Abstract
Maintaining nitric oxide (NO) homeostasis is essential for normal plant physiological processes. However, very little is known about the mechanisms of NO modulation in plants. Here, we report a unique mechanism for the catabolism of NO based on the reaction with the plant hormone cytokinin. We screened for NO-insensitive mutants in Arabidopsis and isolated two allelic lines, cnu1-1 and 1–2 (continuous NO-unstressed 1), that were identified as the previously reported altered meristem program 1 (amp1) and as having elevated levels of cytokinins. A double mutant of cnu1-2 and nitric oxide overexpression 1 (nox1) reduced the severity of the phenotypes ascribed to excess NO levels as did treating the nox1 line with trans-zeatin, the predominant form of cytokinin in Arabidopsis. We further showed that peroxinitrite, an active NO derivative, can react with zeatin in vitro, which together with the results in vivo suggests that cytokinins suppress the action of NO most likely through direct interaction between them, leading to the reduction of endogenous NO levels. These results provide insights into NO signaling and regulation of its bioactivity in plants.
Keywords: flowering, nitration, cross-talk, phytohormone
Nitric oxide (NO) is one of the most widespread signaling molecules in living organisms (1, 2). In plants, NO is involved in the regulation of numerous physiological processes during growth and development and is also an important modulator of disease resistance (2–4). Several laboratories discovered that NO is produced not only from nitrate/nitrite but also from L-arginine (L-Arg), which is the main substrate for NO synthesis in animals (4–6). NO is also a widespread atmospheric pollutant. Therefore, this gas not only is a pivotal player in signal transduction but also has the potential to exert significant deleterious effects by being a pollutant. As an inevitable result, increased NO levels in the atmosphere can influence multiple NO-regulated processes in organisms. Despite the wealth of information gathered from analyses of NO functioning in plants, the molecular processes underlying NO effects in plants are still largely unknown.
NO differs from other signaling molecules by being reactive, lipophilic, and volatile. In fact, chemically, NO is a free radical, and such a reactive molecule is unlikely to interact specifically with a single specific receptor (3). In animals, NO appears to act through the chemical modification of targets. NO can bind to transition metals of metalloproteins (metal nitrosylation). It also can bind covalently to cysteine (S-nitrosylation) and tyrosine (tyrosine nitration) residues (3, 7, 8). Such specific protein modifications are emerging as key mechanistic intermediates for NO signal transduction. In plant cells, NO has also been found to regulate the activity of various target proteins through S- or metal-nitrosylation and probably through tyrosine nitration as well (9–13).
Furthermore, it has been shown that NO takes part in different phytohormone signaling pathways, frequently under the control of hormonal stimuli. For instance, NO functions in auxin-induced signaling pathways driving root growth and developmental processes, operating downstream of auxin through a linear signaling pathway (14). NO acts downstream of abscisic acid in controlling both induction of stomatal closure and inhibition of stomatal opening (6, 15, 16). In addition, NO is also involved in ethylene, gibberellin, salicylic acid, and jasmonic acid signaling (17–20).
Although several lines of evidence point to the involvement of NO in cytokinin signaling (21–24), no study has yet provided definitive proof of a role for NO in cytokinin signaling (25). To date, evidence for an interaction between NO and cytokinin remains rudimentary. In this study, NO-insensitive/hyposensitive screens for continuous NO-unstressed (cnu) mutants in Arabidopsis were performed, and we found that cnu1 was allelic to altered meristem program1 (amp1), a known mutant having high levels of the plant hormone cytokinin. Further studies show that high levels of cytokinin suppress the action of NO most likely through direct biochemical reactions, leading to the reduction of endogenous NO levels. These findings also imply that cytokinins have protective effects against nitrosative stress by acting as NO scavengers. We showed a unique mechanism of hormone–hormone interaction in plants by which two biomolecules combine to form a new product via a direct chemical reaction.
Results
cnu1 Mutant Is Less Sensitive to NO and Flowers Earlier Than WT.
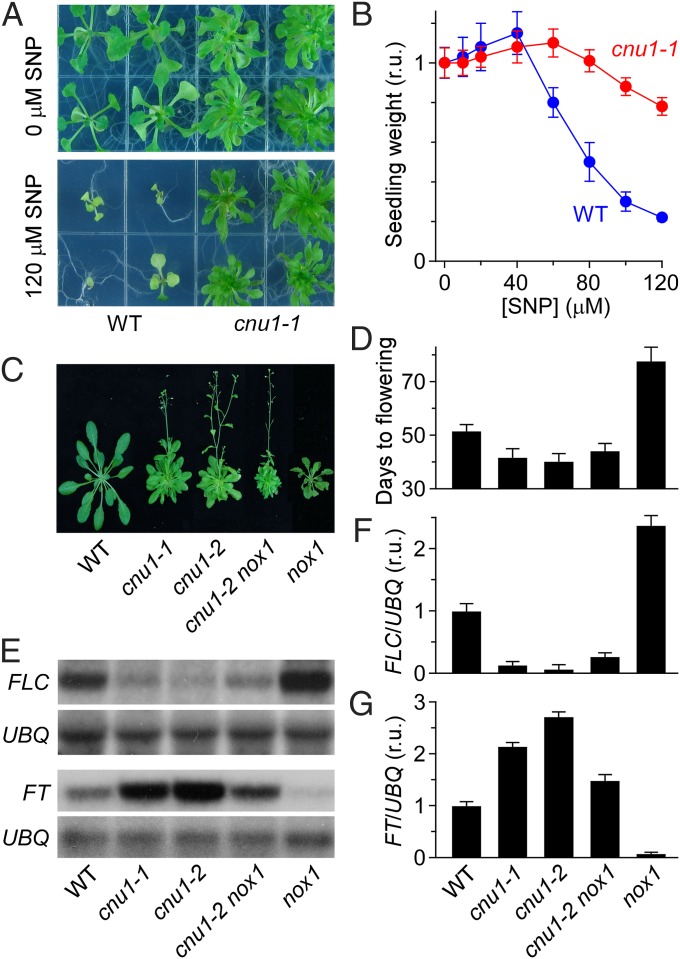
Treating Arabidopsis thaliana seedlings with an NO donor, sodium nitroprusside (SNP), promotes vegetative growth at low concentrations but inhibits growth at high concentrations (4). At 120 µM SNP, vegetative growth is strongly but not completely inhibited. We used this concentration in a screen for mutants with altered NO sensitivity to identify unique components in the NO response pathway. Two independent SNP-insensitive lines were isolated that proved to be allelic based on their failure to complement SNP insensitivity in the F1 progeny. We named these lines cnu (Fig. 1A; Fig. S1). The cnu1-1 mutant was recovered from T-DNA insertional mutagenesis, whereas cnu1-2 was recovered from Ethyl methane sulfonate (EMS) mutagenesis. The phenotypes of the two lines were essentially indistinguishable, and unless indicated specifically, cnu1 refers to cnu1-1. Further genetic analysis indicated that cnu1 harbors a single, recessive, nuclear mutation (Fig. S2C).
