Abstract
Soluble cytosolic carbonic anhydrases (CAs) are well known to participate in pH regulation of the cytoplasm of mammalian cells. Membrane-bound CA isoforms—such as isoforms IV, IX, XII, XIV, and XV—also catalyze the reversible conversion of carbon dioxide to protons and bicarbonate, but at the extracellular face of the cell membrane. When human CA isoform IV was heterologously expressed in Xenopus oocytes, we observed, by measuring H+ at the outer face of the cell membrane and in the cytosol with ion-selective microelectrodes, not only extracellular catalytic CA activity but also robust intracellular activity. CA IV expression in oocytes was confirmed by immunocytochemistry, and CA IV activity measured by mass spectrometry. Extra- and intracellular catalytic activity of CA IV could be pharmacologically dissected using benzolamide, the CA inhibitor, which is relatively slowly membrane-permeable. In acute cerebellar slices of mutant mice lacking CA IV, cytosolic H+ shifts of granule cells following CO2 removal/addition were significantly slower than in wild-type mice. Our results suggest that membrane-associated CA IV contributes robust catalytic activity intracellularly, and that this activity participates in regulating H+ dynamics in the cytosol, both in injected oocytes and in mouse neurons.
Keywords: extracellular carbonic anhydrase, membrane surface H+, ethoxzolamide, cerebellar granule cells, intracellular transport vesicles
Carbonic anhydrases (CAs) are zinc metalloenzymes, which catalyze the reversible hydration of CO2 to yield H+ and HCO3− (1, 2). The CAs are involved in a variety of physiological processes including respiration, water and ion transport, pH regulation, and bone resorption. Alterations in CA expression are therapeutic goals in a number of diseases, such as glaucoma, osteoporosis, epilepsy, and cancer, which has promoted the search for CA inhibitors (3, 4). One or more of various genetically distinct isoforms is expressed nearly ubiquitously in most living cells. Some isoforms are located in the cytosol (e.g., CA I, CA II, CA III, CA VII, and CA XIII), while others express their catalytic activity at the extracellular membrane face (e.g., CA IV, CA IX, CA XII, CA XIV, CA XV). Due to the expression of multiple isoforms at different intra- and extracellular locations, it has been difficult to discriminate the contribution of a single CA isoform in a given location.
Extracellular CA activity has been found in various tissues, including kidney (5), skeletal muscle (6–8), lung (9, 10), heart (11, 12), and brain (13–15). CA IV from kidney and lung was the first membrane-associated CA characterized. CA IV is posttranslationally cleaved and the newly synthesized enzyme anchored by a glycosylphosphatidylinositol (GPI) tail to the luminal face of the membrane in the endoplasmic reticulum (ER) (9, 10). After folding and disulfide bonding in the ER, it moves through the Golgi to the plasma membrane by vesicular transport. Baird et al. (16) characterized the recombinant, 260-amino-acid soluble CA IV resulting from N-terminal and C-terminal truncations, which deleted the leader signal peptide and the sequence for attachment of the GPI anchor, respectively. These studies showed that its catalytic activity is high, like that of the most active isoform CA II, and at physiological pH, it has even greater dehydrase activity than CA II.
We have studied the expression and function of CA IV in injected Xenopus oocytes, which are particularly suited as a heterologous expression system for studying CAs, because they virtually express no intrinsic CA themselves (17–19). In addition, evidence about the catalytic activity of CA in oocytes, either expressed or injected as protein, can be obtained physiologically by measuring intracellular H+ in intact oocytes, and by mass spectrometry of lysed oocytes (20, 21). By measuring H+ with ion-selective microelectrodes in the cytosol and at the outer membrane surface, intra- and extracellular CA activity can be identified and discriminated by the rate and amplitude, respectively, of H+ changes. Our experiments show that CA IV, which is attached to the outer membrane surface as a mature, GPI-anchored protein, also displays robust intracellular activity in the transport vesicles destined eventually to fuse with the plasma membrane and deliver the CA IV to the cell surface. The intracellular activity plays a prominent role in regulation of H+ homeostasis in the cytosol.
Results
Human CA IV Expressed in Frog Oocytes Displays Not only Extracellular, but also Intracellular Activity.
Since most cells express several isoforms of CA, some intra- and some extracellularly (2, 22, 23), we chose Xenopus oocytes, which are virtually free of any CA activity (17–19) to test whether intracellular activity mediated by CA IV could be identified in injected oocytes. We observed that oocytes injected with full-length human CA (hCA) IV-cRNA responded with a fast rise of cytosolic H+ (H+i) upon exposure to CO2/HCO3−–buffered saline, which was inhibited by ethoxzolamide (EZA) in a concentration-dependent manner (Fig. 1 A and B), from 131.2 ± 19.2 nM/min without EZA to 27.0 ± 4.4 nM/min in the presence of 30 µM EZA (n = 5–6). Extracellular CA activity was measured by recording H+ at the extracellular membrane surface (H+s). Addition of CO2/HCO3− elicited a transient fall of H+s, and removal of CO2/HCO3− resulted in a robust, transient rise of H+, which fully recovered within a few minutes (Fig. 1C). This transient H+s rise was also inhibited by EZA in a concentration-dependent manner (Fig. 1 C and D). The amplitude of the H+s transient decreased significantly from 89.1 ± 3.7 nM to 24.7 ± 2.7 nM in 30 µM EZA (n = 5–6). The H+s transient remaining after a presumably complete block of CA enzymatic activity is attributed to the appreciable nonenzymatic CO2 hydration reaction. When the catalytically inactive CA IV mutant V165Y was expressed, cytosolic and surface H+ changes were not different from those of native, noninjected oocytes, and neither was further reduced by 30 µM EZA (Fig. 1 E and F).
