction of gene deletion strains. We thank M. Kirchmair, J. Frisvad, M. Fisher, and D. Henk for providing wild-type strains of P. chrysogenum, T. Stützel and S. Adler for their help with electron microscope preparations, and M. Nowrousian for help with bioinformatic calculations. This work was funded by Sandoz GmbH, the Christian Doppler Society, and the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217943110/-/DCSupplemental.
References
- 1.Keller NP, Turner G, Bennett JW. Fungal secondary metabolism - from biochemistry to genomics. Nat Rev Microbiol. 2005;3(12):937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 2.Adrio JL, Demain AL. Genetic improvement of processes yielding microbial products. FEMS Microbiol Rev. 2006;30(2):187–214. doi: 10.1111/j.1574-6976.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 3.Barber MS, Giesecke U, Reichert A, Minas W. Industrial enzymatic production of cephalosporin-based beta-lactams. Adv Biochem Eng Biotechnol. 2004;88:179–215. doi: 10.1007/b99261. [DOI] [PubMed] [Google Scholar]
- 4.Backus MP, Stauffer JF. The production and selection of a family of strains in Penicillium chrysogenum. Mycologia. 1955;47:429–463. [Google Scholar]
- 5.van den Berg MA, Westerlaken I, Leeflang C, Kerkman R, Bovenberg RA. Functional characterization of the penicillin biosynthetic gene cluster of Penicillium chrysogenum Wisconsin54-1255. Fungal Genet Biol. 2007;44(9):830–844. doi: 10.1016/j.fgb.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Henk DA, et al. Speciation despite globally overlapping distributions in Penicillium chrysogenum: The population genetics of Alexander Fleming’s lucky fungus. Mol Ecol. 2011;20:4288–4301. doi: 10.1111/j.1365-294X.2011.05244.x. [DOI] [PubMed] [Google Scholar]
- 7.Houbraken J, Frisvad JC, Samson RA. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus. 2011;2(1):87–95. doi: 10.5598/imafungus.2011.02.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeill J, et al., editors. International Code of Botanical Nomenclature (Vienna Code): Adopted by the Seventeenth International Botanical Congress, Vienna, Austria, July 2005. 2006. (ARG Gantner, Ruggell, Liechtenstein), Regnum Vegetabile Ed, Vol 146. [Google Scholar]
- 9.Kück U, Pöggeler S. Cryptic sex in fungi. Fungal Biol Rev. 2009;23:86–90. [Google Scholar]
- 10.Hoff B, Pöggeler S, Kück U. Eighty years after its discovery, Fleming’s Penicillium strain discloses the secret of its sex. Eukaryot Cell. 2008;7(3):465–470. doi: 10.1128/EC.00430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni M, Feretzaki M, Sun S, Wang X, Heitman J. Sex in fungi. Annu Rev Genet. 2011;45:405–430. doi: 10.1146/annurev-genet-110410-132536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braumann I, van den Berg M, Kempken F. Repeat induced point mutation in two asexual fungi, Aspergillus niger and Penicillium chrysogenum. Curr Genet. 2008;53(5):287–297. doi: 10.1007/s00294-008-0185-y. [DOI] [PubMed] [Google Scholar]
- 13.Taylor J, Jacobson D, Fisher M. The evolution of asexual fungi: reproduction, speciation and classification. Annu Rev Phytopathol. 1999;37:197–246. doi: 10.1146/annurev.phyto.37.1.197. [DOI] [PubMed] [Google Scholar]
