Abstract
C4 photosynthesis is a series of anatomical and biochemical modifications to the typical C3 pathway that increases the productivity of plants in warm, sunny, and dry conditions. Despite its complexity, it evolved more than 62 times independently in flowering plants. However, C4 origins are absent from most plant lineages and clustered in others, suggesting that some characteristics increase C4 evolvability in certain phylogenetic groups. The C4 trait has evolved 22–24 times in grasses, and all origins occurred within the PACMAD clade, whereas the similarly sized BEP clade contains only C3 taxa. Here, multiple foliar anatomy traits of 157 species from both BEP and PACMAD clades are quantified and analyzed in a phylogenetic framework. Statistical modeling indicates that C4 evolvability strongly increases when the proportion of vascular bundle sheath (BS) tissue is higher than 15%, which results from a combination of short distance between BS and large BS cells. A reduction in the distance between BS occurred before the split of the BEP and PACMAD clades, but a decrease in BS cell size later occurred in BEP taxa. Therefore, when environmental changes promoted C4 evolution, suitable anatomy was present only in members of the PACMAD clade, explaining the clustering of C4 origins in this lineage. These results show that key alterations of foliar anatomy occurring in a C3 context and preceding the emergence of the C4 syndrome by millions of years facilitated the repeated evolution of one of the most successful physiological innovations in angiosperm history.
Keywords: precursor, preadaptation, phylogeny, Poaceae
The evolvability of some traits can influence the capacity of organisms to adapt to environmental changes, but the factors dictating differences in evolvability among groups are often unknown. Climate changes during the Cenozoic included a marked decline of atmospheric CO2 levels around 30 million years ago (1, 2), which lowered the efficiency of photosynthesis in plants (3). Several lineages of flowering plants adapted to the resulting CO2 depletion, particularly in warm, open, and/or dry conditions, by evolving mechanisms that concentrate CO2 around chloroplasts, where the Calvin cycle uses CO2 and energy to produce sugars (4, 5). In the so-called C4 photosynthetic pathway, this increase in internal CO2 concentration results from a spatial segregation of carbon assimilation and reduction (3). The vast majority of C4 plants use a dual-cell system, where the assimilation of CO2 into organic compounds occurs in mesophyll (M) cells of the leaf, whereas carbon reduction by the Calvin cycle, which occurred in the M cells in their C3 ancestors, happens in the vascular bundle sheath (BS).
The C4 pathway requires both a specialized foliar anatomy and the addition of a complex biochemical pathway that assimilates and delivers carbon to the Calvin cycle (3). Despite this complexity, it evolved more than 62 times independently in distantly related groups of flowering plants (5). These recurrent C4 origins were probably facilitated by the existence in most C3 plants of metabolic modules that are suitable for the C4 pathway and can be recruited for this function through relatively few mutations (6, 7). In addition, the photorespiratory pump based on glycine decarboxylase is a likely evolutionary stable intermediate phenotype on the road from C3 to C4 (3, 8). However, C4 origins are not randomly distributed across the plant tree of life; although most large (species rich) clades of plants completely lack C4 taxa, others contain an astonishingly high number of C3-to-C4 transitions (5). This is particularly the case with grasses, in which C4 photosynthesis evolved independently 22–24 times (9). Most grass species belong to either the BEP or PACMAD clade, which contain approximately the same number of species (ca. 5,423 and 5,706, respectively); however, the repeated C4 origins occurred only in the PACMAD clade (9).
The homoplastic tendency of some groups, manifested in the extreme clustering of C4 origins, suggests that several plant lineages possess traits that increase C4 evolvability (10, 11). Such enablers may include ecological factors, as well as genomic and anatomical properties (10). For optimal functioning of the C4 pathway, foliar anatomy must be altered to increase the relative proportion of BS cells and reduce the average path length between M and BS cells (12–15). These modifications guarantee an efficient centripetal increase of CO2 concentration, but they also imply that C4 evolution involved many mutations, unless the C3 ancestors already possessed some C4-like anatomical characters. Putative C4 preadaptations have been detected in C3 taxa closely related to C4 species in small groups of eudicots with a limited number of C4 origins (16–19). Recently, a preliminary survey of the proportion of BS tissue and the interveinal distance (IVD) in different C3 and C4 grasses suggested that a larger proportion of BS tissue characterizes the C3 PACMAD, which are closer to C4 values (20). The same study concluded that short IVD evolved later and was not an important determinant of C4 evolvability (20). In this work, the different traits that, together, determine the amount of BS tissue or the IVD (e.g., number and size of different types of cells) were not measured separately. The species sampling did not include species outside the BEP and PACMAD clades, preventing any inference about the directionality of trait evolution. In addition, the analyses did not use phylogenetic comparative methods, precluding a formal statistical evaluation of the link between foliar characteristics and C4 evolvability.
