Abstract
The present study demonstrates that agonist-mediated activation of α2A adrenergic receptors (α2AAR) is voltage-dependent. By resolving the kinetics of conformational changes of α2AAR at defined membrane potentials, we show that negative membrane potentials in the physiological range promote agonist-mediated activation of α2AAR. We discovered that the conformational change of α2AAR by voltage is independent from receptor-G protein docking and regulates receptor signaling, including β-arrestin binding, activation of G proteins, and G protein-activated inwardly rectifying K+ currents. Comparison of the dynamics of voltage-dependence of clonidine- vs. norepinephrine-activated receptors uncovers interesting mechanistic insights. For norepinephrine, the time course of voltage-dependent deactivation reflected the deactivation kinetics of the receptor after agonist withdrawal and was strongly attenuated at saturating concentrations. In contrast, clonidine-activated α2AAR were switched by voltage even under fully saturating concentrations, and the kinetics of this switch was notably faster than dissociation of clonidine from α2AAR, indicating voltage-dependent regulation of the efficacy. We conclude that adrenergic receptors exhibit a unique, agonist-dependent mechanism of voltage-sensitivity that modulates downstream receptor signaling.
Keywords: FRET, G protein-coupled receptor, adrenoceptor, symphathetic
Adrenergic receptors represent a clinically important group of G protein-coupled receptors (GPCRs). This large family of ligand-gated signaling molecules is involved in many physiological and pathophysiological processes, which represent important pharmacological targets (1) and share common downstream signaling pathways (2). α-adrenergic receptor type 2A (α2AAR) are expressed in neurons of the central nervous system, where they control the regulation of neurotransmitter release (3). Therefore, they are exposed to the electric field of the membrane and may be modulated by alterations in the membrane potential (VM), a phenomenon that is the topic of this study. Indeed, voltage-dependent binding of neurotransmitters to their receptors, or at least voltage-dependent regulation of their downstream signal, has been described for a few other members of the GPCR family, specifically for M1 and M2 muscarinic receptors (4), as well as P2Y1 purinergic receptors (5, 6) and metabotropic glutamate receptors (7). Voltage-dependence of GPCRs has been implicated in fine-tuning of neurotransmitter release (8, 9), in regulation of cardiac excitability (10), and in potentiation of IP3-dependent intracellular Ca2+ signals (6). So far the mechanism underlying this voltage sensitivity of GPCRs is not very well understood; however, the current view is that allosteric regulation of receptor conformations by docking of the G protein seems to be required for voltage sensitivity. Notably, muscarinic M1 and M2 receptors display fast voltage-induced charge movements in the absence of agonists similar to the gating currents of ion channels (11, 12). Recently, conformational changes directly linked to the charge movements have been identified, which propagate to the binding pocket of muscarinic receptors, but do not reflect activation or deactivation of these receptors (13).
In the present study we aimed to directly monitor receptor conformations underlying receptor activation in dependence of VM by using a FRET-based biosensor for α2AAR receptors, termed α2AAR-cam (14). FRET-based biosensors sense the outward movement of transmembrane helix 6 relative to the C-terminal region of helix 8, which is directly linked to receptor activation (15). Therefore, these biosensors represent an ideal tool to study the dynamics of voltage-induced changes in receptor conformations. Here we report that α2AAR activation is voltage-dependent, which in contrast to previously described receptors does not require G-protein docking to the receptor. Consequently, the voltage-dependence of the α2AAR affected not only G-protein signaling but also receptor-mediated β-arrestin signaling. By analysis of the receptor dynamics, we further aimed to differentiate between effects of voltage on affinity and efficacy.
