Abstract
Language production and spatial attention are the most salient lateralized cerebral functions, and their complementary specialization has been observed in the majority of the population. To investigate whether the complementary specialization has a causal origin (the lateralization of one function causes the opposite lateralization of the other) or rather is a statistical phenomenon (different functions lateralize independently), we determined the lateralization for spatial attention in a group of individuals with known atypical right hemispheric (RH) lateralization for speech production, based on a previous large-scale screening of left-handers. We show that all 13 participants with RH language dominance have left-hemispheric dominance for spatial attention, and all but one of 16 participants with left-hemispheric language dominance are RH dominant for spatial attention. Activity was observed in the dorsal fronto-parietal pathway of attention, including the inferior parietal sulcus and superior parietal lobule, the frontal eye-movement field, and the inferior frontal sulcus/gyrus, and these regions functionally colateralized in the hemisphere dominant for attention, independently of the side of lateralization. Our results clearly support the Causal hypothesis about the complementary specialization, and we speculate that it derives from a longstanding evolutionary origin. We also suggest that the conclusions about lateralization based on an unselected sample of the population and laterality assessment using coarse functional transcranial Doppler sonography should be interpreted with more caution.
A striking observation in the human brain is the hemispheric asymmetry of many information-processing functions. Various tasks elicit more brain activity in the left than the right half of the brain, or vice versa. This asymmetry has become particularly clear in the brain-imaging studies of the last two decades. Cerebral lateralization has long been considered a hallmark of human development. However, it now is clear that functional lateralization exists not only in humans but also in a variety of vertebrates such as primates (1, 2), songbirds (3), mice (4), and even in invertebrates such as honey bees (5).
The mechanisms underlying functional lateralization are still unclear, although it seems reasonable to assume that it must have an evolutionary advantage. A series of studies by Rogers and colleagues (6, 7; see also ref. 8) gave some hints about the possible advantages of functional lateralization. They examined the performance of chicks in “dual-task” situations consisting of predator detection, associated with fear response, which is lateralized to the right side of the brain, and pecking, which is associated with left hemisphere (LH) specialization. Rogers and colleagues compared the performance of chicks with strong lateralization and with weak lateralization. The results suggested that strong lateralization of the tasks in different hemispheres, i.e., complementary specialization, resulted in better performance (6).
Although animal studies suggest that functional lateralization has advantages in carrying out simultaneous processing, which may have contributed to the evolution of cognitive lateralization (9), surprisingly little empirical evidence is available for humans. On the one hand, recent imaging techniques have confirmed that language production is left lateralized in the great majority of the population (10, 11), whereas visuospatial attention is right lateralized (12–15). On the other hand, although some studies suggested that a larger degree of hemispheric lateralization is associated with better performance (16, 17), others found negative correlations or no correlation between the degree of laterality and performance (18,19; for a discussion see ref. 20). Also, few advantages of dual-task performance have been reported so far.
An interesting theory about the origin of human laterality was proposed by Kosslyn (21). He reasoned that activities involving the coordination of rapid sequences of precise, ordered operations require unilateral control, because in such cases one needs a single set of commands for both halves of the body. Therefore, these activities are innately lateralized. Kosslyn postulated two unilateral control systems: (i) speech control, which usually is lateralized to the LH, and (ii) shifts of spatial attention in response to environmental stimuli, which commonly are controlled by the right hemisphere (RH). According to Kosslyn, both systems perform best if they are controlled by different hemispheres, in line with the crowding hypothesis, which states that spatial attention performance can be crowded out if language involves regions in the same hemisphere (22–24). These two seeds then cause snowball effects, affecting the laterality of systems/subsystems interacting with them. Individual differences in laterality are assumed to result from the innate biases of the two systems and the degree of information degradation caused by interhemispheric transfer. Here we refer to this theory as the “Causal hypothesis.” Note that this term does not necessarily imply causality between the two systems themselves; they also could derive from a common origin.
An alternative to the Causal hypothesis is the Statistical hypothesis, according to which complementary specialization is a statistical rather than a causal phenomenon (25). Asymmetries of functions reflect innate biases of independent sources, but different functions lateralize independently. Atypical laterality of one function has no consequences for the laterality of the other functions. The Statistical hypothesis seems to be the dominant one at the moment (26–28 and see Discussion).
Although the issue of functional laterality in humans has been seen as a critical question in evolution and development, and the two hypotheses have been discussed in numerous studies, they are not easy to dissociate. Some earlier evidence of atypical colateralization of speech and spatial attention came from clinical cases. The problem, however, is that it is hard to know to what extent functioning has changed as a result of the brain damage (29, 30). Luckily, advances in neuroimaging have opened a new approach to test the issue, by revealing that atypical laterality (i.e., RH language or LH visuospatial attention) can be observed in healthy participants (11, 31). Therefore, a particularly interesting question is what happens to one function (e.g., attention control) in participants who have the other function (speech) lateralized in the nontypical RH. If the Causal hypothesis is correct, we would expect both functions to lateralize atypically, and a division in hemispheric specialization would still be observed. If the Statistical hypothesis is correct, we would expect participants with one atypically lateralized function to have the same hemisphere dominant for both functions, given the low probability of atypical lateralization of the other function.
Among previous studies investigating the relationship between language lateralization and spatial attention lateralization, a few have tested the issue in healthy participants (26–28, 31–34). Interestingly, these studies all failed to find a correlation between language lateralization and spatial attention lateralization, and they interpreted the results as evidence for the Statistical hypothesis. It should be noticed, however, that the Statistical theory predicts that for the majority of individuals with one function atypically lateralized, the other function should be lateralized to the same hemisphere, as mentioned above. This was not the case. In other words, the results favored neither the Causal nor the Statistical hypothesis. In all likelihood, the lack of correlation was caused by a high degree of noise (Discussion).
The following shortcomings may have blurred the findings: (i) very few participants with atypical laterality were tested, and (ii) handedness and hemispheric dominance were sometimes confounded. In student populations, only 1 of 10 left-handers has clear atypical speech dominance (10). This is a very low percentage to find significant correlations in unselected samples. Furthermore, (iii) the control tasks used in some studies either were of too low a level (e.g., fixation) or were not appropriate as baseline (e.g., the task-related hand response was not controlled); (iv) some paradigms were not efficient, possibly eliciting activity that was too low to get a clear lateralization pattern, and the laterality index (LI) therefore was very method dependent; and (v) the functional transcranial Doppler (fTCD) sonography technique used in some studies may have been too coarse. fTCD measures stimulus-related changes in the velocity of blood flow in the vascular territories of cerebral arteries and may not be able to decide the functional lateralization within a particular cerebral region precisely.
In the current study, we carefully examined the lateralization of visuospatial attention by testing a group of individuals with known RH lateralization for speech production, based on a previous large-scale screening of 265 left-handers (35). In that study, we showed that behavioral visual half-field (VHF) tasks are a good screening method to determine language dominance in a large sample of healthy left-handers. Participants were tested first on a word and picture-naming task, and one-fourth of them then were examined for speech lateralization in a silent word-generation task in functional MRI (fMRI). About 80% of participants with a left visual field advantage in both word and picture naming turned out to have atypical right hemispheric speech dominance, whereas all participants with a clear right visual field advantage in the VHF tasks showed left dominance in fMRI. The silent word-generation task has good concordance with Wada test results and is considered the most robust and reliable paradigm for measuring language production (36–38), whereas the Landmark task is widely used as a measure of visuospatial attention. We opted for the Landmark task in the current study because it has limited eye movement and motor demands and is considered a particularly good paradigm for fMRI (14, 39).
