Abstract
Peroxisomes are organelles that perform diverse metabolic functions in different organisms, but a common function is β-oxidation of a variety of long chain aliphatic, branched, and aromatic carboxylic acids. Import of substrates into peroxisomes for β-oxidation is mediated by ATP binding cassette (ABC) transporter proteins of subfamily D, which includes the human adrenoleukodystropy protein (ALDP) defective in X-linked adrenoleukodystrophy (X-ALD). Whether substrates are transported as CoA esters or free acids has been a matter of debate. Using COMATOSE (CTS), a plant representative of the ABCD family, we demonstrate that there is a functional and physical interaction between the ABC transporter and the peroxisomal long chain acyl-CoA synthetases (LACS)6 and -7. We expressed recombinant CTS in insect cells and showed that membranes from infected cells possess fatty acyl-CoA thioesterase activity, which is stimulated by ATP. A mutant, in which Serine 810 is replaced by asparagine (S810N) is defective in fatty acid degradation in vivo, retains ATPase activity but has strongly reduced thioesterase activity, providing strong evidence for the biological relevance of this activity. Thus, CTS, and most likely the other ABCD family members, represent rare examples of polytopic membrane proteins with an intrinsic additional enzymatic function that may regulate the entry of substrates into the β-oxidation pathway. The cleavage of CoA raises questions about the side of the membrane where this occurs and this is discussed in the context of the peroxisomal coenzyme A (CoA) budget.
The peroxisome is the sole site of β-oxidation of fatty acids and related molecules in plants and fungi and is essential in metabolism of very long chain fatty acids and bioactive lipid molecules in mammals. ATP binding cassette (ABC) proteins of subfamily D are required for the transport of these substrates across the peroxisome membrane (1). These are peroxisomal ABC transporter 1 and 2 (Pxa1p/Pxa2p) in yeast, adrenoleukodystrophy protein (ALDP/ABCD1), adrenoleukodystrophy related protein (ALDPR/ABCD2), and the 70 kDa peroxisome membrane protein (PMP70/ABCD3) in mammals, and Comatose (CTS; also known as PED3, PXA1, and AtABCD1) in plants. Defects in ALDP result in X-linked adrenoleukodystrophy, a neurological disorder in which very long chain fatty acids accumulate (2). Similarly, cts mutants are defective in germination and mobilization of stored triacylglycerol (3).
Activation by formation of a CoA thioester is a prerequisite for entry of substrates into β-oxidation (4) but whether ABCD proteins accept free fatty acids or acyl-CoAs has been contentious. Arabidopsis mutants lacking CTS accumulate acyl-CoAs (5) and the basal ATPase activity of the protein is stimulated by acyl-CoAs rather than free fatty acids (6). Studies with yeast cells and trypanosomes suggest that Pxa1p/Pxa2p and the trypanosome ABCD protein GAT1 transport acyl-CoAs (7, 8) and human ALDP, ALDR, and Arabidopsis CTS are all able to complement the Saccharomyces cerevisiae pxa1/pxa2Δ mutant (6, 9, 10). Conversely, in Arabidopsis, genetic evidence indicates that CTS and the peroxisomal long chain acyl-CoA synthetases (LACS)6 and -7 act in the same pathway, suggesting that free fatty acids are transported (11). Moreover, knockdown of the Arabidopsis peroxisomal adenine nucleotide translocators inhibits fatty acid degradation, consistent with a need for intraperoxisomal ATP for fatty acid activation (12).
To reconcile these differences, a model was proposed in which CTS transports fatty acyl-CoA but the CoA moiety is removed either by the transporter or by a peroxisomal thioesterase (11). Recently, complementation of the S. cerevisiae pxa1/pxa2Δ mutant by human ALDP was shown to be dependent upon the presence of the peroxisomal fatty acyl-CoA synthetase, Faa2p (13), indicating that CoA is cleaved during or directly after transport. CoA cleavage was demonstrated by isotopic labeling but the protein responsible for the acyl-CoA thioesterase activity was not identified (13). Peroxisomes of mammals, yeast, and plants contain acyl-CoA thioesterases, which catalyze the hydrolysis of fatty acyl-CoAs to free fatty acids and free coenzyme A (CoASH) but their physiological roles are not well characterized. It has been suggested that these enzymes are important for maintaining CoASH at optimal levels during periods of increased fatty acid oxidation (14).
In this study we demonstrate that CTS requires Faa2p or the targeting of the Arabidopsis synthetases LACS6 or LACS7 to yeast peroxisomes to support β-oxidation of oleate in S. cerevisiae. This functional interaction between transporter and synthetase is also reflected in a physical interaction in planta. We expressed CTS in insect cells and demonstrated that the protein has intrinsic thioesterase activity, which is stimulated by ATP and is essential for fatty acid degradation in yeast and in planta. This resolves the long standing debate about the transport substrate for ABCD transporters and provides a rare example of a transporter that also possesses an intrinsic accessory enzyme activity.