Fig. 1.
The cnu1 mutant is insensitive to NO and flowers early. (A) Effects of an NO donor SNP on plant growth and development. Arabidopsis seedlings were grown on petri dishes containing several concentrations of SNP during long days (16-h light/8-h dark) for 3 wk. It can be seen that cnu1 mutants have started flowering, and WT plants are still in the vegetative stage at 120 μM SNP. (B) The effect of SNP concentration on shoot growth. Fresh weight per seedling was from experiments as in A (mean ± SD; n = 150 seedlings). (C and D) The variation of endogenous NO and cytokinin levels affect the onset of flowering in Arabidopsis. The cnu1 mutant and cnu1-2 nox1 double mutant flowers early. Plants were grown on soil under 12-h light/12-h dark cycles and were photographed (C) after 50 d of growth. The days to flowering (D) from experiments as in C were scored (mean ± SD; n ≥ 30 plants). (E–G) The variation of endogenous NO and cytokinin levels affect the expression of genes that control the floral transition in Arabidopsis. The expression levels of FLC and FT, respectively, in WT, cnu1-1, cnu1-2, cnu1-2 nox1, and nox1 plants (E). Seedlings were grown on MS media under 16-h light/8-h dark cycles for 10–12 d. Leaves were collected 8 h after dawn for total RNA extraction. The FLC mRNA abundance was analyzed by using Northern blot and FT mRNA by RT-PCR. Ubiquitin mRNA (UBQ10) was used as a loading control. Quantification of the effects of endogenous NO and cytokinin on flowering repressor FLC (F) and flowering promoter FT (G) expression, respectively, showed that endogenous NO and cytokinins on flowering control gene expression have the opposite effect. The relative mRNA abundance was normalized to the UBQ levels. The relative mRNA abundance of WT was arbitrarily set to 1 (mean ± SEM; n = 3).
The insensitivity of cnu1 plants to 120 µM SNP, as used for screening, was remarkable. When seedlings were weighed after 3 wk, the dose–response curve confirmed that cnu1 was less responsive to SNP (Fig. 1B). Although the trend to less growth promotion at low SNP concentrations was not significant, the weaker growth inhibition seen in cnu1 at high concentrations of SNP was highly significant. For example, the ratio of seedling weight (cnu1/WT) increased as the SNP concentration increased (Fig. S1A). Additionally, inhibition of growth in cnu1 occurred at a higher concentration (80 µM SNP) than in the WT (50 µM). The cnu1 mutant was therefore less sensitive and less responsive to the NO donor.
Moreover, whether planted in the soil or grown under SNP treatment, cnu1 flowered substantially earlier than WT. Quantitative analyses of flowering time showed that cnu1 indeed flowered earlier under long day conditions (Fig. 1 C and D). Corresponding to these, RNA blot analysis showed that FLOWERING LOCUS C (FLC) expression was all but eliminated, and the FLOWERING TIME (FT) expression was increased in cnu1 (Fig. 1 E–G). Consistent with previous results, growing WT plants in the presence of SNP delayed flowering (4). This response was strongly suppressed in cnu1, both in terms of the days to flowering and in the up-regulation of FLC and the down-regulation of FT (Fig. S1 B–F). Together, these results showed that both seedling growth and flowering time in the cnu1 mutant are insensitive to NO.
CNU1 Is Identical to AMP1.
The CNU1 gene was cloned using a conventional positional cloning approach (Fig. S2A). Map-based cloning identified CNU1 as At3g54720, a gene previously characterized as AMP1 (26, 27). Sequencing analysis of the AMP1 genomic region from cnu1 revealed a G to A point mutation in cnu1-2, which is identical to the mutation in amp1-1. PCR analysis showed that the expected product from the AMP1 locus was detected in cnu1-2 and in WT, but not in cnu1-1, which harbors a T-DNA insertion between primers locating at each end of AMP1 gene (Fig. S2B). When cnu1-1 was crossed with amp1-1, none of the 214 F1 plants analyzed exhibited the WT phenotype, indicating a failure to complement (Fig. S2C). The morphological phenotype of cnu1 closely resembled that described for amp1, including polycotyly, faster rate of leaf initiation, and early flowering time (26, 27). Finally, the amp1 mutant responded poorly to SNP to the same extent as cnu1 did (Fig. S2D). Taken together, these results corroborated the identification of CNU1 as AMP1.