Results
fMRI Results.
One participant had to be excluded from further analyses because of excessive head movements (up to 5.1 mm). The remaining 31 participants made head movements of less than one voxel.
For the word-generation task, a group analysis on all 31 participants showed strong activity in the inferior and middle frontal gyri [peaking in the inferior frontal gyrus (IFG) pars opercularis and extending to the precentral gyrus and insula], the cingulate gyrus, the supplementary motor area (SMA), the inferior parietal lobule, and the cerebellum. For the Landmark task, the group analysis with all participants showed increased activation in the Landmark task condition (LM) vs. the Landmark control condition (LMC) at the inferior parietal sulcus (IPS) and superior parietal lobule (SPL), extending to middle/inferior occipital gyri, and anterior activations in the frontal eye field (FEF, precentral gyrus) and the inferior frontal sulcus (IFS), extending to the inferior and middle frontal gyri (Table 1).
Table 1.
Peak locations and coordinates in the Landmark task (LM > LMC) based on all participants with either LH or RH dominance for language (n = 31, P < 0.05 family-wise error)
| Region | Hemisphere | Peak coordinates MNI (mm) | t value |
| IPS/SPL | R | (42, −49, 49) | 8.75 |
| (28, −63,5 2) | 8.06 | ||
| IPS/SPL | L | (−38, −46, 46) | 8.15 |
| (−18, −66, 56) | 7.22 | ||
| SMA | R+L | (0, 24, 49) | 9.84 |
| Precentral (FEF) | R | (31, −4, 63) | 5.75 |
| (49, 4, 35) | 7.33 | ||
| Precentral (FEF) | L | (−28, −7, 52) | 7.71 |
| (−46, 0, 35) | 6.42 | ||
| IFS/Insula | L | (−38,18,10) | 7.64 |
| IFS/Insula | R | (38, 24, 10) | 8.25 |
| (49, 4, 35) | 7.33 | ||
| (38, 46, 10) | 6.28 | ||
| Middle frontal gyrus | R | (46, 35, 35) | 7.45 |
| Middle occipital gyrus | L | (−38, −88, 0) | 11.08 |
| Inferior occipital gyrus | L | (−28, −84, −14) | 9.05 |
| Lingual | L | (−18, −88, −14) | 9.20 |
| Middle occipital gyrus | R | (32, −77, 24) | 8.94 |
| (38, −84, 4) | 8.02 | ||
| Inferior occipital gyrus | R | (24, −84, −18) | 5.36 |
Individual word-production LIs were calculated for IFG activity using weighted mean LI and bootstrap methods (40, 41). Sixteen participants showed the typical left-lateralized activation pattern (LI > 0.5), 14 participants were right lateralized (LI < −0.5), and one was marginally right lateralized (LI, −0.45) and was considered as right lateralized in further analyses. Group analysis for the participants with typical LH and atypical RH dominance showed clear mirror-reversed patterns (see also ref. 35).
Analysis of the fMRI data in the Landmark task showed that all but two participants had significantly more activation in LM than LMC at the whole-brain level (P < 0.001 uncorrected). The two participants (RH dominant for language) who showed no parietal activity even at a much lower threshold (P < 0.01 uncorrected, k = 10) were excluded from further analyses. Individual LIs were calculated for IPS/SPL, which is considered the critical site for visuospatial attention (14, 42) and also was the most activated region in the current study. For each participant, an LI was calculated on a series of thresholds of t values, and a weighted mean LI then was calculated by attributing a higher weight to higher thresholds. (See Methods for more details.)
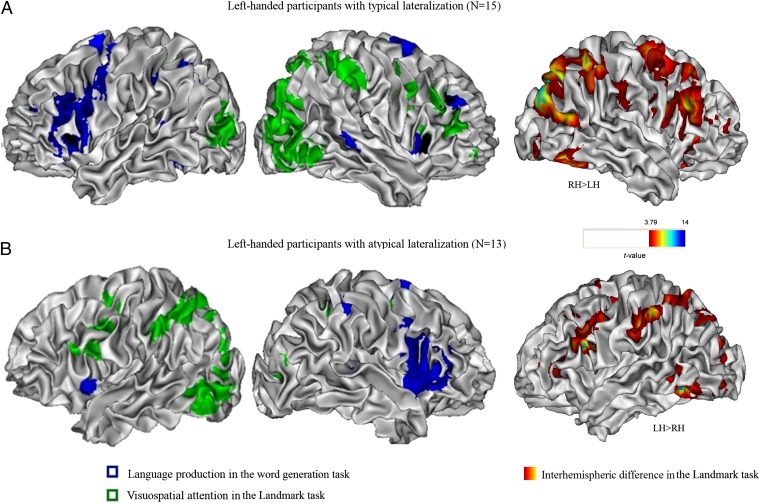
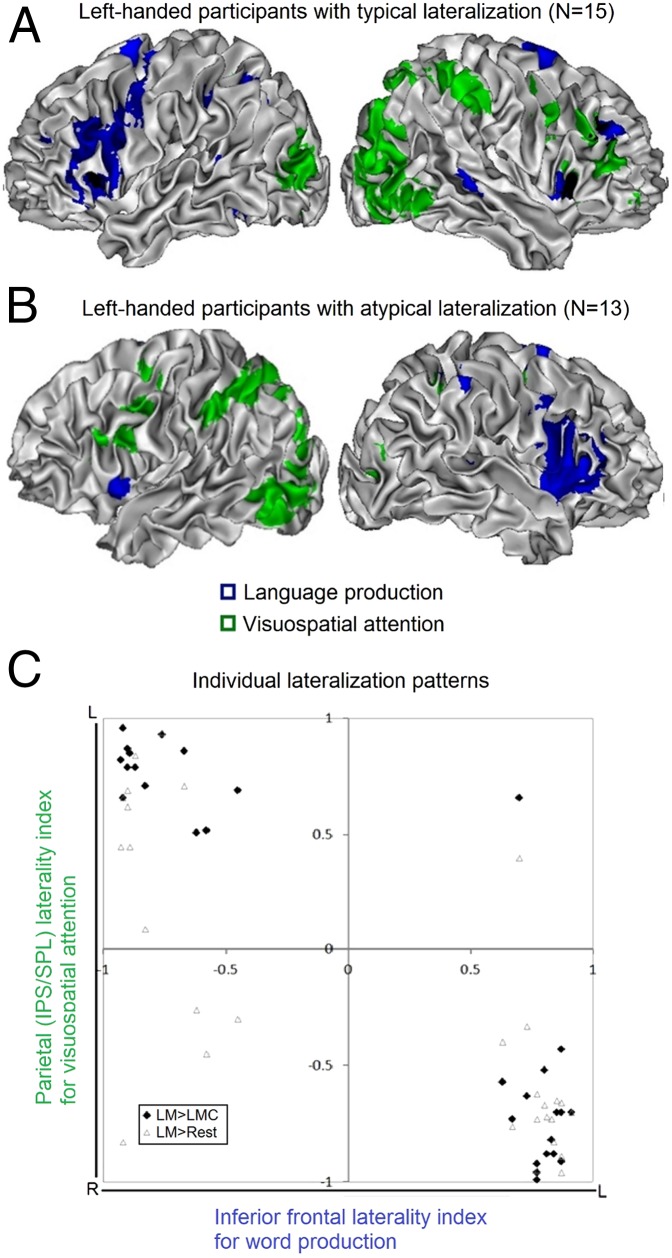
Fig. 1 shows the activation patterns in the word-generation and the Landmark tasks for the participants with typical and atypical speech dominance. All but one participant had language production and spatial attention lateralized to opposite hemispheres, independent of whether the language lateralization pattern was typical. Fifteen of the 16 participants with typical LH language were right dominant for visuospatial attention, and all participants with atypical RH language were left dominant for spatial attention (Fig. 2, red diamonds). In both groups, activations common to language production and spatial attention were seen mostly in the SMA and the bilateral insula, slightly extending to inferior frontal regions, and in the inferior parietal regions (Landmark P < 0.001 inclusively masked by Word generation P < 0.001).