Results
Activation of α2AAR Is Voltage-Dependent.
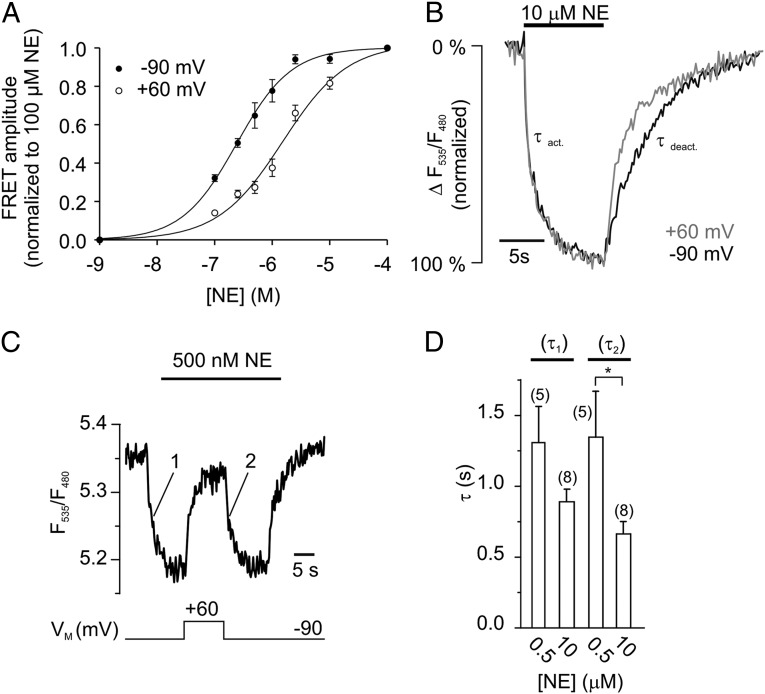
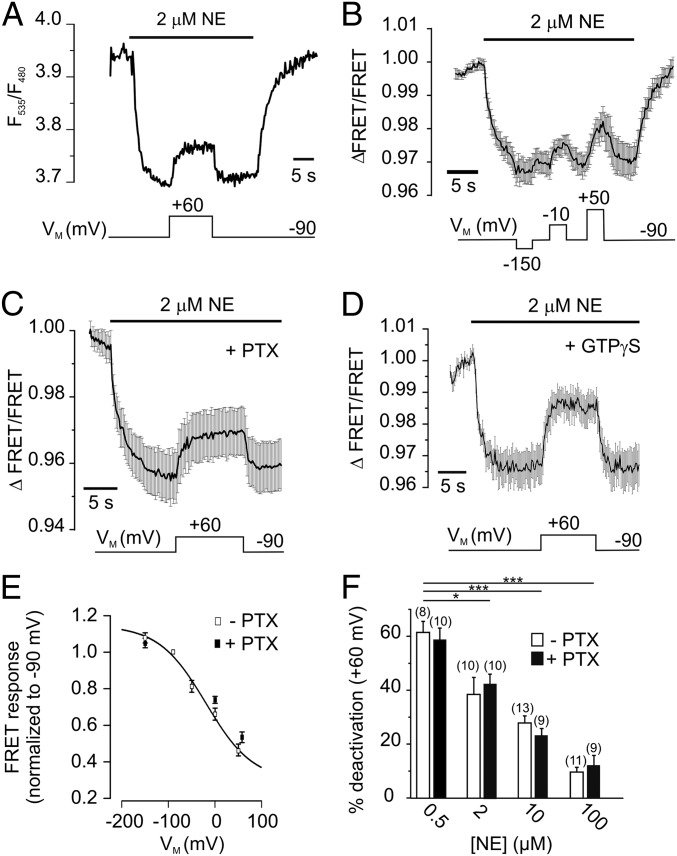
To test for a voltage dependence of α2AAR function, we expressed the FRET-based biosensor α2AAR-cam (14) (Fig. S1) in HEK 293 cells and subjected them to conditions of whole-cell voltage clamp. For these initial experiments we have chosen a norepinephrine (NE) concentration around the EC50 value (e.g., see Fig. 2A, discussed below) that allows monitoring of activation and deactivation of α2AAR-cam in a dynamic range. Application of 2 µM NE at VM = −90 mV resulted in activation of the biosensor, which was measured as a decrease in the FRET ratio (F535/F480) (Fig. 1A). Depolarization of the membrane (+60 mV) caused receptor deactivation, measured as FRET increase that reached a new steady-state level. Subsequent hyperpolarization to −90 mV caused recovery of the FRET ratio, which indicates reactivation of α2AAR-cam. Notably, α2AAR-cam was not voltage-sensitive in the absence of agonist (Fig. S1). To determine the relation between receptor activation and VM, we activated α2AAR-cam with NE, and VM was changed stepwise to various potentials, as indicated in Fig. 1B. A Hyperpolarization of the membrane to −150 mV resulted in a small, but detectable decrease in FRET reflecting receptor activation, whereas stepwise depolarization to −10 mV or +50 mV yielded to gradual increases in FRET, in line with receptor deactivation. A plot of the normalized FRET amplitudes (FRET/FRET−90mV) against VM was fitted to a Boltzmann function and the membrane potential with half-maximal activation of the biosensor was calculated as V0.5 = −20 mV with a slope factor of −50 mV (Fig. 1E, open squares). Voltage-sensitivity of muscarinic M2 receptors has been attributed to a high-affinity, G protein-bound state of the receptor (4, 13). To test whether this holds true for the Gi-coupled α2AAR, we treated HEK 293 cells expressing α2AAR-cam (14), with pertussis toxin (PTX, 50 ng/mL for 3 to 5 h) or applied GTPγS (500 μM) via the patch pipette. Both treatments effectively uncoupled the α2AAR from Gi proteins (Fig. S2). However, α2AAR-cam showed still robust deactivation in PTX- (Fig. 1 C and E) and GTPγS-treated cells (Fig. 1D) in response to depolarization. The effect of depolarization on α2AAR-cam was sensitive to the concentration of NE. We activated the biosensor with various NE concentrations (VM = −90 mV) and determined its deactivation at +60 mV (Fig. 1F). If moderate concentrations were used, we observed strong receptor deactivation (500 nM NE: 61 ± 4.0% deactivation, n = 8), which was almost abolished at saturating concentrations (100 µM NE: 9 ± 1.2% deactivation, n = 6). This effect was very similar in PTX-treated cells (Fig. 1F). These data demonstrate voltage-sensitivity of agonist-induced activation of the α2AAR biosensor with high sensitivity in the range of physiological values for VM.
Fig. 2.
Voltage modulates the affinity of NE-activated receptors. (A) Concentration-response relation of α2AAR-cam at −90 mV and at +60 mV (n = 7–15 per datapoint). (B) The deactivation kinetics of NE-activated α2AAR-cam was faster at +60 mV than at −90 mV (mean of n = 8). (C) The time courses of agonist-induced activation (denoted as 1) and of voltage-induced (denoted as 2) activation were recorded and fitted to a first-order monoexponential decay, giving rise to time constants τ1 and τ2. (D) Summary data of τ1 and τ2 for two different concentrations of NE (mean ± SEM, *P = 0.029).