Fig. 1.
Measurement of cytosolic H+ changes (H+i; A, B, and I) and H+ changes at the outer surface of the cell membrane (H+s; C–H) using H+-selective microelectrodes in Xenopus oocytes, injected with 1 ng CA IV–cRNA (A–D and G–I) in the absence and presence of different concentrations of ethoxyzolamide (EZA, 0.5–30 µM). EZA reduced both the rate of cytosolic H+ rise during addition of CO2/HCO3− (A and B) and the amplitude of the H+s during the removal of CO2/HCO3− (C and D). No H+s transient was observed in oocytes injected with CA IV–V165Y–cRNA, a catalytically inactive mutant, and in native oocytes (E and F). The H+ surface transient was suppressed (G and H) after increasing the H+ buffering capacity of the external solution by raising the concentration of Hepes from 5 to 30 mM, which left the rate of H+i change unaffected (I).
Increasing the extracellular H+ buffer capacity by raising the Hepes concentration from 5 mM to 30 mM suppressed the H+s transients upon removal of CO2/HCO3− but had no effect on the rate of H+i change (Fig. 1 G and I). This result confirms that the fast rate of H+i change, indicative of intracellular CA activity, was independent of the extracellular buffer capacity and of the presence of H+s transients.
Intracellular and Extracellular Activity of CA IV Can Be Dissected by Benzolamide.
The rate of H+i rise during addition of 5% CO2/25 mM HCO3− was also measured in the absence and in the presence of either 0.5–1 µM benzolamide (BZA) or 30 µM EZA (Fig. 2 A and B). The slowly membrane-permeable CA inhibitor BZA decreased the rate of H+i rise by 30%, from 107.1 ± 13.0 to 78.1 ± 10.2 nM/min (n = 11), while the rapidly membrane-permeable CA inhibitor EZA decreased the rate of H+i increase even further to 27 ± 2.1 nM/min, a value close to that obtained after full inhibition of CA activity and in the background level seen in native, noninjected oocytes (Fig. S1E). The amplitude of the H+s transient in CA IV-expressing oocytes was decreased by BZA from 86.7 ± 9.6 to 27.4 ± 1.7 nM (n = 11) and to 20.8 ± 2.0 in 30 µM EZA (n = 9; Fig. 2 C and D).
Fig. 2.
Pharmacological dissection of intra- and extracellular CA activity in oocytes injected with CA IV–cRNA. Cytosolic H+ (H+i) recording (A) and the rate of H+i change (B), and membrane surface H+ (H+s) recording (C) and amplitude of H+s (D) during addition and removal of 5% CO2/25 mM HCO3−, respectively, without inhibitor and in the presence of 0.5–1 µM BZA or 30 µM EZA. The rate of H+i change (E) and the amplitude of H+s (F) were differently affected by prior injection of 1 µM BZA into the oocyte but equally sensitive to application of 30 µM EZA.
We also injected BZA directly into oocytes in a final concentration of about 1 µM to see whether BZA effectively inhibits intracellular CA activity in CA IV–expressing oocytes. Indeed, injection of BZA reduced the rate of H+i rise during addition of CO2/HCO3− from 139.2 to 35.7 nM/min, which was close, but still significantly different from the values obtained in the presence of 30 µM EZA, being 30.6 ± 2.1 nM/min (Fig. 2E). In contrast, BZA injection had no significant effect on the amplitude of the H+s transient upon removal of CO2/HCO3−, being 74.8 ± 9.1 and 59.1 ± 1.7 nM, respectively, and which was reduced in the presence of EZA to 20.7 ± 1.7 nM (n = 7; Fig. 2F). These results indicate that extracellular CA activities are more easily inhibited than intracellular activity by extracellular application of BZA and that intracellular CA activity in CA IV–expressing oocytes is much more sensitive to BZA when the inhibitor had been injected into the oocyte and only the intracellular (i.e., vesicular) membrane has to be crossed by the inhibitor to reach the intravesicular CA IV.
Western blot analysis, biotinylation, immunocytochemistry, and mass spectrometry have recently demonstrated expression of functional, extracellular CA IV in injected oocytes (21). The previous study focused on the expression and extracellular location of CA IV, rather than on the discrimination of extra- and intracellular activity. Here, we specifically looked for evidence for the expression of intracellular CA activity in oocytes injected with CA IV–cRNA. Staining with antibodies against CA IV could be detected in CA IV–cRNA–injected oocytes not only near the cell membrane, but also on entities in the cytosol, presumably on the surfaces of transport vesicle membranes (Fig. 3 A and C). Native oocytes showed virtually no staining (Fig. 3 B and D).
Fig. 3.
