Abstract
The Golgi complex is essential for many aspects of cellular function, including trafficking and sorting of membrane and secretory proteins and posttranslational modification by glycosylation. We observed reversible fragmentation of the Golgi complex in cultured hippocampal neurons cultured in hyperexcitable conditions. In addition, Golgi fragmentation was found in cultured neurons with hyperactivity due to prolonged blockade of GABAA-mediated inhibition or withdrawal of NMDA receptor antagonism. The interplay between neuronal hyperactivity and Golgi structure established in this study thus reveals a previously uncharacterized impact of neuronal activity on organelle structure. This finding may have important roles in protein processing and trafficking in the Golgi as well as effects on neuronal signaling.
Keywords: hyperexcitability, activity-dependent
The Golgi complex is a highly dynamic cellular organelle that processes and sorts membrane proteins during transport from the site of synthesis in the endoplasmic reticulum to the cell surface, secretory vacuoles, or lysosomes. Distinct from other cellular organelles due to its ribbon-like organization of interconnected membrane stacks (1, 2), the Golgi is typically located around the centrosome, where it is positioned by a microtubule-dependent mechanism. The Golgi complex is continuously involved in membrane fusion and fission processes during protein and membrane cargo transport. Despite this dynamic quality, the Golgi complex maintains a distinct morphology with high stability in the number of cisternae per stack (1). However, physiological and pathological conditions are known to change the shape of the Golgi, including disassembly for limited cases such as microtubule reorganization during mitosis (3) or depolymerization by specific drugs (e.g., nocodazole, brefeldin A) that cause Golgi fragmentation into ministacks (4, 5). The interconnected Golgi stacks are rebuilt from the fragments upon exit from mitosis or drug washout.
The Golgi apparatus also undergoes irreversible fragmentation during apoptosis (6), which is due in part to caspase-mediated cleavage of Golgi-associated proteins (7). It is unclear if Golgi fragmentation is causative in cell death pathways or an effect of the signaling cascade. There is some evidence that the Golgi complex acts as a sensor to control entry into apoptosis (6, 8). Golgi fragmentation was also observed in several neurodegenerative pathologies, including Alzheimer’s disease (9), amyotrophic lateral sclerosis (ALS) (10), Creutzfeldt–Jakob disease (11), Niemann–Pick type C (12), Parkinson’s disease (13), and Spinocerebellar ataxia type 2 (14).
In neurons, the Golgi apparatus is not only crucial for proper forward trafficking of ion channels, receptors, and other signaling molecules but also mediates transport of exogenous molecules by retrograde and transsynaptic paths. The Golgi also functions in posttranslational modification of proteins and lipids by glycosylation, with sequential glycosylation reactions performed during trafficking through the Golgi. Consequently, damage to neuronal Golgi structure could have important functional consequences (15).
We observed fragmentation of the Golgi complex in hyperexcitable neurons. We used chronic exposure to slightly elevated potassium ion concentration as depolarizing stimuli. We also found Golgi fragmentation after treatment with increased potassium concentration for 2 d. This fragmentation was reversible upon return to normal culture medium.
We reasoned that if Golgi fragmentation occurs under hyperexcitable conditions, then fragmentation might also take place during prolonged hyperactivity. Therefore, we observed the Golgi complex in cultured hippocampal neurons during increased neuronal activity by prolonged treatment with bicuculline (16) or withdrawal of 2-amino-5-phosphonovaleric acid (APV) (17). Bicuculline blocks GABAA-mediated inhibition, thereby increasing neuronal activity, while APV is a selective NMDA receptor antagonist, and removal of APV after extended exposure results in increased neuronal activity. As a result of either type of increased neuronal activity, we observed fragmentation of the Golgi complex. The observed Golgi complex fragmentation was also reversible, as the interconnected stacks of the Golgi reorganized as neuronal activity returned to control levels.
The observed Golgi fragmentation occurs by a specific mechanism that depends on activation of CaM kinase by calcium. We observed block of Golgi fragmentation in cultured neurons pretreated with CaM kinase inhibitor KN-93. Additionally, Golgi fragmentation can be induced by treatment with okadaic acid, which blocks protein phosphatase 2A (PP2A) and PP1 at concentrations used in this study.
Overall, this report demonstrates reversible, activity-dependent fragmentation of the Golgi complex in neurons. Our findings reveal a unique cell biological consequence of hyperexcitability and increased neuronal activity.
Results
Prolonged Hyperexcitability Fragments the Golgi Complex.