Fig. 1.
The α2AAR is voltage-sensitive. (A) Depolarization reduces the activation of α2AAR-cam. The biosensor was activated with 2 μM NE at −90 mV, reflected as a decrease in the FRET signal. Subsequent depolarization to +60 mV deactivates α2AAR-cam, indicated by a rise in the FRET ratio. The FRET ratio was calculated as F535/F480 (see Fig. S1 for detailed properties of F485 and F535). (B) The FRET response of α2AAR-cam to NE was measured at various membrane potentials as indicated. The trace represents mean ± SEM, n = 4. (C) Voltage-dependence of α2AAR-cam after uncoupling of G proteins by PTX (30 ng/mL for 3 to 5 h, n = 11) or irreversible activation of G proteins by GTPγS (500 μM, n = 6) (D). (E) Normalized FRET amplitudes from α2AAR-cam (FRET/FRET−90mV) were plotted against the membrane potential VM and fitted to a Boltzmann function giving rise to a V0.5 = −20 mV (n = 5–10 cells per datapoint; control: filled squares; PTX: open squares). (F) The magnitude of receptor deactivation at +60 mV was attenuated at high NE concentrations in both control and PTX-treated cells. Data are presented as mean ± SEM of n individual cells (*P = 0.0101, ***P < 0.001).
Depolarization Reduces the Apparent Affinity of the α2AAR for NE.
The voltage sensitivity of the muscarinic M2 receptor was related to changes in apparent affinity for acetylcholine during depolarization of the membrane (11, 12). The fact that deactivation of α2AAR-cam by voltage was reversible (FRET recovery at −90 mV in Fig. 1 B and C) and sensitive to high concentrations of NE (Fig. 1F) led to the hypothesis that voltage modulates the apparent affinity of α2AAR-cam toward NE. Binding studies have shown previously that the degree of conformational change of α2AAR-cam (i.e., the FRET response) is directly related to the amount of agonist bound to the biosensor (14). Therefore, changes in the FRET signal of α2AAR-cam can be used to estimate agonist affinity and agonist binding. We obtained concentration response curves at −90 mV and at +60 mV and calculated EC50 values (Fig. 2A). Indeed, depolarization caused a rightward shift of the curve (Fig. 2A) (EC50, −90 mV: 0.24 μM; EC50, +60 mV: 1.52 μM). This finding would suggest that α2AAR-cam exhibits higher affinity toward NE at −90 mV compared with +60 mV. This higher affinity is accompanied by a slower receptor deactivation after agonist withdrawal. As depicted in Fig. 2B, deactivation at −90 mV (black trace, τdeact: 7.25 ± 1.32 s, n = 8) took about three times longer than the deactivation at +60 mV (gray trace, τdeact: 2.40 ± 0.41 s). To test whether indeed a major part of the observed voltage-dependent receptor activation can be attributed to alterations in affinity, we tested for the dependency of the speed of voltage-induced receptor activation on the agonist concentration.
Using the FRET-based α2AAR-cam biosensor as a readout, we measured the time courses of NE-induced receptor activation (denoted as 1 in Fig. 2C) and of hyperpolarization-induced receptor activation (denoted as 2 in Fig. 2C). In both cases time courses were fitted to a first-order monoexponential decay to calculate the corresponding time constants τ1 and τ2 (Fig. 2 C and D). Both time constants were very similar for a given concentration (Fig. 2D), but became smaller at high agonist concentrations. For control purposes, we confirmed that the time course of agonist-mediated receptor activation is not affected by partial prestimulation of receptors (Fig. S3). These data obtained with α2AAR-cam indicate that receptor activation correlates with binding and release of agonists at negative and positive membrane potentials, respectively.
Properties of the Voltage Dependence of α2A Receptors Depend on Receptor Pharmacology.