Fig. 1.
Language production and visuospatial attention lateralize to different hemispheres, independent of the side of lateralization. Results for participants with (A) typical lateralization or (B) atypical lateralization, for word generation (in blue, word generation against repetition) and for the Landmark task (in green, Landmark against control task). In each panel the left picture shows activation in the left hemisphere, and the middle picture shows activation in the right hemisphere. The group level activations are rendered on the brain of a single participant with typical lateralization, with values of t > 4.98 (P < 0.0001 uncorrected) for the typical group (n = 15) and t > 4.30 (P < 0.001 uncorrected) for the atypical group (n = 13). The right picture in each panel shows the outcome of the additional interhemispheric difference analysis for the Landmark task (in red-yellow-blue, Landmark against control task).
Fig. 2.
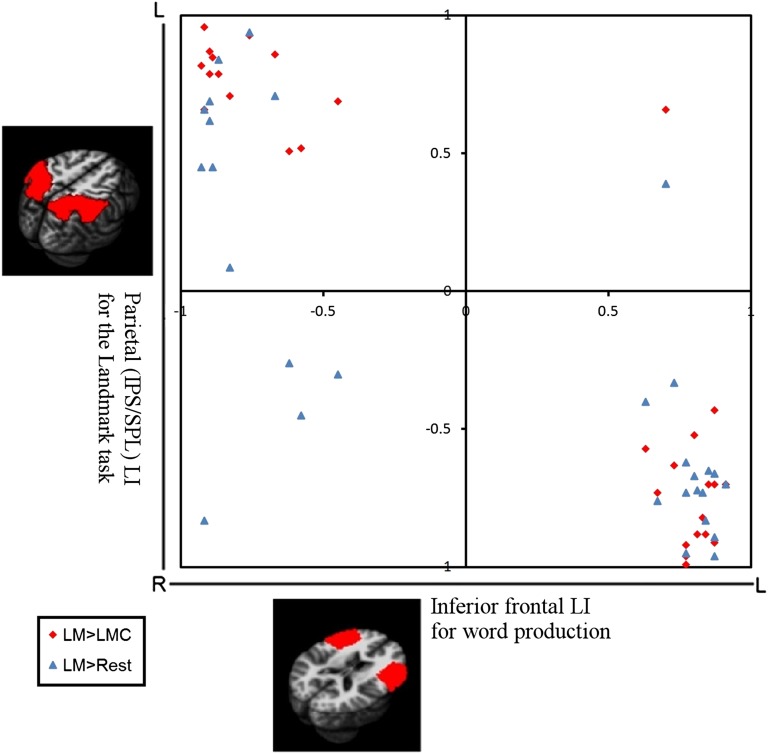
Lateralization patterns for all 29 participants. The red diamonds show the outcome of the comparison of LM and LMC in IPS/SPL with the inferior frontal lateralization of language production (Word generation > Control). All but one participant had language production and spatial attention lateralized to opposite hemispheres, no matter whether the lateralization pattern was typical or atypical (15 participants showed the typical LH language–RH attention pattern, and 13 participants showed the atypical RH language–LH attention pattern). The blue triangles show the outcome of the analysis when the laterality index of the Landmark task is based on a comparison of LM and Rest. This analysis gives less clear data.
Correlation analysis of the individual data showed a high negative correlation between the IPS/SPL lateralization for the Landmark task and the IFG lateralization for word generation (r = −0.93 with all participants included and r = −0.98 without the outlier). This correlation remained marginally significant when the data were limited to the atypical group (r = −0.54, P = 0.056) but not when data were limited to the typical group (r = −0.05, P = 0.86).
To test the hemispheric differences further, we ran an extra analysis for the Landmark task in which we directly compared the amplitude of the hemodynamic response between the two cerebral hemispheres for each participant. (See Methods for more details.) This analysis confirmed the significant RH dominance for the typical group (Fig. 1A, Right) and the significant LH dominance for the atypical group (Fig. 1B, Right). It further showed that functional laterality was observed in widespread parietal and frontal regions and also in the inferior/middle occipital and inferior temporal regions, as well as in the thalamus. The “typical” group showed additional right-lateralized activations in the middle cingulate gyrus (all P < 0.001, cluster P < 0.05). Both groups had a crossed cerebro-cerebellar lateralization pattern. That is, the activation in the posterior cerebellum was contralateral to the cerebral hemisphere dominant for visuospatial attention and was not related to the participant’s handedness.
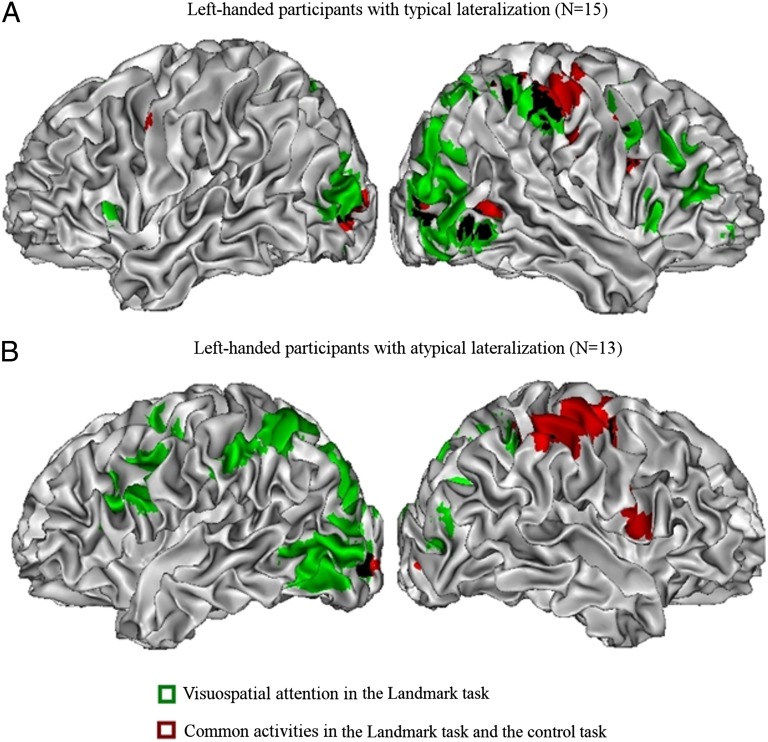
To investigate the importance of a good control condition, we calculated the IPS/SPL LIs for the the LM condition vs. the Rest condition. This time, the correlation with frontal language lateralization (Fig. 2, blue triangles) was much lower (r = −0.76). This result shows the need for a proper baseline condition. The data also were less clear if we limited the analysis to the participants with atypical language lateralization (r = −0.41, n = 13, P = 0.18), because with Rest condition as control some participants showed a bilateral (n = 6) or even contralateral (n = 1) activation pattern. We therefore examined further the common activation in the LM and LMC conditions compared with the Rest condition. Activations common to LM and LMC were present in the right postcentral sulcus extending to the central sulcus and anterior inferior parietal sulcus, the right precentral gyrus, the left/bilateral middle occipital gyri, and the SMA both in participants with typical lateralization and in those with atypical lateralization (Fig. 3, LM > Rest inclusively masked by LMC > Rest, both P < 0.001). The right-lateralized parietal and precentral activations in both groups seem to be largely caused by the finger responses. No significant activity was seen for LMC vs. LM condition (masked by LMC > Rest).