Evidence for cytosolic expression of CA IV in oocytes by immunocytochemistry (A–D) and by mass spectrometry (E and F). Fluorescent labeling of oocytes injected with CA IV–cRNA (A and C) and of native oocytes (B and D) in the confocal microscope (A and B) and in combined fluorescence and transmission mode (C and D). The same settings of the confocal microscope were applied to all images. (E) Original recordings of the degradation of 18O-labeled CO2 as measured by mass spectrometry. The first minute of the recordings shows the spontaneous degradation. The black arrow indicates the addition of oocyte lysate, prepared from 20 cells expressing CA IV–WT (blue) and CA IV–V165Y (orange), respectively, and native oocytes (black). (F) CA activity as measured by mass spectrometry in intact and lysed oocytes expressing CA IV or injected with CA II. For each experiment, 100 intact or 20 lysed oocytes were added to the measuring cuvettete. Catalytic activity was then calculated as activity per single oocyte. (G) Calibration curve for quantification of CA IV in Xenopus oocytes. Catalytic activity of defined amounts of CA IV protein was measured by mass spectrometry and fitted by linear regression to calculate the amount of expressed CA IV. (H) Absolute amount of extra- and intracellular CA IV concentration as calculated from the catalytic activity measured in intact and lysed oocytes, respectively, and the calibrated activity curve.
For determination of CA IV activity with mass spectrometry, we used intact and lysed oocytes that had been injected with CA IV–WT–cRNA or CA IV–V165Y–cRNA, respectively; native, noninjected cells were used as controls (Fig. 3E). In lysates of oocytes expressing CA IV–V165Y, the rate of 18O enrichment did not differ from the rate in lysates of native oocytes, while lysates of CA IV–WT–expressing cells showed robust catalytic activity. To quantify the activity of extra- and intracellular CA IV in oocytes, CA activity was compared in intact and lysed oocytes, respectively, using mass spectrometry. Because 13C18O2 permeability in Xenopus oocytes is relatively low, measurements in intact oocytes should only represent the activity of extracellular CA IV, whereas measurements on lysed oocytes will allow determination of total (extracellular and intracellular) catalytic activity. The data indicate that only 24% of the total CA activity measured in lysates can be attributed to CA IV located extracellularly (on the cell surface) (Fig. 3F). Calibration of the total enzymatic activity in lysates of injected oocytes with CA IV protein (Fig. 3G) revealed that oocytes injected with 1 ng of full-length CA IV–cRNA expresses 1.8 ng of CA IV at the outer face of the plasma membrane, whereas 7.4 ng of active CA IV is located inside the cell (Fig. 3H).
To test whether a known cytosolic, intracellular CA can contribute to CA activity measured in intact oocytes, oocytes were injected with 5 ng cRNA of cytosolic isoform CA II. Comparison with lysed oocytes showed that only 1.5% of the CA II activity was detected by mass spectrometry on intact oocytes. These results support the premise that intracellular CA contributes little to the CA activity measured in intact oocytes and therefore that CA activity measured in intact oocytes by mass spectrometry is virtually entirely attributable to extracellular CA activity.
hCA II Expressed in CA II–cRNA–Injected Frog Oocytes Does Display Cytosolic Activity.
To measure the activity of expressed (as opposed to injected) cytosolic CA, we recorded H+i and H+s in oocytes injected with CA II–cRNA that expresses an isozyme known to be cytosolic (2). The rate of H+i rise upon addition of CO2/HCO3− was similarly fast in CA II–expressing oocytes as in CA IV–expressing oocytes and could similarly be reduced by EZA to values close to those measured in native oocytes (Fig. S1 A and C). The rate of H+i increase could not be inhibited by extracellular application of BZA, but was significantly reduced after injection of BZA. The amplitude of H+s transients upon removal of CO2/HCO3− in CA II–expressing oocytes was similar in amplitude to those recorded in native oocytes (Fig. 1F) and not affected by EZA (Fig. S1 B and D). These results show that expression of cytosolic CA II activity from CA II–cRNA, in contrast to CA IV expressed from CA IV–cRNA, does not result in any significant extracellular catalytic activity. They also validate the approach of using the ion selective electrodes to discriminate intracellular and extracellular CA activity.