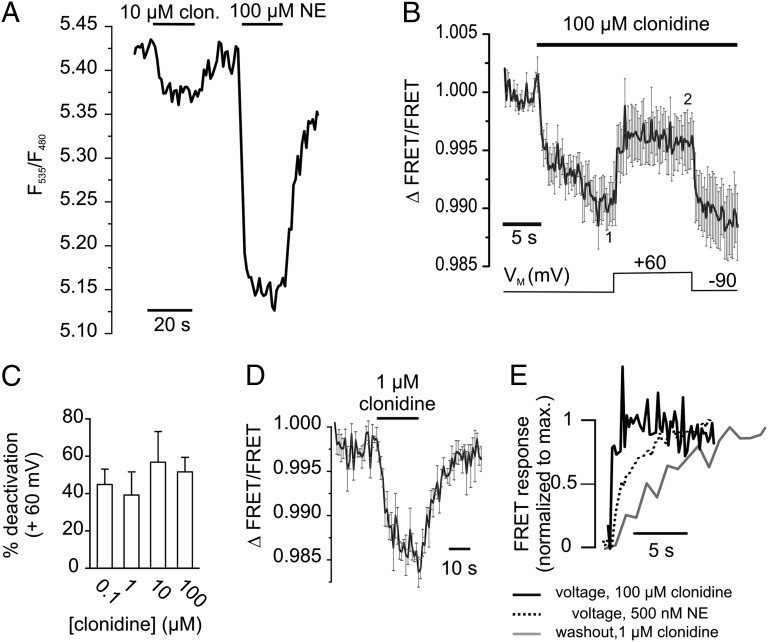
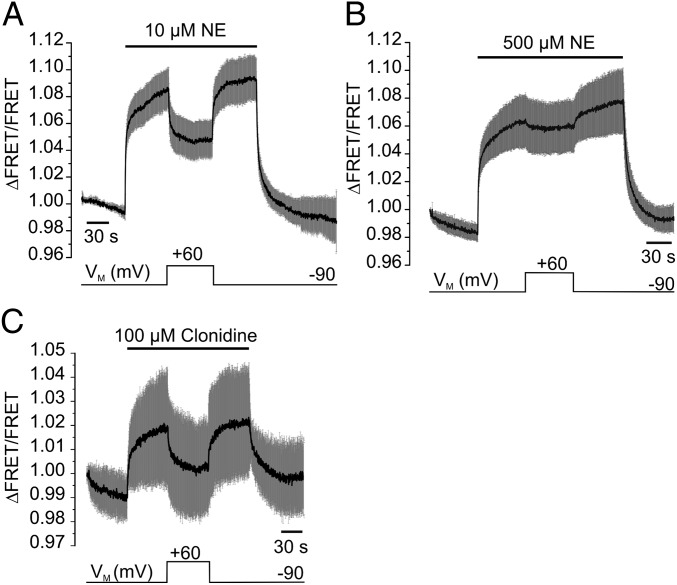
Partial agonists of GPCRs may bind to their receptor with high affinity but display only incomplete intrinsic activity, even under saturating conditions. According to the current model, partial agonists may either arrest the receptor in a partial active conformation or allow a switch of the receptors to active conformations with lower probability than full agonists (16). We aimed to test (i) whether activation of the α2AAR by clonidine, a partial agonist of α2AAR with clinical relevance, also exhibits voltage dependence, and if so, (ii) whether this voltage-dependence can be primarily attributed to alterations in agonist affinity or efficacy. The KD of binding of clonidine to α2AAR expressed in heterologous expression systems has been described to be in the low nanomolar range (17, 18). Stimulation of α2AAR-cam with a saturating clonidine concentration (10 µM) resulted in moderate receptor activation, which was about 30% of the full response obtained with 100 µM NE (Fig. 3A) (14). We then activated the biosensor with an even higher concentration of clonidine (100 µM, VM = −90 mV) and depolarized the membrane. We observed deactivation of the receptor at +60 mV, as reflected by a substantial increase in the FRET signal (> 40% deactivation) (Fig. 3B). Notably, this deactivation by voltage was not sensitive to the extracellular clonidine concentration (Fig. 3C). We measured clonidine evoked alterations in FRET at both potentials (average over 2 s, as denoted in Fig. 3B) and calculated the voltage-induced deactivation (Fig. 3C). Furthermore, the kinetics of deactivation of the biosensor because of depolarization to + 60 mV (Fig. 3B) (100 μM clonidine) were about twofold faster (t0.5 = 0.57 ± 0.11 s, n = 10 vs. 1.22 ± 0.06 s, n = 5) compared with the voltage-induced deactivation of NE-stimulated receptors, and about 10-fold faster than the FRET increase upon washout of 1 µM clonidine (t0.5 = 4.8 ± 0.73 s, n = 4) (Fig. 3 D and E). This result is clearly different from the results obtained with the full-agonist NE, where the kinetics of voltage-induced deactivation always resembled the deactivation kinetics upon washout of the agonist, even at low agonist concentrations (Fig. 2C). These data indicate that the conformation of α2AAR induced by the partial agonist clonidine is sensitive to changes in VM. In contrast to the full-agonist NE, voltage-induced deactivation of clonidine-activated receptors does not involve complete dissociation from the receptor.
Fig. 3.
Voltage sensitivity of clonidine-activated α2AAR. (A) A representative FRET recording of a α2AAR-cam expressing cell exposed to the partial agonist clonidine (10 µM) or the full-agonist NE (100 µM). (B) α2AAR-cam was activated with clonidine and exposed to membrane depolarization as indicated (mean ± SEM, n = 10). (C) The depolarization-induced deactivation of α2AAR-cam at +60 mV was similar over a broad range of clonidine concentrations. (D) FRET response of cells expressing α2AAR-cam stimulated with 1 µM clonidine (mean ± SEM, n = 4). (E) Magnified representation of the kinetics of voltage-induced receptor deactivation in the presence of clonidine (black trace, same data as in B), NE (dashed black trace, n = 5) in comparison with the kinetics of receptor deactivation upon clonidine withdrawal (gray trace; same data as in D). For clarity, average traces are presented as mean without SEM.
Membrane Potential VM Modulates Receptor Signaling.