In the present work, we measured anatomical traits in 157 grasses representing the BEP and PACMAD clades and several outgroups, as well as the diversity of photosynthetic types known within the group. A phylogenetic framework was used to test statistically for the presence of C4 precursors and to locate them on the phylogeny, and the evolution of foliar traits was modeled in a phylogenetic context to (i) reconstruct the leaf modifications that occurred during the transition from C3 to C4 photosynthesis and (ii) identify the leaf traits that might explain the propensity of some grass lineages to evolve C4 photosynthesis.
Results
Phylogenetic Patterns.
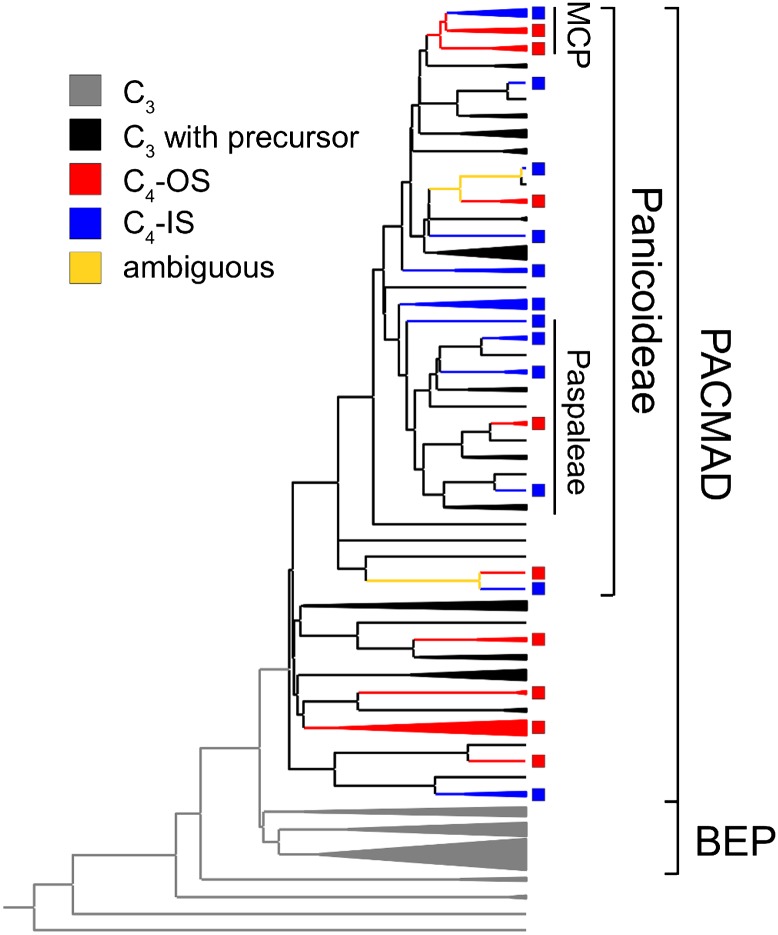
A total of 172 cross-sections were measured, representing 157 species. Six of these represent outgroup taxa (either grasses sister to the BEP-PACMAD clade or members of families affiliated with grasses), 37 C3 BEP, 62 C3 PACMAD, and 52 C4 PACMAD. The two BS layers that characterize all C3 and many C4 grasses [outer sheath (OS) and inner sheath (IS)] were measured separately (Fig. S1). Of the C4 taxa, 26 use the OS for carbon reduction (C4-OS) and 26 use the IS (C4-IS). Only five of the C4-IS species also possessed an OS layer. A total of 17 C4 lineages were sampled out of 22–24 described previously (9). This included five C4-OS lineages, nine C4-IS, and three that contained both C4-IS and C4-OS (Fig. 1). The five C4 lineages not sampled in this study all belong to Paspaleae and are species-poor groups, putatively using a C4-IS pathway (21, 22).
Fig. 1.
Sampled species and phylogenetic distribution of photosynthetic types. This time calibrated phylogeny was used in comparative analyses. The C4-IS and C4-OS groups are indicated by colored squares. Putative ancestral photosynthetic types are indicated as colors on branches. The evolution of a precursor character was identified with the precursor_2 model as implemented in r8s. The main groups are delimited on the right.
The model of C4 evolution that assumed the existence of precursors (precursor_2) was significantly better than the binary model [Akaike information criterion (AIC), 135.5; AIC difference of 4.7] but only slightly better than the model with equal rates of transition from C3 to precursor and from precursor to C4 (precursor_1; difference of AIC, 0.4). In the precursor_2 model, the rate of C3-to-precursor transition was estimated to be 0.003, and the rate of precursor-to-C4 transition was estimated to be 0.016, which suggests that some characteristics evolved a small number of times and favored the subsequent repeated origins of C4 photosynthesis (Fig. 1). A single origin of the precursor was inferred along the branch leading to the PACMAD clade (probability of precursor at the stem, 0.05; at the crown, 1.0) and was followed by multiple transitions to C4.
Description of Variables.
