Abstract
One of the strategies used by organisms to adapt to life under conditions of short energy supply is to use the by-product pyrophosphate to support cation gradients in membranes. Transport reactions are catalyzed by membrane-integral pyrophosphatases (PPases), which are classified into two homologous subfamilies: H+-transporting (found in prokaryotes, protists, and plants) and Na+-transporting (found in prokaryotes). Transport activities have been believed to require specific machinery for each ion, in accordance with the prevailing paradigm in membrane transport. However, experiments using a fluorescent pH probe and 22Na+ measurements in the current study revealed that five bacterial PPases expressed in Escherichia coli have the ability to simultaneously translocate H+ and Na+ into inverted membrane vesicles under physiological conditions. Consistent with data from phylogenetic analyses, our results support the existence of a third, dual-specificity bacterial Na+,H+-PPase subfamily, which apparently evolved from Na+-PPases. Interestingly, genes for Na+,H+-PPase have been found in the major microbes colonizing the human gastrointestinal tract. The Na+,H+-PPases require Na+ for hydrolytic and transport activities and are further activated by K+. Based on ionophore effects, we conclude that the Na+ and H+ transport reactions are electrogenic and do not result from secondary antiport effects. Sequence comparisons further disclosed four Na+,H+-PPase signature residues located outside the ion conductance channel identified earlier in PPases using X-ray crystallography. Our results collectively support the emerging paradigm that both Na+ and H+ can be transported via the same mechanism, with switching between Na+ and H+ specificities requiring only subtle changes in the transporter structure.
Keywords: ion transport, membrane bioenergetics, molecular evolution, proton transport, sodium transport
Electrochemical H+ and Na+ ion gradients are used by organisms to power ATP production and to translocate substances across membranes. These gradients are maintained by primary and secondary H+ and Na+ transporters that consume chemical or light energy. Membrane-integral pyrophosphatases (PPases) are primary pumps that couple the translocation of H+ or Na+ to pyrophosphate (PPi) hydrolysis (1) (Fig. S1). PPi is an abundant by-product of important biosynthetic reactions, and its hydrolysis recycles the cellular Pi pool and provides a thermodynamic pull for PPi-producing reactions. H+-transporting PPase (H+-PPase) is widespread in all domains of life and provides the host with essential energy reserves, particularly during stress and low-energy conditions (2, 3). Accordingly, agricultural plants overexpressing H+-PPase display salt- and drought-tolerant phenotypes (4). Na+-transporting PPase (Na+-PPase) is one of the most commonly used primary Na+ pumps in prokaryotes.
Both PPi hydrolysis and cation transport activities are attributable to a single polypeptide that forms homodimeric PPase (5, 6). Each subunit is organized as two concentric rings formed by six and 10 α-helical segments, respectively (Fig. S2). The central part of the subunit is hydrophilic and forms a gated ion conductance channel through the membrane. On the cytoplasmic side, the channel widens to form the PPi-binding site. Based on conservation of membrane topology and critical amino acid residues, all members of the membrane PPase superfamily appear to share similar folds, but differ in terms of structural details that reflect functional divergence. Thus, membrane PPases are divided into K+-dependent and K+-independent families, based on whether Ala or Lys is present at a specific position (7). K+ and presumably Lys coordinate and activate substrate (5). Replacement of the Na+-PPase Asp-Lys-Glu channel gate (6) with Asp-Lys is possibly the key event in conferring H+ selectivity to plant-type PPases (5), despite the close similarities of the ion conductance channels. Other structural solutions to control the identity of transported ions have additionally been used in the evolutionary history of the PPase superfamily, which includes several independent transitions from Na+ to H+ transport (8).
All previously characterized membrane PPases have been shown to operate as either specific H+ or Na+ transporters. In the current study, we contradict this paradigm with the discovery of a unique subfamily of PPases (Na+,H+-PPases) capable of transporting both Na+ and H+ ions in a noncompetitive manner. Phylogenetic and structural analyses suggest that these enzymes have evolved from Na+-PPases by acquiring several signature residues at the interface of the inner and outer helix rings. These results indicate remarkable structural and functional plasticity in primary transporters and lend strong support to the emerging paradigm that Na+ and H+ ions can be transported via the same mechanism. Moreover, the identification of Na+,H+-PPase genes in major microbes colonizing the human gastrointestinal tract may signify that these symbiotes actively generate and use both Na+ and H+ gradients, and present new targets for drug design.
Results
Expression of Bacteroides vulgatus PPase in Escherichia coli.
Western analysis of inverted membrane vesicles (IMV) isolated from E. coli cells containing an expression plasmid for the membrane-integral PPase gene of B. vulgatus revealed a single protein band reactive to a specific antibody raised against a conserved motif in PPase (1) (Fig. S3A, lane 10). The expressed protein mass was consistent with that of B. vulgatus PPase (Bv-PPase; predicted mass 77.0 kDa). As expected, no antibody-reactive protein was identified in IMV prepared from plasmid-free E. coli cells. Our activity data confirmed the presence of active Bv-PPase in E. coli IMV. Specifically, PPi hydrolysis activity (i) was significantly above the level detected in native E. coli IMV, (ii) exhibited an absolute requirement for Na+, (iii) was further stimulated by K+, (iv) was inhibited by the membrane PPase inhibitor, aminomethylenediphosphonate (AMDP), and (v) was almost completely insensitive to the cytoplasmic E. coli PPase inhibitor, fluoride (Fig. S3B). Similar criteria were used throughout the study to evaluate the expression of various membrane PPases.
Western analysis indicated the presence of an analogous antibody-reactive protein and low PPi hydrolyzing activity (∼7 nmol·min−1·mg−1) in IMV prepared from authentic B. vulgatus cells (Fig. S3B, Inset). This activity was similarly dependent on the presence of Na+ and K+.
Hydrolytic Activity of Bv-PPase.