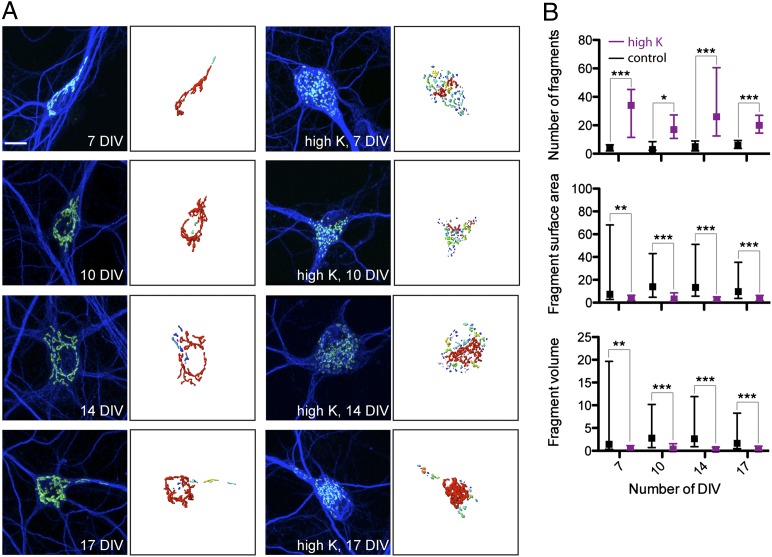
Golgi fragmentation was reported in neurons in many neurodegenerative pathologies (18). One common characteristic of these neurodegenerative diseases is neuronal hyperexcitability (19–26). For example, hyperexcitable motor neurons in an ALS mouse model showed Golgi fragmentation before symptoms of the disease (10). We hypothesized that prolonged hyperexcitability may lead to Golgi fragmentation. To test this, we cultured hippocampal neurons in medium with slightly elevated potassium ion concentration (15 mM compared with 5 mM) to induce hyperexcitability (27). The neurons were immunostained with antibody against the Golgi resident protein GM130 to assess Golgi structure (Fig. 1A). Images were obtained as z-stacks using confocal microscopy. The images were analyzed using Imaris software to quantify the number of distinct fragments comprising the Golgi staining (Fig. 1B). Analysis using Imaris also provided quantification of fragment surface area and volume. In all comparisons [from 7 d in vitro (DIV) to 17 DIV], the neurons cultured in medium with elevated potassium showed Golgi fragmentation compared with normal medium (Fig. 1 A and B). For example, at 17 DIV, neurons cultured in normal medium had a median value of six fragments with interquartile range (IR) = 3.8–9.3 fragments compared with neurons in elevated potassium having 20 fragments with IR = 14–27. Neurons were also cultured in normal medium, then switched to high potassium medium at 14 DIV for 2 d, at which point Golgi fragmentation was observed, whereas no such Golgi fragmentation was evident after the neurons were returned to normal potassium medium for an additional 2 d (Fig. S1).
Fig. 1.
The Golgi complex fragments under hyperexcitable conditions. (A) Neurons were cultured under normal and hyperexcitable conditions (elevated potassium concentration, high K). Immunostaining with anti-GM130 (green) and anti-MAP2 (blue) with 3D reconstruction of anti-GM130 signal. The color of the distinct Golgi fragments corresponds to the relative size of the fragment. (Scale bar: 10 μm.) (B) Quantification of number, surface area (μm2), and volume (μm3) of distinct Golgi fragments from reconstructed anti-GM130 fluorescent signal. Data shown are median and IR (controls: 7 DIV, n = 10; 10 DIV, n = 9; 14 DIV, n = 10; 17 DIV, n = 21; high K: 7 DIV, n = 10; 10 DIV, n = 8; 14 DIV, n = 9; 17 DIV, n = 17).
Golgi Fragmentation also Results from Neuronal Hyperactivity.
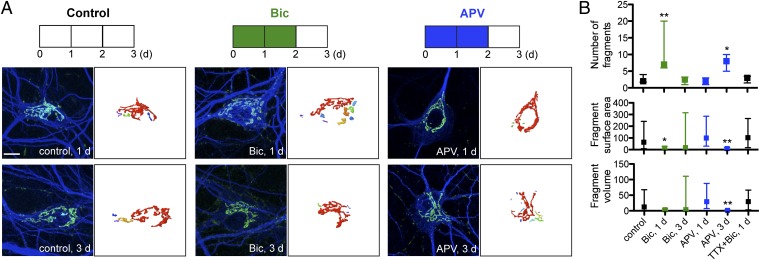
Knowing that Golgi fragmentation results from neuronal hyperexcitability, we wondered if hyperactivity also causes fragmentation of the Golgi complex. Mature cultured neurons (≥21 DIV) were treated with bicuculline for 1–2 d, then bicuculline was removed (Fig. 2A). Bicuculline is a GABAA receptor antagonist, thereby increasing neuronal activity by blocking GABAA-mediated inhibition. Treatment with bicuculline is also a known pharmacological model of seizures (16). Within 1 d of bicuculline exposure (Bic, 1 d), the Golgi complex in the majority of neurons was fragmented, as seen by the increase in the number of distinct fluorescent units within the Golgi mass (at 1 d, median value of seven fragments with IR = 6–20 compared with two fragments with IR = 1.8–4 for mock-treated control, Fig. 2B). During bicuculline treatment (Bic, 1 d), both the surface area (14 μm2 with IR = 5.5–29 for bicuculline compared with 64 μm2 with IR = 8.3–240 for control) and volume (3.5 μm3 with IR = 0.9–7.3 for bicuculline compared with 13 μm3 with IR = 1.1–68 for control) decreased significantly with fragmentation. Removal of bicuculline (Bic, 3 d, Fig. 2A) resulted in regeneration of a less fragmented Golgi ribbon (2.5 fragments with IR = 1–3), with a corresponding increase in fragment surface area (18 μm2 with IR = 6.1–320) and volume (3.8 μm3 with IR = 1.0–110) to mock-treated control levels.