Results
Peroxisomal Acyl-CoA Synthetase Is Essential for CTS-Dependent Fatty Acid β-Oxidation.
Expression of CTS in the S. cerevisiae pxa1/pxa2Δ mutant permitted this strain to metabolize oleate (C18:1) at ∼90% of the wild-type level (Fig. 1A) (6). A mutation, S810N, which compromises CTS-dependent fatty acid β-oxidation in plants (15, 16), also resulted in an inability to support oleate β-oxidation in yeast (Fig. 1A), despite the protein being correctly targeted to peroxisomes (Fig. S1). Expression of wild-type CTS in the pxa1/pxa2/faa2Δ triple mutant resulted in only 12% of the wild-type activity (Fig. 1B), in marked contrast to the β-oxidation activity of cells where CTS was expressed in the pxa1/pxa2Δ background (Fig. 1A), showing dependence of CTS on Faa2p.
Fig. 1.

Peroxisomal acyl-CoA synthetase is required for CTS-dependent transport. The Saccharomyces cerevisiae pxa1/pxa2∆ and pxa1/pxa2/faa2∆ mutants were transformed as indicated. (A and B) Yeast cells grown on oleate medium were incubated with [1-14C] oleic acid (C18:1) and β-oxidation rates were measured. Genotypes are shown to the Left of the graphs. The fox1∆ mutant lacks acyl-CoA oxidase (the first enzyme of β-oxidation) and serves as a negative control. The activity in wild-type cells was taken as a reference (100%) and values are means ± SD of three independent experiments. (C–E) 17,000 × g pellets of oleate-grown pxa1/pxa2/faa2∆ cells expressing LACS6 (C), LACS7 (D), or mistargeted LACS7 (LACS7-PTS; E) were subjected to Nycodenz equilibrium density gradient centrifugation and C8:0-CoA synthetase activity was measured in the different fractions; 3-Hydroxy-CoA dehydrogenase (3-HAD) activity was used as a peroxisomal marker. Activity in each fraction is expressed as a percentage of the total activity measured throughout the gradient.
In Arabidopsis two peroxisomal acyl-CoA synthetases with a broad substrate range are involved in β-oxidation of fatty acids (11, 17). When either of these synthetases was coexpressed with CTS in the triple mutant background, β-oxidation activity was restored to ∼40% (LACS6) and 80% (LACS7) of the wild-type level. Expression of either synthetase in the absence of CTS gave a much smaller rescue of oleate β-oxidation showing that the effect of CTS plus LACS is additive (Fig. 1B). This reflects the ability of yeast to import free fatty acids via an ABCD-independent route that requires Faa2p and that accounts for the residual growth of the pxa1/pxa2Δ mutant on oleate (Fig. 1A) (9, 18).
Complementation was dependent on the correct peroxisomal targeting of the synthetase. The localization and function of LACS6 and -7 were assessed by measuring octanoyl-CoA (C8:0) synthetase activity in different subcellular fractions, because this activity is absent from the cytosol of yeast (18). In pxa1/pxa2/faa2Δ cells transformed with LACS6 or -7, C8:0-CoA synthetase activity was detectable in cell lysates, organelle pellets, and purified peroxisomes but was absent from cells transformed with vector lacking insert (Fig. 1 C–E and Fig. S2 A and B). Removal of the PTS1 and -2 peroxisome targeting signals from LACS7 (LACS7-PTS) resulted in loss of peroxisomal C8:0-CoA synthetase activity and inability to restore β-oxidation of oleate (Fig. 1 B and E). The peak peroxisomal fraction (no. 3) from the gradients shown in Fig. 1 C and D was assayed for C18:1-CoA synthetase activity. This revealed that LACS7 has a much higher ratio of C18:1- to C8:0-CoA synthetase activity (Fig. S2C) as also reported previously (17), thereby explaining the more efficient rescue of oleate β-oxidation by LACS7 compared with LACS6 (Fig. 1B).
CTS Is Present in a Complex with LACS6/7 in Planta.
These results indicate a functional interaction between CTS and the peroxisomal acyl-CoA synthetases LACS6, LACS7, and Faa2p; therefore, evidence for a physical interaction was sought. Membranes were prepared from Arabidopsis thaliana suspension culture cells, solubilized with β-dodecylmaltoside (β-DDM), separated on a blue native gel followed by a denaturing (SDS/PAGE) second dimension and then immunoblotted with an antibody against CTS. The transporter molecular weight (MW) (150 kDa) was found predominantly in a complex of approximately 700 kDa (Fig. 2A). The experiment was repeated using membranes from seedlings that express a CTS–GFP fusion from the native CTS promoter. This construct is functional, as judged by complementation of the cts-1 mutant (Fig. S3A). On a 2D gel, a similar complex of approximately 700 kDa was observed when probed with anti-GFP antibody, although the apparent size of the denatured CTS–GFP species was approximately 170 kDa, as expected from the addition of GFP (28 kDa) (Fig. S3B). The complex is quite stable, because it was also detected in the presence of Triton X-100 (Fig. S3C). When this complex was probed with both anti-CTS and anti-LACS6/7 antibodies (19), two pools of LACS immunoreactivity were seen (Fig. S3C). The most intense migrated at around 150 kDa, the expected size for the dimeric LACS proteins, but a significant immunoreactivity was also detectable in the higher molecular weight fractions between 700 and 1,100 kDa that also contained CTS (Fig. S3C).