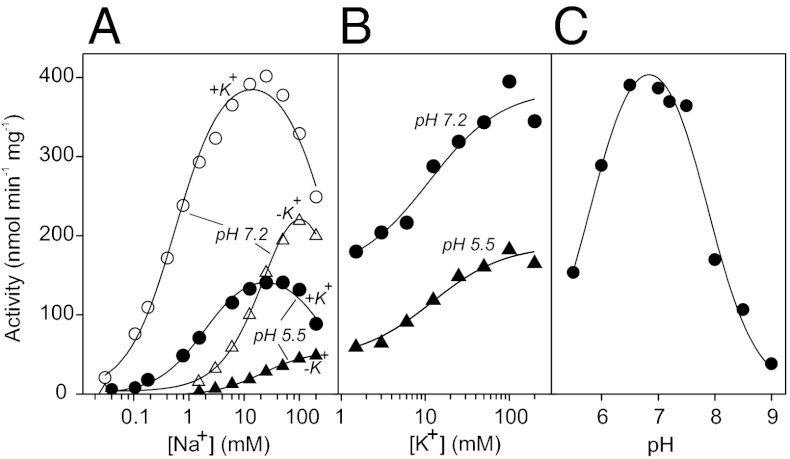
The hydrolytic activity of Bv-PPase, measured at the saturating PPi level (160 μM), revealed a bell-shaped dependence on Na+ concentration, both in the presence and absence of K+ (at pH 7.2; Fig. 1A). In the presence of 50 mM K+, the curve shifted markedly to lower Na+ concentrations, and maximal achievable activity increased. The rate data were quantitatively modeled in terms of Scheme 1, which assumes the binding of one activating and one inhibitory Na+ ion to Bv-PPase, similar to previous studies of Na+-PPases (8). The best-fit parameter values, summarized in Table 1, indicate that at pH 7.2, K+ induces a 60-fold decrease in the dissociation constant for the activating Na+ ion (Ka), no change in inhibitory Na+ ion binding (Ki), and a 1.2-fold increase in the activity of the monosodium complex (V1). The activity values extrapolated to zero Na+ concentration were <1% of the respective maximum activities. Notably, Bv-PPase retained its Na+ dependence at pH 5.5, indicative of an inability of H+ to substitute for Na+ as the activator. In the presence of K+, increased acidity led to a 2.5-fold decrease in V2 and a 3.5-fold increase in Ka, making Bv-PPase a less efficient hydrolase.
Fig. 1.
Hydrolytic activity of Bv-PPase. Dependencies of the PPi hydrolysis rate on Na+ concentration in the absence and presence of 50 mM K+ (A) and K+ concentration in the presence of 50 mM Na+ (B) at two pH values. (C) pH dependence of the PPi hydrolysis rate, measured at 10 mM Na+ and 50 mM K+. PPi concentrations in µM were 1,550, 474, 234, 169, 158, 149, 143, 141, and 140 µM at pH 5.5, 6.0, 6.5, 7.0, 7.2, 7.5, 8.0, 8.5, and 9.0, respectively. The buffers were MES- (pH 5.5–6.5), MOPS- (pH 6.5–8.0), TAPS- (pH 8.0–9.0), or CAPSO-TMA4OH (pH 9.0).
Table 1.
Kinetic parameters for Bv-PPase activation by Na+ and K+
| Activator | pH | Ka, mM | V1, nmol·min−1·mg−1 | V2, nmol·min−1·mg−1 | Ki, mM |
| Na+ | 7.2 | 0.57 ± 0.06/33 ± 3 | <3/<3 | 420 ± 20/360 ± 20 | 340 ± 60/320 ± 30 |
| Na+ | 5.5 | 2.0 ± 0.3/21 ± 2 | <2/<2 | 160 ± 10/56 ± 5 | 310 ± 70/n.d. |
| K+ | 7.2 | 11 ± 3 | 150 ± 10 | 390 ± 20 | |
| K+ | 5.5 | 12 ± 3 | 44 ± 4 | 170 ± 10 |
Parameter values separated by the slashed line were measured in the presence and absence of 50 mM K+, respectively. K+ binding experiments were conducted in the presence of 50 mM Na+. Parameter values are reported with SD. n.d., not determined.
At a fixed Na+ concentration (50 mM), the PPi hydrolysis rate increased 2.6- and 3.9-fold from the basal nonzero level with increasing K+ concentration at pH 7.2 and 5.5, respectively (Fig. 1B). The dependence is well described by Scheme 1 with Ki = ∞, yielding a K+ dissociation constant (Ka) of 11–12 mM. These effects of alkali metal ions on hydrolytic activity collectively indicate that Bv-PPases share a common regulatory mechanism with Na+-PPases. In both cases, Na+ acts as an obligatory activator, whereas K+ modulates catalytic activity by increasing Na+ binding affinity and maximal PPi hydrolysis rate.
The pH profile of Bv-PPase hydrolytic activity was recorded at the saturating substrate concentration and thus characterizes the catalytic constant (Fig. 1C). The symmetrical bell-shaped pH profile indicates that a deprotonated group with a pKa of 5.8 ± 0.1 and protonated group with a pKa of 7.9 ± 0.1 are both required for catalysis. The activity of the optimally protonated enzyme was estimated at 480 ± 30 nmol·min−1·mg−1.
Scheme 1. Alkali cation (M) binding to the enzyme-substrate complex (ES). Ka and Ki are the metal binding constants, and V1 and V2 are the maximal velocities for the corresponding complexes. When M = Na+, V1 = 0 (inactive ES complex) and when M = K+, Ki = ∞ (the ESM2 species is absent).
Na+ and H+ Transport Activities of Bv-PPase.