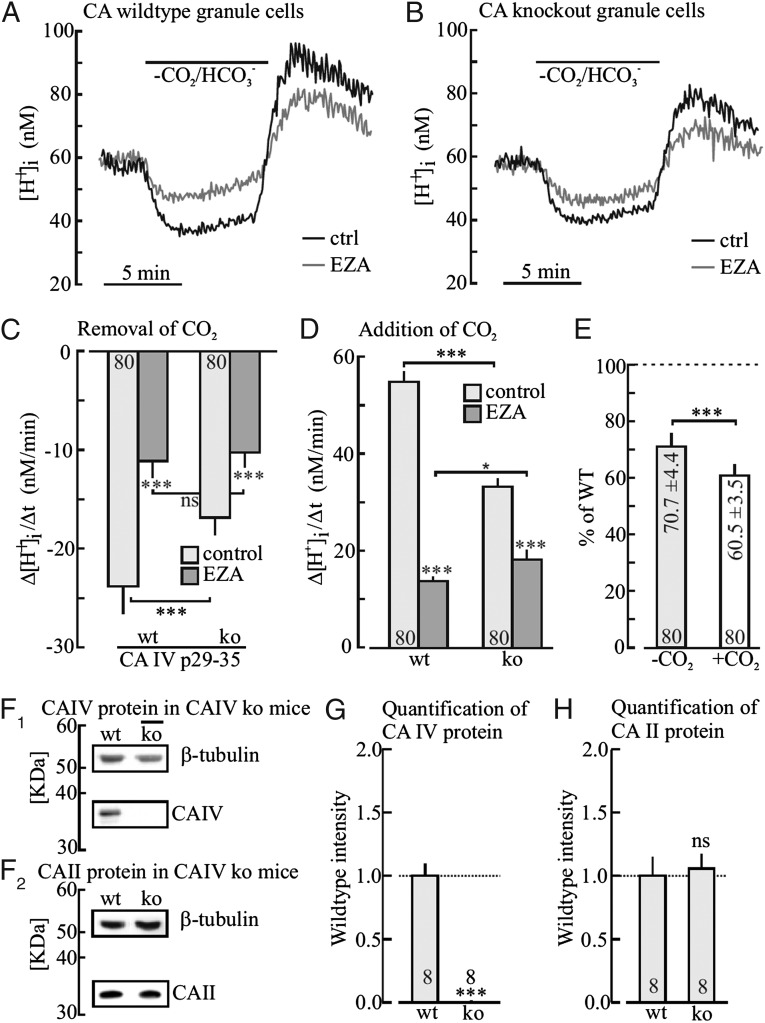
CO2-Dependent Cytosolic H+ Shifts in Cerebellar Granule Cells Are Slowed in CA IV−/− Mice.
Acute cerebellar brain slices of wild-type and CA IV–deficient mice, loaded with the H+-sensitive dye, BCECF, were exposed to a nominally CO2/HCO3−–free saline buffered with Hepes for 8 min (Fig. 4 A and B). The H+ shifts were recorded in granule cells by selecting appropriate regions of interest (ROIs) in the cell somata in saline without and with the CA inhibitor EZA (30 µM). The rates of H+ change during the removal and during the addition of CO2/HCO3− were measured and plotted for WT and mutant mice (Fig. 4 C and D). Note that the rates were reduced to 70% and 60% in CA IV−/− mice, respectively (Fig. 4E). Because EZA is a membrane-permeable CA inhibitor, it inhibits all major intra- and extracellular CA isoforms (3). Western blots for CA IV and CA II expression in the cerebellum showed that no CA IV, but similar amounts of CA II, could be detected in CA IV−/− mice compared with control wild-type mice (Fig. 4 F–H). Therefore, these results suggest significant contribution of CA IV to the total intracellular CA activity in cerebellar granule cells of wild-type mice, implying that the membrane-associated CA IV, presumably facing the interior of the transport vesicles, nonetheless contribute substantially to intracellular H+ dynamics. The contribution of extracellular CA IV on the cell surface to H+ regulation and ion transport has already been widely appreciated (13, 24, 25).
Fig. 4.
Cytosolic H+ shifts in cerebellar granule cells following removal and addition of 5% CO2/25 mM HCO3− before and after inhibition of CA activity by ethoxyzolamide (EZA, 30 µM), as measured in BCECF-loaded acute cerebellar tissue slices of wild-type and in CA IV–deficient mice (A and B). Plot of the rate of H+ shift during removal (C) and during addition (D) of CO2 in the absence and presence of EZA and of the reduction of the rates of H+ shifts in CA IV−/− mice (E). Western blots of CA IV, β-tubulin (F1), CA II, and β-tubulin (F2) in wild-type and knock-out mice, and relative quantification of CA IV (G) and CA II (H) protein levels in CA IV−/− relative to wild-type mice.
Discussion
Studies presented here show that CA isoform IV, a GPI-anchored CA known to be located on the cell surface, not only displays activity at the extracellular membrane surface but also contributes to regulation of H+ dynamics in the cytosol. By expressing CA IV in frog oocytes, which are virtually free of any CA activity in their native state, we could identify intracellular CA activity by measuring the rate of H+i rise with ion selective microelectrodes and also by mass spectrometry. We could also discriminate intracellular and extracellular CA activity by using two CA inhibitors, the freely membrane-permeable EZA and the more slowly membrane-permeable BZA. A cartoon depicting the topology of the GPI-anchored CA IV in the intracellular and extracellular membranes of the oocyte, the location of cytosolic CA II, and the action of the inhibitors EZA and BZA is presented in Fig. S2. In cerebellar neurons in situ, we could identify a highly significant reduction in intracellular CA activity in mice deficient in CA IV compared with that in wild-type mice. Thus, our results suggest that CAs, known to be active extracellularly, also display robust intracellular activity. Therefore, the intracellular CA activity, which is crucial for cellular H+ dynamics and various acid/base transport processes across the cell membrane (20, 26), is not only due to known, soluble cytosolic CAs, but is also attributable to intracellular activity of membrane-associated CAs. This discovery makes it important to take into account the interplay between intracellular and extracellular CA activities to understand their role in physiological processes and to design drugs specific for extracellular CA isoforms.