Fig. 2.

CTS forms a complex with peroxisomal acyl-CoA synthetase in planta. Membrane proteins from Arabidopsis thaliana suspension cultured cells were solubilized with β-dodecyl maltoside. (A) Solubilized membrane proteins were subjected to blue-native PAGE (in first dimension) followed by denaturing SDS/PAGE and immunoblotting for CTS. (B) Solubilized membrane proteins were separated in a sucrose step gradient. Fractions were collected from the top of the gradient and subjected to immunoblotting with a range of antibodies as indicated. T, total membranes. Positions of molecular weight markers (sizes in kilodaltons) are shown above (first dimension or sucrose gradient) and to the Left (second dimension) of A and B. (C) Solubilized membrane proteins were subjected to immunoprecipitation using anti-CTS beads, separated by SDS/PAGE, and immunoblotted with anti-LACS and anti-CTS antibody. Lane 2, immunoprecipitate; lane 1, mock immunoprecipitation without protein extract; lane 3, immunoblot of protein extract probed with anti-LACS and anti-CTS antibody, to show positions of LACS and CTS. Figures to the Left indicate positions of molecular weight markers (sizes in kilodaltons).
Detergent-solubilized membranes were fractionated on a sucrose gradient followed by SDS/PAGE and immunoblotting. CTS sedimented in a broad peak with maximum intensity in fractions 6–9, corresponding to complexes >700 kDa (Fig. 2B). The same fractions were probed with antibodies against LACS6/7 and the β-oxidation enzymes acyl-CoA oxidase 1 (ACX1), multifunctional protein 2 (MFP2), and 3-ketoacyl-CoA thiolase (KAT2). MFP2 and KAT2 sedimented in the lightest fractions of the gradient, and showed no comigration with CTS, as would be expected for soluble proteins. However, both LACS and ACX1 showed a distinct minor peak in fraction 8, which also contained CTS.
Whereas comigration of LACS proteins with CTS is suggestive of their being part of the same complex, this does not provide evidence for a physical interaction between the two. Therefore, solubilized membranes were immunoprecipitated with anti-CTS antibodies, and the immunoprecipitates probed with anti-LACS6/7 and anti-CTS antibodies (Fig. 2C). Because the antibody chains from the immunoprecipitation (IP) react with the secondary antibody in the blot, a mock IP was carried out without extract but with anti-CTS antibodies (lane 1) and processed alongside the extract containing sample (lane 2). Bands present in lane 2 but not lane 1 represent proteins specifically immunopreciptated by the anti-CTS antibody. The approximately 70-kDa band that is absent from control lane 1 but is present in lane 2 comigrates with native LACS as detected by immunoblotting of the extract without prior IP (Fig. 2C, lane 3).
CTS Exhibits Intrinsic Thioesterase Activity.
CTS was expressed in insect cells to investigate its biochemical properties. Expression of the active, full-length protein was demonstrated by the presence in membrane preparations of immunoreactive protein of MW approximately 150 kDa and detection of ATPase activity that was much higher than uninfected controls (Fig. 3 A and F), and was inhibited by AlFx as previously described (6). Insect cell membranes containing CTS exhibited thioesterase activity toward 18:0-CoA, which was absent from membranes prepared from uninfected cells (Fig. 3B). ATP stimulated C18:0-CoA thioesterase activity more than twofold. C14:0-CoA thioesterase exhibited about 60% of the activity of C18:0-CoA (Fig. 3B). In the presence of ATP, acyl-CoA thioesterase activity exhibited Michaelis–Menten behavior; the Km for C18:0 CoA was 1.05 ± 0.16 μM, Vmax 38.15 ± 7.27 nmol⋅mg CTS−1⋅s−1, and quantification of CTS levels by immunoblotting permitted estimation of kcat at 5.7 s−1 (Fig. 3C).
Fig. 3.