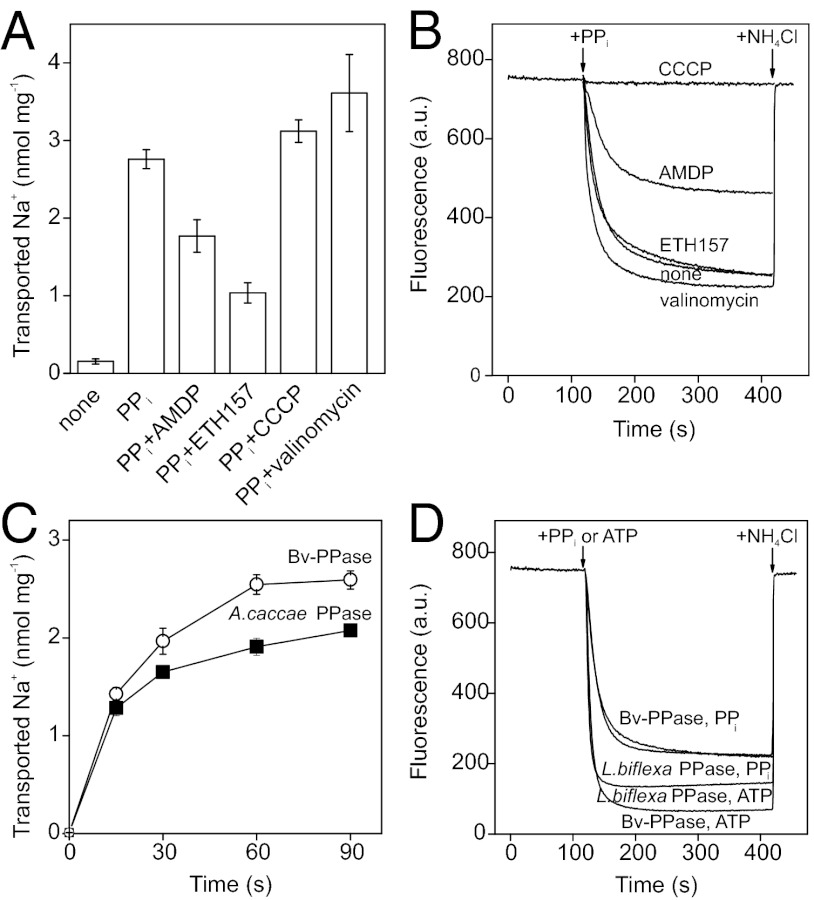
All membrane PPases requiring Na+ for activity operate as primary Na+ transporters (1, 8, 9). Accordingly, addition of PPi to the reaction medium caused accumulation of 22Na+ in Bv-PPase IMV, but not in native E. coli IMV (Fig. 2A). Na+ accumulation was inhibited by AMDP and the Na+ ionophore, N,N′-dibenzyl-N,N′-diphenyl-1,2-phenylenedioxy-diacetamide (ETH157), but was unaffected (or slightly stimulated) by the H+ ionophore, carbonyl cyanide m-chlorophenylhydrazone (CCCP) and the K+ ionophore, valinomycin. Ionophores mediate the diffusion of Na+ and H+ ions across membranes, resulting in dissipation of the respective ion gradients (ETH157 and CCCP) or electric potential difference (valinomycin).
Fig. 2.
Transport activities of Bv-PPase. (A) Na+ accumulated in IMV in 1 min after PPi addition. (B) H+ transport, determined using ACMA fluorescence. The following additions were made where indicated: 100 μM AMDP, 5 μM CCCP, 2 μM valinomycin, 20 μM ETH157. (C) Comparison of Na+ transport activities of Bv-PPase and A. caccae Na+-PPase. Hydrolytic activities of Bv-PPase and A. caccae Na+-PPase were 15 and 16 nmol·min−1·mg−1, respectively. (D) Comparison of H+ transport activities of Bv-PPase and L. biflexa H+-PPase. Hydrolytic activities of Bv-PPase and L. biflexa H+-PPase under these conditions were 34 and 31 nmol·min−1·mg−1, respectively. The error bars in A and C are the SDs of three measurements.
All membrane PPases characterized to date mediate either Na+ or H+ transport. Surprisingly, however, Bv-PPase was able to mediate both processes. Addition of PPi to the IMV suspension triggered time-dependent acidification of IMV lumen, as indicated by the quenching of 9-amino-6-chloro-2-methoxyacridine (ACMA) fluorescence (Fig. 2B). Fluorescence was rapidly restored to the initial level upon disruption of the H+ gradient by the addition of ammonium chloride or more slowly restored upon consumption of all PPi. Similar results were obtained using acridine orange as the H+ gradient indicator (Fig. S4A). Moreover, under similar conditions, we found that IMV harboring the Na+-PPase of Methanosarcina mazei (Mm-PPase) were unable to transport H+ ions over a pH range of 5.5–9 (Fig. S4B); this finding is consistent with previous results (1).
ACMA fluorescence quenching in IMV hydrolyzing PPi was considerably diminished in the presence of AMDP or CCCP, and absent in native E. coli IMV lacking membrane PPase (Fig. 2B). ETH157 exerted no significant effect, whereas valinomycin, which dissipates the membrane potential by allowing free transmembrane diffusion of K+, stimulated both Na+ and H+ transport into Bv-PPase IMV (Fig. 2 A and B). The ionophore data exclude the possibility that either the Na+ or H+ gradient formed by Bv-PPase is generated by secondary antiport effects, e.g. by a Na+/H+ antiporter. Instead, it appears that Bv-PPase directly transports both Na+ and H+ ions via an electrogenic process.
In view of the difficulties in estimating absolute H+ transport rates and PPi/cation coupling ratios for both transport activities, an indirect comparison was made. The initial slope and amplitude of Na+ accumulation by Bv-PPase were similar to those generated by the exclusively Na+-transporting Anaerostipes caccae PPase (8), which exhibited nearly identical PPi hydrolysis activity in IMV (Fig. 2C). A similar correlation with PPi hydrolysis activity was observed upon comparing the rates of H+ transport by Bv-PPase and the exclusive H+-transporting Leptospira biflexa PPase (8) (Fig. 2D). These data suggest similar PPi/cation coupling ratios for the Bv-PPase–catalyzed Na+ and H+ transport reactions, and rule out the possibility that one of the activities always predominates in vivo.
Relationship Between Na+ and H+ Transport.
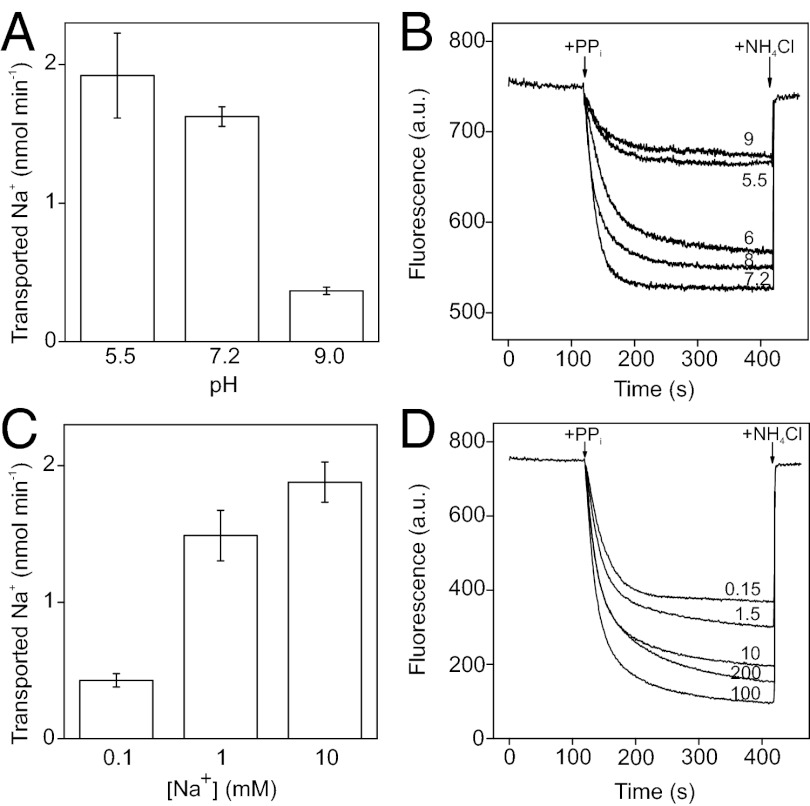
Na+ and H+ cotransport may occur as independent competing reactions or simultaneous processes associated with each catalytic cycle. In the case of the former mechanism, H+ transport would be reduced relative to Na+ transport at increasing pH and Na+ concentrations, and conversely, Na+ transport would be inhibited at decreasing pH and Na+ concentrations. In contrast, intrinsic simultaneous transport of both ions would suggest that pH and Na+ concentration do not exert apparent control over the ratio of transport activities.