AMP1 encodes a putative glutamate carboxypeptidase, but its biological role remains unclear (26). In amp1, most likely, the enlarged meristem leads to an increase in the rate of cytokinin biosynthesis by some unknown mechanism. Thus far, several amp1 allelic mutants (amp1-1, pt/hpt, and cop2, etc.) have been isolated, and all show properties associated with an increased level of cytokinin biosynthesis. The increased cytokinin in these mutants is mainly zeatin class, which increased by about sevenfold (26–29). We found that the cnu1 mutant behaved similarly.
Zeatin Rescues the nox1 Phenotypes Resulting from High Levels of Endogenous NO.
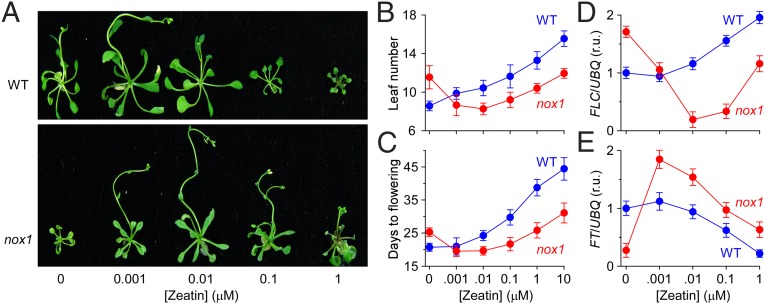
Is cnu1 less responsive to NO because of its elevated endogenous cytokinin levels? If so, then exogenous zeatin should rescue the delayed flowering phenotype of nox1, which results from the increased endogenous NO level (4). In fact, low concentrations (i.e., 0.001–1 μM) of zeatin promoted nox1 flowering significantly (Fig. 2 A–C). Consistently, the expression of flowering repressor FLC was reduced and the expression of flowering promoter FT was increased in a dose-dependent manner in response to added zeatin in nox1 (Fig. 2, D and E). Opposite responses were elicited in WT (Fig. 2), consistent with earlier reports (30). Indeed, we found that only 1 nM zeatin showed a slight effect of promoting flowering in WT. With zeatin levels >10 nM, the responses of nox1 and WT were generally parallel, but with a weaker response for nox1 at any given zeatin dose. Amelioration of the nox1 delayed flowering phenotypes by zeatin is consistent with the idea that elevated cytokinin levels suppress responsiveness to NO.
Fig. 2.
Exogenous zeatin rescues the late-flowering phenotype in nox1 resulting from elevated NO. (A) The cytokinin zeatin rescues the late-flowering phenotype in nox1. WT and nox1 seedlings were grown on MS media containing several concentrations of trans-zeatin under 16-h light/8-h dark cycles and were photographed after 25 d of growth. (B and C) Quantification of flowering time measured as the days to flowering (C) and the number of rosette leaves (B) (mean ± SD; n = 180 seedlings) from plants grown as in A. (D and E) Quantification of the FLC (D) and the FT (E) expression in response to zeatin treatments, respectively, using the methods as described in Fig. 1. Seedlings were grown on media containing several concentrations of trans-zeatin under long days for 10–12 d. The relative mRNA abundance was normalized to the UBQ levels. The relative mRNA abundance of WT at 0 μM zeatin was arbitrarily set to 1 (mean ± SEM; n = 3).
Zeatin was also able to rescue the seedling growth phenotype of nox1 (Fig. S3 A and B). Three-week-old nox1 seedlings were about one-half the weight of WT seedlings, whereas nox1 plants grown on 1 or 10 µM zeatin had about the same weight as untreated WT plants. This result is notable because the cytokinin decreased growth in WT. Almost 1,000 times more zeatin is required to suppress the vegetative growth of nox1 than that of WT. These results are again consistent with a meaningful interaction between cytokinin and NO. In addition, reticulate leaf is one of the typical phenotypes seen in nox1 (4) and cue1 (31) mutants. Treatment with a high concentration of SNP (120 μM) makes reticulate leaves appear in WT and also leads to a decreased chlorophyll content (4). Interestingly, when nox1 was treated with zeatin, its reticular veins gradually disappeared as the zeatin concentration increased, traits tending to be more WT-like (Fig. S3C). When the zeatin concentration reached 1 μM and above, reticular veins could no longer be seen in nox1. Consistent with this, nox1 chlorophyll content also increased as the concentration of zeatin increased (Fig. S3D).
Together, it appears that exogenous zeatin can rescue almost all of the phenotypes of nox1 caused by elevated levels of endogenous NO in a dose-dependent manner. It is likely that cytokinin antagonizes NO either through functional antagonism or by reducing endogenous NO levels.
cnu1 nox1 Double Mutant Has a Low Endogenous Level of NO and Flowers Early.