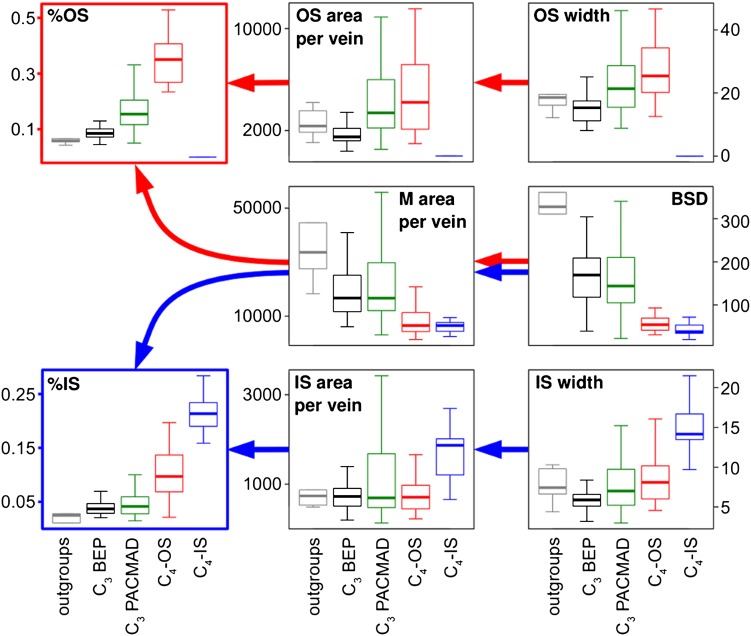
The individual anatomical trait values all overlapped between C3 and C4 taxa, but C4-OS had higher proportions of OS tissue [%OS = OS area/(OS area + M area)] and C4-IS higher proportions of IS tissue [%IS = IS area/(IS area + M area)] (Fig. 2). The larger %IS and %OS values result mainly from a decreased amount of M tissue per vein in both C4-OS and C4-IS compared with C3 taxa, which is the consequence of a reduction in BS distance (BSD). The area values of OS tissue per vein and the width of OS cells largely overlapped between C4-OS species and C3 taxa. By contrast, the area of IS tissue per vein tends to be larger in C4-IS than in C4-OS and C3 taxa and this is attributable mainly to an increase of the width of IS cells in C4-IS. The other variables are not consistently different between C3 and C4 taxa (Fig. S2).
Fig. 2.
Distribution of trait values among clades and photosynthetic types. The distribution of eight variables is summarized by boxplots individually for the outgroups (gray; n = 6), C3 BEP (black; n = 37), C3 PACMAD (green; n = 62), C4-OS (red; n = 26), and C4-IS (blue; n = 26). Boxes indicate the 25th and 75th percentiles, and whiskers indicate the extreme values still within 1.5 interquartile range of the upper and lower quartiles. Arrows indicate inferred effect, with the changes linked to C4-OS evolution in red and the changes linked to C4-IS evolution in blue.
The C3 BEP and C3 PACMAD do not differ in amount of M tissue per vein, which is reduced compared with the outgroups (Fig. 2). However, C3 PACMAD have greater areas of OS tissue per vein compared with C3 BEP, which is attributable to larger OS cells. For these two traits, C3 PACMAD do not differ from C4-OS species. This results in %OS values in C3 PACMAD that are intermediate between C3 BEP and C4-OS (Fig. 2). The C3 BEP show smaller IS and OS sizes and, consequently, lower areas of OS and IS per vein compared with the outgroups (Fig. 2).
Comparative Analyses.
The evolutionary dynamics of several characters were best explained by a stabilizing selection [Ornstein–Uhlenbeck (OU)] model with a single optimum when considering C3 taxa only (Table 1). However, M area per vein and BSD evolved around different optima (OU models) in the outgroups compared with the BEP-PACMAD clade (Table 1). Similarly, the optima of OS area per vein, OS width and IS width were reduced in BEP compared with both the C3 PACMAD and outgroups, and the optima of %OS and leaf thickness were increased in C3 PACMAD relative to both the BEP clade and the outgroups, whereas the optimum of the ratio of thicknesses was decreased (Table 1). Within PACMAD, the mean vein area was best modeled by an OU model with a single optimum (Table 1). The optima of M area per vein and leaf thickness were decreased in C4 (both C4-IS and C4-OS) compared with C3 PACMAD. C4-IS had a lower optimum for OS per vein and OS width but a higher optimum for IS per vein and IS width. Finally, %IS, %OS, and BSD were best modeled with one optimum per photosynthetic type (Table 1).
Table 1.