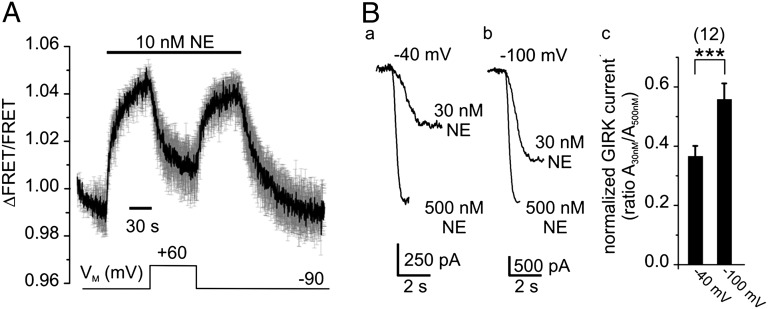
Because the α2AAR is voltage-sensitive, it is important to test whether the membrane potential modulates receptor signaling. We assessed the function of nonlabeled, wild-type α2AAR by directly measuring G-protein activation using a FRET-based assay between Gαi1-YFP, Gβ1-Cerulean, and Gγ2 (Fig. S2) (19). This assay allows us not only to relate VM with G-protein activation, but also to characterize the voltage-dependence of native receptors, the function of which is not altered because of fusion with fluorescent proteins. Based on the observation that heterologously expressed α2AAR exhibit a robust spare-receptor phenomenon (20), cells were stimulated with much lower NE concentrations than in experiments using the receptor-biosensor. A concentration of 10 nM NE was chosen to activate Gαi proteins to submaximal levels. Application of NE (VM = −90 mV) resulted in a rise of the FRET signal that reflects activation of Gαi proteins (Fig. 4A) (19). Depolarization of the membrane (+60 mV) led to deactivation of G proteins by more than 50% and subsequent hyperpolarization resulted in reactivation of the G protein. In contrast, stimulation of the α2AAR with 1 µM NE resulted in maximal G-protein activation that was much less sensitive to depolarization of the membrane (8.2 ± 2.4% deactivation, n = 6). The kinetics of voltage-dependent deactivation of the G protein was similar to deactivation upon removal of NE (Fig. 4A). Voltage also modulated the activity of G protein-activated, inwardly rectifying K+ (GIRK) channels. Application of NE resulted in moderate (30 nM) or maximal activated (0.5 μM) inward currents, which were measured and compared at a holding potential of −40 mV (Fig. 4 B, a) and −100 mV (Fig. 4 B, b). The fraction of GIRK channels activated by 30 nM NE relative to those activated by 0.5 µM NE (ratio A30nM/A500nM) was much larger at −100 mV than at −40 mV (Fig. 4 B, c), suggesting potentiation of GIRK currents because of a higher affinity of the α2AAR. We conclude that the membrane potential modulates receptor function and that hyperpolarization of the membrane enhances receptor signaling via Gαi proteins.
Fig. 4.
The membrane potential VM modulates G protein signaling of the α2AAR. (A) Single-cell FRET measurements of HEK 293 cells transfected with the G-protein subunits Gαi1-YFP, Gβ1-Cerulean, Gγ2, and the wild-type α2AAR were performed under conditions of voltage-clamp using the indicated voltage and superfusion protocol. (mean ± SEM, n = 3). (B) Representative inward K+ currents of HEK 293 cells expressing wild-type α2AAR and GIRK channels evoked by 30 nM or 500 nM NE, measured at −40 mV (a) or −100 mV (b). (c) The GIRK current ratio (A30nM/A500nM) indicates that the response to 30 nM NE is potentiated at −100 mV. ***P ≤ 0.001.
Binding of β-Arrestins to α2A Receptors Depends on VM.
Many GPCRs display reduced signaling if they are chronically stimulated with agonists. In many cases this phenomenon, termed homologous receptor desensitization, is initiated by receptor phosphorylation via G-protein receptor kinases (GRKs) (21). β-Arrestins bind to receptors that are activated and phosphorylated (22), which leads to G-protein uncoupling and initiates endocytosis of the receptors (23). Because β-arrestins specifically distinguish between active and inactive receptor conformations, we tested whether the β-arrestin/receptor interaction is affected by VM. We measured recruitment of β-arrestins to α2AAR with a FRET assay that uses α2AAR-YFP and Turquoise-β-arrestin 2 (Fig. S4) (22). Activation of the receptor with 10 µM NE at −90 mV resulted in a large increase in FRET ratio (Fig. 5A), which reflects binding of β-arrestin to the activated α2AAR (22). Depolarization of the membrane to +60 mV reduced the FRET signal substantially and subsequent hyperpolarization back to −90 mV led to recovery of the FRET signal, indicating voltage-induced receptor reactivation and rebinding of β-arrestin. Of note, application of a saturating NE concentration (500 µM) resulted in a FRET signal that was almost insensitive to depolarization (Fig. 5B). These results correspond very well with those observed with α2AAR-cam and indicate that β-arrestins sense voltage-dependent alterations in receptor activity. We further tested whether the partial agonist clonidine also induces an active receptor conformation that is recognized by β-arrestins. Application of 100 µM clonidine induced a rise in FRET ratio, indicating that this partial agonist is able to recruit β-arrestins to the activated receptor. The shape of the FRET signal was similar to the one of NE-induced recruitment of β-arrestins (Fig. 5C). One remarkable difference was that depolarization caused a substantial reduction of the FRET signal at +60 mV, indicating dissociation of β-arrestin from the receptor. Subsequent hyperpolarization led to fast of reappearance of the FRET signal, reflecting rebinding of β-arrestin. These results are the first to demonstrate voltage dependence of receptor-mediated β-arrestin signaling.
Fig. 5.