The effects of pH on Na+ and H+ transport into Bv-PPase IMV are presented in Fig. 3 A and B. Owing to the potentially complex dependence of membrane and dye properties on pH, we did not attempt to interpret the data quantitatively. Nevertheless, changes in the H+ concentration apparently had no effect on H+ transport capability that significantly differed from effects of variations in H+ concentration on PPi hydrolysis (compare Figs. 3B and 1C). Na+ transport activities were similarly correlated with hydrolytic activity in neutral and alkaline buffers, but at acidic pH, the relative Na+ transport activity appeared slightly elevated (Fig. 3A).
Fig. 3.
Lack of competition between Na+ and H+ transport reactions. Dependencies of Na+ and H+ transport on pH (A and B, respectively) and Na+ concentration (C and D, respectively). Values of pH and Na+ concentration are shown in B and D as curve labels. The Na+ transport reaction time was 15 s. The buffers used were MES- (pH 5.5), MOPS- (pH 6.0, 7.2, and 8.0), or CAPSO-TMA (pH 9.0). Na+ transport was measured with 1 mM Na+, 50 mM K+, and the following Mg2+/TMA4⋅PPi concentrations: 13.1/9.8, 6.6/1.0, and 6.5/0.8 mM at pH 5.5, 7.2, and 9.0, respectively. H+ transport measurements were performed in the presence of 10 mM Na+, 50 mM K+, and the following Mg2+/TMA4⋅PPi concentrations: 8.8/4.65, 6.6/1.42, 5.8/0.47, 5.7/0.43, and 5.7/0.42 mM at pH 5.5, 6.0, 7.2, 8.0, and 9.0, respectively. Other conditions were as described for Fig. 2.
Na+ concentration dependencies of Na+ and H+ transport activities at pH 7.2 (Fig. 3 C and D) also appeared to follow the pattern of dependence of hydrolytic activity (Fig. 1A). Analogous results were obtained with Bv-PPase IMV in the absence of K+ (Fig. S5), indicating that Bv-PPase does not require K+ for transport activity. The collective effects of pH and Na+ concentration on Bv-PPase transport activities fail to support a simple competitive model of H+ and Na+ transport. Instead, transportation of Na+ and H+ appear to be intrinsically coupled in the catalytic cycle.
Defining the Subfamily of Na+,H+-PPases.
To estimate the proportion of membrane PPases in which Na+ and H+ transport are intrinsically coupled, we constructed a Bayesian phylogeny of the protein superfamily using a previously described approach (8). The sequence set was supplemented with Bv-PPase, and a few similar sequences identified in the National Center for Biotechnology Information (NCBI) nonredundant protein sequence (nr) databank (10). The resulting tree reiterated the conclusion that the K+-dependent PPase family can be phylogenetically classified into four major clades originating at nodes A, B, C, and D (Fig. 4A) (8). Bv-PPase homologs that clustered within this family (in the node C clade) displayed the closest phylogenetic relationship with M. mazei Na+-PPase. To comprehensively map phylogenetic diversity near Bv-PPase, we performed a second round of phylogenetic analyses using a representative set of sequences (37 taxa) clustering to clade C in the preliminary analysis. This step allowed us to obtain a longer block of reliably aligned residues (656 vs. 333), which facilitated high-quality reconstruction of the phylogeny, as indicated by the generally high clade credibility values (Fig. 4B).
Fig. 4.
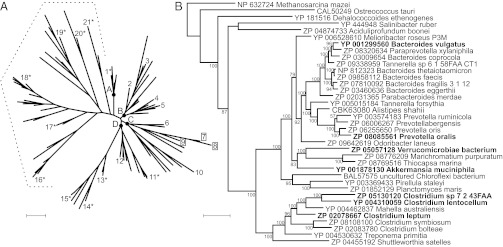
Phylogeny of membrane PPase protein sequences. (A) Experimentally characterized Na+-PPases are labeled with plain numbers, Na+,H+-PPases with squares, and H+-PPases with asterisks. The dotted line highlights the K+-independent subfamily, whereas letters A, B, C, and D indicate the nodes of different clades within the K+-dependent subfamily. The taxon numbers 6 and 7 represent M. mazei Na+-PPase and Bv-PPase, respectively. Other taxon names are provided in Table S2. (B) Phylogeny of the Na+,H+-PPase subfamily. The tree was rooted to M. mazei Na+-PPase. The enzymes characterized in this study are indicated in bold. Nodes are supplemented with clade credibility values. (Scale bars: 0.2 substitutions per residue.)
Based on the tree obtained, we selected a comprehensive set of divergent sequences to experimentally verify the distribution of transport specificities within the Bv-PPase clade. We attempted to express PPase genes from Akkermansia muciniphila (Am-PPase), Clostridium leptum (Cl-PPase), Clostridium lentocellum PPase (Clen-PPase), Prevotella oralis (Po-PPase), Salinibacter ruber, Dehalococcoides ethenogenes, Ostreococcus tauri, and Planctomyces maris in E. coli. Among these enzymes, Am-PPase, Cl-PPase, Clen-PPase, and Po-PPase were isolated in the active form (Fig. S3). Characterization of these PPases revealed an absolute requirement for Na+ for activity, which was further stimulated by K+ (Fig. S3B). Importantly, the four PPases mediated PPi-energized H+ and Na+ transport activities (Fig. S6), supporting the existence of a unique PPase subfamily (Na+,H+-PPases) capable of transporting both cations.