- 14.Seidl V, Seibel C, Kubicek CP, Schmoll M. Sexual development in the industrial workhorse Trichoderma reesei. Proc Natl Acad Sci USA. 2009;106(33):13909–13914. doi: 10.1073/pnas.0904936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289(5477):307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- 16.Magee PT, Magee BB. Through a glass opaquely: The biological significance of mating in Candida albicans. Curr Opin Microbiol. 2004;7(6):661–665. doi: 10.1016/j.mib.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Dyer PS, O’Gorman CM. Sexual development and cryptic sexuality in fungi: Insights from Aspergillus species. FEMS Microbiol Rev. 2012;36(1):165–192. doi: 10.1111/j.1574-6976.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 18.Dyer PS, O’Gorman CM. A fungal sexual revolution: Aspergillus and Penicillium show the way. Curr Opin Microbiol. 2011;14(6):649–654. doi: 10.1016/j.mib.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Debuchy R, Berteaux-Lecellier V, Silar P. Mating systems and sexual morphogenesis in Ascomycetes. In: Borkovich KA, Ebbole DJ, editors. Cellular and Molecular Biology of Filamentous Fungi. Washington, DC: ASM Press; 2010. pp. 501–535. [Google Scholar]
- 20.O’Gorman CM, Fuller HT, Dyer PS. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature. 2009;457(7228):471–474. doi: 10.1038/nature07528. [DOI] [PubMed] [Google Scholar]
- 21.Pöggeler S, O’Gorman CM, Hoff B, Kück U. Molecular organization of the mating-type loci in the homothallic Ascomycete Eupenicillium crustaceum. Fungal Biol. 2011;115(7):615–624. doi: 10.1016/j.funbio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Molowitz R, Bahn M, Hock B. The control of fruiting body formation in the ascomycete Sordaria macrospora Auersw. by arginine and biotin: A two-factor analysis. Planta. 1976;128:143–148. doi: 10.1007/BF00390315. [DOI] [PubMed] [Google Scholar]
- 23.Frisvad JC, Thrane U, Samson RA, Pitt JI. Important mycotoxins and the fungi which produce them. Adv Exp Med Biol. 2006;571:3–31. doi: 10.1007/0-387-28391-9_1. [DOI] [PubMed] [Google Scholar]
- 24.Hoff B, Kamerewerd J, Sigl C, Zadra I, Kück U. Homologous recombination in the antibiotic producer Penicillium chrysogenum: Strain DeltaPcku70 shows up-regulation of genes from the HOG pathway. Appl Microbiol Biotechnol. 2010;85(4):1081–1094. doi: 10.1007/s00253-009-2168-4. [DOI] [PubMed] [Google Scholar]
- 25.Sigl C, Handler M, Sprenger G, Kürnsteiner H, Zadra I. A novel homologous dominant selection marker for genetic transformation of Penicillium chrysogenum: Overexpression of squalene epoxidase-encoding ergA. J Biotechnol. 2010;150(3):307–311. doi: 10.1016/j.jbiotec.2010.09.941. [DOI] [PubMed] [Google Scholar]
- 26.Klix V, et al. Functional characterization of MAT1-1-specific mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and nonessential sexual regulators. Eukaryot Cell. 2010;9(6):894–905. doi: 10.1128/EC.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoletti M, et al. Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Curr Biol. 2007;17(16):1384–1389. doi: 10.1016/j.cub.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Lee T, Lee YW, Yun SH, Turgeon BG. Shifting fungal reproductive mode by manipulation of mating type genes: obligatory heterothallism of Gibberella zeae. Mol Microbiol. 2003;50(1):145–152. doi: 10.1046/j.1365-2958.2003.03694.x. [DOI] [PubMed] [Google Scholar]
- 29.Wada R, et al. Presence and functionality of mating type genes in the supposedly asexual filamentous fungus Aspergillus oryzae. Appl Environ Microbiol. 2012;78(8):2819–2829. doi: 10.1128/AEM.07034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bidard F, et al. Genome-wide gene expression profiling of fertilization competent mycelium in opposite mating types in the heterothallic fungus Podospora anserina. PLoS ONE. 2011;6(6):e21476. doi: 10.1371/journal.pone.0021476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pöggeler S, et al. Microarray and real-time PCR analyses reveal mating type-dependent gene expression in a homothallic fungus. Mol Genet Genomics. 2006;275(5):492–503. doi: 10.1007/s00438-006-0107-y. [DOI] [PubMed] [Google Scholar]