Fig. 3.
Lateralization patterns of visuospatial attention in the Landmark task (in green, Landmark against control task), and right-lateralized activations common to the LM and the LMC (in red, both against Rest condition). All participants are left-handed, either (A) with typical lateralization or (B) with atypical lateralization, and responses were made with the left hand. (A) Fifteen participants with typical lateralization. (B) Thirteen participants with atypical lateralization.
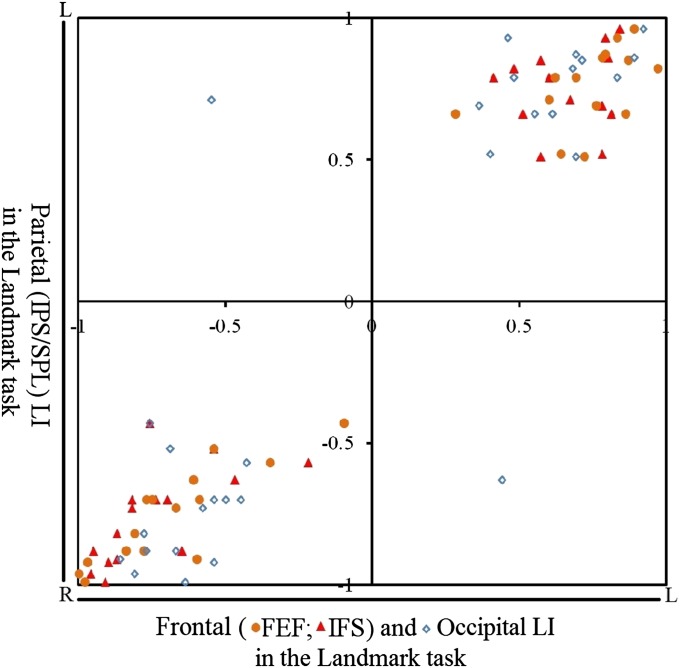
Finally, to investigate further the laterality of the fronto-parietal network underlying visuospatial attention, LIs were calculated for other regions that were significantly activated in the current study and that in the literature are considered to play an important role, mainly the FEF, the IFS, and the visual cortex. For the FEF, we defined a symmetric sphere around the peaks of FEF activation based on the group analysis of all the participants (center at x = ±30, y = −6, z = 58, with a radius of 12 mm). For the IFS activation, which was more extensive, we used a broader anatomically defined symmetrical region of interest (ROI), taking the inferior and middle frontal gyri as we did for the IPS activations. We also defined a symmetric occipital ROI including the middle and inferior occipital gyri. As expected, we found that in the Landmark task the lateralization of the IPS/SPL activation was highly positively correlated to the activation in all these regions (IFS: r = 0.98; FEF: r = 0.98; occipital: r = 0.83), as shown in Fig. 4.
Fig. 4.
The laterality of frontal (FEF and IFS) and occipital activations are correlated to the laterality of parietal (IPS/SPL) activations in the Landmark task.
Behavioral Results for the Landmark Task.
To see how brain activation was related to performance in the Landmark task, we analyzed the behavioral data of this task. They showed that the LMC condition was easier than the LM condition. Participants made 16.1% errors on average in the LM condition and 1.8% in the LMC condition (paired t test, P < 0.001). They also were faster in the LMC condition [mean response time (RT) = 574 ms] than in the LM condition (mean RT = 778 ms; paired t test P < 0.001). To examine whether this pattern was the same for both laterality groups, a two-way ANOVA was run on the RTs with the variables Lateralization pattern (typical or atypical) and Condition (LM or LMC). This analysis returned an effect of task [F(1,26) = 154.52, P < 0.001], no effect of lateralization pattern [F(1,26) = 0.55, P = 0.47], and no interaction [F(1,26) = 0.075, P = 0.79].
Also of interest is the finding that the participants showed a slight rightward bias in the LM condition. They observed a deviation from the center to the right more accurately than a deviation to the left. This bias was particularly seen with the deviations close to the center and was observed both in the group with typical laterality (93.1 ± 6.1% midline responses at the center, 67.6 ± 25.4% midline responses at the closest right location, and 30.0 ± 17.5% midline responses at the closest left location) and the group with atypical laterality (84.5 ± 18.6% midline responses at the center, 76.9 ± 30.1% midline responses at the closest right location, and 28.2 ± 23.9% midline responses at the closest left location). Two-way repeated-measures ANOVAs (left vs. right location × distance to the center) on all six noncenter locations confirmed that each group showed an effect of direction (P < 0.003 for the atypical lateralization group, and P < 0.001 for the typical lateralization group). In addition, there was a main effect of distance (P < 0.001 for both groups) and an interaction effect (P = 0.0011 for atypical lateralization and P < 0.001 for typical lateralization), indicating that the right bias was most salient at the locations closest to the center.
Discussion
In the current study, we carefully examined a group of 13 left-handed participants with atypical speech dominance on the Landmark task, and we observed that all were atypically LH dominant for spatial attention. In contrast, all but one of the 16 participants with typical LH speech dominance were RH dominant for spatial attention. These results are largely consistent with the predictions of the Causal hypothesis, because, given the low probability of atypical lateralization of either function (11, 32), the opposite asymmetries are unlikely to be to the result of chance, as claimed by the Statistical hypothesis. The question arises: Why has the pattern not been observed before?
Reexamination of the Previous Studies.