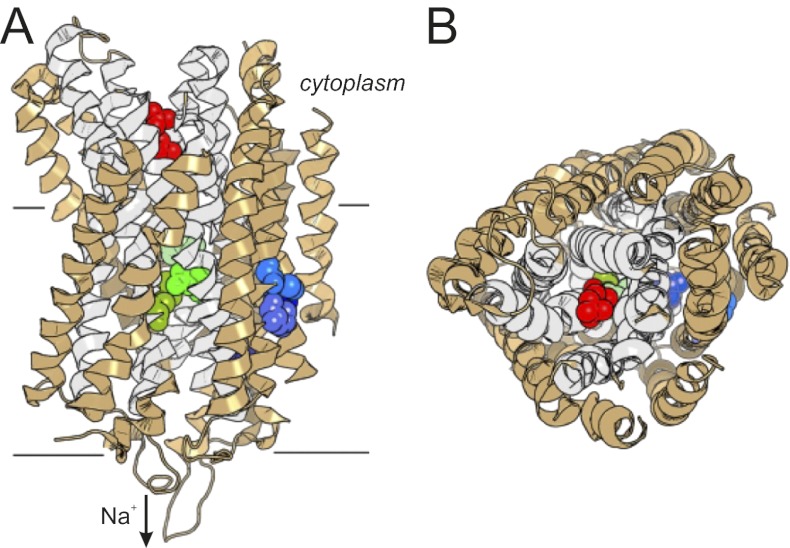
Manual and computer-aided (Sequence Harmony) (11) sequence comparisons of Na+,H+-PPases with Na+-PPases and H+-PPases revealed that the residues known to form binding sites for Mg2+, K+, and PPi, and ion conductance channels in the 3D structures of plant-type H+-PPase (5) and Na+-PPase (6) are nearly identical in all three groups of enzymes (Fig. S7); the only exception is the gate Glu residue, which in plant-type H+-PPases has moved one helical turn deeper into the membrane, to position 301 (Vigna radiata H+-PPase numbering) (8). However, the analysis led to the identification of residues at four positions strongly conserved in Na+,H+-PPases and their homologs, but not other PPases—specifically, Asp146, Thr/Ser90, Phe94, and Met176 (Bv-PPase numbering; Fig. S7). Based on the positions of the corresponding residues in V. radiata and Thermotoga maritima PPase structures (5, 6), these residues were localized as follows (Fig. 5): Asp146 is present in outer ring helix 4 in the middle of the membrane; Thr90 and Phe94 are in adjacent helix 3 and possibly interact directly with Asp146; and Met176 is close to the periplasmic end of helix 5, 10–14 Å away from the above triad.
Fig. 5.
Two views of the ribbon model of the T. maritima Na+-PPase subunit (PDB ID code 4AV6) (6). Four amino acids occupying the positions of the proposed Na+,H+-PPase T/F/D/M signature residues (shades of blue), Asp243, Glu246, and Lys707 gate residues (shades of green), and two phosphates (red) bound in the hydrolysis site are shown in a sphere representation. The inner circle helices are colored in gray, and the rest of the molecule in light brown. The left side of the subunit in A and B forms contacts with the other subunit. The figure was created with PyMOL Molecular Graphics System, version 1.5.0.4 (Schrödinger, LLC).
Interestingly, PPases from Verrucomicrobiae bacterium (Vb-PPase) and Clostridium sp. 7_2_43FAA (Cs-PPase) phylogenetically localize to the Na+,H+-PPase subfamily but harbor substitutions in three or four of the putative signature residues, respectively (Fig. 4B; Fig. S7). Characterization of the transport specificity of these enzymes provided a feasible test for the validity of the signature residues. Both enzymes were isolated in the active form in E. coli IMV (Fig. S3). Data from our transport assays indicate that Vb-PPase and Cs-PPase operate as exclusive Na+ transporters (Fig. S6). Notably, Cs-PPase is the closest homolog of Clen-PPase (70% sequence identity, no gaps), which transports both Na+ and H+, but lacks the typical Na+,H+-PPase signature residues and thus exhibits only Na+ transport activity.
Discussion
In the current study, we have defined a unique subfamily of PPi-energized ion pumps, designated Na+,H+-PPases, capable of transporting both Na+ and H+ ions. This finding contradicts the earlier view that membrane PPases exclusively pump either H+ or Na+, but complements the recent identification of an A1Ao ATPase from the methanogenic archaeon Methanosarcina acetivorans, which apparently transports both Na+ and H+ ions under physiological conditions (12).
Sequence analyses facilitated the identification of four residues that have coevolved and apparently control transport specificity within the Na+,H+-PPase subfamily. Three of these residues (Thr90, Phe94, and Asp146; Bv-PPase numbering) possibly form a triad of interacting residues in the membrane-spanning part of α-helices 3 and 4, whereas Met176 resides close to the periplasmic end of α-helix 5. Helices 3–6 form a tightly interacting bundle, suggesting that the introduction of polar (Thr90 and Asp146) and bulky (Phe94 and Met176) residues at the helix interfaces causes repositioning of the helices to avoid steric clashes. Importantly, helices 5 and 6, which line the ion conductance channel, harbor two gate-forming residues (Asp243 and Glu246). Accordingly, we hypothesize that the H+ transport capability was acquired in Na+-PPases via an allosteric evolutionary pathway, which linked distal substitutions to the reconfiguration of the core catalytic machinery via rigid body movements of interconnected α-helices. Compared with direct substitutions, allosteric remodeling of the gate and/or transport channel appears to permit the enzyme to maintain its Na+ transport activity, rather than inducing a complete change in the identity of the transported ion.
Na+,H+-PPase maintains its H+ and Na+ transport activities over a wide range of pH and Na+ concentrations without apparent competition between the transported ions. The proton fails to substitute for Na+ as the enzyme activator. Furthermore, the PPi/cation coupling ratios appear similar for the Na+ and H+ transport reactions. Therefore, we propose that Na+,H+-PPases concurrently transport both Na+ and H+ ions during each catalytic cycle. Given that PPi hydrolysis in vivo liberates 20–25 kJ/mol energy (corresponding to a 200- to 260-mV membrane potential difference) (13), thermodynamics allows simultaneous transport of the two ions in membranes generating low or moderate electrochemical potential gradients, such as those in fermentative bacteria (14). Consistent with this viewpoint, H+/PPi transport ratios of 1–2 have been reported for H+-PPases (15–17). Alternatively, H+ and Na+ transport may occur in different subunits of dimeric PPase, and may therefore require one PPi molecule per cation. This mechanism may involve preexisting or induced structural asymmetry in the enzyme dimer, such that binding of H+ or Na+ to one subunit elicits structural changes in the other subunit, allowing it to bind preferentially to the alternative ion. Asymmetry is not evident in the crystal structures of the monospecific H+- and Na+-PPases (5, 6), but may arise in allosterically remodeled Na+,H+-PPase.