Modeling of evolution among taxonomic groups and photosynthetic types
| Trait | C3 only |
PACMAD only (C3 and C4) |
||||
| Model* | Groups† | Optima | Model* | Groups† | Optima | |
| %OS | OU2 | P [out+B] | 0.18 [0.09] | OU3 | C3 OS IS | 0.18/0.40/−0.01 |
| %IS | OU | — | 0.05 | OU3 | C3 OS IS | 0.05/0.10/0.22 |
| M per vein | OU2 | Out [B+P] | 38,260 [17,371] | OU2 | C3 [OS+IS] | 18,331 [6,698] |
| BSD | OU2 | Out [B+P] | 326 [133] | OU3 | C3 OS IS | 140/57/42 |
| OS per vein | OU2 | B [out+P] | 1,586 [3,534] | OU2 | IS [C3+OS] | −1 [4,774] |
| OS width | OU2 | B [out+P] | 14.1 [21.6] | OU2 | IS [C3+OS] | −0.8 [24.2] |
| IS per vein | OU | — | 852 | OU2 | IS [C3+OS] | 3,080 [943] |
| IS width | OU2 | B [out+P] | 6.0 [7.6] | OU2 | IS [C3+OS] | 19.4 [7.9] |
| Mean vein area | OU | — | 1,050 | OU | — | 1,049 |
| Leaf thickness | OU2 | P [out+B] | 153 [111] | OU2 | C3 [OS+IS] | 146 [114] |
| Ratio of thicknesses | OU2 | P [out+B] | 0.44 [0.55] | OU2 | OS [C3+IS] | 0.27 [0.44] |
B, BEP; C3, C3 PACMAD; IS, C4-IS; OS, C4-OS; OU2, OU with different optima for two groups; OU3, OU with different optima for three groups; out, outgroups; P, PACMAD.
*Best model determined by likelihood ratio tests and Akaike information criteria.
†Groups with significantly different optima. Dashes indicate that a single optimum is favored.
Variation in the amount of M tissue per vein is explained by a combination of changes in BSD, thickness, ratio of thicknesses, and mean area of veins (R2 = 0.94), with BSD and thickness alone explaining most of the variation (R2 = 0.92). Changes in OS area are driven by changes in the width of OS cells, thickness, mean area of veins, and BSD (R2 = 0.95), with OS cell width and thickness explaining most of the variation (R2 = 0.94). Finally, modifications of IS area are attributable to a combination of changes in IS cell width, thickness and mean vein area (R2 = 0.96), with most of the variance explained by IS cell width and vein area (R2 = 0.96).
Ancestral Reconstructions.
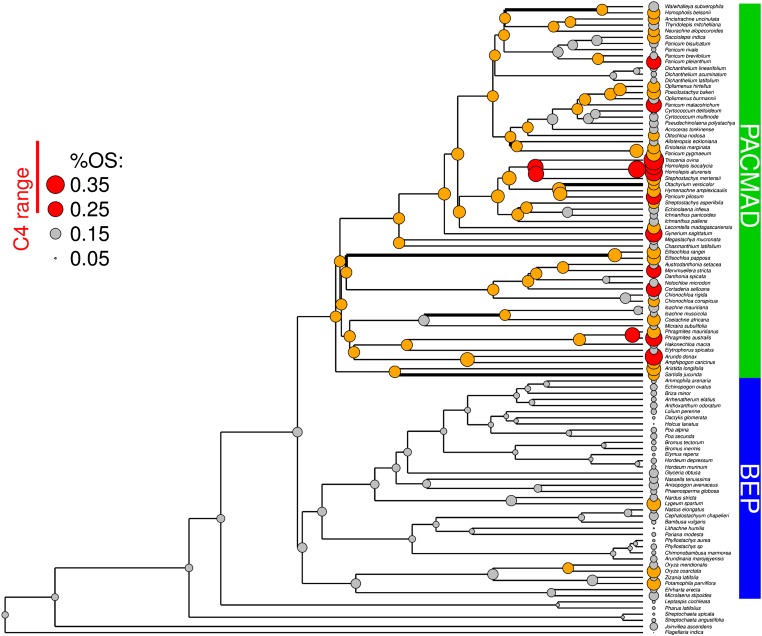
The ancestral reconstructions indicate that a higher %OS evolved at the base of the PACMAD clade, as hypothesized previously (20), and was maintained with further increases in multiple clades that led to the emergence of C4-like %OS in several parts of the phylogeny (Fig. 3). This increase of %OS was attributable to an increase of OS cell size, which is especially large in several PACMAD lineages, whereas in the BEP clade, the Bambusoideae and Pooideae lineages are generally characterized by very small OS cells (Fig. S3). By contrast, %IS shows smaller differences between BEP and C3 PACMAD lineages, and C4-like values evolved only a small number of times: three in the PACMAD and one in the BEP (Fig. S4). However, larger IS cells are more frequent in PACMAD; in BEP, they occurred only in one species (Lygeum spartum; Fig. S5). Small BSD values characterize the whole BEP-PACMAD clade, but large values appeared repeatedly within both BEP and PACMAD (Fig. S6).
Fig. 3.
Evolution of %OS in grasses. The measured and inferred values of %OS are mapped on a calibrated phylogeny of the C3 grasses included in this study. The diameter of the dots is proportional to %OS values. The values in the C4 range are indicated in red and those outside the C4 range but above the threshold that promotes C4-OS evolution are in orange. Branches where the C3 to C4-OS transitions occurred are thicker.