Fig. 2.
Prolonged treatment with bicuculline (20 μM) or removal of APV (200 μM DL-APV) fragments the Golgi complex. (A) Immunostaining of cultured hippocampal neurons (≥21 DIV) with cis-Golgi marker anti-GM130 (green) and anti-MAP2 (blue) with 3D reconstruction of Golgi staining. (Scale bar: 10 μm.) (B) Quantification of number, surface area (μm2), and volume (μm3) of distinct Golgi fragments from reconstructed anti-GM130 fluorescent signal. Application of TTX (1 μM) before bicuculline to block synaptic transmission. Data shown are median and IR (control, n = 10; Bic, 1 d, n = 7; Bic, 3 d, n = 6; APV, 1 d, n = 9; APV, 3d, n = 7; TTX+Bic, 1 d, n = 5). For Bic, 1 d, P < 0.1.
A similar observation of Golgi fragmentation was detected for neurons after removal of APV (Fig. 2A). APV is a selective NMDA receptor antagonist, and removal of APV after extended exposure results in increased neuronal activity (17). Neurons were treated with APV for 2 d, then replaced with conditioned medium without APV for 1 d before immunostaining with anti-GM130. Increased neuronal activity from removal of APV resulted in Golgi fragmentation in the neurons (eight fragments with IR = 5–10; Fig. 2B), with a concomitant decrease in fragment size (surface area of 9.0 μm2 with IR = 2.8–18, volume of 1.5 μm3 with IR = 0.4–3.4). The number of fragments decreased to untreated levels 3–4 d after removal, showing reversibility as neuronal activity decreases. Thus, we observed activity-dependent fragmentation of the Golgi complex using two different means (bicuculline and APV withdrawal) to increase neuronal activity in cultured rat hippocampal neurons.
To demonstrate that the Golgi fragmentation requires neuronal activity, we inhibited synaptic activity by pretreating neurons for 20 min with tetrodotoxin (TTX), then added bicuculline. After 1 d of cotreatment with TTX and bicuculline, there was no change in the number (three fragments with IR = 2–4; Fig. 2B) or size (surface area of 100 μm2 with IR = 18–270, volume of 30 μm3 with IR = 3.3–66) of Golgi fragments. Thus, the Golgi fragmentation requires an increase in neuronal synaptic activity.
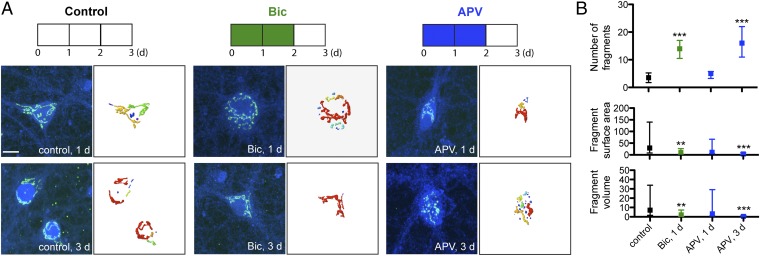
To ensure the observed fragmentation was not an effect only on GM130, we repeated the immunostaining using anti-TGN38 (Fig. 3A). Whereas GM130 is a protein marker for the cis region of the Golgi, TGN38 is located in the trans Golgi. For TGN38, the number of Golgi fragments was 14 (IR = 11–17) after 1 d bicuculline treatment, 16 (IR = 11–22) for 1 d after APV withdrawal (APV, 3 d), and 4 (IR = 2–5) for untreated control (Fig. 3B). This increase in Golgi fragments also occurred with a decrease in fragment surface area (10 μm2 with IR = 3.6–27 for bicuculline, 3.8 μm2 with IR = 1.7–8.6 for APV withdrawal, 29 μm2 with IR = 7.9–140 for control) and volume (2.0 μm3 with IR = 0.5–7.3 for bicuculline, 0.5 μm3 with IR = 0.2–1.7 for APV withdrawal, 7.2 μm3 with IR = 1.6–34 for control).
Fig. 3.
Golgi fragmentation is visualized with the trans-Golgi marker TGN38 as well as the cis-Golgi marker GM130. (A) Immunostaining of hippocampal neurons (≥21 DIV) with anti-TGN38 (green) and anti-MAP2 (blue) with 3D reconstruction of anti-TGN38 signal. (Scale bar: 10 μm.) (B) Quantification of number, surface area (μm2), and volume (μm3) of distinct Golgi fragments from reconstructed anti-TGN38 fluorescent signal. Data shown are median and IR (control, n = 10; Bic, 1 d, n = 9; APV, 1 d, n = 8; APV, 3d, n = 7).
Golgi Fragmentation from Hyperactivity Is Reversible.