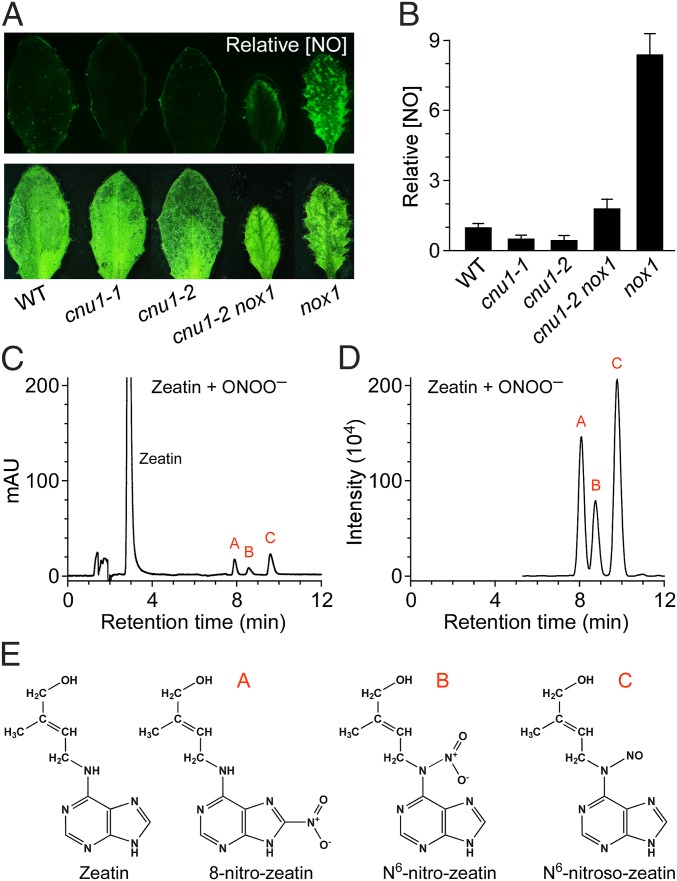
To further explore the relationship between elevated cytokinin levels and diminished responsiveness to NO observed in cnu1, we quantified NO levels using the NO-sensitive dye, 4,5-diaminofluorescein diacetate (DAF-2DA) (Fig. 3 A and B). As previously reported (4), the NO level in nox1 was nearly 10 times higher than that of WT. Interestingly, it was found that levels of NO in cnu1-1 and cnu1-2 were lower than that of WT, and the NO level in the cnu1 nox1 double mutant was only slightly elevated compared with that of WT. Compared with nox1, the content of NO in the cnu1-2 nox1 double mutant decreased significantly: about 30% that in nox1. These results demonstrate that elevated levels of cytokinin exert a prominent negative effect on the levels of NO, an effect that might explain the diminished responsiveness to NO in cnu1/amp1.
Fig. 3.
Excessive NO nitrates the adenine group of cytokinins and leads to the reduction of endogenous NO levels. (A) The endogenous NO levels in WT, cnu1-1, cnu1-2, cnu1-2 nox1, and nox1. Leaves were stained with DAF-2DA (Upper). (Lower) White-light images are shown. A total of 50 leaves were analyzed for each genotype in three independent experiments, and similar results were obtained. (B) Quantification of the relative NO levels for various genotypes grown in A. The relative NO level of WT was arbitrarily set to 1 (mean ± SD; n = 50). (C and D) HPLC-UV and HPLC-MS/MS profile of the reaction products of peroxynitrite with trans-zeatin in test tube at pH 9.5. The reaction solution of trans-zeatin and peroxynitrite (Zeatin + ONOO–) was run through HPLC and monitored by UV absorption (C) or MS/MS detection (D). Similar results were seen from more than 10 independent experiments. (E) The chemical structures of zeatin and the identified reaction products. Peaks A, B, and C in HPLC-UV (C) and HPLC-MS/MS (D) profile correspond to 8-nitro-trans-zeatin, N6-nitro-trans-zeatin, and N6-nitroso-trans-zeatin, respectively.
In addition, the cnu1-2 nox1 double mutants flowered much early and had lower expression of FLC and higher expression of FT than nox1 (Fig. 1 C–G), implying that CNU1 acts downstream of the production of NO.
These results strongly suggested that the elevated levels of endogenous cytokinins made the content of NO decrease by an unknown mechanism, thus alleviating the inhibitory effect of NO in cnu1-2 nox1. It is possible that cytokinin alters NO levels directly.
Peroxynitrite and Zeatin React Chemically In Vitro.
In cells, NO is short lived because it reacts rapidly/readily with the free radical, superoxide (O2−), to form the reactive molecule peroxynitrite (ONOO−), a powerful oxidant that mediates numerous cellular injuries (7, 32). Compared with NO, ONOO− is longer lived, but it does react with a variety of molecules, including adenine, guanine, and xanthine nucleosides (33, 34). Natural cytokinins are all adenine derivatives and have the molecular structure needed to react directly with ONOO−.
We first studied the reaction of zeatin and ONOO− in vitro. An equimolar (0.2 mM) mixture of trans-zeatin and ONOO− was combined at a range of pH from 4.3 to 10.5. The resulting products were then analyzed by reverse-phase (RP)-HPLC with detection using either UV absorption or mass spectrometry. In addition to unreacted zeatin, three peaks were detected (Fig. 3 C and D). The source of peroxynitrite, synthesized either from nitrite and H2O2 (35) or from isoamyl nitrite and H2O2 (36), did not affect the formation of these peaks. However, the peaks were absent when trans-zeatin was mixed with previously decomposed peroxynitrite or when zeatin was mixed with sodium nitrite and H2O2 either alone or in combination. Similar results were obtained over a pH range from 4 to 12.
The products that were formed by a reaction of zeatin with peroxynitrite were designated as A, B, and C according to the order of elution. At neutral pH, products A, B, and C had absorbance maxima at 360, 280, and 300 nm, respectively. Product A, but not products B and C, underwent a hypsochromic shift with decreasing pH and a bathchromic shift with increasing pH (Fig. S4), which has been reported to be a characteristic of 8-nitroguanine and 8-nitroxanthine (33, 35). This observation implies nitrate addition on the purine ring of product A. The three products were studied further by collision-induced dissociation of the protonated molecular ions, which showed the same m/z 265 (M+H)+, 529 (2M+H)+ for peak A and peak B and m/z 249 (M+H)+, 497 (2M+H)+ for peak C (Figs. S5–S7). From this, the molecular formulas of A and B were determined to be C10H12O3N6, and of C to be C10H12O2N6.