The K+-dependent membrane PPase subfamily is a functionally divergent group that includes both Na+- and H+-transporting enzymes. Reconstruction of the evolutionary history of the subfamily indicates that the ancestral enzyme possibly operated as a Na+ pump, and that a transition to H+ specificity evolved in several independent enzyme lineages (8). Based on the topology of the phylogenetic tree, conserved regulation by Na+/K+, relatively limited structural diversity (≥46% pairwise sequence identity), and host species similarities (see below), it is predicted that Na+,H+-PPases descend from Na+-PPases. Interestingly, the evolutionary pathway of the Na+,H+ pump may be readily reversible, as indicated by the subsequent loss of H+ transport capability in Cs-PPase and Vb-PPase. In previously identified Na+→H+ specificity shifts, the critical mechanism appeared to involve relocation of a conserved Glu in the primary and tertiary structure (8), which forms part of the cytoplasmic gate of the ion conductance channel in Na+-PPases (6). In contrast, Na+,H+-PPases acquired the H+ transportation function as a result of multiple distant mutations that remodeled the gate and/or transport channel. Thus, the existence of Na+,H+-PPases can be attributed to an alternative evolutionary route from ancestor Na+-PPases to Na+,H+-PPases, independent of the route(s) leading to H+-PPases.
Based on the four Na+,H+-PPase signature residues (Thr90, Phe94, Asp146, and Met176; Bv-PPase numbering), 104 of 1,192 membrane PPase sequences contained in the nonredundant NCBI Protein Database (as of June 2012) are predicted to belong to the Na+,H+-PPase subfamily. Therefore, the subfamily is smaller and structurally less divergent than the Na+-PPase subfamily (262 sequences), perhaps reflecting the late evolutionary emergence and stringent host specialization of Na+,H+-PPases. All Na+,H+-PPase–harboring organisms are bacteria, and most of them live as abundant colonists in various parts of the gastrointestinal tract in humans and other mammals (Table S1). The predominant characteristics of this environment, which shapes the bioenergetic machinery of the bacteria and apparently exerts positive selection pressure for Na+,H+-PPases, include anaerobiosis and intense niche competition (18). Overall, more than 90% of presumed Na+,H+-PPase host species are anaerobic. Thus, the use of PPi energy to support both H+ and Na+ gradients appears to represent a means of adapting to environmental challenges, requiring mobilization of all available energy resources. This theory is consistent with data showing that overexpression of H+-PPase in plants improves their survival under stress conditions (4). Accordingly, genetically enhanced expression of Na+,H+-PPase or inhibition of such expression by appropriate drugs may provide a promising means to facilitate or impede the survival of the host organism, respectively.
Within the ubiquitous ATPase world, Na+,K+-ATPase (19) and Na+-coupled rotary ATP synthases (20–22) can transport H+ instead of Na+, but only at low pH and/or Na+ concentrations, whereas H+-coupled ATPases are apparently incapable of translocating Na+ (23). M. acetivorans A1A0 ATP synthase appears to be the only other enzyme known to be capable of generating both Na+ and H+ gradients under physiological conditions (12). However, the Na+ and H+ transport activities of this unique ATPase are not tightly coupled, allowing H+ transport and ATP hydrolysis to proceed in the absence of Na+ at pH 5. In contrast, Na+,H+-PPase retains its Na+ transport activity and Na+ dependence of hydrolytic activity in acidic media.
In summary, we have characterized a unique subfamily of membrane-integral PPases that transport both Na+ and H+ ions in a noncompetitive manner and are widespread in the bacterial world. Our findings provide support for the hypothesis that selection pressure favors membrane H+ coupling vs. Na+ coupling in modern organisms (24–27). Furthermore, we conclude that (i) changes in transport specificity can be achieved in different ways and require only minor rearrangements in protein structure; (ii) the evolutionary pathway from Na+ to H+ transport is not strictly unidirectional, and may involve recursions; (iii) Na+ and H+ transport proceed via highly similar mechanisms; and (iv) Na+,H+-PPase represents an attractive model transporter for further detailed investigations.
Materials and Methods
Expression of Recombinant Membrane PPases.
Genes encoding various membrane-integral PPases were amplified from genomic DNA and inserted into the multiple cloning site of the pET36b(+) expression vector (Novagen) under T7 promoter control. Amino acid substitutions were engineered with inverse PCR using Phusion DNA Polymerase (Finnzymes), and mutated genes were inserted into the pET36b(+) vector. PPase-encoding regions of the final expression constructs were sequenced.
Membrane PPase genes were expressed in E. coli, and inverted membrane vesicles were isolated using the French Press procedure, as described previously (28). IMV were suspended in storage buffer [10 mM morpholinopropanesulfonic acid (MOPS)-tetramethylammonium (TMA) hydroxide (pH 7.2), 1 mM MgCl2, 750–900 mM sucrose, 5 mM DTT, 50 µM EGTA], frozen in liquid N2, and stored at –85 °C. The protein content of IMV was estimated with the Bradford assay (29).
Membrane PPase protein expression was verified using Western blot analysis, with minor modifications to a published protocol (1). Briefly, 2 µg of protein per sample in SDS loading buffer [0.07 M Tris⋅HCl (pH 6.8), 11% (vol/vol) glycerol, 2% (vol/vol) SDS, 2.5 mM DTT, and 0.25 mg/mL OrangeG] was loaded onto an SDS/PAGE gel. PPase bands were visualized on a nitrocellulose membrane with an Odyssey Infrared Imager (Li-Cor) using rabbit antiserum raised against a conserved amino acid motif in PPases and fluorescently labeled secondary antibody [IRDye 800CW Donkey Anti-Rabbit IgG (H + L) Highly Cross Adsorbed (Li-Cor)].
Hydrolytic and Transport Activity Assays.