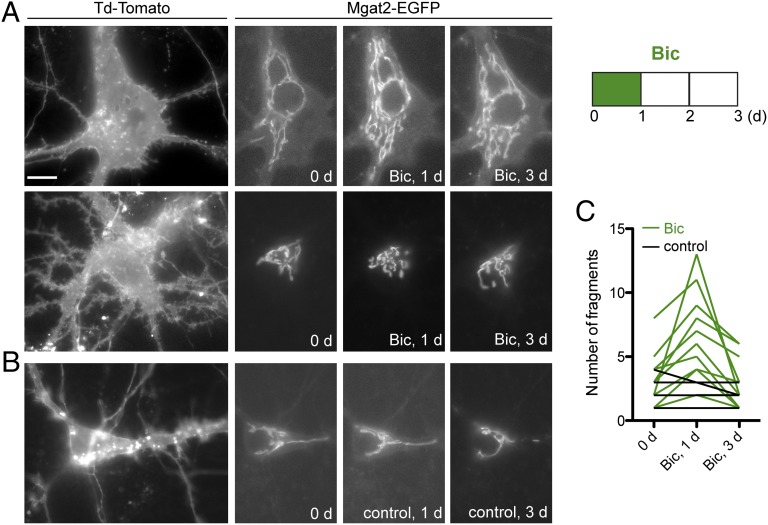
The experiments shown in Figs. 2 and 3 suggest that the Golgi fragmentation is reversible upon return to normal neuronal activity. Additionally, we checked the neurons during Golgi fragmentation conditions (both during bicuculline and after APV washout) for signs of apoptosis and found the neurons remain healthy with intact mitochondria and nuclei (Fig. S2). Nonetheless, we wanted to observe the reversibility of the Golgi fragmentation, so we turned to live cell time-lapse imaging of cultured hippocampal neurons cotransfected with fluorescently labeled Golgi enzyme Mgat2 (Mgat2–EGFP) and myristoylated Td-Tomato (to visualize neuron morphology). The somatic region of individual neurons was imaged before and 1 d after treatment with bicuculline. With addition of bicuculline, Mgat2–EGFP localization showed some fragmentation (Fig. 4A shows two example neurons and Fig. 4B shows a mock-treated control). After 1 d of bicuculline treatment, the medium was removed and replaced with preconditioned normal medium. Following return to normal medium, the neurons were imaged 2 d later to observe reversal of the Golgi fragmentation. The summary data of individual neurons (Fig. 4C) shows the trend of reversal of Golgi fragmentation upon return to normal neuronal activity after bicuculline-induced hyperactivity.
Fig. 4.
Golgi fragmentation occurs during bicuculline treatment and reverses after return to normal medium. Cultured hippocampal neurons were transfected with Mgat2–EGFP and myristoylated Td-Tomato. Individual neurons were imaged, then treated with bicuculline for 1 d. Bicuculline was removed and neurons were imaged again after 2 d in normal medium. (A) Examples of two neurons with fragmentation of the Golgi complex after 1 d with bicuculline, then reversal of fragmentation 2 d after bicuculline removal. (Scale bar: 15 μm.) (B) Example control neuron showing change in Mgat2–EGFP signal but lack of fragmentation. (C) Summary of data from bicuculline-treated (green, n = 12) and control (black, n = 4) neurons.
Activity-Dependent Golgi Fragmentation Requires CaM Kinase Activation.
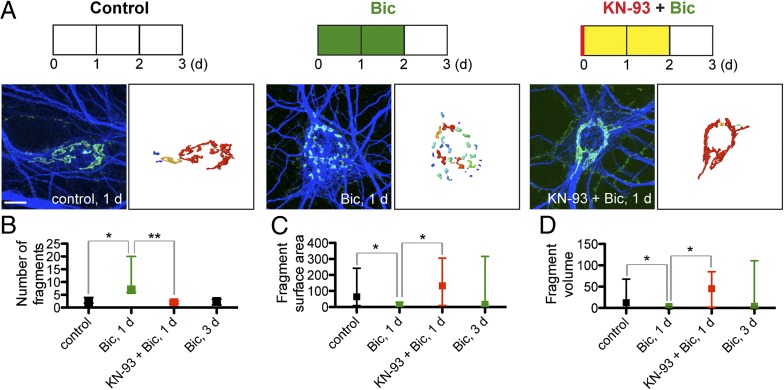
Knowing increased neuronal activity leads to an increase in intracellular calcium, we hypothesized that a calcium-dependent pathway may lead to the Golgi fragmentation. We found that pretreatment of cultured neurons with the CaM kinase II/IV inhibitor KN-93 blocks Golgi fragmentation by bicuculline treatment. Using the same conditions of mature cultured hippocampal neurons as used in Fig. 1, KN-93 was added 20 min before addition of bicuculline (Fig. 5A). As visualized by immunostaining of the Golgi marker GM130, CaM kinase inhibition with KN-93 blocked Golgi fragmentation (Fig. 5B), with coupled preservation of fragment size (Fig. 5 C and D). Thus, the observed neuronal activity-dependent Golgi fragmentation occurs via a specific CaM kinase II/IV–dependent pathway.
Fig. 5.