To understand our findings better, we reexamined the observations reported in previous brain-imaging studies. The number of these investigations is limited, because most studies on atypical functional asymmetry focused on language [e.g., to find out whether brain imaging could replace the Wada test for clinical purposes; see Chee, et al. (43), among others]. The most extensive study comparing word generation and the Landmark task was run by Badzakova-Trajkov, et al. (26). They examined 155 participants (48 left-handed) with fMRI. Of these, only five were RH dominant for language (word generation against fixation) if the threshold is set at LI < −0.5. If the same threshold is used for the Landmark task, one of the five participants was LH dominant, one was RH dominant, and the remaining three had bilateral activation. Flöel, et al. (31) used fTCD to examine a selection of 10 participants with LH language dominance and 10 participants with RH dominance from a cohort of 326 healthy volunteers. They observed that all participants with LH language dominance had RH dominance on a line bisection task (comparison of bisection vs. rest). Four of the 10 participants with RH dominance for language also showed RH dominance for line bisection. However, two of the latter group were among the three least lateralized participants on the language and the bisection tasks. Other cases of uncrossed laterality were reported (or replicated) in fMRI studies (32, 34). Again, the patterns of uncrossed dominance were more in line with bilateral than unilateral control (LIs between 0 and −0.5), except for one right-hander with RH language and RH attention, reported by Jansen, et al. (34). Whitehouse and Bishop (27) examined 75 participants using fTCD. They found no participant with a complete reversal of the typical organization (RH language and LH attention), but 16 participants had both language and spatial attention in the same hemisphere. However, if the results are thresholded at |LI| > 0.5, as before, only one of the 16 participants showed colateralization of both tasks. Rosch, et al. (28) also used fTCD and showed no relationship between language and visuospatial lateralization in 20 right-handed participants. In our view, however, these data are more illustrative of the limits of fTCD than anything else, given that 5 of the 20 strongly right-handed participants supposedly had an RH dominance for language and 5 of 20 participants supposedly had an LH dominance on the Landmark task. These results do not agree with the findings from previous fMRI studies.
All in all, it seems to us that previous authors may have been too ready to adhere to the Statistical hypothesis. They did not make a distinction between degree of laterality (or rather, between clear and unclear laterality patterns) and side of laterality, a problem that is likely when unselected samples are used, given the rarity of atypical laterality. In addition, most of the studies did not use a proper control task, which is needed to parse out irrelevant activity arising from hand-related responses. In particular the fTCD studies compared an LM condition with a Rest condition. As shown in Fig. 4, both the LM and the LMC conditions strongly activated the hemisphere contralateral to the responding hand, compared with the Rest condition. This activation spreads from the postcentral sulcus/gyrus to the precentral gyrus and anterior IPS, and is in line with hand-related activations shown in previous studies (44, 45). As can be seen in our results, as well as in other recent imaging studies, the activation resulting from finger movement is very close to the IPS and even overlaps in anterior IPS. Therefore, it must be controlled for carefully. Still, most of the studies discussed above did not include a control task eliciting a hand response, and some studies mixed left- and right-hand responses in the Landmark task, making the findings difficult to interpret.
Finally, authors may want to be more careful when calculating LI indices. As indicated by Wilke and colleagues (40, 41), a multithresholded bootstrapped method is less affected by interindividual differences in activation strength. It is also advised to ensure that there is enough activity in the ROIs (46). Otherwise, LIs may be based largely on noise. This precaution is particularly important when the experiment uses a low-efficiency paradigm and when the activity is weak.
Lateralization of the Dorsal Fronto-Parietal Attention Network.
In our study, we found crossed lateralization in all but one participant for speech production and the Landmark task, even though the brain areas involved in both tasks have very little overlap. Previous studies have suggested two networks underlying visuospatial attention. The first is the dorsal fronto-parietal network, including part of IPS/SPL and the superior frontal cortex, which is active during voluntary (“top-down”) attention. The second is a ventral system, including the temporo-parietal junction and IFG, which is used to direct attention to salient events (“bottom-up” attention) (42, 47). In our study, the Landmark task predominantly activated the dorsal fronto-parietal network, which reflects the top-down nature of the task. In the frontal cortex, we observed clear activation in the FEF and IFS, which functionally colateralized with IPS/SPL. Although the FEF is considered a typical site in the network, we cannot completely exclude the possibility that part of the activity resulted from the extra eye movements elicited by the Landmark task to judge the exact midline, as compared with the LMC task. On the other hand, given that eye movements can be considered as overt shifts in attention controlled by the same network as covert attention (48), the interpretation largely remains the same. The IFS/inferior frontal junction (IFJ) activation also is in line with the results of previous studies (49), which have considered this area as an additional region related to the dorsal network. Intriguingly, the IFS/IFJ region also is involved in the dorsal attention network related to resting-state activity, which is independent of spatial attention (47). This involvement may indicate that the asymmetry of this area is part of an even wider asymmetry and that the laterality of speech production is not the driving force behind the reversal of the dorsal fronto-parietal network but itself is the outcome of a deeper asymmetry with a longstanding evolutionary origin (compare the lateralities documented in other species).
It also should be noted that the Landmark task was used initially to disambiguate the contribution of perceptual biases from motor biases in bisection (50). It involves not only processes related to shifting visuospatial attention to a location and sustaining it there but also processes related to the perceptual judgment of localization. In the current study, the control task also involved shifting and sustaining of attention and some perceptual judgment but was easier than the Landmark task (as indicated by the overall accuracy and mean reaction time). Therefore the fMRI activity in the Landmark task as compared with the control task may reflect not only spatial attention but also perceptual judgment. The use of perceptual judgment may explain why we observed activation in the extrastriate cortex, which functionally colateralized with the dorsal attention network. This region has been associated with the top-down influences on the early visual processing related to the midline assessment of the Landmark task (14). The possible involvement of perceptual judgment in the Landmark task does not undermine the capability of the present study to distinguish between the Causal and the Statistical hypotheses of hemispheric asymmetry, but it indicates that the Landmark paradigm may measure more than pure visuospatial attention.
Intriguingly, the divergent hemispheric dominances of the participants in the brain imaging data did not translate to the behavioral results of the Landmark task. Both groups of participants showed a slight rightward bias. To determine whether the right bias could (partly) result from the left-handedness of our participants, we tested a different group of 13 right-handed persons who at a group level were supposed to be right dominant for visuospatial attention. We tested them twice with the paradigm described above, once in a normal upright position in front of a computer screen and once in an MRI simulator. Surprisingly, the right-handed group also showed a rightward bias in the scanner (90.1 ± 8.5% midline responses at the center, 52.6 ± 36.6% midline responses at the closest right location, and 32.3 ± 22.9% midline responses at the closest left location). Furthermore, this bias shifted to the left when the participants performed the same task in front of a computer, at a distance of 50 cm (with the length of the horizontal line reduced to 8.2 cm, to achieve the same visual angle as in the scanner). At the computer there were 89.5 ± 9.8% midline responses at the center, 19.2 ± 15.0% midline responses at the closest right location, and 28.2 ± 21.9% midline responses at the closest left location. Researchers previously have observed that the usual leftward bias in the Landmark task with near stimuli reverses to a rightward bias when the stimulus is placed outside the participants’ reaching space (51, 52). The most likely explanation for the rightward bias in the scanner, but not in front of a computer, is that the Landmark stimulus is experienced in far space when participants are lying in the scanner (with the stimulus 1.1 m from eye position and no possibility of touching the stimulus). In other words, although the Landmark task is considered a particularly good paradigm for fMRI (14, 39) because of its limited eye movement and motor demands, it may not be fully the same in the scanner as in the laboratory in front of a computer. The far-space experience of the stimulus in the scanner may be another reason why we saw enhanced bilateral activation in the occipital and medial occipitotemporal cortex (53); this activation should be taken into account in further fMRI studies.
Implications for Genetic Models of Handedness.
One of the reasons why the Statistical hypothesis is dominant at present is that it is in line with genetic models of hand preference, as proposed by Annett (54) and McManus (55). Therefore, our finding of crossed laterality is likely to have implications for these models as well.