CA IV, which displays its extracellular catalytic activity on the plasma membrane surface, is anchored to the cell surface membrane by a GPI lipid moiety that is attached in the ER (16) and the GPI-anchored CA IV is delivered to the cell surface by the secretory pathway (i.e., by vesicular transport). Antibodies against CA IV produce staining not only at the cell membrane, but also stain entities in the cytosol of permeabilized oocytes. Our studies suggest that CA IV already exhibits enzymatic activity in the secretory vesicles that will deliver it to the cell surface plasma membrane. Mass spectrometry studies using intact and lysed oocytes allow discrimination between the extracellular and intracellular enzymatic activity and actually indicate that the intracellular activity is the predominant component of the total CA IV produced in CA IV–cRNA–injected oocytes.
In a previous study, we demonstrated expressed CA IV at the oocyte plasma membrane by biotinylation of cell surface CA IV, and by antibody staining of expressed and injected CA IV (21). In addition, CA activity was demonstrated in lysed oocytes injected with CA IV–cRNA via mass spectrometry. By measuring membrane surface H+ with extracellular H+-selective microelectrodes, which shows a characteristic, EZA-sensitive, transient acidification upon the removal of CO2/HCO3−, functional extracellular CA activity could also be demonstrated in intact CA IV–cRNA–injected oocytes (21). These studies confirmed the functional extracellular expression of CA IV. We now show that CA activity at the outer membrane surface of oocytes injected with CA IV–cRNA is sensitive to EZA and even more sensitive to BZA, which inhibited CA activity in low micromolar concentrations. Also, we observed that BZA eventually penetrated the cell membrane and also reduced cytosolic CA activity, as was reported previously (27). Nonetheless, the diffusion through the membrane was sufficiently slow to allow us to discriminate extra- and intracellular CA activity. Others had exploited the limited velocity of BZA in crossing cell membranes to block extracellular CA activity preferentially without abolishing the activity of intracellular, cytosolic CAs (13, 28, 29). Direct injection of BZA blocked all intracellular CA activity, whether oocytes were injected with CA IV–cRNA or with CA II–cRNA (3, 30). We also showed previously that when soluble, truncated CA IV was injected into oocytes as protein, its cytosolic CA activity could be clearly identified and calibrated by mass spectrometry and by intracellular H+ shifts (21). What is particularly exciting here is the demonstration that full-length, membrane-associated CA IV expressed from CA IV–cRNA also displays catalytic activity intracellularly, as if in the “cytosol“ of oocytes, following injection of CA IV–cRNA.
It has been documented that the GPI anchor of cell surface proteins is first made on the cytoplasmic face of the ER (31) and then translocated to the lumen where it is transferred to the carboxyl terminus of newly synthesized proteins that contain a recognition sequence for cleavage and receipt of the GPI anchor (32). The membrane topology of the newly synthesized GPI-anchored proteins like CA IV is preserved on the vesicles bound to the cell surface (33, 34). Previous studies showed that one can make a secretory form of CA IV by deleting the cleavage and anchoring sequence (35). However, if the newly synthesized CA IV remains inside the cell, addition of the GPI anchor is an essential step in allowing exit from the ER and realization of enzymatic activity. Failure to cleave the peptide sequence and add the GPI anchor led to rapid degradation in the ER (36).
Studies reported here show that nearly 80% of the total CA IV expressed in oocytes is on intracellular membranes. If this CA IV faces the interior of the secretory vesicles and its activity modulates the H+ of the cytosol, as the current studies suggest, it is likely that ion transporters and/or ion channels that allow access of the CA to its substrates, CO2 and H2O, and its products, H+ and HCO3−, to the cytosol are present in many of the same vesicles. The concept that membrane CAs and other transport proteins operate in enclosed secretory vesicles to modulate cytosolic pH is a unique one that could be tested experimentally. For example, if all intracellular CA IV faces the lumen of such vesicles, it should be inaccessible to fully membrane-impermeant CA inhibitors (27) and to inhibitory antibodies that are injected into CA IV–expressing oocytes.
Granule cells of acute mouse cerebellar cortex slices showed typical, fast intracellular H+ shifts upon the removal and addition of CO2/HCO3− at the same extracellular solution pH (7.4). These H+ shifts are due to the diffusion of CO2 out and into the cells, resulting in a decrease and increase in the cytosolic H+ concentration. The rates of these H+ shifts are enhanced by CA activity. In neurons and many other cell types in the brain and other tissues, CA II is the major cytosolic CA isoform (37, 38). Blocking cytosolic CA activity results in a reduction of the rate of H+ change during removal and addition of CO2/HCO3− (Fig. 4) (39).
Functional extracellular CA activity was found in hippocampal slices, where the enzyme was implicated in the regulation of excitatory transmission (40), and again in hippocampal neurons, where the anion exchanger AE3 activity was enhanced by enzymatic activity (29), and for H+ buffering (41). None of these studies addressed the intracellular activity of CA IV. In mice deficient for extracellular CA IV, the reduction in the rate of the cytosolic H+ shifts, as recorded here in cerebellar neurons, was not expected. The robust decreases of 30% and 40% upon removal and addition of CO2/HCO3− observed in granule cells of CA IV−/− mice suggest that CA IV contributes substantially to the total intracellular CA activity in cerebellar neurons. We could exclude a decrease in the expression of CA II in CA IV−/− mice. Thus, the observations in oocytes that CA IV has both intracellular as well as extracellular activity are not unique to that system, but have more general relevance.