The structures of the three products were further characterized by 1H and 13C NMR, including 2D NMR 1H-1H Correlation Spectroscopy (COSY) and Heteronuclear Multiple Quantum Correlation (HMQC) experiments, mainly on the basis of a comparison with zeatin (Table S1). The 13C NMR spectrum of A showed pronounced downfield shifts in the resonances of two of the carbons: C-5 and C-8. The C-8 shift was 20 ppm downfield, consistent with enhanced electron withdrawal. In a long-range effect, the shift of C-5 in 8-nitroxanthine was about 4 ppm downfield, along with the disappearance of the C8-H around 8.1 ppm, indicating that the C8-H of zeatin was substituted with a nitro group. Furthermore, the absence of carbon-proton correlation of C8 and C8-H supported the substitution position of a nitro group at C8. The 1H NMR spectrum of B and C showed 0.8 ppm downfield shifts in the resonances of the same H10, along with the downfield shifts of the C10 of 12 ppm for B and 1 ppm for C, which indicate that the N6-H of zeatin was substituted with a nitro group (B) or a nitroso group (C) (Table S1). The HMQC spectra of B and C supported the substitution position at N6.
In conclusion, we identified compound A as 8-nitro-trans-zeatin, compound B as N6-nitro-trans-zeatin, and compound C as N6-nitroso-trans-zeatin (Fig. 3E). In addition, we also detected the formation of three reaction products at low (50 μM) concentrations of trans-zeatin and ONOO− and found that the ONOO− concentrations were the yield-limiting factor of this reaction (Fig. S8 A–D). Similarly, N6-isopentenyl adenine (iP), a kind of cytokinin, could also react chemically with peroxynitrite in vitro and form three products: 8-nitro-isopentenyl adenine, N6-nitro-isopentenyl adenine, and N6-nitroso-isopentenyl adenine, respectively.
NO and Cytokinin Interact Directly In Vivo.
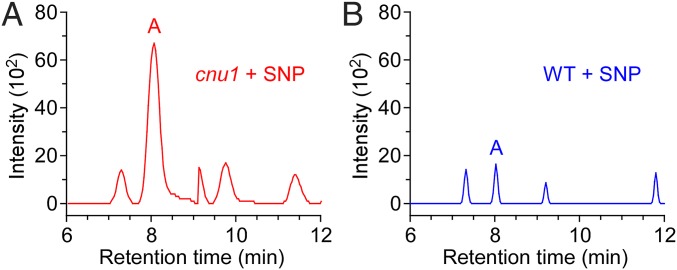
We determined whether a direct interaction between ONOO− and zeatin exists in vivo. An extract from Arabidopsis seedlings treated with 100 µM SNP was analyzed by HPLC-MS/MS (Fig. 4 A and B). A peak with a retention time matching product A was found in SNP-treated cnu1 and WT, which gave an identical mass spectrum as 8-nitro-trans-zeatin, confirming that ONOO− can react with zeatin in vivo. Noteworthy, the abundance of this compound was much higher in SNP-treated cnu1. These results support our hypothesis that the weakened effectiveness of NO in cnu1/amp1 is caused by a direct reaction between cytokinin and ONOO−. This reaction leads to the reduction of endogenous NO level. Only when the endogenous cytokinin level was inadequate to react with NO, was the inhibitory effect of NO exhibited in cnu1. Note that, considering the effect of pH on the trans-zeatin and ONOO− reaction products in vitro (Fig. S8E), theoretically, products B and C cannot be detected in the neutral to slightly acidic environment found in the plant cytosol. Clearly, 8-nitro-trans-zeatin is not the only product resulted from the SNP treatment, considering there was a peak between the retention time 9–10 min in the cnu1 mutant that was absent in WT (Fig. 4A).
Fig. 4.
Peroxynitrite and cytokinin can interact directly in vivo. HPLC-MS/MS profile of the extract from the cnu1 (A) and WT (B) seedlings treated with 100 μM SNP. Arabidopsis seedlings were grown on petri dishes containing 100 μM SNP for 2 wk. Peak A corresponds to the main product A (8-nitro-trans-zeatin). The intensity of peak A from SNP-treated WT (WT + SNP) was much lower than that from SNP-treated cnu1 (cnu1 + SNP).
Cytokinin-Like Activity of the Derivatized Zeatins.
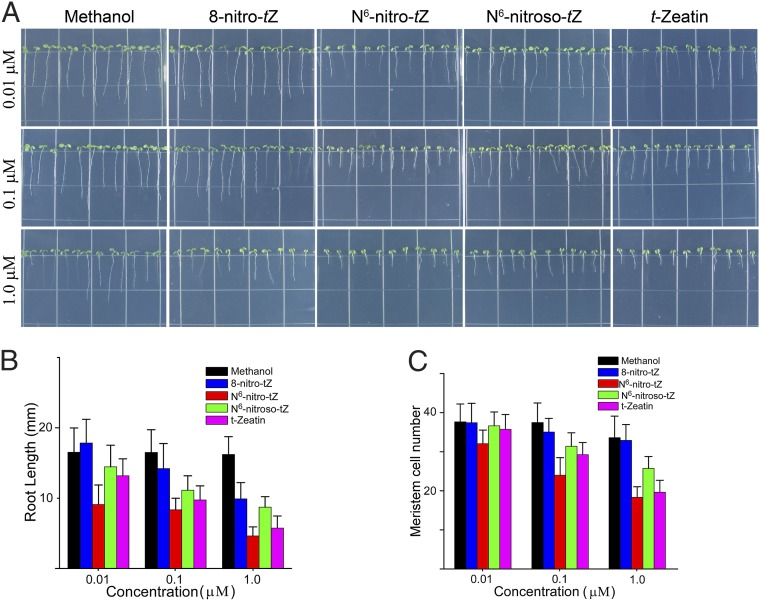
Our explanation for the diminished responsiveness of cnu1 to NO is that cytokinins essentially act like a sponge, catabolizing peroxinitrite and thereby decreasing its ambient level within the cell. However, the derivatization of zeatin will also reduce the levels of cytokinin, and therefore, the phenotype of cnu1/amp1 treated with NO might also depend on the lowering of cytokinin levels. To test this, we assayed the effects of the three derivatized zeatins on root elongation and meristem cell number, both processes known to be inhibited by cytokinins (30, 37). Interestingly, although the N6-nitro- and the N6-nitroso-zeatin were approximately as effective as zeatin itself, the 8-nitro-zeatin was less effective. At 0.01 μM, 8-nitro-trans-zeatin showed almost no inhibitory effect (Fig. 5). Insofar as 8-nitro-trans-zeatin is predicted to be the dominant product in the acidic to neutral plant cell environment (Fig. S8E) and was in fact the major product detected (Fig. 4), these results suggest that treatment of plants with NO might bring about a net decrease in cytokinin activity and also suggest that the formation of 8-nitro-trans-zeatin can contribute to alleviating the inhibition of growth and development by NO in Arabidopsis.