Genetic models of hand preference aim to explain the prevalence and co-occurrence of handedness and cerebral dominance (54, 55). Among other things, they have to explain why the congruence of speech laterality and hand preference is higher among right-handers (95% LH speech control) than among left-handers (10–25% RH speech control in unselected samples). An influential suggestion (56) is that hand preference is controlled by an allele, which can be either right-biased (the D variant) or not biased (the C variant). People with DD alleles are assumed always to be right-handed and LH-dominant for speech; people with CC alleles are at random both for hand preference and speech dominance; and people with DC alleles have a 75% chance of right-handedness and LH speech-dominance). A good fit of the data is obtained when the proportion of the C variant in the population is estimated to be around 0.155.
Importantly, McManus’s model assumes that atypical dominance of hand control and speech control are the result of chance and therefore should be statistically independent. Consistent biases are expected only for people with DD alleles. Therefore the finding that all 13 participants with RH language control showed LH dominance in the dorsal fronto-parietal network is unexpected, unless one accepts that the C allele initially allows plasticity, so that laterality of one core function (either language or visuospatial attention) increases the chances of crossed asymmetry of the other function to avoid crowding, as hypothesized by Kosslyn (21) and several authors before him (22–24). The findings that crossed lateralization is not 100% (see the deviating person in Fig. 2) and also is less present in people with bilateral control are in line with the presence of some element of chance.
Link Between Function and Anatomy.
Another question our findings raise is whether atypical functional asymmetries are associated with atypical anatomical asymmetries. Recent tractography studies suggest that visuospatial attention depends largely on a fronto-parietal pathway that corresponds to the second branch of the superior longitudinal fasciculus described in monkeys (SLF II) (57–59). Thiebaut de Schotten, et al. (58) further showed a positive correlation between the laterality of this parieto-frontal connection and visuospatial performances in a line-bisection task, even though this finding was limited to some degree by the narrow range of LIs used (i.e., lack of strong lateralization). Intriguingly, some researchers also suggested that language lateralization is linked to more extensive fronto-temporal connectivity along the SLF (including the arcuate fasciculus) (60, but see 61). Therefore, given that our results lend support to the Causal hypothesis, it would be of interest to investigate further the asymmetry of these structural fibers and their relationship with functional lateralization.
To conclude, the current study examined the relationship between the functional lateralization of language production and that of visuospatial attention, in left-handers with typical or atypical language lateralization. Our results strongly support the Causal hypothesis—a function becomes localized to one hemisphere because the other hemisphere already has taken responsibility for the other function. Together with evidence from previous studies in other fields, we think that the lateralization of language and spatial attention are dependent and have a longstanding evolutionary origin, because both functions perform better with a single, unilateral control center and because crossed lateralization avoids crowding.
Methods
Participants.
Thirty-two subjects (28 females and four males; age, 18–29 y; mean age, 20.4 y) participated in the current study. They were selected from a large group of 265 left-handers who had been tested for atypical language laterality (35). On the basis of two behavioral VHF tasks (word and picture naming) and an fMRI word-generation task (see below), 16 were classified as being LH dominant, 14 participants were known to be RH dominant for language, and one was bilateral (with a predominance of the RH). One participant had to be excluded from the analyses because of excessive head movements in the scanner.
All participants were Dutch-speaking students from Ghent university or higher education schools (with minimum 12 y of education), with no history of neurologic, medical, psychiatric problems, or abnormal brain morphology. All had normal or corrected-to-normal vision. All participants reported writing and drawing with the left hand, and handedness was assessed with the Edinburgh handedness inventory (62) combined with a questionnaire about eyedness, earedness, and footedness (63). All participants fulfilled the conditions for scanning according to the guidelines of the Ethics Committee of the Ghent University Hospital and gave their written informed consent before participation.
Tasks and Stimuli.
Word-generation task.
A word-generation task was used to measure the lateralization of language production (26, 36, 64). The task consisted of an active condition and a control condition. In each active block, a letter (b, d, k, l, m, n, p, r, s, or t) was presented in the middle of the screen for 15 s, during which participants were asked to produce silently as many words starting with that letter as possible. In control blocks, participants saw the letter string “baba,” which is not a word in Dutch, and were asked to repeat this nonword silently for 15 s. Ten active and 10 control blocks were alternated with 20 rest blocks in which a horizontal line instructed the participants to relax. A practice phase was run outside the scanner.
Landmark task.
The lateralization of visuospatial attention was measured with the Landmark task. The task consisted of six active blocks (the Landmark task condition, hereafter LM), six control blocks (hereafter LMC), and six fixation blocks as a low-level baseline condition [the procedure followed by Ciçek, et al. (65)]. Each LM or LMC block was preceded by an instruction screen for 4 s indicating which task was to be performed. Blocks consisted of 12 trials in a randomized order and lasted for 21.6 s. On each trial a horizontal line (15 cm long, subtending a visual angle of 8°) was presented for 1.6 s, together with a short vertical line. In the LM blocks, the vertical line was centered on the horizontal line, either exactly at the middle of the horizontal line (in 50% of the trials) or slightly deviated to the left or to the right (in the remaining 50% of the trials). Three distances were used: 2.5, 5.0, or 7.5% of the length of the horizontal line. Participants were asked to decide whether the line bisection was exact. They were instructed to press a button on the response box with the left index finger if the bisecting line was exactly in the middle and to press another button with the left middle finger if it was not. In the LMC blocks, the stimuli were identical to the LM condition except that in 50% of the trials the short vertical line was placed perpendicular on the horizontal line and in the other 50% trials it was placed slightly above the horizontal line and did not make contact with it. Participants were asked to decide whether the short vertical line made contact with the horizontal line (pressing a button on the response box with the left index finger) or did not (pressing with the left middle finger). Two hundred ms after the offset of the stimulus the next trial started. LM blocks and LMC blocks were presented alternatively, and between them six fixation blocks were included, during which participants were asked to rest.
fMRI Data Acquisition.
Imaging data were acquired on a 3-T Siemens Trio MRI scanner (Siemens Medical Systems) at the Ghent University Hospital with an eight-channel rf head coil. Stimuli were presented using Presentation software (Neurobehavioral Systems) and projected onto a translucent screen. Participants viewed the screen via a mirror mounted on the head coil in front of their eyes. A high-resolution structural T1 image was collected at the beginning, using MPRAGE sequence [TR = 1,550 ms, TE = 2.39 ms, image matrix = 256 × 256, field of view (FOV) = 220 mm, flip angle = 90°, voxel size = 0.9 × 0.9 × 0.9 mm3]. Functional images were obtained by using a T2*-weighted gradient-echo EPI sequence (TR = 2,630 ms, TE = 35 ms, image matrix = 64 × 64, FOV = 224 mm, flip angle = 80°, slice thickness = 3.0 mm, distance factor = 17%, voxel size = 3.5 × 3.5 × 3.5 mm3).
fMRI Data Analyses.
fMRI data were analyzed using SPM5 (www.fil.ucl.ac.uk). The first four functional images of each session were eliminated to obtain magnetization equilibrium. The remaining functional images were slice-time corrected, spatially aligned, and coregistered to the individual T1. All functional images and structural image then were normalized to the standard MNI T1 template, and the normalized functional images were smoothed using a Gaussian kernel of isotropic 10-mm FWHM and were high-pass filtered at 128 s.
For each participant and experiment, the data were modeled using boxcar functions convolved with a canonical hemodynamic response function. Six parameters capturing participants’ head movements were included in the model as additional regressors of no interest. Statistical parametric maps for effects of interests were calculated by applying corresponding contrasts to the parameter estimates. The individual results for each contrast then were entered into a second-level random-effects group analysis.