- 32.Mayrhofer S, Pöggeler S. Functional characterization of an alpha-factor-like Sordaria macrospora peptide pheromone and analysis of its interaction with its cognate receptor in Saccharomyces cerevisiae. Eukaryot Cell. 2005;4(4):661–672. doi: 10.1128/EC.4.4.661-672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsueh YP, Xue C, Heitman J. A constitutively active GPCR governs morphogenic transitions in Cryptococcus neoformans. EMBO J. 2009;28(9):1220–1233. doi: 10.1038/emboj.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler TJ, DeSimone SM, Mitton MF, Kurjan J, Raper CA. Multiple sex pheromones and receptors of a mushroom-producing fungus elicit mating in yeast. Mol Biol Cell. 1999;10(8):2559–2572. doi: 10.1091/mbc.10.8.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cross F, Hartwell LH, Jackson C, Konopka JB. Conjugation in Saccharomyces cerevisiae. Annu Rev Cell Biol. 1988;4:429–457. doi: 10.1146/annurev.cb.04.110188.002241. [DOI] [PubMed] [Google Scholar]
- 36.Houbraken J, Frisvad JC, Samson RA. Sex in Penicillium series Roqueforti. IMA Fungus. 2010;1(2):171–180. doi: 10.5598/imafungus.2010.01.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arabatzis M, Velegraki A. Sexual reproduction in the opportunistic human pathogen Aspergillus terreus. Mycologia. 2012 doi: 10.3852/11-426. 10.3852/11-426. [DOI] [PubMed] [Google Scholar]
- 38.Dyer PS, Paoletti M. Reproduction in Aspergillus fumigatus: Sexuality in a supposedly asexual species? Med Mycol. 2005;43(Suppl 1):S7–S14. doi: 10.1080/13693780400029015. [DOI] [PubMed] [Google Scholar]
- 39.Lang GI, Murray AW, Botstein D. The cost of gene expression underlies a fitness trade-off in yeast. Proc Natl Acad Sci USA. 2009;106(14):5755–5760. doi: 10.1073/pnas.0901620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawksworth DL, et al. The amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2(1):105–112. doi: 10.5598/imafungus.2011.02.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoustra S, Rundle HD, Dali R, Kassen R. Fitness-associated sexual reproduction in a filamentous fungus. Curr Biol. 2010;20(15):1350–1355. doi: 10.1016/j.cub.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 42.van den Berg MA, et al. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat Biotechnol. 2008;26(10):1161–1168. doi: 10.1038/nbt.1498. [DOI] [PubMed] [Google Scholar]
- 43.Kück U, Hoff B. New tools for the genetic manipulation of filamentous fungi. Appl Microbiol Biotechnol. 2010;86(1):51–62. doi: 10.1007/s00253-009-2416-7. [DOI] [PubMed] [Google Scholar]
- 44.Hoff B, et al. Two components of a velvet-like complex control hyphal morphogenesis, conidiophore development, and penicillin biosynthesis in Penicillium chrysogenum. Eukaryot Cell. 2010;9(8):1236–1250. doi: 10.1128/EC.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv. 2004;22(3):189–259. doi: 10.1016/j.biotechadv.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Metin B, Findley K, Heitman J. The mating type locus (MAT) and sexual reproduction of Cryptococcus heveanensis: Insights into the evolution of sex and sex-determining chromosomal regions in fungi. PLoS Genet. 2010;6(5):e1000961. doi: 10.1371/journal.pgen.1000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bullock WO, Fernandez JM, Short JM. 1987. XL1-Blue: A high-efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. BioTechniques 5:376–379.
- 48.Sambrook J, Russell DW. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2001. [Google Scholar]
- 49.Kamerewerd J, Zadra I, Kürnsteiner H, Kück U. PcchiB1, encoding a class V chitinase, is affected by PcVelA and PcLaeA, and is responsible for cell wall integrity in Penicillium chrysogenum. Microbiology. 2011;157(Pt 11):3036–3048. doi: 10.1099/mic.0.051896-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.