Rates of PPi hydrolysis were estimated from continuous recordings of Pi production obtained with a flow-through phosphate analyzer at 25 °C (30). Unless indicated otherwise, the reaction medium contained 160 µM TMA4⋅PPi, 5.3 mM MgCl2, 10 mM NaCl, 50 mM KCl, 40 µM EGTA, and 0.1 M MOPS-TMA hydroxide (pH 7.2).When the buffer pH was changed, the amounts of Mg2+ and PPi were adjusted to maintain the concentration of the active substrate species, Mg2PPi, at 100 µM, as in the pH 7.2 solution.
PPi-energized H+ transport into IMV was assayed by measuring fluorescence quenching of the pH-sensitive probe ACMA or acridine orange, upon addition of 470 µM TMA4⋅PPi at 25 °C (8). Unless stated otherwise, the reaction medium contained 20 mM MOPS-TMA hydroxide (pH 7.2), 78 mM TMA4⋅Cl, 50 mM KCl, 10 mM NaCl, 5.8 mM MgCl2, 8 µM EGTA, 2 µM ACMA or acridine orange, and 0.3 mg/mL protein. Where required, the amount of TMA4⋅Cl was adjusted to maintain Cl− concentration at 150 mM. Before initiation of the reaction, the medium was incubated for 4 min in a fluorometer (LS-50; PerkinElmer) sample holder to ensure stable initial fluorescence. In the H+ and Na+ transport reactions used to quantify the effect of ionophores, cations were added as sulfate salts.
Na+ transport into IMV was assayed by determining the accumulation of 22Na+ in IMV at 23 °C with a membrane filtration procedure (8). Unless stated otherwise, the reaction mixture contained 0.1 M MOPS-TMA hydroxide (pH 7.2), 90 mM TMA4Cl, 50 mM KCl, 5 mM MgCl2, 1 mM NaCl (including 0.3–4.5 µCi 22Na), and 1–5 mg/mL protein. The reaction was initiated by the addition of 1 mM TMA4⋅PPi and terminated with 20 mM EDTA (adjusted to pH 7.2 with NaOH). Where needed, the amount of TMA4⋅Cl was varied to maintain Cl− concentration at 150 mM. Na+ transport activity data were corrected for background values (typically <10% of the PPi signal) measured without PPi in the reaction mixture.
Established Na+- and K+-free reagents (28) were used to assess the dependence of activities on Na+ and K+. The concentration of contaminating Na+ ions in the assay medium was estimated to be ∼30 μM using atomic absorption spectrometry. MES, MOPS, TAPS, and CAPSO buffers adjusted with TMA-hydroxide were used in pH studies. The buffer concentration used was 0.02 M (H+ transport) or 0.1 M (PPi hydrolysis and Na+ transport). Other details of the assay procedures were as described previously (8).
Kinetic Data Analysis.
The dependencies of PPi hydrolysis rate (v) on Na+ and K+ concentrations were analyzed in terms of Scheme 1, which assumes that binding of the first Na+ ion stimulates hydrolysis, whereas binding of the second Na+ ion inhibits the reaction. K+ binding only modulates hydrolytic activity (V1 ≠ V2). Assuming rapid equilibrium cation binding, the rate equation for the mechanism in Scheme 1 is provided by Eq. 1. The parameter values were determined by fitting the equation to rate data using Scientist software (MicroMath).
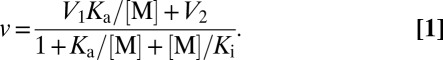 |
The pH profiles of hydrolytic activity were analyzed using
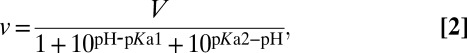 |
where pKa1 and pKa2 represent the pKa values for two protein groups, and V is the pH-independent activity value.
Phylogenetic Analysis.
Two sequence sets, designated set A and B, were retrieved from the NCBI Protein Sequence Databank with BLAST (10). Set A represented full sequence divergence of the membrane PPase protein superfamily, and set B represented the sequence space near Bv-PPase. Set B contained only sequences displaying close similarity and phylogenetic relationship to Bv-PPase and Mm-PPase, relative to other characterized PPases. Protein sequences were aligned using default settings in MUSCLE version 3.8 (31). Alignments were manually refined by eliminating redundant sequences and sequence regions including indels and ambiguously aligned residues. The resulting alignment block A contained 121 taxa and 333 residue columns, whereas block B had 37 taxa and 656 residue columns. Phylogenetic trees were generated using the program MrBayes 3.1.2 (32). The K+ dependence signature sequence of membrane PPases was taken into account by supplementing the alignment block A with another partition containing 398 columns of 0 or 1 character, depending on whether the full protein sequence harbored Ala (indicative of K+ dependence) or Lys (indicative of K+ independence), respectively, at position 460 (Carboxydothermus hydrogenoformans PPase numbering) (7). For the global tree (Fig. 4A), the topology was estimated using the information content of both partitions, whereas the amino acid alignment partition defined the branch lengths. The Bv-PPase clade tree (Fig. 4B) was calculated directly from block B. In each case, four independent phylogenetic analyses were initiated from random trees and run for 10 (A) or 5 million (B) generations (temperature option 0.15) on a computer cluster provided by the CSC–IT Center for Science Ltd. (Espoo, Finland). The mean SD of split frequencies between independent MrBayes runs dropped below 0.01 during the first quarter of the run, indicating convergence of phylogenetic analysis.
Supplementary Material
Acknowledgments
We are grateful to Tiina Pettersson (University of Turku) for skillful technical assistance in cloning P. maris PPase and Dr. Mark Hart (Scottish Association for Marine Science, Dunstaffnage Marine Laboratory) for providing genomic DNA of V. bacterium DG1235. Genomic DNA from Clostridium sp. strain 7_2_43FAA, HM-36D was obtained through the National Institutes of Health (NIH) Biodefense and Emerging Infections Research Resources Repository, National Institute of Allergy and Infectious Diseases, as part of the Human Microbiome Project. This work was supported by Academy of Finland Grant 139031, Russian Foundation for Basic Research Grant 12-04-01002, and grants from the Ministry of Education and the Academy of Finland (for the National Graduate School in Informational and Structural Biology).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217816110/-/DCSupplemental.