Pretreatment with CaMK II/IV inhibitor KN-93 blocks bicuculline-induced Golgi fragmentation. (A) Immunostaining of hippocampal neurons (≥21 DIV) with anti-GM130 (green) and anti-MAP2 (blue) with 3D reconstruction of anti-GM130 signal. The color of the distinct Golgi fragments corresponds to the relative size of the fragment. (Scale bar: 10 μm.) (B) Quantification of number of distinct Golgi fragments from reconstructed anti-GM130 fluorescent signal. Data shown are median and IR (control, n = 10; Bic, 1 d, n = 7; KN-93+Bic, 1 d, n = 5; Bic, 3 d, n = 6). (C and D) Quantification of surface area (μm2) and volume (μm3) of Golgi fragments from reconstructed images.
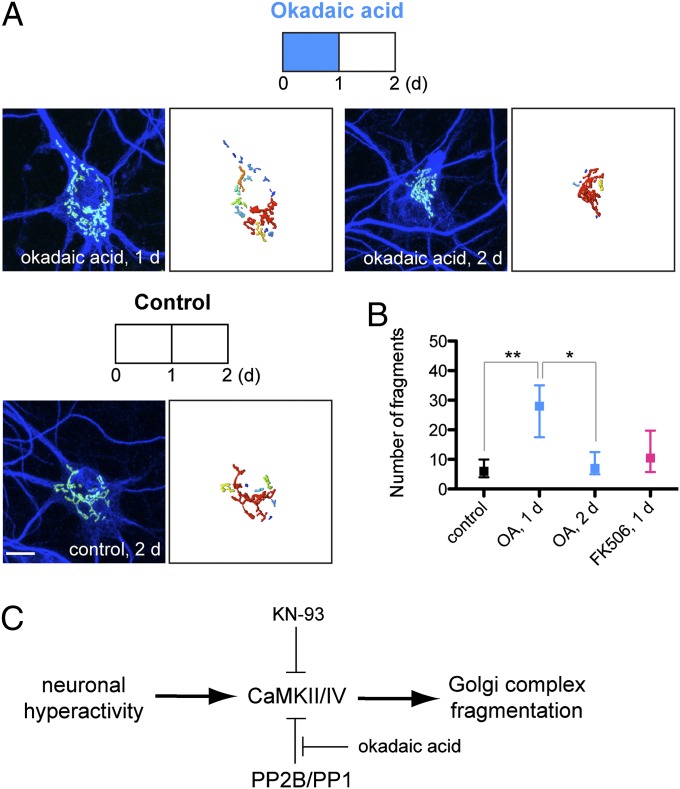
Knowing that protein phosphatases can dephosphorylate CaMKII (28–30) and CaMKIV (31, 32), we also examined the effect of protein phosphatase inhibitors okadaic acid and FK506 on Golgi structure. After inhibiting protein phosphatases with okadaic acid (10 nM) for 1 d (Fig. 6A), the Golgi complex fragmented (28 fragments with IR = 18–35 for okadaic acid treatment compared with six fragments with IR = 4–10 for control; Fig. 6B). The okadaic acid was then removed from the culture medium for an additional day, and we observed reversal of the Golgi fragmentation (seven fragments with IR = 5–13). However, inhibiting PP2B with FK506 (100 nM) did not cause Golgi fragmentation. Because okadaic acid inhibits PP2A (IC50 = 0.2–1 nM) and PP1 (IC50 = 3 nM) at the concentration used in his study, inhibiting PP2A and/or PP1 may allow phosphorylation of target proteins by CaMKII/CaMKIV, which can lead to Golgi fragmentation.
Fig. 6.
Okadaic acid causes Golgi fragmentation in cultured hippocampal neurons. Cultured hippocampal neurons (17 DIV) were treated with okadaic acid (10 nM) or FK506 (100 nM) for 1 d, then returned to normal culture medium for an additional day. (A) Immunostaining with anti-GM130 (green) and anti-MAP2 (blue) with 3D reconstruction of anti-GM130 signal. The color of the distinct Golgi fragments corresponds to the relative size of the fragment. (Scale bar: 10 μm.) (B) Quantification of the number of distinct Golgi fragments from reconstructed anti-GM130 fluorescent signal. Data shown are median and IR (control, n = 11; OA, 1 d, n = 10; OA, 2 d, n = 9). (C) Proposed Golgi fragmentation pathway during neuronal hyperactivity.
These experiments suggest that the Golgi fragmentation induced by prolonged hyperactivity occurs via CaMKII and/or CaMKIV, which in turn may be modulated by PP2A and/or PP1 (Fig. 6C). Future experiments on these pathways, beyond the scope of this report, would elucidate details of this mechanism.
Discussion
We observed Golgi fragmentation during prolonged hyperexcitability induced by elevated potassium. Moreover we studied fragmentation of the Golgi complex in cultured hippocampal neurons with increased neuronal activities by prolonged treatment with bicuculline or withdrawal of APV. Distinct from irreversible Golgi fragmentation during apoptosis (33), this activity-dependent fragmentation was reversible, as reorganization of the Golgi stacks occurred after washout of the drug for return to normal activity. We also found inhibition of this Golgi fragmentation by blocking CaM kinase, thereby implicating this pathway in the underlying mechanism of the observed fragmentation. Moreover, our findings reveal a unique cell biological consequence of neuronal activity and hyperexcitability on Golgi structure.
Implications for Hyperactivity and Seizures.