Individual LIs were calculated for the IFG (taking IFG pars opercularis and pars triangularis as ROI) and the IPS (taking the inferior parietal lobule and SPL as ROI) separately for the word-generation and the Landmark task. The ROIs were symmetric by overlapping the original left and right automated anatomical labeling regions (66) and their LH-RH flipped images. LIs were calculated using the LI Toolbox (40) with a Bootstrap method (41). This method involves the calculation of 20 equally sized thresholds from 0 to the maximum t value. At each threshold, 100 bootstrapped samples with a resampling ratio of k = 0.25 are taken in the left and right ROIs. All 10,000 possible LI combinations then are calculated from these samples for surviving voxels on the left and the right, with the formula [(L − R)/(L + R)]. Only the central 50% of data are kept to exclude statistical outliers. Finally, a weighted mean LI is calculated for each individual from all LIs weighted with their corresponding threshold (Eq. S1) (see also 26, 36). The relationships between the LIs of the different ROIs were examined within and between tasks.
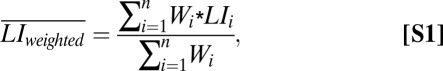 |
where Wi is the t threshold at which the image was thresholded to generate the value of LIi.
To investigate the asymmetry patterns of activation in the Landmark task further, we also performed an analysis based on direct interhemispheric comparisons of signal magnitude (67). A symmetric EPI template was constructed by taking the average of the original MNI EPI template and its left–right reversed image. For each participant, the parameters required to normalize the image spatially to the symmetric EPI then were calculated from the mean of the original spatially normalized EPI time series, and these parameters were applied to the contrast image of interest. We then created images representing hemispheric difference by subtracting the amplitude of the hemodynamic response for each voxel in the RH from its corresponding voxel in the LH. The images of individual hemispheric difference then were entered into group-level t tests.
Acknowledgments
We thank an anonymous reviewer for the suggestion that we conduct a quantitative analysis based on direct interhemispheric comparisons for each participant. This research was made possible by an Odysseus grant awarded by the Government of Flanders (Belgium) to M.B.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 1158 (volume 110, number 4).
This article is a PNAS Direct Submission.
References
- 1.Hauser MD, Andersson K. Left hemisphere dominance for processing vocalizations in adult, but not infant, rhesus monkeys: Field experiments. Proc Natl Acad Sci USA. 1994;91(9):3946–3948. doi: 10.1073/pnas.91.9.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopkins WD, Russell JL, Cantalupo C, Freeman H, Schapiro SJ. Factors influencing the prevalence and handedness for throwing in captive chimpanzees (Pan troglodytes) J Comp Psychol. 2005;119(4):363–370. doi: 10.1037/0735-7036.119.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cynx J, Williams H, Nottebohm F. Hemispheric differences in avian song discrimination. Proc Natl Acad Sci USA. 1992;89(4):1372–1375. doi: 10.1073/pnas.89.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehret G. Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature. 1987;325(6101):249–251. doi: 10.1038/325249a0. [DOI] [PubMed] [Google Scholar]
- 5.Letzkus P, et al. Lateralization of olfaction in the honeybee Apis mellifera. Curr Biol. 2006;16(14):1471–1476. doi: 10.1016/j.cub.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 6.Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci. 2005;28(4):575–589, discussion 589–633. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- 7.Rogers LJ, Zucca P, Vallortigara G. Advantages of having a lateralized brain. Proc Biol Sci. 2004;271(Suppl 6):S420–S422. doi: 10.1098/rsbl.2004.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sovrano VA, Dadda M, Bisazza A. Lateralized fish perform better than nonlateralized fish in spatial reorientation tasks. Behav Brain Res. 2005;163(1):122–127. doi: 10.1016/j.bbr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Levy J. The mammalian brain and the adaptive advantage of cerebral asymmetry. Ann N Y Acad Sci. 1977;299:264–272. doi: 10.1111/j.1749-6632.1977.tb41913.x. [DOI] [PubMed] [Google Scholar]
- 10.Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52(5):1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- 11.Knecht S, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 12.Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10(4):309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 13.Kinsbourne M. Mechanism of unilateral neglect. In: Jeannerod M, editor. Neurophysiological and Neuropsychological Aspects of Spatial Neglect. Amsterdam: Elsevier Science; 1987. pp. 69–86. [Google Scholar]
- 14.Fink GR, et al. Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology. 2000;54(6):1324–1331. doi: 10.1212/wnl.54.6.1324. [DOI] [PubMed] [Google Scholar]
- 15.Fink GR, Marshall JC, Weiss PH, Zilles K. The neural basis of vertical and horizontal line bisection judgments: An fMRI study of normal volunteers. Neuroimage. 2001;14(1 Pt 2):S59–S67. doi: 10.1006/nimg.2001.0819. [DOI] [PubMed] [Google Scholar]
- 16.Bach S, et al. Early emergence of deviant frontal fMRI activity for phonological processes in poor beginning readers. Neuroimage. 2010;53(2):682–693. doi: 10.1016/j.neuroimage.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 17.Chiarello C, Welcome SE, Halderman LK, Leonard CM. Does degree of asymmetry relate to performance? An investigation of word recognition and reading in consistent and mixed handers. Brain Cogn. 2009;69(3):521–530. doi: 10.1016/j.bandc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 18.van Ettinger-Veenstra HM, et al. Right-hemispheric brain activation correlates to language performance. Neuroimage. 2010;49(4):3481–3488. doi: 10.1016/j.neuroimage.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 19.Lust JM, et al. Driving performance during word generation—testing the function of human brain lateralization using fTCD in an ecologically relevant context. Neuropsychologia. 2011;49(9):2375–2383. doi: 10.1016/j.neuropsychologia.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Boles DB, Barth JM. “Does degree of asymmetry relate to performance?” A critical review. Brain Cogn. 2011;76(1):1–4. doi: 10.1016/j.bandc.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Kosslyn SM. Seeing and imagining in the cerebral hemispheres: A computational approach. Psychol Rev. 1987;94(2):148–175. [PubMed] [Google Scholar]
- 22.Lansdell H. Verbal and nonverbal factors in right-hemisphere speech: Relation to early neurological history. J Comp Physiol Psychol. 1969;69(4):734–738. doi: 10.1037/h0028306. [DOI] [PubMed] [Google Scholar]
- 23.Levy J. Possible basis for the evolution of lateral specialization of the human brain. Nature. 1969;224(5219):614–615. doi: 10.1038/224614a0. [DOI] [PubMed] [Google Scholar]
- 24.Teuber HL. Why two brains? In: Schmidts FO, Worden FG, editors. The Neurosciences: Third Study Program. Cambridge, MA: MIT Press; 1974. pp. 71–74. [Google Scholar]
- 25.Bryden MP. Laterality: Functional Asymmetry in the Intact Brain. New York: Academic; 1982. [Google Scholar]