Recombinant CTS has fatty acyl-CoA thioesterase activity. Membranes were isolated from sf9 cells expressing wild-type and mutant forms of CTS. (A) ATPase activity in membranes from control (noninfected) and CTS-expressing cells, in the presence and absence of AlFx. (B) Acyl-CoA thioesterase activity of membranes expressing wild-type CTS with different acyl-CoA substrates (20 μM) in the presence and absence of ATP. Activity was absent from membranes from control (noninfected) cells. (C) The 18:0-CoA thioesterase activity as a function of substrate concentration (in the presence of ATP). Values represent means ± SD (n = 4). (D) Relative 18:0-CoA thioesterase activities of wild-type CTS and S810N mutant in the presence and absence of ATP. (E) Relative ATPase activities of CTS and S810N mutant. (F) Ponceau-stained protein profile of membranes expressing wild-type CTS and S810N mutant, and corresponding immunoblot (α-CTS) stained with anti-CTS antibody.
A mutant, ped3-4, has been described, which is a cts missense mutation, S810N. In planta this mutant germinates but cannot break down fatty acids and establish (15, 16) and it failed to complement the S. cerevisiae pxa1/pxa2Δ mutant for oleate β-oxidation (Fig. 1A). Membranes from insect cells expressing CTSS810N exhibited approximately 70% of WT CTS-dependent ATPase activity but only 40% thioesterase activity (Fig. 3 D and E). No thioesterase activity was detected in the absence of ATP, indicating that this activity retained nucleotide sensitivity, similar to the wild type (Fig. 3D).
Discussion
Previous studies have provided indirect evidence in favor of both acyl-CoAs and free fatty acids as transport substrates of ABCD proteins. It was proposed that the solution to this paradox is that the CoA moiety is cleaved during transport (11), and indirect evidence based on isotope labeling patterns of yeast cells is consistent with this (13). The current study provides direct evidence that CTS has an ATP-stimulated thioesterase activity although the protein has no obvious homology with known thioesterases. Previously described acyl CoA thioesterases are not stimulated by ATP, thus this appears to be a unique type of activity. By analogy with thioesterases of known structure and mechanism, catalytic residues could potentially include either conserved acidic, hydroxyl, histidine, and amide side chains, as in the “hot-dog fold” thioesterases, or the triad of a nucleophilic residue, histidine and acidic residue found in α/β hydrolase-type thioesterases (20). Members of the clade of plant, yeast, and metazoan ABCD transporters known to be involved in peroxisomal fatty acid import (21) contain highly conserved residues of these types at a number of positions. In human ALDP some of these have been implicated in X-linked adrenoleukodystrophy, attesting to their functional importance. Future work will systematically explore the role of these residues in CTS, in the context of our structural model of a lumen-facing conformation of the protein (16).
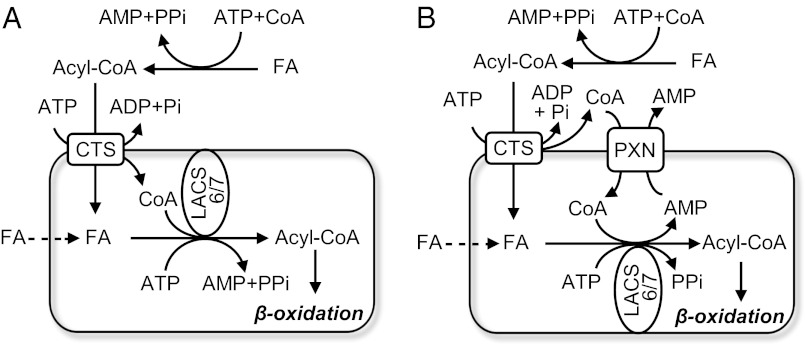
A functional and physical interaction between the peroxisomal ABC transporter CTS and the peroxisomal acyl-CoA synthetases LACS6 and LACS7 could also be demonstrated. Thus, CTS (and probably the other peroxisomal ABCD transporters) cleaves acyl-CoA as part of the transport cycle, with the free fatty acid requiring subsequent reactivation by peroxisomal acyl-CoA synthetases (Fig. 4). Vectorial acylation is a common feature of fatty acid transport across membranes. In mammals, fatty acids are transported into mitochondria by conversion to acyl carnitine by carnitine acyl transferases and reesterification to CoA in the matrix; thus, the mitochondrion maintains a separate CoA pool from the cytosol. Uptake of fatty acids into yeast cells and adipocytes requires cooperation of a long chain acyl CoA synthetase and a fatty acyl transport protein (FATP) that may itself also possess acyl CoA synthetase activity (22). Recently S. cerevisiae Fat1p was shown to be dually targeted to both the plasma membrane and the peroxisomal membrane, and to form a functional interaction with Pxa1p/Pxa2p, which is required for the stability of the former (13). The activity of Fat1p explains the ability of faa2Δ cells to metabolize long chain fatty acid (LCFA) in the presence of Pxa1p/Pxa2p (Fig. 1B). CTS cannot substitute for Pxa1p/Pxa2p when Faa2p is also absent, suggesting that it is unable to interact functionally with Fat1p (Fig. 1B). HsABCD1 and HsABCD2 are similarly unable to rescue the pxa1/pxa2/faa2 triple mutant (13).