Prolonged treatment with bicuculline or removal of APV after extended exposure is known to increase neuronal activity. For example, firing rates of bicuculline-treated neurons rise significantly for acute treatment, then decline to control levels after extended time of washout (34). Also removal of APV after prolonged exposure results in increased action potential firing (17). Such changes in firing rates suggest homeostatic regulation in response to changes in neuronal activity, although underlying cell biological consequences of such hyperactivity are unknown. In this study we demonstrate activity-dependent fragmentation of the Golgi apparatus in neurons by treatment with bicuculline or removal of APV. It remains to be determined whether this Golgi fragmentation is a pathological consequence of increased activity or a mechanism of cell survival. Regardless, disassembly of the Golgi complex is a hitherto unreported cellular mechanism that manifests in some hyperactivity models, which mimic seizures and epilepsy. Additionally, we observed Golgi fragmentation during prolonged hyperexcitability from increased potassium concentration, which may be similar to Golgi fragmentation observed in hyperexcitable neurons in some neurodegenerative diseases. Better understanding of this basic phenomenon of Golgi fragmentation and its implications will provide insight into the cell biological consequences of neuronal hyperexcitability and hyperactivity.
Implications of Golgi Fragmentation on Protein Trafficking.
The Golgi complex has important functions in protein processing and sorting. It fulfills a central role in protein trafficking that follows protein synthesis and processing in the endoplasmic reticulum and precedes secretion or transport to the cell surface or lysosomes. Therefore, disassembly of Golgi structure has potential implications on intracellular protein processing and trafficking.
In polarized cells such as neurons, sorting machinery of the Golgi complex segregates protein cargo into distinct vesicles for subcellular localization. Transport and targeting of proteins to the axon and dendrites is crucial to maintain neuronal cell polarity. Considering these roles of the Golgi complex, alteration of Golgi structure could impair the highly regulated programs of protein processing and sorting to neuronal compartments. Interestingly, fragmentation of the Golgi apparatus occurs during several neurodegenerative diseases, which are characterized by deficient axonal transport and accumulation of protein aggregates in the cell body (15). Although activity-dependent Golgi fragmentation in neuronal soma could broadly alter protein trafficking in neurons, it will be important to determine if there are differential effects on axonal and dendritic proteins. Presumably some dendritic proteins could be synthesized locally and processed by Golgi outposts (35–37). Whether Golgi outposts are affected under activity-dependent Golgi fragmentation conditions is another interesting open question.
Implications for Intracellular Calcium.
Calcium is an important second messenger within cells, where many cellular functions are regulated by the concentration of free cytosolic calcium. Calcium levels are altered by influx from the extracellular space by activation of ion channels or release from intracellular pools in the endoplasmic reticulum, mitochondria, and Golgi complex (38–41). Because the integrity of the Golgi complex is required for normal calcium signaling and homeostasis (42, 43), fragmentation of the Golgi may release calcium from its stores and modify calcium signaling. Release of calcium from intracellular pools is important for synaptic plasticity (44, 45), secretion (46), and neurite growth (47) and also impacts neurodegeneration (48). Thus, in addition to affecting protein trafficking, neuronal activity-dependent Golgi fragmentation may cause calcium release, resulting in further impacts on calcium signaling.
Materials and Methods
Materials.
Chemical reagents used include bicuculline, DL-APV, TTX, KN-93, and okadaic acid (Tocris). Immunologic reagents used include mouse anti-GM130 (BD), mouse anti-rat TGN38 (BD), and rabbit anti-MAP2 (Chemicon) antibodies. Goat anti-mouse Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 647 secondary antibodies (Invitrogen) were also used.
Primary Cultures of Hippocampal Neurons.
The use and care of animals in this study follows the guideline of the Institutional Animal Care and Use Committee at the University of California, San Francisco. Hippocampal neuron primary cultures from 19-d embryonic rats were prepared as previously described (49). Coverslips (Warner Instruments) were pretreated with nitric acid and precoated with poly-L-lysine (0.1 mL/mL; Sigma-Aldrich). Each 12-mm coverslip was plated with 5 × 104. Neurons were maintained in Neurobasal medium (Invitrogen) containing B27 extract (Invitrogen), 0.5 mM glutamine, 100 units of penicillin, and 100 μg/mL of streptomycin. For hyperexcitable neurons, cultures were maintained in medium with an additional 10 mM KCl. For transient transfection, neurons in culture at 9 DIV were treated with Opti-MEM containing Mgat2-EGFP plasmid, myristoylated Td-Tomato plasmid, and Lipofectamine 2000 (Invitrogen).
Induction of Neuronal Activity in Primary Hippocampal Cultures and Immunocytochemistry with Golgi Markers.