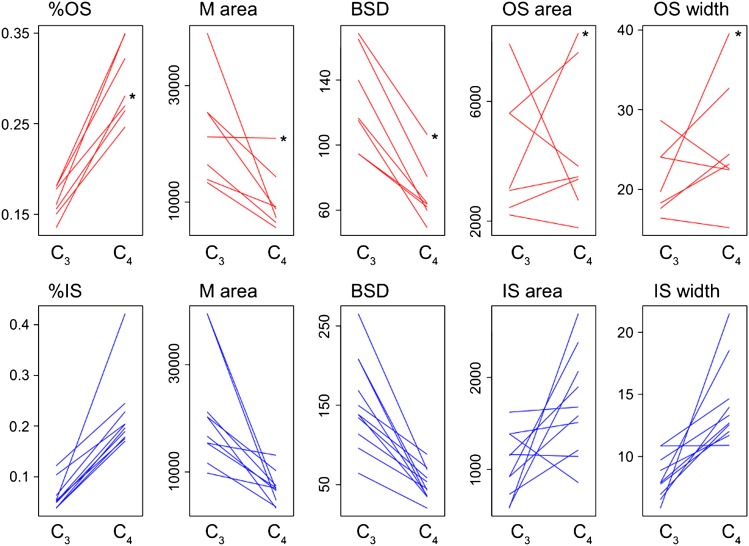
The inferred transitions between C3 and C4-OS involved on average a 1.8-fold increase of %OS (Fig. 4). This was attributable to a strong decrease in the amount of M per vein (except in Alloteropsis), which was driven by a strong reduction in BSD. The size of OS cells and, consequently, the amount of OS tissue per vein were not significantly increased, except in the transition that gave rise to the C4-OS Alloteropsis. The evolution of a C4-IS type from C3 ancestors required more dramatic changes, with, on average, a 4.0-fold increase in %IS. This resulted from a large reduction of BSD, which strongly reduced the amount of M per vein and, in most cases, an increase of IS width and consequently IS area per vein.
Fig. 4.
Changes of anatomical traits inferred during C4 evolution. The inferred change in five important traits is indicated for all C3 to C4-OS and all C3 to C4-IS (in blue) transitions. Each line connects the values of the most recent C3 ancestor (on the left) and the first C4 descendant (on the right). The asterisks indicate changes that led to the Alloteropsis cimicina lineage.
Modeling of Anatomical Enablers.
The model that assumed an increase of the probability of being sister to a C4-OS lineage above a given %OS ratio was significantly better than the null model (χ2 = 12.8; df = 1; P < 0.001). The optimal threshold for a shift of transition rates was 0.15, which appeared at the base of the PACMAD clade and in some BEP (Fig. 3). Above this value, the rate of transition to C4-OS was 0.010. The evolvability of C4-OS was also adequately explained by the size of OS cells alone (χ2 = 5.3; df = 1; P = 0.021), with an OS width above 15.7 leading to a transition rate of 0.005. Finally, BSD also explained the evolvability of C4-OS (χ2 = 6.4; df = 1; P = 0.012), with a BSD below 196 leading to a rate of transition of 0.006. Similarly, the evolvability of C4-IS was explained by %IS, IS width, and BSD (χ2 = 5.1, χ2 = 5.9, and χ2 = 4.1, respectively; df = 1; P = 0.022, P = 0.015, and P = 0.042, respectively), with a %IS above 0.003, an IS width above 6.2, and a BSD below 233 leading to a rate of transition of 0.005.
Discussion
Anatomical Factors Increase C4 Evolvability in PACMAD.
Variation in the evolvability of the C4-OS type among clades of Poaceae and affiliated taxa is well explained by differences in %OS. Higher %OS in C3 plants apparently facilitates the evolution of C4 photosynthesis by reducing the distance between the C3 and C4 phenotypes but also potentially by giving access to transitional stages with a selective advantage over the C3 condition. The exact evolutionary path between C3 photosynthesis and the C4-associated biochemistry is not resolved with confidence, but the photorespiratory pump based on glycine decarboxylase is often seen as an intermediate stage between C3 and C4 photosynthesis (3, 8, 18). Abundant OS tissue is instrumental in allowing its evolution because it implies that a decrease of glycine decarboxylase in M cells will not be detrimental, as enough of the enzyme will still be expressed in OS cells (3). Once a photorespiratory pump is fixed by natural selection, the biochemical components of the C4 pathway can be gradually acquired and optimized until a highly efficient C4 trait emerges. All enzymes of the C4 pathway already exist in C3 plants, and C4 compatible promoter sequences are widespread in angiosperms (6, 7). The evolution of the C4 trait can consequently require relatively few mutations in plants with a suitable leaf anatomy, which, in grasses, largely includes members of the PACMAD clade (Fig. 3).