Fig. 4.
Models showing role of fatty acyl-CoA cleavage in peroxisomal import of substrates for β-oxidation in Arabidopsis. Fatty acids (FAs) are activated to CoA esters extraperoxisomally by as-yet unidentified acyl-CoA synthetases. The ABC transporter, CTS, binds fatty acyl-CoAs on the cytosolic side of the peroxisomal membrane, the CoA moiety is cleaved by CTS, and free FAs are imported into the peroxisome. Transport is energized by ATP hydrolysis. In model A, CoA is released in the peroxisome lumen, where it is reesterified to acyl-CoAs by the action of peroxisomal acyl-CoA synthetases LACS6 and LACS7. FA-CoAs then enter the β-oxidation pathway. The peroxisomal ATP pool is supplied by peroxisomal adenine nucleotide translocators, PNC1 and PNC2 (not shown for clarity). Alternatively, in model B, CoA is released on the cytosolic side of the peroxisomal membrane and must enter peroxisomes via the PXN carrier, probably in exchange for AMP. In both cases, a proportion of FA may cross the peroxisomal membrane by a CTS-independent route and is activated by LACS6/7 (indicated by dashed line).
The S810N mutant has markedly reduced thioesterase activity and is unable to support oleate β-oxidation when expressed in the S. cerevisiae pxa1/pxa2Δ mutant, demonstrating that the thioesterase activity is important for biological function in vivo. Ser810 is predicted to be located in the second transmembrane (TM) helix of the second TM domain (i.e., TM helix 8 of the complete CTS molecule, which is a fused heterodimer) and the equivalent residue in ALDP (Ala141) is mutated in some X-ALD patients (www.x-ald.nl), supporting its functional importance. In plants, the S810N mutant (originally described as ped3-4; ref. 15) germinates slowly but cannot degrade its oil bodies or establish (15, 16). The germination defect of mutants with a severe block in β-oxidation has been ascribed to an inability to metabolize the jasmonic acid precursor, 12-oxophytodienoic acid (OPDA) (23), a plant hormone, which is thought to be transported into the peroxisome by CTS for β-oxidation (24). It is possible that cleavage of activated OPDA is less severely affected by the S810N mutation compared with fatty acyl-CoAs and that the residual thioesterase activity in ped3-4 may be sufficient to reduce OPDA concentrations below the physiological threshold for germination, or alternatively that free OPDA can be accepted by the transporter.
CoA cleavage by CTS might appear to be an energetically wasteful process, because two additional high energy phosphate bonds are expended for every fatty acid converted to a CoA thioester following import into the peroxisome. However, energy expenditure is frequently used as a means of regulation or control, e.g., GTP-dependent proof reading in protein synthesis. Which side of the membrane CoA is released is unknown but has important implications for the CoA budget. Release on the cytosolic side would prevent the peroxisome acting as a sink for CoA, especially during periods of high flux through β-oxidation, but would imply an independent uptake route for this cofactor (Fig. 4). The Arabidopsis peroxisome membrane protein PXN was originally identified as an NAD+ transporter (25) but has recently been shown to be able to transport CoA (26). Surprisingly, pxn knockout mutants were still able to degrade fatty acids, albeit at a reduced rate (25), suggesting the existence of more than one route for CoA import. Thus, it is possible that the thioesterase activity of CTS releases the CoA moiety on the luminal side of the peroxisome membrane (Fig. 4). This scenario implies that a mechanism for CoA removal exists, such as degradation via peroxisomal nudix hydrolases (27).
Sucrose gradients and native gels revealed that the majority of CTS resides in a complex of >700 kDa. Because of the effect of the detergent on protein migration it is impossible to size accurately but is indicative of an oligomer of three or more CTS molecules. This is consistent with other ABC proteins including PMP70 and ALDP, which form homooligomers (28). A subpopulation of LACS and ACX1 proteins was associated with the CTS complex, which is in accordance with the previously reported membrane association of LACS6/7 (19). Although the functional significance of a physical interaction between ALDP and very long chain acyl-CoA synthetase has been disputed (1, 4), here we provide strong evidence for a physical and functional interaction between CTS and LACS6/7 in Arabidopsis. Because CTS, as a broad specificity transporter, appears to be a gateway through which the bulk of β-oxidation substrates must pass (1, 6), the cleavage and reesterification of transported molecules could allow the channelling or prioritizing of substrates for metabolism via induction, activation, or even specific recruitment of distinct acyl-CoA synthetases. Plants, in particular, contain a large family of acyl-activating enzymes, several of which are targeted to the peroxisome (29).
There are a number of examples of transporters that interact functionally with enzymes to create membrane transport metabolons involved in substrate channelling and metabolic regulation (30) and several transporters, particularly in prokaryotes, have acquired related secondary enzyme activities by fusion of additional domains (31). However, CTS, and perhaps the other ABCD proteins, appears to be a rare example of a polytopic membrane transporter with an intrinsic additional enzymatic function that controls entry of diverse substrates to a metabolic pathway.