Our studies clearly suggest that CA IV, although expressed on the cell surface (extracellular) as a GPI-anchored mature enzyme, can also display catalytic activity in intracellular vesicles. It will be interesting to study whether the other extracellular CA isoforms (CA IX, CA XII, CA XIV, CA XV), which are anchored by membrane spanning domains and have variable-length cytosolic tails, but also likely have CA domains facing the interior of secretory vesicles, also display intracellular activity. Another question is whether this intracellular activity can be observed in other cell types in addition to neurons. Expression of these catalytic activities intracellularly in transport vesicles may vary, not only among the particular isozymes but also with cell type, depending on whether relevant accessory proteins, such as proton, HCO3−, and possibility other transporters, are also present in the same transport vesicles in a given cell type. This possibility is especially interesting for those members of the CA gene family, whose products and their interacting proteins are thought to form a functional complex (“metabolon”). The oocyte system seems ideal to help identify and dissect such functional interactions and also determine whether metabolons have an intracellular function similar to or distinct from their putative roles at the cell surface.
Materials and Methods
Constructs, Oocytes, and Injection of cRNA.
The human CA IV cDNA (CA IV) and human CA II cDNA (CA II) were subcloned into the oocyte expression vector pGEM-He-Juel, which contains the 5′ and the 3′ untranscribed regions of the Xenopus β-globulin flanking the multiple cloning site. Xenopus oocytes of the stages V and VI were injected with 12 ng and 1–2 ng of cRNA coding either for human CA II-WT or human CA IV-WT, respectively, or 1–2 ng of the catalytically inactive mutant CA IV–V165Y, dissolved in diethylpyrocarbonate (DEPC)–H2O. Measurements were carried out 3–6 d after injection of cRNA. The oocyte saline had the following composition (in mM): NaCl, 82.5; KCl, 2.5; CaCl2, 1; MgCl2, 1; Na2HPO4, 1; and Hepes, 5, titrated with NaOH to a pH of 7.4. In the bicarbonate-containing saline, NaCl was replaced by an equivalent amount of NaHCO3 and the solution was aerated with 5% (vol/vol) CO2.
Acute Cerebellar Brain Slices.
Brain slices were prepared from CA IV–deficient mice of the B6.129S1-Car4tm1Sly/J strain and their wild-type littermates at postnatal day (P) 14–20. All animal procedures were approved by the Landesuntersuchungsamt Rheinland-Pfalz, Koblenz (23 177-07/A07-2-003 §6).
H+ Measurements.
For measurement of intracellular H+ (H+i) in granule cells, acute cerebellar tissue slices prepared from wild-type and mutant mice deficient in CA IV (CA IV−/−) were loaded with the H+-sensitive dye 2′,7′-bis-(carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF-AM, 1 µM; Molecular Probes) in Ca2+-reduced artificial cerebral spinal fluid (ACSF) for 30 min. For measurement of H+i and membrane potential in oocytes, double-barreled microelectrodes were used; the manufacture and application have been described in detail previously (42, 43). As described previously (44), optimal pH changes were detected when the electrode was located near the inner surface of the plasma membrane.
Determination of CA IV Activity.
Activity of CA IV was determined by monitoring the 18O depletion of doubly labeled 13C18O2 through several hydration and dehydration steps of CO2 and HCO3− at 25 °C (45, 46). For calibration, enzymatic activity was determined for 50, 100, 200, and 300 ng of isolated CA IV protein directly added to the cuvettete. Based on this calibration, total extra- and intracellular CA IV concentrations were calculated separately from the catalytic activity measured in intact and lysed CA IV–expressing oocytes.
Immunohistochemical Analysis of CA IV.
To determine the localization of CA IV, oocytes expressing CA IV (1 ng RNA) as well as native control oocytes were fixed for 20 min in 4% Histofix (Carl Roth, GmbH + Co. KG) in PBS. Oocytes were permeabilized at –25 °C with 100% methanol for 20 min and were embedded in 4% low-melt agarose (PEQLAB Biotechnologie GmbH). Oocytes were cut into 150-µm-thick slices on a conventional microtome (Campden Instruments). The sections stained against CA IV and of native oocytes were analyzed with a confocal laser-scanning microscope (LSM 700, Carl Zeiss GmbH) with the same settings in parallel.
Western Blot Analysis.
For comparison of protein levels of expressed CA II and CA IV, Western blot analyses were performed. Mouse cerebelli were lysed by sonication in 2% SDS solution with protease inhibitor (Complete Mini EDTA-free, Roche). Total protein content was determined using BCA protein assay kit (Pierce, Fisher Scientific GmbH). Quantification of proteins was carried out with the software Quantity One 4.5 (Bio-Rad Laboratories GmbH).
Calculation and Statistics.
Statistical values are presented as means ± SEM (S.E.M.). For calculation of significance in differences, Student’s t test or, if possible, a paired t test was used. Significance level is represented in the figures is as follows: *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
Full details of all materials and methods are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Marc A. Ilies for supplying BZA. This work has been supported by Deutsche Forschungsgemeinschaft (DFG) Grant DE 231/24-1; by DFG-Graduiertenkolleg 845; and by the Membrane Biology Priority Group RIMP, Land Rheinland-Pfalz (to J.W.D.). W.S.S. and A.W. are supported by National Institutes of Health Grant GM034182 (to W.S.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221213110/-/DCSupplemental.