- 26.Badzakova-Trajkov G, Häberling IS, Roberts RP, Corballis MC. Cerebral asymmetries: Complementary and independent processes. PLoS ONE. 2010;5(3):e9682. doi: 10.1371/journal.pone.0009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitehouse AJ, Bishop DV. Hemispheric division of function is the result of independent probabilistic biases. Neuropsychologia. 2009;47(8-9):1938–1943. doi: 10.1016/j.neuropsychologia.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosch RE, Bishop DVM, Badcock NA. Lateralised visual attention is unrelated to language lateralisation, and not influenced by task difficulty - a functional transcranial Doppler study. Neuropsychologia. 2012;50(5):810–815. doi: 10.1016/j.neuropsychologia.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suchan J, Karnath HO. Spatial orienting by left hemisphere language areas: A relict from the past? Brain. 2011;134(Pt 10):3059–3070. doi: 10.1093/brain/awr120. [DOI] [PubMed] [Google Scholar]
- 30.Fischer RS, Alexander MP, Gabriel C, Gould E, Milione J. Reversed lateralization of cognitive functions in right handers. Exceptions to classical aphasiology. Brain. 1991;114(Pt 1A):245–261. [PubMed] [Google Scholar]
- 31.Flöel A, et al. Language and spatial attention can lateralize to the same hemisphere in healthy humans. Neurology. 2001;57(6):1018–1024. doi: 10.1212/wnl.57.6.1018. [DOI] [PubMed] [Google Scholar]
- 32.Flöel A, et al. Atypical hemispheric dominance for attention: Functional MRI topography. J Cereb Blood Flow Metab. 2005;25(9):1197–1208. doi: 10.1038/sj.jcbfm.9600114. [DOI] [PubMed] [Google Scholar]
- 33.Jansen A, et al. Determining the hemispheric dominance of spatial attention: Acomparison between fTCD and fMRI. Hum Brain Mapp. 2004;23(3):168–180. doi: 10.1002/hbm.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen A, Flöel A, Menke R, Kanowski M, Knecht S. Dominance for language and spatial processing: Limited capacity of a single hemisphere. Neuroreport. 2005;16(9):1017–1021. doi: 10.1097/00001756-200506210-00027. [DOI] [PubMed] [Google Scholar]
- 35.Van der Haegen L, Cai Q, Seurinck R, Brysbaert M. Further fMRI validation of the visual half field technique as an indicator of language laterality: A large-group analysis. Neuropsychologia. 2011;49(10):2879–2888. doi: 10.1016/j.neuropsychologia.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18(2):423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 37.Cai Q, Paulignan Y, Brysbaert M, Ibarrola D, Nazir TA. The left ventral occipito-temporal response to words depends on language lateralization but not on visual familiarity. Cereb Cortex. 2010;20(5):1153–1163. doi: 10.1093/cercor/bhp175. [DOI] [PubMed] [Google Scholar]
- 38.Whitehouse AJ, Badcock N, Groen MA, Bishop DV. Reliability of a novel paradigm for determining hemispheric lateralization of visuospatial function. J Int Neuropsychol Soc. 2009;15(6):1028–1032. doi: 10.1017/S1355617709990555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCourt ME, Olafson C. Cognitive and perceptual influences on visual line bisection: Psychophysical and chronometric analyses of pseudoneglect. Neuropsychologia. 1997;35(3):369–380. doi: 10.1016/s0028-3932(96)00143-1. [DOI] [PubMed] [Google Scholar]
- 40.Wilke M, Lidzba K. LI-tool: A new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163(1):128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 41.Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33(2):522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 43.Chee MW, Buckner RL, Savoy RL. Right hemisphere language in a neurologically normal dextral: A fMRI study. Neuroreport. 1998;9(15):3499–3502. doi: 10.1097/00001756-199810260-00030. [DOI] [PubMed] [Google Scholar]
- 44.Simon O, et al. Automatized clustering and functional geometry of human parietofrontal networks for language, space, and number. Neuroimage. 2004;23(3):1192–1202. doi: 10.1016/j.neuroimage.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Verhagen L, Dijkerman HC, Medendorp WP, Toni I. Cortical dynamics of sensorimotor integration during grasp planning. J Neurosci. 2012;32(13):4508–4519. doi: 10.1523/JNEUROSCI.5451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinel P, Dehaene S. Beyond hemispheric dominance: Brain regions underlying the joint lateralization of language and arithmetic to the left hemisphere. J Cogn Neurosci. 2010;22(1):48–66. doi: 10.1162/jocn.2009.21184. [DOI] [PubMed] [Google Scholar]
- 47.Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bisley JW. The neural basis of visual attention. J Physiol. 2011;589(Pt 1):49–57. doi: 10.1113/jphysiol.2010.192666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sylvester CM, Jack AI, Corbetta M, Shulman GL. Anticipatory suppression of nonattended locations in visual cortex marks target location and predicts perception. J Neurosci. 2008;28(26):6549–6556. doi: 10.1523/JNEUROSCI.0275-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milner AD, Harvey M, Roberts RC, Forster SV. Line bisection errors in visual neglect: Misguided action or size distortion? Neuropsychologia. 1993;31(1):39–49. doi: 10.1016/0028-3932(93)90079-f. [DOI] [PubMed] [Google Scholar]
- 51.Longo MR, Lourenco SF. On the nature of near space: Effects of tool use and the transition to far space. Neuropsychologia. 2006;44(6):977–981. doi: 10.1016/j.neuropsychologia.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Cowey A, Small M, Ellis S. Left visuo-spatial neglect can be worse in far than in near space. Neuropsychologia. 1994;32(9):1059–1066. doi: 10.1016/0028-3932(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 53.Weiss PH, Marshall JC, Zilles K, Fink GR. Are action and perception in near and far space additive or interactive factors? Neuroimage. 2003;18(4):837–846. doi: 10.1016/s1053-8119(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 54.Annett M. Left, Right, Hand and Brain: The Right Shift Theory. Hove, UK: Erlbaum; 1985. [Google Scholar]
- 55.McManus IC. 1985. Handedness, language dominance and aphasia: A genetic model. Psychol Med Monogr 8(Suppl):1–40.
- 56.McManus IC. 1991. The inheritance of left-handedness. Ciba Foundation Symposium 162: Biological Asymmetry and Handedness, eds Bock GR, Marsh J (Wiley, Chichester, UK) pp 251–281.
- 57.Thiebaut de Schotten M, et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309(5744):2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- 58.Thiebaut de Schotten M, et al. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14(10):1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- 59.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford Univ Press; 2006. [Google Scholar]
- 60.Powell HW, et al. Hemispheric asymmetries in language-related pathways: A combined functional MRI and tractography study. Neuroimage. 2006;32(1):388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Vernooij MW, et al. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: A combined fMRI and DTI study. Neuroimage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 62.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 63.Brysbaert M. Lateral preferences and visual field asymmetries: appearances may have been overstated. Cortex. 1994;30(3):413–429. doi: 10.1016/s0010-9452(13)80338-3. [DOI] [PubMed] [Google Scholar]
- 64.Hunter ZR, Brysbaert M. Visual half-field experiments are a good measure of cerebral language dominance if used properly: Evidence from fMRI. Neuropsychologia. 2008;46(1):316–325. doi: 10.1016/j.neuropsychologia.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Ciçek M, Deouell LY, Knight RT. Brain activity during landmark and line bisection tasks. Front Hum Neurosci. 2009;3:7. doi: 10.3389/neuro.09.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 67.Stevens MC, Calhoun VD, Kiehl KA. Hemispheric differences in hemodynamics elicited by auditory oddball stimuli. Neuroimage. 2005;26(3):782–792. doi: 10.1016/j.neuroimage.2005.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]