Methods
Plasmids.
Details of plasmid construction, mutagenesis, and primers are given in SI Methods and Table S1.
Yeast Expression.
Yeast strains are given in Table S2. Yeast growth, β-oxidation, subcellular fractionation, and assays for 3-hydroxy-CoA dehydrogenase and acyl-CoA synthetase activity were performed as described (6, 13) and in SI Methods.
Plant Membrane Extraction.
Arabidopsis suspension culture cells were collected by centrifugation (1,000 × g, 15 min), washed twice in cold PBS and once in extraction buffer (EB) [50 mM Tris⋅HCl pH 8.2, 20% (vol/vol) glycerol, 2 mM EDTA, 1 mM PMSF, 5% (wt/vol) plant protease inhibitor; Sigma]. The cell pellet was ground in liquid N2 and the frozen powder resuspended on ice at 1 mg/mLin EB. Following filtration through Miracloth, the extract was clarified by centrifugation (3,000 × g, 5 min at 4 °C) and membranes were collected by centrifugation at 25,000 × g for 20 min at 4 °C. The pellet was resuspended in EB.
Two-Dimensional Gels.
Membranes were resuspended at 1 mg/mL in solubilization buffer [50 mM Tris⋅HCl pH 8.2, 200 mM NaCl, 20% (vol/vol) glycerol, 1.5 mM MgCl2, 3% (vol/vol) DDM, 5% (wt/vol) plant protease inhibitor (Sigma), 2 mg/mL Pefabloc] and incubated on ice for 1 h. Insoluble material was removed by centrifugation at 100,000 × g for 30 min. Solubilized material was loaded on a 4–16% (wt/vol) Blue Native gel (Invitrogen) following manufacturer's recommendations. A single lane was applied to a 10% (wt/vol) SDS/PAGE gel and sealed by addition of 6% (wt/vol) acrylamide mix. Following electrophoresis, proteins were transferred to PVDF and detected with either CTS or GFP antibody as described in ref. 6.
Sucrose Gradients.
Solubilized membrane proteins (200 μL) were layered onto a 25–60% (wt/vol) sucrose step gradient (step volume of 250 μL; step increment, 5%). Gradients were centrifuged overnight at 214,000 × g at 4 °C. Fractions (180 μL) were collected and precipitated with 17% (wt/vol) TCA for 20 min on ice. The precipitated proteins were collected by centrifugation at 17,000 × g for 30 min at 4 °C. Pellets were resuspended in 25 μL 1 M Tris⋅HCl, pH 9.4. Following immunoblotting for CTS, membranes were stripped and probed with antisera raised to LACS6/7 (19), ACX1 (32), thiolase (33), and MFP2 (34). The absence of any residual signal was confirmed followed each stripping step.
Immunoprecipitation.
Solubilized membrane proteins were diluted at 1 mg/mL in IP buffer [10 mM Tris⋅HCl pH 7.4, 150 mM NaCl, 0.3% (wt/vol) DDM] and incubated with preequilibrated protein A beads (Thermo Scientific Pierce) for 1 h at 4 °C. The beads were removed by centrifugation for 1 min at 1,000 × g and the supernatant was diluted twice in IP buffer. A total of 10 μL of affinity-purified CTS antibody was added and the mix was incubated at 4 °C overnight. A total of 50 μL of protein A beads was then added and incubated for a further 3 h at 4 °C. The beads were pelleted at 1,000 × g for 1 min and washed four times with IP buffer. The immunoprecipitated proteins were eluted with SDS PAGE sample buffer.
Insect Cell Expression.
Recombinant bacmids encoding CTS or its S810N mutant were prepared using the Bac-to-Bac system (Invitrogen), according to the manufacturer’s instructions. Sf9 insect cells (Spodoptera frugiperda) were infected with virus (multiplicity of infection = 3) and cultured with serum-free SF900-SFM medium (Invitrogen) in a shaking incubator at 100 rpm, 28 °C for 72 h. The cells were collected by centrifugation at 1,000 × g for 10 min and incubated in a hypoosmotic buffer [5 mM Tris⋅HCl pH 7.4, containing Mini complete protease inhibitor-EDTA (Roche)] for 15 min at 4 °C. A total of 3 mM DTT was then added and cells were further disrupted using 30 strokes of a tight-fitting Dounce homogenizer. The extract was diluted in homogenization buffer (5 mM Tris⋅HCl pH 7.4, 0.5 mM MgCl2, 5 mM EGTA, 1 mM PMSF) and centrifuged at 2,000 × g for 20 min at 4 °C. Membranes were pelleted at 100,000 × g for 1 h at 4 °C. The pellet was then resuspended in resuspension buffer [20 mM Tris pH 7.4, 200 mM NaCl, 1.5 mM MgCl2, 20% (vol/vol) glycerol; 2 mM DTT, plant protease inhibitor mix (Sigma), 2 mg/mL Pefabloc], homogenized in a small Dounce homogenizer, and aliquots were flash frozen in liquid nitrogen before storing at −80 °C. CTS expression levels were estimated by quantitative immunoblotting.