References
- 1.Malinen AM, Belogurov GA, Baykov AA, Lahti R. Na+-pyrophosphatase: A novel primary sodium pump. Biochemistry. 2007;46(30):8872–8878. doi: 10.1021/bi700564b. [DOI] [PubMed] [Google Scholar]
- 2.Serrano A, Pérez-Castiñeira JR, Baltscheffsky M, Baltscheffsky H. H+-PPases: yesterday, today and tomorrow. IUBMB Life. 2007;59(2):76–83. doi: 10.1080/15216540701258132. [DOI] [PubMed] [Google Scholar]
- 3.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3(3):251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 4.Gaxiola RA, Palmgren MG, Schumacher K. Plant proton pumps. FEBS Lett. 2007;581(12):2204–2214. doi: 10.1016/j.febslet.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Lin SM, et al. Crystal structure of a membrane-embedded H+-translocating pyrophosphatase. Nature. 2012;484(7394):399–403. doi: 10.1038/nature10963. [DOI] [PubMed] [Google Scholar]
- 6.Kellosalo J, Kajander T, Kogan K, Pokharel K, Goldman A. The structure and catalytic cycle of a sodium-pumping pyrophosphatase. Science. 2012;337(6093):473–476. doi: 10.1126/science.1222505. [DOI] [PubMed] [Google Scholar]
- 7.Belogurov GA, Lahti R. A lysine substitute for K+. A460K mutation eliminates K+ dependence in H+-pyrophosphatase of Carboxydothermus hydrogenoformans. J Biol Chem. 2002;277(51):49651–49654. doi: 10.1074/jbc.M210341200. [DOI] [PubMed] [Google Scholar]
- 8.Luoto HH, Belogurov GA, Baykov AA, Lahti R, Malinen AM. Na+-translocating membrane pyrophosphatases are widespread in the microbial world and evolutionarily precede H+-translocating pyrophosphatases. J Biol Chem. 2011;286(24):21633–21642. doi: 10.1074/jbc.M111.244483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biegel E, Müller V. A Na+-translocating pyrophosphatase in the acetogenic bacterium Acetobacterium woodii. J Biol Chem. 2011;286(8):6080–6084. doi: 10.1074/jbc.M110.192823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 11.Pirovano W, Feenstra KA, Heringa J. Sequence comparison by sequence harmony identifies subtype-specific functional sites. Nucleic Acids Res. 2006;34(22):6540–6548. doi: 10.1093/nar/gkl901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlegel K, Leone V, Faraldo-Gómez JD, Müller V. Promiscuous archaeal ATP synthase concurrently coupled to Na+ and H+ translocation. Proc Natl Acad Sci USA. 2012;109(3):947–952. doi: 10.1073/pnas.1115796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinonen JK. Biological Role of Inorganic Pyrophosphate. Boston: Kluwer; 2001. [Google Scholar]
- 14.Kakinuma Y. Inorganic cation transport and energy transduction in Enterococcus hirae and other streptococci. Microbiol Mol Biol Rev. 1998;62(4):1021–1045. doi: 10.1128/mmbr.62.4.1021-1045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanishi Y, Yabe I, Maeshima M. Patch clamp analysis of a H+ pump heterologously expressed in giant yeast vacuoles. J Biochem. 2003;134(4):615–623. doi: 10.1093/jb/mvg184. [DOI] [PubMed] [Google Scholar]
- 16.Sosa A, Celis H. H+/PPi stoichiometry of membrane-bound pyrophosphatase of Rhodospirillum rubrum. Arch Biochem Biophys. 1995;316(1):421–427. doi: 10.1006/abbi.1995.1056. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt AL, Briskin DP. Energy transduction in tonoplast vesicles from red beet (Beta vulgaris L.) storage tissue: H+/substrate stoichiometries for the H+-ATPase and H(+)-PPase. Arch Biochem Biophys. 1993;301(1):165–173. doi: 10.1006/abbi.1993.1129. [DOI] [PubMed] [Google Scholar]
- 18.Walter J, Ley R. The human gut microbiome: Ecology and recent evolutionary changes. Annu Rev Microbiol. 2011;65:411–429. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 19.Hara Y, Yamada J, Nakao M. Proton transport catalyzed by the sodium pump. Ouabain-sensitive ATPase activity and the phosphorylation of Na,K-ATPase in the absence of sodium ions. J Biochem. 1986;99(2):531–539. doi: 10.1093/oxfordjournals.jbchem.a135509. [DOI] [PubMed] [Google Scholar]
- 20.Dimroth P. Primary sodium ion translocating enzymes. Biochim Biophys Acta. 1997;1318(1-2):11–51. doi: 10.1016/s0005-2728(96)00127-2. [DOI] [PubMed] [Google Scholar]
- 21.Reidlinger J, Müller V. Purification of ATP synthase from Acetobacterium woodii and identification as a Na+-translocating F1F0-type enzyme. Eur J Biochem. 1994;223(1):275–283. doi: 10.1111/j.1432-1033.1994.tb18992.x. [DOI] [PubMed] [Google Scholar]
- 22.Laubinger W, Dimroth P. The sodium ion translocating adenosinetriphosphatase of Propionigenium modestum pumps protons at low sodium ion concentrations. Biochemistry. 1989;28(18):7194–7198. doi: 10.1021/bi00444a010. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Fillingame RH. Changing the ion binding specificity of the Escherichia coli H+-transporting ATP synthase by directed mutagenesis of subunit c. J Biol Chem. 1995;270(1):87–93. doi: 10.1074/jbc.270.1.87. [DOI] [PubMed] [Google Scholar]
- 24.Mulkidjanian AY, Galperin MY, Koonin EV. Co-evolution of primordial membranes and membrane proteins. Trends Biochem Sci. 2009;34(4):206–215. doi: 10.1016/j.tibs.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulkidjanian AY, Galperin MY, Makarova KS, Wolf YI, Koonin EV. Evolutionary primacy of sodium bioenergetics. Biol Direct. 2008;3:13. doi: 10.1186/1745-6150-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulkidjanian AY, Dibrov P, Galperin MY. The past and present of sodium energetics: May the sodium-motive force be with you. Biochim Biophys Acta. 2008;1777(7-8):985–992. doi: 10.1016/j.bbabio.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holm NG, Baltscheffsky H. Links between hydrothermal environments, pyrophosphate, Na+, and early evolution. Orig Life Evol Biosph. 2011;41(5):483–493. doi: 10.1007/s11084-011-9235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belogurov GA, et al. Membrane-bound pyrophosphatase of Thermotoga maritima requires sodium for activity. Biochemistry. 2005;44(6):2088–2096. doi: 10.1021/bi048429g. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Baykov AA, Avaeva SM. A simple and sensitive apparatus for continuous monitoring of orthophosphate in the presence of acid-labile compounds. Anal Biochem. 1981;116(1):1–4. doi: 10.1016/0003-2697(81)90313-4. [DOI] [PubMed] [Google Scholar]
- 31.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.