The %OS is itself a complex trait, which results from variation in the number and size of cells of different tissues. The main determinants of %OS are BSD and OS width, and both partially explain the evolvability of C4-OS. A small BSD evolved at the base of the BEP-PACMAD clade, and despite important variation in both clades, it still characterizes most extant members of these groups (Fig. S6). This decrease of BSD occurred through a reduction of the number of M cells between OSs without a significant change in the size of these cells (Fig. S7). A small BSD is, however, not sufficient to confer large %OS, which also requires large OS cells. Enlarged OS cells are inferred for the deepest nodes in our phylogeny, which suggests that the ancestor of grasses possessed OS cells that were compatible with C4 anatomy (Fig. S3). A reduction of OS width occurred in most of the lineages of the BEP clade, with even the partial degradation of OS in several Pooideae. The independent changes in BSD and OS width led to the restriction of large %OS to PACMAD, contributing to the recurrent C4-OS origins in this clade millions of years later.
Transitions to C4 Anatomy Involved a Further Reduction of BSD.
Compared with C3 plants, C4-OS species are characterized by higher %OS, which can be achieved through a decrease of M area or an increase of OS area. A decrease of M area must involve a reduction in the number and/or size of M cells, whereas an increase of OS area must occur through an enlargement of OS cells. In plants, the majority of changes in organ size are attributable to mutations in the control of cell proliferation (23), which suggests that mutations affecting the number of cells are more frequent than those affecting their size. Congruently, in all C4-OS, a smaller BSD was achieved by a reduction in the average distance between veins present in the C3 ancestor through a reduction of the number of M cells between veins (Fig. S7). This indicates that the capacity to rapidly reduce BSD is instrumental in the evolution of a C4-suitable foliar anatomy. The relatively high lability of this trait guarantees a recurrent emergence of lower BSD and a fertile ground for natural selection. In contrast, the width of OS cell was not consistently affected during the transition from C3 to C4-OS, and the selective optima for this trait are not different in C3 and C4-OS taxa (Table 1). Cell-size variation among plant species is generally a function of genome size (24) and an increased size of a given type of cell is often achieved through the postmitotic duplication of the plant’s genome (25). Mutations drastically affecting OS width are, thus, probably infrequent, which limits the capacity of natural selection to fix larger OS cells.
Case of C4-IS.
The evolvability of the C4-IS type is increased in groups with enlarged IS cells and shorter BSD. Compared with C4-OS, the C4-IS phenotype is more distant from the C3 ancestral condition, and C4-like values of %IS are extremely rare in both BEP and PACMAD. The only C4-IS lineage outside Panicoideae (Aristida spp.) evolved from a group with a C4-like %IS (Fig S4), and the independent C4-IS lineages of Panicoideae all reduced their BSD through the addition of minor veins between the veins present in the C3 ancestor (Fig. S7) (21), mirroring the evolution of C4 anatomy in the Flaveria (Asteraceae) (26). These minor veins are composed almost exclusively of BS tissue and some species even evolved BS-like cells that punctuate the M without being associated with any vein (21, 27, 28). Their addition resulted in a very dramatic increase of %IS and likely strongly contributed to the recurrent C4 origins in Panicoideae. However, these additional veins per se did not generate the high %IS values observed in extant taxa, which were possible only through a further increase of IS width.
C4-IS evolution would have involved fewer changes if it happened from a C4-OS instead of a C3 ancestor. In some groups, the transition seems to have occurred directly from C3 to C4-IS [e.g., Neurachninae (29)], but a C3 to C4-OS transition followed by a change to C4-IS must have occurred at least once in the MCP clade, which contains a C4-IS group (Cenchrinae) nested within an otherwise C4-OS clade (Melinidinae and Panicinae; Fig. 1) and possibly also in Alloteropsis (11). In addition, the C3 Homolepis spp. have enlarged OS, filled with centripetal chloroplasts (Fig. S8). Whether these are involved in some kind of photorespiratory pump is unknown, but Homolepis is closely related to several C4-IS taxa, some of which still produce starch in OS cells (9, 21), which might suggest that this C3 group with a C4-OS like anatomy is similar to the ancestor that produced C4-IS descendants. The transition from C3 to C4-OS results in an increase of %IS because the decrease of BSD during C4-OS evolution reduces the M area per vein but also because the enlargement of OS cells is associated with increased IS cell size (phylogenetic linear model; R2 = 0.30), possibly because the two traits share some genetic determinism. Together with the capacity of Panicoideae to produce additional veins, a passage through C4-OS might consequently have strongly facilitated the evolution of the C4-IS type.