Fig. 5.
Cytokinin-like activity of products A, B, and C. (A) Root phenotypes of seedlings grown under long days for 1 wk on the indicated treatments. WT seeds were grown on MS media each containing several concentrations of 8-nitro-trans-zeatin, N6-nitro-trans-zeatin, N6-nitroso-trans-zeatin, trans-zeatin, and methanol, respectively. All compounds were dissolved in methanol, which is shown at the same concentration as a control. (B) The root length from experiments as in A was analyzed with Image J software (mean ± SD; n = 160 seedlings). (C) Effects of these three products on the root-meristem cell number. Root-meristem size was expressed as the number of cortex cells in a file extending from the quiescent center (QC) to the first elongated cell. The meristem sizes of roots treated with these three products were analyzed using the number of cortex cells (mean ± SD; n = 160 seedlings).
Discussion
The interactions between NO and other phytohormones are critical to plant growth, development, and environmental responsiveness. In general, hormone–hormone interactions occur at a biosynthetic level, a signaling level, or via a common action on a specific process (38). Herein, we integrated biological and chemical analysis to show that NO can interact directly with cytokinins, most likely through NO nitrating cytokinins, further modulating each other’s homeostatic levels and bioactivity. This interaction is a unique mechanism of NO–hormone interaction in plants by which two bioactive molecules combine to form new products via a chemical reaction.
During the last few years, the accumulated knowledge on NO and cytokinin physiological effects and response pathways have been adding pieces to the puzzle of the relationship between NO and cytokinin. The synergistic effects of NO and cytokinin in the regulation of photomorphogenesis (2), growth (4), and senescence (2) as opposed to their antagonistic effects in the control of seed germination (30, 39), flowering (4, 40, 41), and stomatal closure (42, 43) in Arabidopsis illustrate some of the complexities of the NO–cytokinin interaction. Multilevel or multitype interactions may be involved in the cross-talk between NO and cytokinin. Previously, it was reported that the exogenous application of cytokinins to tobacco, parsley, maize, and Arabidopsis cell cultures leads to a rapid stimulation of NO release, suggesting a positive correlation between cytokinin and NO production (22–24). However, conversely, our data show that elevated cytokinin endogenously not only failed to induce NO production but also decreased the content of NO in vivo (Fig. 3 A and B). In addition, Wilhelmova et al. (44) also found a negative correlation between cytokinin levels and NO production using transgenic tobacco plants with genetically increased or decreased levels of cytokinins. Clearly, the observations from the experiments applying exogenous NO or cytokinins are inconsistent with the results obtained using mutants or transgenic plants with altered NO or cytokinin levels.
Exogenously applied NO or cytokinin may not faithfully replicate the function of endogenous NO or cytokinin and may have side effects in plants. Furthermore, the application of a high concentration of cytokinins may result in stresses (inhibiting/damaging effects), and most all abiotic and biotic stresses induce the production of NO (3). Therefore, it is difficult to determine whether the production of NO is induced by cytokinin itself or by a cytokinin-induced stresses. The reported concentrations of cytokinins that can induce NO biosynthesis have been shown to be rather high and themselves might cause cell damage, whereas a highly effective (but not damaging) cytokinin concentration [4 μM 6-benzylaminopurine (BA)] did not induce NO release from suspension-cultured Arabidopsis cells (22). Further evidence from the work of She and Song (43) showed that added cytokinins [≤0.6 μM 6-BA or Kinetin (KT)] not only reduced NO levels in guard cells caused by SNP treatment in the light but also eliminated NO that had been generated by dark, and then promoted the closed stomata reopening, just as was seen with the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO). Thus, the genetic-based approach, i.e., analysis of mutants with altered endogenous NO or cytokinin levels, is likely to be more accurate for dissecting the nature of the interactions between NO and cytokinin in Arabidopsis.
Even more importantly, while dissecting the nature of interaction between NO and cytokinin, we should also give special attention to the unique chemical properties of NO and its derivatives, especially their strong oxidant activities.