Recruitment of β-arrestins to α2AAR is voltage-sensitive. (A) HEK 293 cells transfected with α2AAR-YFP, Turquoise-β-arrestin 2 and GRK2 are subjected to single-cell FRET recording under conditions of voltage clamp using the indicated voltage and superfusion protocol (10 µM NE, or 500 µM (depicted in B; mean ± SEM, n = 5). (C) The FRET response evoked by clonidine is voltage-dependent even under saturating concentrations (mean ± SEM, n = 6).
Discussion
The present study demonstrates that the α2AAR is sensitive to the voltage across the plasma membrane. Important unique findings are: (i) that the α2AAR exhibits an inherent voltage dependence, which is independent of G-protein coupling; (ii) that voltage affects agonist affinity or efficacy, depending on the agonist; and (iii) that downstream receptor signaling, in particular recruitment of β-arrestins, is modulated by VM.
α2AAR exhibits an inherent voltage dependence.
We used a FRET-based biosensor of the α2AAR to analyze the relation between VM and agonist-induced conformational changes underlying receptor activation. This biosensor reports receptor activation in real time, and therefore represents a more direct tool to study voltage-sensitivity of GPCRs than previous approaches, which relied on measurements of gating currents, intracellular Ca2+ signals, or activation of GIRK channels (24). We found that hyperpolarization of the membrane promotes activation and depolarization induced deactivation of the α2AAR (Fig. 1 B and D). The activation/voltage relation of the α2AAR followed a Boltzmann-function (Fig. 1E). From its slope we calculated a half-maximal activation of the receptor at VM = −20 mV that is right in the physiological range with a charge movement of z = 0.5. The latter is similar to corresponding z-values of 0.55 and 0.85 obtained from gating currents of muscarinic receptors (11, 12). A striking result of the present study is that the α2AAR exhibits voltage-sensitivity in the absence of functional Gi/o proteins: α2AAR-cam displayed robust voltage-dependence in PTX- (Fig. 1C) or GTPγS-treated cells (Fig. 1D) over a wide-range of membrane potentials (Fig. 1E) or agonist concentrations (Fig. 1F). This lower affinity for NE at positive voltages without a G protein docked to the receptor is not in line with the current model of voltage-sensitivity in GPCRs, which is based on the observation that voltage selectively affects the high-affinity state of the receptor, which requires coupling to G proteins. Indeed, such voltage-induced changes in receptor affinity were abolished by PTX treatment in case of the muscarinic M2 receptor for acetylcholine (7, 13). Because the voltage-sensitivity of the α2AAR-cam is clearly present in PTX-treated cells, we conclude that α-adrenergic receptors exhibit voltage-sensitivity via an alternative mechanism that is independent from G-protein coupling.
Voltage affects agonist affinity or efficacy.
So far the regulation of GPCRs by voltage was mechanistically linked to a voltage-dependent change in affinity for the ligand (8, 11–13). In the present study we confirm such a mechanism for NE-activated α2AAR and provide kinetic data that suggest that voltage-dependent conformational changes underlying receptor activation require association or dissociation of NE. Two lines of experimental evidence support the concept that voltage regulates the affinity toward NE: First, membrane depolarization induces a shift in the concentration response curve for receptor activation (Fig. 2A), resulting in a fivefold reduction of affinity at +60 mV compared with −90 mV. Second, the kinetics of NE-induced receptor deactivation was threefold slower at −90 mV than at +60 mV (Fig. 2B). Voltage-induced deactivation of α2AAR was detectable only if the concentration of NE was in the dynamic range of the receptor-activation curve (Fig. 2A) and abolished at high concentrations of NE (Fig. 1F), arguing against alteration of the efficacy of NE by voltage. This effect was evident at the level of the receptor and at downstream signaling levels. As expected from the previously described pronounced spare-receptor phenomenon of α2AAR-mediated G-protein activation (20, 25), NE concentrations sufficient to achieve full activation of G proteins (Fig. 4A) were much lower compared with those needed to induce maximal recruitment of β-arrestin (Fig. 5B). It is important to note that the FRET signal of the receptor-biosensor reflects receptor conformation in response to agonist binding and directly correlates with the binding curve (14). Therefore, the changes in EC50 values by voltage are likely to reflect changes in affinity toward NE (Fig. 2A).
In the case of clonidine, two lines of direct evidence point toward a voltage-dependent modulation of agonist efficacy rather than agonist affinity: First, the voltage-dependent regulation of α2AAR activity was not antagonized by a saturating concentration of clonidine (100 µM, up to > 1,000 KD) (Fig. 3B). Therefore, it can be ruled out that the voltage-dependence of the clonidine response is exclusively the result of a dramatic alteration of the affinity. Second, we observed a fourfold faster time course of voltage-induced deactivation of clonidine-stimulated α2AAR-cam (100 µM) over NE-stimulated α2AAR-cam (500 nM) (Fig. 3E). Notably, the deactivation kinetics by voltage was also 10-fold faster than withdrawal of clonidine from the receptor (washout) (Fig. 3C). This finding is in contrast to both deactivation modes of the biosensor activated with the full-agonist NE (Fig. 2). Such a voltage-dependent modulation of agonist efficacy has been proposed for P2Y1 receptors for which a voltage-dependent conversion of orthosteric antagonists into ligands with agonistic properties has been described. The latter can be interpreted as a regulation of drug efficacy (6).