Conclusions
Identifying the determinants of relative evolvability among clades is key to understanding the drivers of biological diversification. Most flowering plants possess metabolic modules suitable for C4 photosynthesis, and yet C4 origins are tightly clustered only in a handful of lineages. Here, we provide comparative statistical evidence that C4 evolvability is determined, in large part, by leaf anatomy. Both OS and IS cell sizes determine the probability of transition to C4-OS and C4-IS, respectively, with an additional effect of BSD. Previous work has suggested that C4-like anatomy evolved in PACMAD through adaptation to specific environments (20), but our comparative analyses, instead, emphasize the importance of stochastic historical processes. A reduction of BSD happened at the base of the BEP-PACMAD clade, leading to the coexistence of shorter BSD and large IS and OS cells in the common ancestor of the BEP and PACMAD clades. The foliar anatomy of this common ancestor might, thus, have been compatible with C4 photosynthesis, but the atmospheric CO2 levels at this time were high, limiting the advantage of C4 photosynthesis. Subsequently, OS and IS cells became smaller in most BEP, possibly through selection, and this apparently suppressed the capacity of these plants to later evolve C4 photosynthesis. When atmospheric CO2 decreased tens of millions of years after the split of the BEP and PACMAD clades (2, 30–32), a combination of shorter BSD and large OS/IS cells existed only in members of the PACMAD clade, limiting C4 evolution to this lineage. Changes in the number and size of different cells during the early history of grasses might have had limited importance at the time but allowed photosynthetic diversification when environmental changes opened new ecological niches during the Oligocene, highlighting the importance of historical contingency in evolutionary and ecological adaptation.
Materials and Methods
Species were sampled to maximize the number of independent C4 origins and their closest C3 relatives, while maintaining a balanced phylogenetic sampling as established in previous work (9). The species were incorporated in a previously published dataset based on three plastid markers (9), and the phylogenetic relationships between the species sampled for anatomy were extracted. This phylogenetic tree was used for comparative analyses. It was also used to test for the existence of C4 precursors, following a recently developed approach (33), which compares models that assume the existence of an unidentified precursor (precursor_1 and precursor_2 models) to several binary models. Transitions between C3 and C4 photosynthesis are not allowed and can only occur between C3 photosynthesis and a precursor state and then between the precursor state and C4 photosynthesis (33).
Cross-sections of the middle part of mature leaves were photographed and used to measure several areas and lengths that were averaged over multiple veins (Fig. S1). C4 species were classified into those using the OS tissue (C4-OS) and those using the IS (C4-IS) for carbon reduction, based on the localization of starch production. The relative proportions of OS and IS tissues were calculated as %OS = OS area/(OS area + M area) and %IS = IS area/(IS area + M area), respectively. The best evolutionary model was determined for each anatomical variable, and the ancestral values of the anatomical traits were reconstructed on the phylogenetic tree. The effect of anatomical traits on C4-OS and C4-IS evolvability was tested with models that allow transitions only above (or below) a given value of the anatomical trait. These models were compared with the null models (transition rates independent from anatomical traits) through likelihood ratio tests. The methods are detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Rosa Cerros, Russell Hall, Surrey Jacobs, Simon Malcomber, and Steve Renvoize for their help in acquiring useful samples. This work was funded by Marie Curie International Outgoing Fellowship 252568 (to P.-A.C.), National Science Foundation (NSF) Division of Environmental Biology Grant 0920147 (to J.T.C.), Agence Nationale de la Recherche Grant 10LABX-41 (to G.B.), and NSF Division of Integrative Organismal Systems Grant 0843231 (to E.J.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.A.K. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. HF558456–HF558529) and Dataset S1, the phylogenetic matrix, and the phylogenetic tree have been deposited in the Dryad repository (http://dx.doi.org/10.5061/dryad.6j9r7).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216777110/-/DCSupplemental.
References
- 1.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292(5517):686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 2.Beerling DJ, Royer DL. Convergent Cenozoic CO2 history. Nat Geosci. 2011;4(7):418–420. [Google Scholar]
- 3.Sage RF, Sage TL, Kocacinar F. Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol. 2012;63:19–47. doi: 10.1146/annurev-arplant-042811-105511. [DOI] [PubMed] [Google Scholar]
- 4.Christin PA, Osborne CP, Sage RF, Arakaki M, Edwards EJ. C4 eudicots are not younger than C4 monocots. J Exp Bot. 2011;62(9):3171–3181. doi: 10.1093/jxb/err041. [DOI] [PubMed] [Google Scholar]
- 5.Sage RF, Christin PA, Edwards EJ. The C4 plant lineages of planet Earth. J Exp Bot. 2011;62(9):3155–3169. doi: 10.1093/jxb/err048. [DOI] [PubMed] [Google Scholar]