In this study, by dissecting the effects of high levels of cytokinins on the NO insensitivity in cnu1, we provided several lines of evidence to demonstrate that cytokinins down-regulate endogenous NO levels by reacting directly with ONOO− in Arabidopsis, which also explains the underlying mechanism of the NO insensitivity in the cnu1 mutant. First, we observed that exogenously applied zeatin rescued almost all of the phenotypes of nox1, including the delayed flowering phenotype, caused by elevated levels of endogenous NO (Fig. 2; Fig. S3). Second, the cnu1 nox1 double mutant displayed a significantly decreased level of endogenous NO and flowered early, implying that cytokinins function as a negative regulator of NO (Figs. 1 C–G and 3 A and B). Third, chemical data showed that ONOO− was able to react with trans-zeatin to generate three products across a pH range from 4.3 to 10.5 (Fig. 3; Figs. S5–S8E). In the normal plant cell environment (pH 5.5–7.5), 8-nitro-trans-zeatin was the dominant product and had almost no biological activity (Fig. S8E; Fig. 5). Finally, 8-nitro-trans-zeatin was detected in the tissue extract from SNP-treated Arabidopsis plants, and its content in SNP-treated cnu1 was much higher than that in SNP-treated WT plants (Fig. 4). The evidence for a direct interaction between ONOO− and zeatin in vivo also exists. It is important to point out that the product formed by the reaction of zeatin with ONOO− in vivo is much less than the trace amount of zeatin; thus far, it is almost impossible to purify sufficient products from the extract for analysis, so the identity of the product can only be determined by detecting the peaks of HPLC-MS/MS. The data presented here indicate that the type of interaction between NO and cytokinins should be the effect of one of the substances on the level of another. It is achieved through chemical combination of two bimolecules, rather than cytokinin regulation of NO synthesis. However, it should be noted that the direct reaction between NO and cytokinins in plant cells might occur only in a limited range because the NO content in cnu1-2 nox1 was not reduced completely to the WT level (Fig. 3 A and B).
The fact that NO and cytokinins can react chemically also implies that cytokinins can modulate NO homeostasis and have protective effects against reactive nitrogen species in plant cells. It has been described that NO possesses both cytotoxic and cyto-protecting/stimulating dual properties, which depends on the concentration and the local biochemical microenvironment in plant cells (45). The wide variety of sources of NO and its possible effects suggest the need for detoxification mechanisms to modulate its level and functioning. An increasing number of reports have implicated nonsymbiotic hemoglobins as the key enzymatic system for NO scavenging in plants (9). Based on the results of this study, it appears that plants possess more than one way to attenuate high NO stress. In addition to hemoglobin, cytokinins are also involved, at least to some extent, in modulation of NO levels in plants. This finding provides an efficient mechanism for a rapid removal of toxic levels of NO. The existence of a variety of NO modulation reactions indicates that specific NO detoxification mechanisms may be involved in the fine control of the level and functions of NO under specific plant conditions. Interestingly, previous studies have also reported that cytokinins can have protective effects against the damage caused by reactive oxygen species (46, 47). Accordingly, we believe that, as a biomolecule with antioxidant properties, cytokinins reacting directly with reactive nitrogen species to scavenge excessive NO is reasonable.
Taken together, we revealed a unique mechanism for NO–hormone interaction in plants whereby excess NO nitrates the adenine group of cytokinins to decrease its own endogenous level, implying that cytokinins have protective effects against nitrosative stress by scavenging NO. These findings provide insights into NO signaling and homeostasis in plants.
Materials and Methods
Plant materials and growth conditions, isolation and positional identification of cnu mutants, analysis of flowering time and mRNA abundance, detection of endogenous NO levels, chemical details about the purification and characterization of products formed by the reaction of trans-zeatin and peroxynitrite, and other procedures are fully described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Jim Siedow (Duke University) and Tobias I. Baskin (University of Massachusetts) for critical discussions and reading of the manuscript. This work was supported by Ministry of Science and Technology of China Grants 2013CB967300 and 2007CB948200 and grants from the Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality [PHR(IHLB)] (to Y.-K.H.), grants from Monsanto (to Z.-M.P.), and National Natural Science Foundation of China (NSFC) Grant 31171167 (to W.-Z.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213235110/-/DCSupplemental.
References
- 1.Schmidt HH, Walter U. NO at work. Cell. 1994;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 2.Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 3.Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signaling in plants. Annu Rev Plant Biol. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- 4.He Y, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305(5692):1968–1971. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- 5.Asai S, Ohta K, Yoshioka H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell. 2008;20(5):1390–1406. doi: 10.1105/tpc.107.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozano-Juste J, León J. Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis. Plant Physiol. 2010;152(2):891–903. doi: 10.1104/pp.109.148023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106(6):675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 8.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 9.Perazzolli M, et al. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell. 2004;16(10):2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feechan A, et al. A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci USA. 2005;102(22):8054–8059. doi: 10.1073/pnas.0501456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindermayr C, Durner J. S-Nitrosylation in plants: Pattern and function. J Proteomics. 2009;73(1):1–9. doi: 10.1016/j.jprot.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Corpas FJ, del Río LA, Barroso JB. Need of biomarkers of nitrosative stress in plants. Trends Plant Sci. 2007;12(10):436–438. doi: 10.1016/j.tplants.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Tada Y, et al. Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science. 2008;321(5891):952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen WW, et al. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 2010;154(2):810–819. doi: 10.1104/pp.110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45(1):113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 16.Freschi L, et al. Nitric oxide mediates the hormonal control of Crassulacean acid metabolism expression in young pineapple plants. Plant Physiol. 2010;152(4):1971–1985. doi: 10.1104/pp.109.151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gniazdowska A, Dobrzyńska U, Babańczyk T, Bogatek R. Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta. 2007;225(4):1051–1057. doi: 10.1007/s00425-006-0384-z. [DOI] [PubMed] [Google Scholar]