Downstream signaling of clonidine-activated receptors was also modulated by VM. α2AAR, which were activated by 100 µM clonidine, were substantially inhibited by depolarization of the membrane (receptor/β-arrestin interaction) (Fig. 5C). These results obtained for NE and clonidine imply that the responsiveness of the α2AAR to VM strongly depends on receptor pharmacology and led us to speculate about differences in the conformational flexibility of receptors in dependence of bound ligand: the full-agonist NE may induce strong receptor activation by keeping the receptor in an inflexible, active conformation. As interpreted from the deactivation time courses in Fig. 2, voltage-induced deactivation of the receptor would then require removal of NE from the binding pocket. On the other hand, the partial agonist clonidine may bind differently to the binding pocket, thereby inducing a more flexible conformation with less intrinsic activity (Fig. 3). Thus, membrane voltage could influence the receptor conformation with clonidine still bound, and thereby modulating agonist efficacy. Alternatively, efficacy would also be affected if voltage would cause movement of clonidine within the binding pocket without dissociation from the receptor.
Downstream receptor signaling is modulated by VM.
The voltage-dependence of the α2AAR was not restricted to the biosensor itself, but also transduced to downstream cellular signaling. For the wild-type α2AAR we could demonstrate that Gi protein activation and GIRK channels are modulated by voltage and that this modulation reflects the voltage-dependence of the receptor-biosensor (Fig. 4). This finding is in agreement with previous studies that demonstrated voltage-dependent modulation of G-protein mediated signaling pathways, such as the regulation of G-protein regulated K+-channels or Ca2+-activated Cl− channels and the liberation of Ca2+ from intracellular Ca2+ stores (4, 7, 10, 26). A unique aspect from this study is the finding that the recruitment of β-arrestin to active receptors, which induces G protein-independent receptor signaling pathways, also exhibits voltage-dependence. The binding of β-arrestin to the α2AAR was facilitated at negative and attenuated at positive membrane potentials (Fig. 5). It is generally accepted that β-arrestin binding to the GPCR prevents simultaneous binding of G proteins (23). The fact that recruitment of β-arrestins by α2AAR is voltage-dependent underscores our observation that G-protein docking to the receptor is not required for the voltage-sensitivity of the receptor (Fig. 1).
So far the actual voltage sensor of GPCRs has not been identified. Based on the small calculated z-values it has been proposed that putative voltage sensors contain, if at all, only few charged residues, and do not share similarities with the sequences of known classic voltage sensors (27). The simplest mechanism for a voltage-dependence would be that depolarization simply drives charged agonists (such as NE or ACh) out of the binding pocket of the receptor. At least two experimental observations challenge such a mechanism: First, the response of α2AAR to depolarization clearly differs between NE and clonidine, although both agonists are assumed to be equally charged when bound to the receptor. Second, it has been shown that ACh shows opposite effects upon depolarization, depending on the subtype of muscarinic receptors (4).
Future development of voltage-insensitive α2AAR mutants will help to unravel a physiological function of the voltage sensitivity of this receptor.
Methods
Cell Culture and Transfection of HEK 293 Cells.
Human embryonic kidney (HEK 293) cells were cultured using standard conditions (14) and transfected with Effectene reagent (Qiagen) using the following cDNAs (per dish with 6 cm ∅). For G-protein activation: Murine α2AAR (0.5 µg), rat Gαi1-(91)-YFP (0.8 µg), human Cerulean-Gβ1 (0.5 µg), and human Gγ2 (0.2 µg). For arresstin recruitment: Murine α2AAR-YFP (0.6 µg), murine Turquoise-β-arrestin 2 (0.8 µg), and human GPCR kinase 2 (0.6 µg). To measure GIRK currents: Murine α2AAR (0.5 µg) and a bicistronic plasmid expressing GIRK1 and GIRK4 subunits (1 μg). A stable cell line expressing the biosensor α2AAR-cam (14) was generated by transfecting HEK 293 cells with 0.5 µg cDNA and subsequent selection for stable cell clones with G 418. For fluorescence measurements, HEK 293 cells were split on sterile, poly-L-lysine–coated glass coverslips and analyzed the next day.
Fluorescence Microscopy and Electrophysiology.
FRET signals between CFP and YFP were recorded from single cells using an inverted microscope, as described previously (14). Briefly, CFP was excited with brief light flashes and emitted donor fluorescence (F480) and acceptor fluorescence (F535) were collected with photodiodes (TILL Photonics Dual Emission System). Individual traces representing F535 and F480 were acquired at 1- or 5-Hz sampling rate and displayed using Patchmaster software (v2X52, HEKA). FRET was calculated as ratio F535/F480 (termed FRET ratio) and corrected for photobleaching (Fig. S1). The membrane potential VM was controlled simultaneously using an EPC-7 amplifier (HEKA). GIRK currents were measured in the whole-cell configuration as inward currents (holding potential: −40 mV, \x{2212}90 mV, or \x{2212}100 mV; calculated EK = 0 mV) (Fig. S2). During experiments, patched cells were continuously superfused with extracellular buffer or agonist-containing solution. The membrane potential VM and application of agonists were controlled with Patchmaster software. For further experimental details see Fig. S5.
Analysis of Charge Movements, Activation Kinetics, and Concentration-Dependent Responses.