- 6.Hibberd JM, Quick WP. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature. 2002;415(6870):451–454. doi: 10.1038/415451a. [DOI] [PubMed] [Google Scholar]
- 7.Brown NJ, et al. Independent and parallel recruitment of preexisting mechanisms underlying C₄ photosynthesis. Science. 2011;331(6023):1436–1439. doi: 10.1126/science.1201248. [DOI] [PubMed] [Google Scholar]
- 8.Hylton CM, Rawsthorne S, Smith AM, Jones DA. Glycine decarboxylase is confined to the bundle-sheath cells of leaves of C3-C4 intermediate species. Planta. 1988;175(4):452–459. doi: 10.1007/BF00393064. [DOI] [PubMed] [Google Scholar]
- 9.Grass Phylogeny Working Group II New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol. 2012;193(2):304–312. doi: 10.1111/j.1469-8137.2011.03972.x. [DOI] [PubMed] [Google Scholar]
- 10.Sage RF. Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Biol. 2001;3(3):202–213. [Google Scholar]
- 11.Christin PA, Freckleton RP, Osborne CP. Can phylogenetics identify C4 origins and reversals? Trends Ecol Evol. 2010;25(7):403–409. doi: 10.1016/j.tree.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Hattersley PW. Characterization of C4 type leaf anatomy in grasses (Poaceae). Mesophyll: Bundle sheath area ratios. Ann Bot (Lond) 1984;53(2):163–179. [Google Scholar]
- 13.Dengler NG, Dengler RE, Donnelly PM, Hattersley PW. Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): Bundle sheath and mesophyll surface area relationships. Ann Bot (Lond) 1994;73(3):241–255. [Google Scholar]
- 14.Dengler NG, Nelson T. In: C4 Plant Biology. Sage RF, Monson RK, editors. San Diego: Academic; 1999. pp. 133–172. [Google Scholar]
- 15.Muhaidat R, Sage RF, Dengler NG. Diversity of Kranz anatomy and biochemistry in C4 eudicots. Am J Bot. 2007;94(3):362–381. doi: 10.3732/ajb.94.3.362. [DOI] [PubMed] [Google Scholar]
- 16.Marshall DM, et al. Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis. Plant J. 2007;51(5):886–896. doi: 10.1111/j.1365-313X.2007.03188.x. [DOI] [PubMed] [Google Scholar]
- 17.McKown AD, Dengler NG. Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae) Am J Bot. 2007;94(3):382–399. doi: 10.3732/ajb.94.3.382. [DOI] [PubMed] [Google Scholar]
- 18.Christin PA, et al. Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution. 2011;65(3):643–660. doi: 10.1111/j.1558-5646.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 19.Muhaidat R, Sage TL, Frohlich MW, Dengler NG, Sage RF. Characterization of C₃—C₄ intermediate species in the genus Heliotropium L. (Boraginaceae): Anatomy, ultrastructure and enzyme activity. Plant Cell Environ. 2011;34(10):1723–1736. doi: 10.1111/j.1365-3040.2011.02367.x. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths H, Weller G, Toy LF, Dennis RJ. You’re so vein: Bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant Cell Environ. 2012 doi: 10.1111/j.1365-3040.2012.02585.x. [DOI] [PubMed] [Google Scholar]
- 21.Renvoize SA. A survey of leaf-blade anatomy in grasses XI. Paniceae. Kew Bull. 1987;42(3):739–768. [Google Scholar]
- 22.Giussani LM, Cota-Sánchez JH, Zuloaga FO, Kellogg EA. A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. Am J Bot. 2001;88(11):1993–2012. [PubMed] [Google Scholar]
- 23.Powell AE, Lenhard M. Control of organ size in plants. Curr Biol. 2012;22(9):R360–R367. doi: 10.1016/j.cub.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Símová I, Herben T. Geometrical constraints in the scaling relationships between genome size, cell size and cell cycle length in herbaceous plants. Proc Biol Sci. 2012;279(1730):867–875. doi: 10.1098/rspb.2011.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugimoto-Shirasu K, Roberts K. “Big it up”: Endoreduplication and cell-size control in plants. Curr Opin Plant Biol. 2003;6(6):544–553. doi: 10.1016/j.pbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.McKown AD, Dengler NG. Shifts in leaf vein density through accelerated vein formation in C4 Flaveria (Asteraceae) Ann Bot (Lond) 2009;104(6):1085–1098. doi: 10.1093/aob/mcp210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renvoize SA. A survey of leaf-blade anatomy in grasses III. Garnotieae. Kew Bull. 1982;37(3):497–500. [Google Scholar]
- 28.Dengler NG, Donnelly PM, Dengler RE. Differentiation of bundle sheath, mesophyll, and distinctive cells in the C4 grass Arundinella hirta (Poaceae) Am J Bot. 1996;83(11):1391–1405. [Google Scholar]
- 29.Christin PA, et al. Multiple photosynthetic transitions, polyploidy, and lateral gene transfer in the grass subtribe Neurachninae. J Exp Bot. 2012;63(17):6297–6308. doi: 10.1093/jxb/ers282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagani M, Zachos JC, Freeman KH, Tipple B, Bohaty S. Marked decline in atmospheric carbon dioxide concentrations during the Paleogene. Science. 2005;309(5734):600–603. doi: 10.1126/science.1110063. [DOI] [PubMed] [Google Scholar]
- 31.Christin PA, et al. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Curr Biol. 2008;18(1):37–43. doi: 10.1016/j.cub.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 32.Vicentini A, Barber JC, Aliscioni SS, Giussani LM, Kellogg EA. The age of the grasses and clusters of origins of C4 photosynthesis. Glob Change Biol. 2008;14(12):2963–2977. [Google Scholar]
- 33.Marazzi B, et al. Locating evolutionary precursors on a phylogenetic tree. Evolution. 2012;66(12):3918–3930. doi: 10.1111/j.1558-5646.2012.01720.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.