References
- 1.Maren TH. Carbonic anhydrase: Chemistry, physiology, and inhibition. Physiol Rev. 1967;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- 2.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 3.Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7(2):168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 4.Winum JY, Innocenti A, Scozzafava A, Montero JL, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isoforms I and II and transmembrane, tumor-associated isoforms IX and XII with boronic acids. Bioorg Med Chem. 2009;17(10):3649–3652. doi: 10.1016/j.bmc.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Wistrand PJ, Knuuttila KG. Renal membrane-bound carbonic anhydrase. Purification and properties. Kidney Int. 1989;35(3):851–859. doi: 10.1038/ki.1989.63. [DOI] [PubMed] [Google Scholar]
- 6.Waheed A, Zhu XL, Sly WS, Wetzel P, Gros G. Rat skeletal muscle membrane associated carbonic anhydrase is 39-kDa, glycosylated, GPI-anchored CA IV. Arch Biochem Biophys. 1992;294(2):550–556. doi: 10.1016/0003-9861(92)90724-b. [DOI] [PubMed] [Google Scholar]
- 7.Wetzel P, Gros G. Inhibition and kinetic properties of membrane-bound carbonic anhydrases in rabbit skeletal muscles. Arch Biochem Biophys. 1998;356(2):151–158. doi: 10.1006/abbi.1998.0762. [DOI] [PubMed] [Google Scholar]
- 8.Gros G, et al. Extracellular, extravascular carbonic anhydrase in skeletal muscle. Ann N Y Acad Sci. 1984;429:412–414. doi: 10.1111/j.1749-6632.1984.tb12367.x. [DOI] [PubMed] [Google Scholar]
- 9.Waheed A, Zhu XL, Sly WS. Membrane-associated carbonic anhydrase from rat lung. Purification, characterization, tissue distribution, and comparison with carbonic anhydrase IVs of other mammals. J Biol Chem. 1992;267(5):3308–3311. [PubMed] [Google Scholar]
- 10.Zhu XL, Sly WS. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990;265(15):8795–8801. [PubMed] [Google Scholar]
- 11.Scheibe RJ, et al. Expression of membrane-bound carbonic anhydrases IV, IX, and XIV in the mouse heart. J Histochem Cytochem. 2006;54(12):1379–1391. doi: 10.1369/jhc.6A7003.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sender S, et al. Localization of carbonic anhydrase IV in rat and human heart muscle. J Histochem Cytochem. 1998;46(7):855–861. doi: 10.1177/002215549804600709. [DOI] [PubMed] [Google Scholar]
- 13.Tong CK, Brion LP, Suarez C, Chesler M. Interstitial carbonic anhydrase (CA) activity in brain is attributable to membrane-bound CA type IV. J Neurosci. 2000;20(22):8247–8253. doi: 10.1523/JNEUROSCI.20-22-08247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkkila S, et al. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem. 2000;48(12):1601–1608. doi: 10.1177/002215540004801203. [DOI] [PubMed] [Google Scholar]
- 15.Svichar N, Esquenazi S, Waheed A, Sly WS, Chesler M. Functional demonstration of surface carbonic anhydrase IV activity on rat astrocytes. Glia. 2006;53(3):241–247. doi: 10.1002/glia.20277. [DOI] [PubMed] [Google Scholar]
- 16.Baird TT, Jr, Waheed A, Okuyama T, Sly WS, Fierke CA. Catalysis and inhibition of human carbonic anhydrase IV. Biochemistry. 1997;36(9):2669–2678. doi: 10.1021/bi962663s. [DOI] [PubMed] [Google Scholar]
- 17.Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol. 1998;274(2 Pt 1):C543–C548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- 18.Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3- cotransporter. J Biol Chem. 2007;282(18):13508–13521. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- 19.Becker HM, Deitmer JW. Nonenzymatic proton handling by carbonic anhydrase II during H+-lactate cotransport via monocarboxylate transporter 1. J Biol Chem. 2008;283(31):21655–21667. doi: 10.1074/jbc.M802134200. [DOI] [PubMed] [Google Scholar]
- 20.Becker HM, Klier M, Schüler C, McKenna R, Deitmer JW. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc Natl Acad Sci USA. 2011;108(7):3071–3076. doi: 10.1073/pnas.1014293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klier M, et al. Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II. J Biol Chem. 2011;286(31):27781–27791. doi: 10.1074/jbc.M111.255331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkkila S. An overview of the distribution and function of carbonic anhydrase in mammals. EXS. 2000;2000(90):79–93. doi: 10.1007/978-3-0348-8446-4_4. [DOI] [PubMed] [Google Scholar]
- 23.Imtaiyaz Hassan M, Shajee B, Waheed A, Ahmad F, Sly WS. Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem. 2012 doi: 10.1016/j.bmc.2012.04.044. 10.1016/j.bmc.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Brion LP, Suarez C, Zhang H, Cammer W. Up-regulation of carbonic anhydrase isozyme IV in CNS myelin of mice genetically deficient in carbonic anhydrase II. J Neurochem. 1994;63(1):360–366. doi: 10.1046/j.1471-4159.1994.63010360.x. [DOI] [PubMed] [Google Scholar]