The voltage dependence of gating-charge movement was determined by fitting a single Boltzmann function to the normalized degree of receptor activation values (FRETxmV/FRET−90mV) (Fig. 1E). The kinetics of receptor activation was fitted to a first-order exponential decay and corresponding τ-values were analyzed. To obtain concentration response curves and EC50 values, the normalized FRET response (FRET/FRET100μM) of α2AAR-cam was measured at −90 mV and +60 mV, plotted against (NE) and fitted to a sigmoid curve. For details on curve fitting see Fig. S5.
Statistics.
All data represent individual observations or an average of individual recordings and are presented as mean ± SEM of n individual cells. Data were statistically analyzed using Student t test and differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Cornelius Krasel for providing reagents for β-arrestin assays. This work was funded in part by Deutsche Forschungsgemeinschaft SFB 593, TP13 (to M.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212656110/-/DCSupplemental.
References
- 1.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 3.Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402(6758):181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003;278(25):22482–22491. doi: 10.1074/jbc.M301146200. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Pinna J, et al. Direct voltage control of signaling via P2Y1 and other Galphaq-coupled receptors. J Biol Chem. 2005;280(2):1490–1498. doi: 10.1074/jbc.M407783200. [DOI] [PubMed] [Google Scholar]
- 6.Gurung IS, Martinez-Pinna J, Mahaut-Smith MP. Novel consequences of voltage-dependence to G-protein-coupled P2Y1 receptors. Br J Pharmacol. 2008;154(4):882–889. doi: 10.1038/bjp.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohana L, Barchad O, Parnas I, Parnas H. The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. J Biol Chem. 2006;281(34):24204–24215. doi: 10.1074/jbc.M513447200. [DOI] [PubMed] [Google Scholar]
- 8.Kupchik YM, et al. A novel fast mechanism for GPCR-mediated signal transduction—Control of neurotransmitter release. J Cell Biol. 2011;192(1):137–151. doi: 10.1083/jcb.201007053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parnas H, et al. Depolarization initiates phasic acetylcholine release by relief of a tonic block imposed by presynaptic M2 muscarinic receptors. J Neurophysiol. 2005;93(6):3257–3269. doi: 10.1152/jn.01131.2004. [DOI] [PubMed] [Google Scholar]
- 10.Moreno-Galindo EG, et al. Relaxation gating of the acetylcholine-activated inward rectifier K+ current is mediated by intrinsic voltage sensitivity of the muscarinic receptor. J Physiol. 2011;589(Pt 7):1755–1767. doi: 10.1113/jphysiol.2010.204115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Chaim Y, et al. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444(7115):106–109. doi: 10.1038/nature05259. [DOI] [PubMed] [Google Scholar]
- 12.Navarro-Polanco RA, et al. Conformational changes in the M2 muscarinic receptor induced by membrane voltage and agonist binding. J Physiol. 2011;589(Pt 7):1741–1753. doi: 10.1113/jphysiol.2010.204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekel N, Priest MF, Parnas H, Parnas I, Bezanilla F. Depolarization induces a conformational change in the binding site region of the M2 muscarinic receptor. Proc Natl Acad Sci USA. 2012;109(1):285–290. doi: 10.1073/pnas.1119424109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilardaga J-P, Bünemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol. 2003;21(7):807–812. doi: 10.1038/nbt838. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen SGF, et al. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469(7329):175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warne T, et al. The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature. 2011;469(7329):241–244. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pohjanoksa K, et al. α2-adrenoceptor regulation of adenylyl cyclase in CHO cells: Dependence on receptor density, receptor subtype and current activity of adenylyl cyclase. Eur J Pharmacol. 1997;335(1):53–63. doi: 10.1016/s0014-2999(97)01154-0. [DOI] [PubMed] [Google Scholar]
- 18.Kukkonen JP, Renvaktar A, Shariatmadari R, Akerman KEO. Ligand- and subtype-selective coupling of human alpha-2 adrenoceptors to Ca++ elevation in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1998;287(2):667–671. [PubMed] [Google Scholar]
- 19.Bünemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci USA. 2003;100(26):16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bünemann M, Bücheler MM, Philipp M, Lohse MJ, Hein L. Activation and deactivation kinetics of α 2A- and α 2C-adrenergic receptor-activated G protein-activated inwardly rectifying K+ channel currents. J Biol Chem. 2001;276(50):47512–47517. doi: 10.1074/jbc.M108652200. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson SSG. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53(1):1–24. [PubMed] [Google Scholar]
- 22.Krasel C, Bünemann M, Lorenz K, Lohse MJ. β-arrestin binding to the β2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J Biol Chem. 2005;280(10):9528–9535. doi: 10.1074/jbc.M413078200. [DOI] [PubMed] [Google Scholar]
- 23.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32(9):521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008;29(8):421–429. doi: 10.1016/j.tips.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Hein P, Frank M, Hoffmann C, Lohse MJ, Bünemann M. Dynamics of receptor/G protein coupling in living cells. EMBO J. 2005;24(23):4106–4114. doi: 10.1038/sj.emboj.7600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Pinna J, Gurung IS, Mahaut-Smith MP, Morales A. Direct voltage control of endogenous lysophosphatidic acid G-protein-coupled receptors in Xenopus oocytes. J Physiol. 2010;588(Pt 10):1683–1693. doi: 10.1113/jphysiol.2009.183418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9(4):323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.