Cultured hippocampal neurons (≥21 DIV) were treated with bicuculline (20 μM) or DL-APV (200 μM). For TTX or KN-93 pretreatment, TTX (1 μM) or KN-93 (5 μM) was added 20 min before bicuculline. For okadaic acid, 17 DIV neurons were treated with okadaic acid (10 nM). For hyperexcitable conditions, KCl (10 mM) was added. Neurons were fixed with 4% (wt/vol) sucrose/4% (wt/vol) formaldehyde in PBS for 10 min at room temperature. Samples were washed three times with PBS for 10 min each, then treated with block solution (5% (vol/vol) normal goat serum in PBS) with 0.1% Triton X-100 for 1 h. Primary antibodies against GM130 or TGN38 and MAP2 were diluted (1:500 for anti-GM130 or anti-TGN38 and 1:1,000 for anti-MAP2) in block solution and applied for 2 h. Cells were washed three times with PBS for 10 min each. Secondary antibodies were diluted (1:1,000 each) in block solution and applied for 1 h. Samples were washed three times with PBS. The coverslips were mounted on glass slides. Images were acquired on a Leica SP5 confocal microscope, with z-stacks of 0.17 μm. Imaris software was used to quantify distinct Golgi fragments and for size analysis. Images from 3D reconstruction shown in figures (Figs. 1A, 2A, 3A, 5A, 6A and Fig. S1) are color-coded (only for ease of visualization of fragments) with spectrum coloring of red (largest fragment) to violet (for the smallest).
Live Time-Lapse Imaging.
Cotransfected neurons (Mgat2-EGFP and myristoylated Td-Tomato) were imaged by epifluorescence microscopy at 15 DIV. Then at least half of the conditioned medium was removed and saved, and bicuculline (20 μM) was added to the cells. After 1 d, the same neurons were imaged before removal of the bicuculline-containing medium and replacement with the conditioned medium. Two days later the cells were imaged again. The numbers of distinct fragments of Mgat2–EGFP signal were counted and compared with mock-treated cultures.
Data Analysis.
Results are reported as median and IR; means and SD were not used, as the datasets are not normally distributed. Comparisons of group medians were performed with nonparametric Kruskal–Wallis with Dunns posttest using Prism 5 (GraphPad Software), with differences considered significant at P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001 in all graphs).
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health National Research Service Award postdoctoral fellowship (to D.A.T.) and National Institute of Mental Health Grant MH065334. Y.N.J. and L.Y.J. are Howard Hughes Medical Institute investigators.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220978110/-/DCSupplemental.
References
- 1.Polishchuk RS, Mironov AA. Structural aspects of Golgi function. Cell Mol Life Sci. 2004;61(2):146–158. doi: 10.1007/s00018-003-3353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rambourg A, Clermont Y. Three-dimensional electron microscopy: Structure of the Golgi apparatus. Eur J Cell Biol. 1990;51(2):189–200. [PubMed] [Google Scholar]
- 3.Warren G, Malhotra V. The organisation of the Golgi apparatus. Curr Opin Cell Biol. 1998;10(4):493–498. doi: 10.1016/s0955-0674(98)80064-1. [DOI] [PubMed] [Google Scholar]
- 4.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: Insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogalski AA, Bergmann JE, Singer SJ. Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J Cell Biol. 1984;99(3):1101–1109. doi: 10.1083/jcb.99.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslan JE, Thomas G. Death by committee: Organellar trafficking and communication in apoptosis. Traffic. 2009;10(10):1390–1404. doi: 10.1111/j.1600-0854.2009.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sesso A, et al. Structural elements common to mitosis and apoptosis. Tissue Cell. 1999;31(3):357–371. doi: 10.1054/tice.1999.0042. [DOI] [PubMed] [Google Scholar]
- 8.Nakagomi S, et al. A Golgi fragmentation pathway in neurodegeneration. Neurobiol Dis. 2008;29(2):221–231. doi: 10.1016/j.nbd.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun K-H, et al. Novel genetic tools reveal Cdk5’s major role in Golgi fragmentation in Alzheimer’s disease. Mol Biol Cell. 2008;19(7):3052–3069. doi: 10.1091/mbc.E07-11-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stieber A, et al. The fragmented neuronal Golgi apparatus in amyotrophic lateral sclerosis includes the trans-Golgi-network: Functional implications. Acta Neuropathol. 1998;95(3):245–253. doi: 10.1007/s004010050794. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai A, et al. Fragmentation of the Golgi apparatus of the ballooned neurons in patients with corticobasal degeneration and Creutzfeldt-Jakob disease. Acta Neuropathol. 2000;100(3):270–274. doi: 10.1007/s004010000182. [DOI] [PubMed] [Google Scholar]
- 12.Lin P, et al. Calnuc binds to Alzheimer’s beta-amyloid precursor protein and affects its biogenesis. J Neurochem. 2007;100(6):1505–1514. doi: 10.1111/j.1471-4159.2006.04336.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujita Y, Ohama E, Takatama M, Al-Sarraj S, Okamoto K. Fragmentation of Golgi apparatus of nigral neurons with alpha-synuclein-positive inclusions in patients with Parkinson’s disease. Acta Neuropathol. 2006;112(3):261–265. doi: 10.1007/s00401-006-0114-4. [DOI] [PubMed] [Google Scholar]
- 14.Huynh DP, Yang H-T, Vakharia H, Nguyen D, Pulst SM. Expansion of the polyQ repeat in ataxin-2 alters its Golgi localization, disrupts the Golgi complex and causes cell death. Hum Mol Genet. 2003;12(13):1485–1496. doi: 10.1093/hmg/ddg175. [DOI] [PubMed] [Google Scholar]
- 15.Fan J, et al. Golgi apparatus and neurodegenerative diseases. Int J Dev Neurosci. 2008;26(6):523–534. doi: 10.1016/j.ijdevneu.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Heyer EJ, Nowak LM, Macdonald RL. Membrane depolarization and prolongation of calcium-dependent action potentials of mouse neurons in cell culture by two convulsants: Bicuculline and penicillin. Brain Res. 1982;232(1):41–56. doi: 10.1016/0006-8993(82)90609-6. [DOI] [PubMed] [Google Scholar]