ATPase Assays.
ATPase activity was assayed according to ref. 35. Briefly, insect cell membranes expressing wild-type CTS or S810N mutant were mixed with buffer containing 10 mM Tris⋅HCl pH 7.4, 0.1 mM EGTA, 1 mM DTT, 15 mM MgSO4, 2 mM ouabain and 10 mM sodium azide (final concentrations) in the presence of 10 mM AlFx when indicated and incubated for 3 min at 37 °C (total volume, 50 μL.). The reaction was started by the addition of ATP and incubated for 30 min at 37 °C.
Thioesterase Assays.
Acyl-CoA thioesterase activity of CTS-expressing insect cell membranes was measured spectrophotometrically using 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB). Membranes were mixed at a final concentration of 0.3 mg/mL with 200 μM DTNB, 4 μM BSA, 10 mM ATP in 50 mM potassium phosphate buffer, pH 8.0 in the presence of various concentrations of acyl-CoAs (Avanti Polar Lipids). The reaction was started by the addition of membranes and specific activities were determined by measuring the change in absorbance at 412 nm for 10 min. Σ = 17,780 M−1⋅cm−1.
Supplementary Material
Acknowledgments
We thank Ian Graham, Greg Howe, Luigi DeBellis, and Doug Muench for antibodies; Amelia Lesiuk and Vincent Agboh for assistance with insect cell culture; and Tom Lanyon-Hogg for advice on thioesterase assays. This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) [Grants BB/F007108/1 (to F.L.T.) and BB/F007299/1 (to A.B. and S.A.B.)]. Rothamsted Research receives grant-aided support from the BBSRC of the United Kingdom.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218034110/-/DCSupplemental.
References
- 1.Theodoulou FL, Holdsworth M, Baker A. Peroxisomal ABC transporters. FEBS Lett. 2006;580(4):1139–1155. doi: 10.1016/j.febslet.2005.12.095. [DOI] [PubMed] [Google Scholar]
- 2.Kemp S, Theodoulou FL, Wanders RJ. Mammalian peroxisomal ABC transporters: From endogenous substrates to pathology and clinical significance. Br J Pharmacol. 2011;164(7):1753–1766. doi: 10.1111/j.1476-5381.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theodoulou FL, Eastmond PJ. Seed storage oil catabolism: A story of give and take. Curr Opin Plant Biol. 2012;15(3):322–328. doi: 10.1016/j.pbi.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Watkins PA, Ellis JM. Peroxisomal acyl-CoA synthetases. Biochim Biophys Acta. 2012;1822(9):1411–1420. doi: 10.1016/j.bbadis.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Footitt S, et al. Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 2002;21(12):2912–2922. doi: 10.1093/emboj/cdf300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyathi Y, et al. The Arabidopsis peroxisomal ABC transporter, comatose, complements the Saccharomyces cerevisiae pxa1 pxa2∆ mutant for metabolism of long-chain fatty acids and exhibits fatty acyl-CoA-stimulated ATPase activity. J Biol Chem. 2010;2285(39):29892–29902. doi: 10.1074/jbc.M110.151225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verleur N, Hettema EH, van Roermund CW, Tabak HF, Wanders RJ. Transport of activated fatty acids by the peroxisomal ATP-binding-cassette transporter Pxa2 in a semi-intact yeast cell system. Eur J Biochem. 1997;249(3):657–661. doi: 10.1111/j.1432-1033.1997.00657.x. [DOI] [PubMed] [Google Scholar]
- 8.Igoillo-Esteve M, Mazet M, Deumer G, Wallemacq P, Michels PA. Glycosomal ABC transporters of Trypanosoma brucei: Characterisation of their expression, topology and substrate specificity. Int J Parasitol. 2011;41(3–4):429–438. doi: 10.1016/j.ijpara.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 9.van Roermund CW, et al. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22(12):4201–4208. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- 10.van Roermund CW, Visser WF, Ijlst L, Waterham HR, Wanders RJ. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid β-oxidation. Biochim Biophys Acta. 2011;1811(3):148–152. doi: 10.1016/j.bbalip.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Fulda M, Schnurr J, Abbadi A, Heinz E, Browse J. Peroxisomal Acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell. 2004;16(2):394–405. doi: 10.1105/tpc.019646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linka N, et al. Peroxisomal ATP import is essential for seedling development in Arabidopsis thaliana. Plant Cell. 2008;20(12):3241–3257. doi: 10.1105/tpc.108.062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Roermund CW, et al. Peroxisomal fatty acid uptake mechanism in Saccharomyces cerevisiae. J Biol Chem. 2012;287(24):20144–20153. doi: 10.1074/jbc.M111.332833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkby B, Roman N, Kobe B, Kellie S, Forwood JK. Functional and structural properties of mammalian acyl-coenzyme A thioesterases. Prog Lipid Res. 2010;49(4):366–377. doi: 10.1016/j.plipres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, et al. Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid beta-oxidation. Plant Cell Physiol. 2002;43(1):1–11. doi: 10.1093/pcp/pcf023. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich D, et al. Mutations in the Arabidopsis peroxisomal ABC transporter COMATOSE allow differentiation between multiple functions in planta: Insights from an allelic series. Mol Biol Cell. 2009;20(1):530–543. doi: 10.1091/mbc.E08-07-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulda M, Shockey J, Werber M, Wolter FP, Heinz E. Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid beta-oxidation. Plant J. 2002;32(1):93–103. doi: 10.1046/j.1365-313x.2002.01405.x. [DOI] [PubMed] [Google Scholar]
- 18.Hettema EH, et al. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 1996;15(15):3813–3822. [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi H, et al. Molecular characterization of an Arabidopsis acyl-coenzyme a synthetase localized on glyoxysomal membranes. Plant Physiol. 2002;130(4):2019–2026. doi: 10.1104/pp.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantu DC, Chen Y, Reilly PJ. Thioesterases: A new perspective based on their primary and tertiary structures. Protein Sci. 2010;19(7):1281–1295. doi: 10.1002/pro.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyathi Y, et al. Pseudo half-molecules of the ABC transporter, COMATOSE, bind Pex19 and target to peroxisomes independently but are both required for activity. FEBS Lett. 2012;586(16):2280–2286. doi: 10.1016/j.febslet.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 22.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta. 2012;1821(5):852–857. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dave A, et al. 12-oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell. 2011;23(2):583–599. doi: 10.1105/tpc.110.081489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodoulou FL, et al. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 2005;137(3):835–840. doi: 10.1104/pp.105.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernhardt K, Wilkinson S, Weber AP, Linka N. A peroxisomal carrier delivers NAD+ and contributes to optimal fatty acid degradation during storage oil mobilization. Plant J. 2012;69(1):1–13. doi: 10.1111/j.1365-313X.2011.04775.x. [DOI] [PubMed] [Google Scholar]
- 26.Agrimi G, Russo A, Pierri CL, Palmieri F. The peroxisomal NAD+ carrier of Arabidopsis thaliana transports coenzyme A and its derivatives. J Bioenerg Biomembr. 2012;44(3):333–340. doi: 10.1007/s10863-012-9445-0. [DOI] [PubMed] [Google Scholar]
- 27.Antonenkov VD, Hiltunen JK. Transfer of metabolites across the peroxisomal membrane. Biochim Biophys Acta. 2012;1822(9):1374–1386. doi: 10.1016/j.bbadis.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Guimarães CP, et al. Mouse liver PMP70 and ALDP: Homomeric interactions prevail in vivo. Biochim Biophys Acta. 2004;1689(3):235–243. doi: 10.1016/j.bbadis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Shockey J, Browse J. Genome-level and biochemical diversity of the acyl-activating enzyme superfamily in plants. Plant J. 2011;66(1):143–160. doi: 10.1111/j.1365-313X.2011.04512.x. [DOI] [PubMed] [Google Scholar]
- 30.Moraes TF, Reithmeier RAF. Membrane transport metabolons. Biochim Biophys Acta. 2012;1818(11):2687–2706. doi: 10.1016/j.bbamem.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Reddy VS, Shlykov MA, Castillo R, Sun EI, Saier MH., Jr The major facilitator superfamily (MFS) revisited. FEBS J. 2012;279(11):2022–2035. doi: 10.1111/j.1742-4658.2012.08588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schilmiller AL, Koo AJK, Howe GA. Functional diversification of acyl-coenzyme A oxidases in jasmonic acid biosynthesis and action. Plant Physiol. 2007;143(2):812–824. doi: 10.1104/pp.106.092916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Germain V, et al. Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 2001;28(1):1–12. doi: 10.1046/j.1365-313x.2001.01095.x. [DOI] [PubMed] [Google Scholar]
- 34.Chuong SD, Mullen RT, Muench DG. Identification of a rice RNA- and microtubule-binding protein as the multifunctional protein, a peroxisomal enzyme involved in the beta -oxidation of fatty acids. J Biol Chem. 2002;277(4):2419–2429. doi: 10.1074/jbc.M109510200. [DOI] [PubMed] [Google Scholar]
- 35.Chifflet S, Torriglia A, Chiesa R, Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: Application to lens ATPases. Anal Biochem. 1988;168(1):1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.