- 18.Zottini M, et al. Salicylic acid activates nitric oxide synthesis in Arabidopsis. J Exp Bot. 2007;58(6):1397–1405. doi: 10.1093/jxb/erm001. [DOI] [PubMed] [Google Scholar]
- 19.Xu MJ, Dong JF, Zhu MY. Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol. 2005;139(2):991–998. doi: 10.1104/pp.105.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethke PC, et al. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143(3):1173–1188. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherer GFE, Holk A. NO donors mimic and NO inhibitors inhibit cytokinin action in betalaine accumulation in Amaranthus caudatus. Plant Growth Regul. 2000;32:345–350. [Google Scholar]
- 22.Carimi F, et al. NO signalling in cytokinin-induced programmed cell death. Plant Cell Environ. 2005;28:1171–1178. [Google Scholar]
- 23.Tun NN, Holk A, Scherer GF. Rapid increase of NO release in plant cell cultures induced by cytokinin. FEBS Lett. 2001;509(2):174–176. doi: 10.1016/s0014-5793(01)03164-7. [DOI] [PubMed] [Google Scholar]
- 24.Tun NN, Livaja M, Kieber JJ, Scherer GF. Zeatin-induced nitric oxide (NO) biosynthesis in Arabidopsis thaliana mutants of NO biosynthesis and of two-component signaling genes. New Phytol. 2008;178(3):515–531. doi: 10.1111/j.1469-8137.2008.02383.x. [DOI] [PubMed] [Google Scholar]
- 25.Romanov GA, Lomin SN, Rakova NY, Heyl A, Schmülling T. Does NO play a role in cytokinin signal transduction? FEBS Lett. 2008;582(6):874–880. doi: 10.1016/j.febslet.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Helliwell CA, et al. The Arabidopsis AMP1 gene encodes a putative glutamate carboxypeptidase. Plant Cell. 2001;13(9):2115–2125. doi: 10.1105/TPC.010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhury AM, Letham S, Craig S, Dennis ES. amp1: A mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 1993;4:907–916. [Google Scholar]
- 28.Saibo NJ, et al. A comparative analysis of the Arabidopsis mutant amp1-1 and a novel weak amp1 allele reveals new functions of the AMP1 protein. Planta. 2007;225(4):831–842. doi: 10.1007/s00425-006-0395-9. [DOI] [PubMed] [Google Scholar]
- 29.Nogué F, Hocart C, Letham DS, Dennis ES, Chaudhury AM. Cytokinin biosynthesis is higher in the Arabidopsis amp1 mutant. Plant Growth Regul. 2000;32:267–273. [Google Scholar]
- 30.Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18(1):40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Culligan K, Dixon RA, Chory J. CUE1: A mesophyll cell-specific positive regulator of light-controlled gene expression in Arabidopsis. Plant Cell. 1995;7(10):1599–1610. doi: 10.1105/tpc.7.10.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem. 2000;275(42):32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- 33.Sodum RS, Fiala ES. Analysis of peroxynitrite reactions with guanine, xanthine, and adenine nucleosides by high-pressure liquid chromatography with electrochemical detection: C8-nitration and -oxidation. Chem Res Toxicol. 2001;14(4):438–450. doi: 10.1021/tx000189s. [DOI] [PubMed] [Google Scholar]
- 34.Jena NR, Mishra PC. Formation of 8-nitroguanine and 8-oxoguanine due to reactions of peroxynitrite with guanine. J Comput Chem. 2007;28(8):1321–1335. doi: 10.1002/jcc.20607. [DOI] [PubMed] [Google Scholar]
- 35.Yermilov V, et al. Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16(9):2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 36.Uppu RM. Synthesis of peroxynitrite using isoamyl nitrite and hydrogen peroxide in a homogeneous solvent system. Anal Biochem. 2006;354(2):165–168. doi: 10.1016/j.ab.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Dello Ioio R, et al. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr Biol. 2007;17(8):678–682. doi: 10.1016/j.cub.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 38.Teale WD, et al. Auxin as a model for the integration of hormonal signal processing and transduction. Mol Plant. 2008;1(2):229–237. doi: 10.1093/mp/ssn006. [DOI] [PubMed] [Google Scholar]
- 39.Bethke PC, Libourel IG, Jones RL. Nitric oxide reduces seed dormancy in Arabidopsis. J Exp Bot. 2006;57(3):517–526. doi: 10.1093/jxb/erj060. [DOI] [PubMed] [Google Scholar]
- 40.Werner T, et al. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15(11):2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura C, et al. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004;16(6):1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.García-Mata C, Lamattina L. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 2001;126(3):1196–1204. doi: 10.1104/pp.126.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.She XP, Song XG. Cytokinin- and auxin-induced stomatal opening is related to the change of nitric oxide levels in guard cells in broad bean. Physiol Plant. 2006;128:569–579. [Google Scholar]
- 44.Wilhelmová N, et al. The effect of plant cytokinin hormones on the production of ethylene, nitric oxide, and protein nitrotyrosine in ageing tobacco leaves. Biofactors. 2006;27(1-4):203–211. doi: 10.1002/biof.5520270118. [DOI] [PubMed] [Google Scholar]
- 45.Perazzolli M, Romero-Puertas MC, Delledonne M. Modulation of nitric oxide bioactivity by plant haemoglobins. J Exp Bot. 2006;57(3):479–488. doi: 10.1093/jxb/erj051. [DOI] [PubMed] [Google Scholar]
- 46.Gidrol X, Lin WS, Dégousée N, Yip SF, Kush A. Accumulation of reactive oxygen species and oxidation of cytokinin in germinating soybean seeds. Eur J Biochem. 1994;224(1):21–28. doi: 10.1111/j.1432-1033.1994.tb19990.x. [DOI] [PubMed] [Google Scholar]
- 47.Causin HF, et al. Changes in hydrogen peroxide homeostasis and cytokinin levels contribute to the regulation of shade-induced senescence in wheat leaves. Plant Sci. 2009;177(6):698–704. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.