- 17.Chung HJ, et al. G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation. Proc Natl Acad Sci USA. 2009;106(2):635–640. doi: 10.1073/pnas.0811685106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonatas NK, Stieber A, Gonatas JO. Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. J Neurol Sci. 2006;246(1-2):21–30. doi: 10.1016/j.jns.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 19.D’Arcangelo G, et al. Glutamatergic neurotransmission in a mouse model of Niemann-Pick type C disease. Brain Res. 2011;1396:11–19. doi: 10.1016/j.brainres.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Martin LJ, Chang Q. Inhibitory synaptic regulation of motoneurons: A new target of disease mechanisms in amyotrophic lateral sclerosis. Mol Neurobiol. 2012;45(1):30–42. doi: 10.1007/s12035-011-8217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minkeviciene R, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29(11):3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noebels J. A perfect storm: Converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(Suppl 1):39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamburin S, et al. Abnormal sensorimotor integration is related to disease severity in Parkinson’s disease: A TMS study. Mov Disord. 2003;18(11):1316–1324. doi: 10.1002/mds.10515. [DOI] [PubMed] [Google Scholar]
- 25.Yokota T, et al. Electrophysiological features of central motor conduction in spinocerebellar atrophy type 1, type 2, and Machado-Joseph disease. J Neurol Neurosurg Psychiatry. 1998;65(4):530–534. doi: 10.1136/jnnp.65.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokota T, Yoshino A, Hirashima F, Komori T, Miyatake T. Increased central motor tract excitability in Creutzfeldt-Jakob disease. J Neurol Sci. 1994;123(1-2):33–37. doi: 10.1016/0022-510x(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 27.Rannals MD, Kapur J. Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of GABA(A) receptors. J Neurosci. 2011;31(48):17701–17712. doi: 10.1523/JNEUROSCI.4476-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blitzer RD, et al. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280(5371):1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- 29.Bradshaw JM, Kubota Y, Meyer T, Schulman H. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc Natl Acad Sci USA. 2003;100(18):10512–10517. doi: 10.1073/pnas.1932759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura Y, Sogawa Y, Yamauchi T. Protein phosphatase 1 is involved in the dissociation of Ca2+/calmodulin-dependent protein kinase II from postsynaptic densities. FEBS Lett. 1999;446(2-3):239–242. doi: 10.1016/s0014-5793(99)00226-4. [DOI] [PubMed] [Google Scholar]
- 31.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87(7):1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 32.Reece KM, Mazalouskas MD, Wadzinski BE. The Balpha and Bdelta regulatory subunits of PP2A are necessary for assembly of the CaMKIV.PP2A signaling complex. Biochem Biophys Res Commun. 2009;386(4):582–587. doi: 10.1016/j.bbrc.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker A, et al. Golgi fragmentation during Fas-mediated apoptosis is associated with the rapid loss of GM130. Biochem Biophys Res Commun. 2004;316(1):6–11. doi: 10.1016/j.bbrc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391(6670):892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 35.Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23(15):6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torre ER, Steward O. Protein synthesis within dendrites: Glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. J Neurosci. 1996;16(19):5967–5978. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye B, et al. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130(4):717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276(31):29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 39.Martone ME, Edelmann VM, Ellisman MH, Nef P. Cellular and subcellular distribution of the calcium-binding protein NCS-1 in the central nervous system of the rat. Cell Tissue Res. 1999;295(3):395–407. doi: 10.1007/s004410051246. [DOI] [PubMed] [Google Scholar]
- 40.Pinton P, Pozzan T, Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998;17(18):5298–5308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Southall TD, et al. Novel subcellular locations and functions for secretory pathway Ca2+/Mn2+-ATPases. Physiol Genomics. 2006;26(1):35–45. doi: 10.1152/physiolgenomics.00038.2006. [DOI] [PubMed] [Google Scholar]
- 42.Cifuentes F, et al. A ryanodine fluorescent derivative reveals the presence of high-affinity ryanodine binding sites in the Golgi complex of rat sympathetic neurons, with possible functional roles in intracellular Ca(2+) signaling. Cell Signal. 2001;13(5):353–362. doi: 10.1016/s0898-6568(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 43.Vanoevelen J, et al. Cytosolic Ca2+ signals depending on the functional state of the Golgi in HeLa cells. Cell Calcium. 2005;38(5):489–495. doi: 10.1016/j.ceca.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Greer PL, Greenberg ME. From synapse to nucleus: Calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59(6):846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59(6):861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 46.Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22(4):496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011;12(4):191–203. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wojda U, Salinska E, Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. 2008;60(9):575–590. doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]
- 49.Shi S-H, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112(1):63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.