- 25.Shah GN, et al. Carbonic anhydrase IV and XIV knockout mice: Roles of the respective carbonic anhydrases in buffering the extracellular space in brain. Proc Natl Acad Sci USA. 2005;102(46):16771–16776. doi: 10.1073/pnas.0508449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem. 2001;276(51):47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 27.Supuran CT, Casini A, Scozzafava A. In: Carbonic Anhydrase: Its Inhibitors and Activators. Supuran CT, Scozzafava A, Conway J, editors. Boca Raton, FL: CRC Press; 2004. pp. 67–149. [Google Scholar]
- 28.Swenson ER, Grønlund J, Ohlsson J, Hlastala MP. In vivo quantitation of carbonic anhydrase and band 3 protein contributions to pulmonary gas exchange. J Appl Physiol. 1993;74(2):838–848. doi: 10.1152/jappl.1993.74.2.838. [DOI] [PubMed] [Google Scholar]
- 29.Svichar N, et al. Carbonic anhydrases CA4 and CA14 both enhance AE3-mediated Cl–HCO3- exchange in hippocampal neurons. J Neurosci. 2009;29(10):3252–3258. doi: 10.1523/JNEUROSCI.0036-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain A, Madhesia D. Heterocyclic compounds as carbonic anhydrase inhibitor. J Enzyme Inhib Med Chem. 2011;27(6):773–783. doi: 10.3109/14756366.2011.617882. [DOI] [PubMed] [Google Scholar]
- 31.Vidugiriene J, Menon AK. The GPI anchor of cell-surface proteins is synthesized on the cytoplasmic face of the endoplasmic reticulum. J Cell Biol. 1994;127(2):333–341. doi: 10.1083/jcb.127.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Udenfriend S, Kodukula K. How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem. 1995;64:563–591. doi: 10.1146/annurev.bi.64.070195.003023. [DOI] [PubMed] [Google Scholar]
- 33.Rivier AS, et al. Exit of GPI-anchored proteins from the ER differs in yeast and mammalian cells. Traffic. 2010;11(8):1017–1033. doi: 10.1111/j.1600-0854.2010.01081.x. [DOI] [PubMed] [Google Scholar]
- 34.Vallet V, Pfister C, Loffing J, Rossier BC. Cell-surface expression of the channel activating protease xCAP-1 is required for activation of ENaC in the Xenopus oocyte. J Am Soc Nephrol. 2002;13(3):588–594. doi: 10.1681/ASN.V133588. [DOI] [PubMed] [Google Scholar]
- 35.Waheed A, Okuyama T, Heyduk T, Sly WS. Carbonic anhydrase IV: Purification of a secretory form of the recombinant human enzyme and identification of the positions and importance of its disulfide bonds. Arch Biochem Biophys. 1996;333(2):432–438. doi: 10.1006/abbi.1996.0412. [DOI] [PubMed] [Google Scholar]
- 36.Okuyama T, Waheed A, Kusumoto W, Zhu XL, Sly WS. Carbonic anhydrase IV: Role of removal of C-terminal domain in glycosylphosphatidylinositol anchoring and realization of enzyme activity. Arch Biochem Biophys. 1995;320(2):315–322. doi: 10.1016/0003-9861(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 37.Cammer WB, Brion LP. Carbonic anhydrase in the nervous system. EXS. 2000;2000(90):475–489. doi: 10.1007/978-3-0348-8446-4_24. [DOI] [PubMed] [Google Scholar]
- 38.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83(4):1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 39.Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Prog Neurobiol. 1996;48(2):73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 40.Fedirko N, Avshalumov M, Rice ME, Chesler M. Regulation of postsynaptic Ca2+ influx in hippocampal CA1 pyramidal neurons via extracellular carbonic anhydrase. J Neurosci. 2007;27(5):1167–1175. doi: 10.1523/JNEUROSCI.3535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen JC, Chesler M. pH transients evoked by excitatory synaptic transmission are increased by inhibition of extracellular carbonic anhydrase. Proc Natl Acad Sci USA. 1992;89(16):7786–7790. doi: 10.1073/pnas.89.16.7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker HM, Bröer S, Deitmer JW. Facilitated lactate transport by MCT1 when coexpressed with the sodium bicarbonate cotransporter (NBC) in Xenopus oocytes. Biophys J. 2004;86(1 Pt 1):235–247. doi: 10.1016/S0006-3495(04)74099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deitmer JW. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J Gen Physiol. 1991;98(3):637–655. doi: 10.1085/jgp.98.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bröer S, et al. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333(Pt 1):167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverman DN. Carbonic anhydrase: Oxygen-18 exchange catalyzed by an enzyme with rate-contributing proton-transfer steps. Methods Enzymol. 1982;87:732–752. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- 46.Sültemeyer DF, Fock HP, Canvin DT. Mass spectrometric measurement of intracellular carbonic anhydrase activity in high and low C(i) cells of chlamydomonas: Studies using O exchange with C/O labeled bicarbonate. Plant Physiol. 1990;94(3):1250–1257. doi: 10.1104/pp.94.3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.